Abstract
Background
Chronic pruritus is frequently seen in daily dermatological practice and is associated with marked impact on quality of life. Research on the use of gabapentin and oral antidepressants in daily dermatological practice is scarce.
Objective
To evaluate the efficacy and safety of gabapentin and oral antidepressants in patients with chronic pruritus in daily clinical practice.
Methods
A prospective observational single-center cohort study was conducted including adult patients with chronic pruritus and an indication for systemic treatment between June 2016 and May 2019.
Results
Systemic treatment with gabapentin and/or antidepressants was initiated in 31 patients with severe chronic pruritus (median average pruritus NRS score 7.0), in which most cases no underlying origin was identified (83.9%). In patients treated with gabapentin 900–1800 mg/day (N = 25), median average pruritus NRS decreased to 5.5 (IQR 3.0) after 4 weeks and remained stable up to 24 weeks of treatment. Efficacy of antidepressants was variable, with the highest response after initiation of amitriptyline, nortriptyline, and mirtazapine. Side effects were frequently observed in both gabapentin and antidepressant treatments; however, were mostly mild and temporary.
Limitations
This was a single-site observational study, with limited sample size.
Conclusion
Treatment with gabapentin and antidepressants should be considered in patients with chronic pruritus unresponsive to conventional treatment.
Introduction
Chronic pruritus is frequently seen in daily dermatological practice, and can be caused by various cutaneous, systemic, neurogenic and psychiatric disorders (Citation1–3). When no specific causal disease is identified, it is referred to as chronic pruritus of unknown origin (CPUO) (Citation4). Chronic pruritus is associated with a negative impact on quality of life, and can lead to sleep deprivation, anxiety and depression (Citation5,Citation6). Due to the heterogenic pathogenesis and lack of effective treatments, management of chronic pruritus can be a challenge for clinicians (Citation7,Citation8). According to the current guidelines for chronic pruritus, systemic treatment such as gabapentin and oral antidepressants should be considered in patients unresponsive to conventional treatment, such as topical treatment, antihistamines and phototherapy (Citation9,Citation10). However, evidence for both gabapentin and oral antidepressants in daily dermatological practice is scarce. Observational studies, case series and case reports mainly focused on pruritus due to a specific origin only, such as pruritus due to chronic kidney disease or paraneoplastic pruritus. To effectively educate patients in daily practice on treatment outcomes and side effects, more information on efficacy and safety of gabapentin and antidepressants in daily dermatological practice is needed. The aim of this study was to evaluate the efficacy and safety of gabapentin and oral antidepressants (Selective Serotonin Reuptake Inhibitors (SSRI’s), Tricyclic Antidepressants (TCA’s) and/or atypical antidepressants) in patients with chronic pruritus in daily clinical practice.
Methods
Study design and patients
A prospective observational single-center cohort study was conducted at the Department of Dermatology, Radboud university medical center, Nijmegen, the Netherlands between June 2016 and May 2019. Adult patients (aged ≥ 18 years) with chronic pruritus (defined as pruritus present for a minimum of 6 weeks) that could not be resolved by treatment of an underlying cause, and/or were unresponsive to first-line therapies for chronic pruritus, were eligible for inclusion. Patient and clinical characteristics were organized using CASTOR Electronic Data Capture, a secure web-based data management application in compliance with Good Clinical Practice (GCP) standards. Ethical approval was waived by the medical ethical committee Arnhem-Nijmegen (2016–2606). All participants provided written informed consent.
Procedures
Patients were treated with either gabapentin or oral antidepressants (SSRIs, TCAs, or mirtazapine), depending on both patient and physician preference. As this is an observational study in daily practice, patients were allowed to use concomitant topical treatment. At baseline, the following patient and clinical characteristics were collected: age, gender, ethnicity, skin type, atopic diathesis (defined as presence of atopic dermatitis, allergic asthma, or allergic rhinitis), presence of co-morbidities and concomitant medications, duration of pruritus, localization of pruritus, sleep deprivation caused by pruritus, and therapeutic history. Visits were scheduled after 4, 12 and 24 weeks, or more when needed. At each visit, pruritus intensity and adverse events were documented. Pruritus intensity was measured using 11-point numeric rating scales (NRS) for average and peak pruritus over the previous 24 h. In addition, the Dermatology Life Quality Index (DLQI) and the Hospital Anxiety and Depression Scale (HADS) were recorded to quantify the impact of chronic pruritus.
