Abstract
Hidradenitis suppurativa (HS) is a chronic autoinflammatory follicular disease, affecting body areas that are rich in apocrine glands. Moderate-to-severe HS may severely impair patients’ quality of life also because the available therapies are often unsatisfactory. Several lines of evidence suggest that inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-17 play a pivotal role in the physiopathology of HS. TNF-α inhibitors have long been used with benefit in moderate-severe forms of HS. However, several monoclonal antibodies against IL-17 isoforms are currently being investigated for HS. We report the case of a 50-year-old man with long-standing HS and concomitant palmo-plantar psoriasis treated with brodalumab after failure of various TNF-α inhibitors. The HS lesions and the patient’s quality of life improved steadily over time until week-136. Interestingly, the clinical benefit was confirmed by radiological improvement with MRI evaluation. Our case report demonstrates the long-term efficacy and safety of brodalumab in HS encouraging the use of drugs to inhibit the T helper-type 17 immune axis, especially in cases of HS refractory to therapy with TNF-α inhibitors.
Introduction
Hidradenitis suppurativa (HS) is a chronic autoinflammatory follicular disease, affecting body areas that are rich in apocrine glands. HS is clinically characterized by nodules, painful abscesses and draining sinuses with possible progression to scarring, leading to a significant disease burden with a large impact on physical and emotional aspects of patient’s life. Several lines of evidence suggest that inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin (IL)-17 play a pivotal role in the physiopathology of HS (Citation1, Citation2). TNF-α inhibitors have long been used with benefits in moderate-severe forms of HS (Citation3). However, several monoclonal antibodies against IL-17 isoforms are currently investigated for HS (Citation4–6).
We report the long-term maintenance of effectiveness and safety of brodalumab in a patient with concomitant HS and palmo-plantar psoriasis. Interestingly, the clinical benefit was confirmed by radiological improvement with MRI evaluation.
Case report
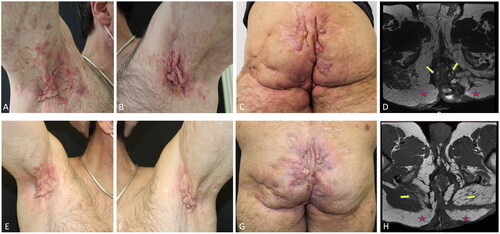
A 50-year-old man who has been affected by HS for more than 20 years was referred to our attention. He had no particular family or medical history, normal body weight (BMI ≅ 22 kg/m2) and was a moderate smoker (∼10 cigarettes per day). He also complained of a 15-year history of palmo-plantar psoriasis unresponsive to topical corticosteroids. On clinical examination, axillae, inguinal, perineal and buttock regions were affected by multiple painful abscesses, numerous erythematous-edematous subcutaneous nodules, confluent mesh-like scars and draining sinus tracts with a purulent discharge (). Fissured erythematous plaques were present on the palms and soles. The patient reported a pain Visual Analog Scale (VAS) of 10 and a Dermatology Life Quality Index (DLQI) of 28. HS was classified as Hurley stage III with 20 nodules, 6 abscesses and 6 draining sinus tracts with an International Hidradenitis Suppurativa Severity Score System (IHS4) of 56. The patient underwent a Magnetic Resonance Imaging (MRI) with contrast of the lower abdomen and pelvis that showed multiple fistulous tracts, abscesses and an important serum-hematical fluid collection inside the loco-regional tissues (). The patient had undergone topical/systemic antibiotics and multiple surgical interventions which resulted in limited and transitory benefit. Thereafter, the patient underwent several biologic therapies, including etanercept for 12 months, infliximab for 5 years, and adalimumab for 3 years, all with an initial but unsatisfactory improvement that was lost over time. Given the failure of many treatments and the coexistence of HS and psoriasis we started brodalumab in combination with acitretin. Brodalumab 210 mg was administered as subcutaneous injections at weeks 0, 1, and 2 followed by 210 mg every other week while oral acitretin 10 mg was given daily. Psoriatic lesions improved in a short time and acitretin was interrupted after 24 weeks, while HS skin manifestations gradually improved with a reduction in erythema, edema, and pus discharge in the affected regions. At week-12, the patient achieved the Hidradenitis Suppurativa Clinical Response (HiSCR) and an IHS4 of 20, reaching at week-24 an IHS4 of 15 and at week-48 an IHS4 of 10 with marked improvement in pain (VAS 3/10) and in quality of life (DLQI 8). The improvement was confirmed by a follow-up MRI without contrast of the lower abdomen and pelvis at week-96 showing muscular fibro-adipose involution associated with a significant reduction of the fluid component within the loco-regional tissues, compared to baseline (). At week-136 we observed complete remission of HS active manifestations in the axilla and a low disease activity in inguinal and perineal areas, obtaining an IHS4 of 5 (). Pain VAS was 1 and DLQI 4. Treatment was well tolerated, and the patient did not experience side effects.