Analysis
Demographic data of study patients were displayed as medians and interquartile ranges (IQRs) for continuous variables, and numbers and percentages for categorical variables (No (%)). If patients were treated with both gabapentin and (different) antidepressants, they were considered two individual subjects. Multiple occurrences of the same adverse event in a single subject were counted once.
Results
From June 2016 to November 2018, 40 patients were eligible for inclusion in this study. A total of nine patients were excluded as systemic treatment could not be initiated because of patient’s decisions (e.g., scared of side effects) (N = 5), co-morbidities (N = 2) or lost to follow-up (N = 2). Systemic treatment was initiated in 31 patients (74.2% male), with a median age of 66.0 years (IQR 22.0), and with a median duration of pruritus at baseline of 2 years (IQR 9.0). Four patients (12.9%) had a history of chronic pruritus due to dermatological conditions that could not be resolved by conventional treatment options: atopic dermatitis (N = 2), red scrotum syndrome (N = 1), and lichen sclerosus (N = 1). In addition, one patient (3.2%) had chronic pruritus due to notalgia paresthetica . In the remaining 26 patients (83.9%), no underlying origin of pruritus was identified (CPUO). None of the included patients had serum creatinine, aspartate aminotransferase, or alanine aminotransferase levels higher than twice the upper limit of normal. Patients were previously treated with topical therapy (e.g., topical corticosteroids, menthol, and capsaicin) (100%), antihistamines (87.1%), phototherapy (48.4%) and/or oral corticosteroids (25.8%). At baseline, median average pruritus NRS was 7.0 (IQR 2.0), median peak pruritus NRS was 8.0 (IQR 1.0), median DLQI score was 13.0 (IQR 12.0), and median HADS score was 14.0 (IQR 17.0). All baseline characteristics are shown in .
Table 1. Demographic and clinical characteristics of patients treated with systemic medication (N = 31) at baseline.
Gabapentin
Gabapentin was initiated in 25 patients, with a median age of 64.0 (IQR 24.0) years and a median duration of pruritus of 2.0 (IQR 9.5) years (Supplemental Figure 1). Gabapentin was initiated at a dose of 300 mg/day, which was increased up to a maximum of 1800 mg/day in three divided doses. Gabapentin dosage was adjusted in patients with mild renal insufficiency. At baseline, median average pruritus NRS was 7.0 (IQR 2.0), median Peak Pruritus NRS (PP-NRS) was 8.0 (IQR 1.0) and median DLQI score was 13.0 (IQR 10.0). After 4 weeks of treatment, median average pruritus NRS decreased to 5.5 (IQR 3.0), PP-NRS to 7.0 (IQR 5.0) and DLQI to 6.0 (IQR 8.0) (, Supplemental Table I). In 5 patients, a ≥ 4-point reduction in PP-NRS was observed. Efficacy of gabapentin remained stable up to 24 weeks of treatment, with median average pruritus NRS of 4.5 (IQR 5.0) after 12 weeks and 5.0 (IQR 4.0) after 24 weeks. Median DLQI scores slightly increased up to 9.5 (IQR 12.0) after 12 weeks en 10.5 (IQR 11.0) after 24 weeks. We observed no improvement in HADS at 4, 12, and 24 weeks compared to baseline.
Figure 1. Effectiveness outcomes during 24 weeks of gabapentin treatment. (A) Average pruritus NRS and Peak Pruritus NRS; (B). DLQI.