Figure 1. HS manifestations of the Axillary and gluteal regions at baseline (A–C) and after 136 weeks of treatment with brodalumab (E–G). MRI of the lower abdomen and pelvis performed at baseline showed widespread morphologic and signal intensity alterations in the subcutaneous soft tissue, localized in the gluteal region bilaterally (yellow arrows). in addition, numerous abscesses mainly involved the gluteus maximus muscle (red stars) but smaller lesions also affected the intergluteal fold and the scrotal region, extending to the pararectal location (D). MRI of the lower abdomen and pelvis performed after 96 weeks demonstrated a reduction in the thickness of the subcutaneous adipose tissue that was associated with fibroadipose involution of the gluteus maximus muscle bilaterally (yellow arrows). fibrotic areas with sporadic abscesses and drainage tunnels are found in the adipose tissue of the ischioanal fossa (red stars). However, a significant reduction of the fluid component was recognized in the locoregional soft tissue compared to the previous examination (H).
Discussion
Hidradenitis suppurativa and psoriasis are both chronic inflammatory skin diseases that are clinically different but share several pathogenic features. TNF-α, IL-17 and IL-23 play a pivotal role in psoriasis and seem to be also relevant in HS (Citation1–4). IL-17A, IL-17C, IL-17F and IL-23 have been identified in the lesional tissue of patients with HS, therefore drugs aimed at inhibiting the T helper (Th) type 17 immune axis appear to be very promising for the treatment of HS (Citation5). Several monoclonal antibodies against IL-17 isoforms are currently investigated in clinical trials for HS (Citation5–9). Particularly, secukinumab and bimekizumab showed their efficacy in two double-blind, placebo-controlled, randomized trials (Citation6, Citation7).
Brodalumab, an IL-17RA antagonist with the ability to block IL-17A, IL-17C and IL-17F, represents a potentially advantageous drug compared to other agents that block only IL-17A or IL-17A/IL-17F (Citation9, Citation10).
Few real-life experiences of HS treated with brodalumab are reported in the literature showing encouraging results () (Citation10–15). In 2019, Tampouratzi et al. first described a patient with both HS and psoriasis vulgaris treated with brodalumab 210 mg every 2 weeks. At 16 weeks, the patient exhibited a significant improvement in both conditions, achieving an IHS4 of 3 and a PASI of 1.5, starting from baseline IHS4 and PASI scores of 10 and 18.5, respectively (Citation11). In 2020, Frew et al. demonstrated the clinical efficacy and safety of brodalumab in a cohort of 10 patients with moderate-to-severe HS (Citation10). The clinical response was rapid, and all patients achieved a HiSCR at weeks 12 and 24. No adverse events were reported, however in 2 out of 10 patients there was a recurrence of draining tunnels after the loading dose period. In 2021, the same authors evaluated brodalumab administration weekly in a cohort of 10 patients with moderate-to-severe HS, including 7 patients of the previous report. HiSCR was achieved at week 4 by the entire cohort of patients and no cyclical recurrences of draining tunnels and adverse events were documented during the 24 weeks of treatment (Citation12). Arenbergerova et al. described a patient with gluteal HS successfully treated with brodalumab every 2 weeks. The result in terms of effectiveness and improvement in the patient’s quality of life after only 12 weeks was impressive, with a reduction of the IHS4 score from 62 to 18 and of DLQI from 17 to 5 (Citation13). Additionally, Yoshida et al. described a patient with concomitant plaque psoriasis and HS treated with brodalumab 210 mg every 2 weeks. At 12 months, the patient achieved satisfactory improvement in psoriasis and HS. No adverse effects or recurrences were noted in the subsequent follow-up up to 1.5 years (Citation14). Kearney et al. recently reported 8 patients treated with brodalumab 210 mg every other week, who had failed at least one biologic. Seven patients reported a reduction in disease activity with mean DLQI variation from 20.6 to 16.8 at week 16. One patient experienced primary failure after 16 weeks and was started on infliximab therapy. Three patients experienced secondary failure and were switched to guselkumab (Citation15).
Table 1. Patients’ demographics, disease characteristics and brodalumab treatment outcome in all reported patients.
Herein, we report a very long maintenance of effectiveness and safety of brodalumab in a patient with concomitant HS and palmoplantar psoriasis. Interestingly, the clinical benefit was associated with a clear radiological improvement, emphasizing the importance of MRI in the follow-up of HS patients in selected cases. In fact MRI should not be considered as a routine imaging examination for all HS patients, but it should be performed in patients with severe disease involving deep areas of gluteal and perineal region. MRI improves diagnostic accuracy and assessment of therapeutic response in the aforementioned localizations since it may better define the characteristics of fistula tracts that are difficult to assess with clinical examination and ultrasonography.
In total, including our case, 25 HS patients successfully treated with brodalumab have been reported in the literature (). Although a precise definition of the follow-up period in the reported cases sometimes is lacking, our case seems characterized by a longer follow-up period. Although further studies will be needed to assess the long-term profile of brodalumab in HS patients, our positive experience encourages the potential therapeutic utility of blocking the IL-17 pathway in this disease.