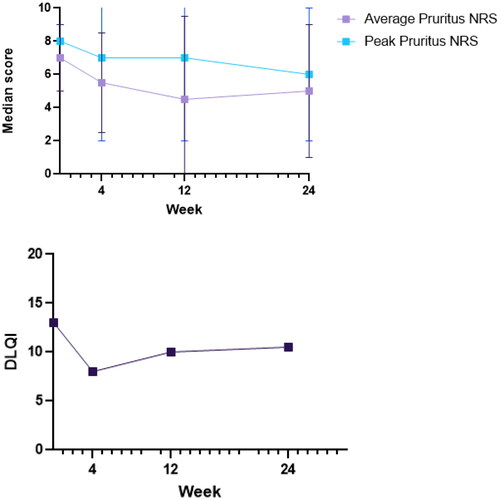
Side effects of gabapentin were experienced by 22 of 25 patients (88.0%). The most common side effects were vertigo and drowsiness (both reported by 7 out of 25 patients), followed by fatigue (4/25), headache (3/25), nausea (2/25), gastro-intestinal pain (2/25) and weight gain (2/25). Most adverse events were mild, and mostly present in the first few days of treatment. A total of 16 patients (64.0%) discontinued treatment during follow-up, due to side effects (N = 6) or inefficacy (N = 10). Another 2 patients were lost to follow-up.
Antidepressants
In 19 patients with chronic pruritus (62.5% male, median age 66.0 (IQR 19.0) years), treatment with oral antidepressants was initiated. Patients were treated with SSRIs (N = 5), TCAs (N = 10) or atypical antidepressants (N = 6). In five patients, treatment with another type of antidepressant was initiated following discontinuation of their first treatment option due to side effects or inefficacy (e.g., SSRI followed by TCA treatment). At baseline, median average pruritus NRS score was 8.0 (IQR 2.0), median maximum pruritus NRS score was 8.0 (IQR 2.0) and median DLQI score was 14.0 (IQR 11.0).
SSRI treatment with paroxetine was initiated in five patients, all with CPUO. Paroxetine was initiated at 10 mg/day, and increased up to a maximum of 50 mg/day. All patients discontinued treatment during follow-up after a median of 10 weeks (range 3–18 weeks). Treatment was discontinued due to inefficacy (N = 3) or side effects (N = 2). Only one patient experienced a decrease in itch symptoms, with a 2-point decrease in average NRS pruritus after 4 weeks, and another 2-point decrease at week 12, however, was discontinued because of side effects. Reported side effects included fatigue (N = 2), drowsiness (N = 1), headache (N = 1), sexual dysfunction (N = 1), agitation (N = 1), nausea (N = 1), diarrhea (N = 1) and gastro-intestinal pain (N = 1). Two patients experienced symptoms associated with antidepressant discontinuation syndrome, including nausea, headache, and electric shock-like sensations.
TCA treatment was initiated in 10 patients, including amitriptyline (N = 3), nortriptyline (N = 3) and doxepin (N = 4). Patients had an underlying dermatologic origin of pruritus (N = 2), notalgia paresthethica (N = 1), or CPUO (N = 7). Amitriptyline and nortriptyline were initiated at 10 or 25 mg/day in 6 patients, and increased up to a maximum of 100 mg/day. Amitriptyline was initiated in 3 patients, with a slight decrease in average pruritus NRS (2-point reduction), after 2 weeks of treatment. The remaining 2 patients discontinued treatment within 2 weeks due to side effects. Nortriptyline was initiated in 3 patients, including 2-point reduction in average pruritus NRS in 2 patients and 4-point reduction in 1 patient after 12 weeks of treatment. However, all patients experienced side effects including xerostomia (N = 3), headache (N = 2) and hyperhidrosis (N = 2). Doxepin was initiated in 4 patients at 10 mg/day and increased up to 25 mg/day. In 2 patients, doxepin was not successful in reduction of pruritus, with stable or slight increase in average and peak pruritus NRS. The other 2 patients did not make week 4 analysis because of discontinuation due to side effects (xerostomia and sleep impairment).
Mirtazapine, an atypical antidepressant, was initiated in 6 patients, starting at 7.5 or 15 mg/day, and increased up to a maximum of 30 mg/day. In one patient, average pruritus NRS decreased over 50%, and this effect remained stable up to 24 weeks of treatment. In 3 other patients, pruritus improved during the first 4–8 weeks with a 2-point (N = 2) or 1-point (N = 1) reduction in average pruritus NRS, however, complaints of itch increased during follow-up. In the remaining patient, mirtazapine did not reduce average pruritus or PP-NRS. Patients reported drowsiness (N = 3), fatigue (N = 2), headache (N = 2), and xerostomia (N = 2) most frequently as side effects following mirtazapine treatment.
In five patients in which treatment with antidepressants was discontinued due to side effects (N = 2) or inefficacy (N = 3), a different type of antidepressants was initiated. This was only successful in one case, in which a patient responded to nortriptyline (4-point reduction in average pruritus NRS) after previous treatment failure on paroxetine.
Discussion
This single-center, prospective observational study focused on efficacy and safety of systemic treatment in chronic pruritus in daily dermatological practice. Among most patients included in our cohort, no underlying origin of pruritus was identified (83.9%), and consisted of patients with severe pruritus (median pruritus NRS at baseline 7.0) with significant impact on quality of life (median DLQI at baseline 13.0) (Citation11). Symptoms of pruritus and dermatological related quality of life (DLQI) improved after initiation of gabapentin treatment (900-1800mg/day), with a ≥ 2 point reduction in average pruritus in 11 out of 25 treated patients (44%). In addition, in 5 out of 25 patients (20%) PP-NRS was reduced by ≥4 points, which is considered clinically meaningful (Citation12,Citation13). The main improvement of pruritus was observed in the first 4 weeks, and remained stable up to 24 weeks of treatment. Therefore, a treatment duration of 4 weeks could potentially serve as a minimum period for assessing the response to gabapentin therapy, which is consistent with findings in a previous observational study evaluating pregabalin in patients with chronic pruritus (Citation14). The effect of antidepressants on chronic pruritus was variable, with the highest response after initiation of amitriptyline and nortriptyline (≥2 point reduction in average pruritus NRS in 4/6 patients at week 4) or mirtazapine (≥2 point reduction in average pruritus NRS in 3/6 patients at week 4). Even though there is evidence available for efficacy of paroxetine treatment in chronic pruritus due to various origins (Citation15), we only observed improvement of pruritus in 1 out of 5 patients. Although the effect on pruritus after initiation of gabapentin or antidepressants may appear modest, it can be of meaningful significance at the individual patient level, and therefore both gabapentin and antidepressants should be considered as a therapeutic option in chronic pruritus unresponsive to first-line treatment options.
However, side effects were common after initiation of both gabapentin and antidepressants, resulting in considerable rates of discontinuation. After initiation of gabapentin, most adverse events were mild and particularly present in the first few days of treatment, with vertigo and drowsiness (both reported by 7 out of 25 patients) reported most frequently. Even though previous clinical trials for gabapentin in neuropathic pain did not find a higher incidence of these adverse reactions in older adults compared to a younger population (Citation16), clinicians should be careful in (older) patients with preexisting dizziness or somnolence, with adjusted dosing, patients instructions and close follow-up.
In a previous study, it was found that CPUO patients with increased eosinophil counts (defined as an absolute eosinophil count greater than 0.30 K/mm3) might be less likely to benefit from gabapentin treatment (Citation17). Therefore, we checked our cohort in retrospect on presence of eosinophilia in all patients treated with gabapentin (data available in 19 out of total of 25 patients). However, we found a higher percentage of patients with eosinophilia in patients with a treatment response (defined as ≥2 point reduction in average pruritus) compared to all patients treated with gabapentin, and could not confirm the previous observation of eosinophilia as a possible predictor of treatment success.