Disclosure statement
M.C. Fargnoli has served on advisory boards, received honoraria for lectures and/or research grants from AMGEN, Almirall, Abbvie, Boehringer-Ingelheim, BMS, Galderma, Kyowa Kyrin, Leo Pharma, Pierre Fabre, UCB, Lilly, Pfizer, Janssen, MSD, Novartis, Sanofi-Regeneron, Sunpharma. M. Esposito has served as a speaker/board member for Abbvie, Almirall, Biogen, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis. M. Bruni has served as a speaker/board member for Abbvie, Almirall, Novartis. E. Vagnozzi has no conflicts of interests to declare.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Van der Zee HH, de Ruiter L, van den Broecke DG, et al. Elevated levels of tumour necrosis factor (TNF)-a, interleukin (IL)-1b and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-a and IL-1b. Br J Dermatol. 2011;164(6):1–4. doi:10.1111/j.1365-2133.2011.10254.x.
- Matusiak Ł, Szczęch J, Bieniek A, et al. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017;76(4):670–675. doi:10.1016/j.jaad.2016.10.042.
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375(5):422–434. Aug 4 PMID: 27518661. doi:10.1056/NEJMoa1504370.
- Frew JW, Marzano AV, Wolk K, et al. A systematic review of promising therapeutic targets in hidradenitis suppurativa: a critical evaluation of mechanistic and clinical relevance. J Invest Dermatol. 2021;141(2):316–324.e2. doi:10.1016/j.jid.2020.06.019.
- Navrazhina K, Frew JW, Grand D, et al. Interleukin-17RA blockade by brodalumab decreases inflammatory pathways in hidradenitis suppurativa skin and serum. Br J Dermatol. 2022;187(2):223–233. Aug Epub 2022 Jun 2. PMID: 35191018; PMCID: PMC9356983. doi:10.1111/bjd.21060.
- Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet. 2023;401(10378):747–761. Mar 4 doi:10.1016/S0140-6736(23)00022-3. Epub 2023 Feb 3. PMID: 36746171.
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, Placebo-Controlled randomized clinical trial. JAMA Dermatol. 2021;157(11):1279–1288. Nov 1 Erratum in: JAMA Dermatol. 2021 Nov 1;157(11):1384. PMID: 34406364; PMCID: PMC8374742 doi:10.1001/jamadermatol.2021.2905.
- Witte-Handel E, Wolk K, Tsaousi A, et al. The IL-1 pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol. 2019;139(6):1294–1305. doi:10.1016/j.jid.2018.11.018.
- Kashetsky N, Mufti A, Alabdulrazzaq S, et al. Treatment outcomes of IL-17 inhibitors in hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2022;26(1):79–86. Jan-Feb Epub 2021 Aug 8. PMID: 34365863. doi:10.1177/12034754211035667.
- Frew JW, Navrazhina K, Grand D, et al. The effect of subcutaneous brodalumab on clinical disease activity in hidradenitis suppurativa: an open-label cohort study. J Am Acad Dermatol. 2020;83(5):1341–1348. Nov Epub 2020 May 13. PMID: 32416208; PMCID: PMC7572493. doi:10.1016/j.jaad.2020.05.007.
- Tampouratzi E, Kanni T, Katsantonis J, et al. Case report: treating a co-existence of hidradenitis suppurativa and psoriasis with different therapeutic approaches. F1000Res. 2019;8:2002. Nov 26 PMID: 33456757; PMCID: PMC7791350. doi:10.12688/f1000research.21216.2.
- Frew JW, Navrazhina K, Sullivan-Whalen M, et al. Weekly administration of brodalumab in hidradenitis suppurativa: an open-label cohort study. Br J Dermatol. 2021;184(2):350–352. Feb Epub 2020 Sep 24. PMID: 32974912. doi:10.1111/bjd.19478.
- Arenbergerova M, Arenberger P, Marques E, et al. Successful treatment of recalcitrant gluteal hidradenitis suppurativa with brodalumab after anti-TNF failure. Int J Dermatol. 2020;59(6):733–735. Jun Epub 2020 Feb 3. PMID: 32012238. doi:10.1111/ijd.14792.
- Yoshida Y, Oyama N, Iino S, et al. Long-standing refractory hidradenitis suppurativa responded to a brodalumab monotherapy in a patient with psoriasis: a possible involvement of Th17 across the spectrum of both diseases. J Dermatol. 2021;48(6):916–920. doi:10.1111/1346-8138.15807.
- Kearney N, Hughes R, Kirby B. Treatment of hidradenitis suppurativa with brodalumab in biologic treatment failures - experiences from a specialty clinic. Clin Exp Dermatol. 2023;48(7):790–792. Apr 5:llad130. Epub ahead of print. PMID: 37017188. doi:10.1093/ced/llad130.