As we conducted an observational study in daily clinical practice, our study lacked a control group or parallel treatment group to compare. In addition, our study was limited by a small sample size and high level of discontinuation, which is also a reflection of daily clinical practice. More research in randomized controlled trials is needed to further investigate efficacy of both treatment groups.
To conclude, due to its heterogenic origin, complex pathogenesis and limited therapeutic options with current no registered treatment available, treatment of pruritus remains a challenge, especially in chronic and/or severe cases. In this prospective study in daily clinical practice, we aimed to provide information on efficacy and side effects of gabapentin and antidepressants in this population, to inform patients more adequately. Even though there was modest efficacy after initiation of gabapentin and antidepressants, this can be of clinical relevance to individual patients, and therefore gabapentin and antidepressants should be considered as a treatment option in patients with chronic pruritus.
Supplemental Material
Download Zip (198.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med. 2013;368(17):1–4. doi:10.1056/NEJMcp1208814.
- Kopyciok ME, Stander HF, Osada N, et al. Prevalence and characteristics of pruritus: a one-week cross-sectional study in a German dermatology practice. Acta Derm Venereol. 2016;96(1):50–55. doi:10.2340/00015555-2166.
- Weisshaar E, Szepietowski JC, Darsow U, et al. European guideline on chronic pruritus. Acta Derm Venereol. 2012;92(5):563–581. doi:10.2340/00015555-1400.
- Kim BS, Berger TG, Yosipovitch G. Chronic pruritus of unknown origin (CPUO): uniform nomenclature and diagnosis as a pathway to standardized understanding and treatment. J Am Acad Dermatol. 2019;81(5):1223–1224. doi:10.1016/j.jaad.2019.06.038.
- Halvorsen JA, Dalgard F, Thoresen M, et al. Itch and mental distress: a cross-sectional study among late adolescents. Acta Derm Venereol. 2009;89(1):39–44. doi:10.2340/00015555-0554.
- Dalgard F, Lien L, Dalen I. Itch in the community: associations with psychosocial factors among adults. J Eur Acad Dermatol Venereol. 2007;21(9):1215–1219. doi:10.1111/j.1468-3083.2007.02234.x.
- Pereira MP, Stander S. Chronic pruritus: current and emerging treatment options. Drugs. 2017;77(9):999–1007. doi:10.1007/s40265-017-0746-9.
- Stander S, Pogatzki-Zahn E, Stumpf A, et al. Facing the challenges of chronic pruritus: a report from a multi-disciplinary medical itch Centre in Germany. Acta Derm Venereol. 2015;95(3):266–271. doi:10.2340/00015555-1949.
- Weisshaar E, Szepietowski JC, Dalgard FJ, et al. European S2k guideline on chronic pruritus. Acta Derm Venereol. 2019;99(5):469–506. doi:10.2340/00015555-3164.
- Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5(4):e42. doi:10.1097/itx.0000000000000042.
- Stander S, Augustin M, Reich A, et al. Pruritus assessment in clinical trials: consensus recommendations from the International Forum for the Study of Itch (IFSI) special interest group scoring itch in clinical trials. Acta Derm Venereol. 2013;93(5):509–514. doi:10.2340/00015555-1620.
- Riepe C, Osada N, Reich A, et al. Minimal clinically important difference in chronic pruritus appears to be dependent on baseline itch severity. Acta Derm Venereol. 2019;99(13):1288–1290. doi:10.2340/00015555-3332.
- Reich A, Riepe C, Anastasiadou Z, et al. Itch assessment with visual analogue scale and numerical rating scale: determination of minimal clinically important difference in chronic itch. Acta Derm Venereol. 2016;96(7):978–980. doi:10.2340/00015555-2433.
- Park JM, Jwa SW, Song M, et al. Efficacy and safety of pregabalin for the treatment of chronic pruritus in Korea. J Dermatol. 2012;39(9):790–791. doi:10.1111/j.1346-8138.2012.01572.x.
- Kouwenhoven TA, van de Kerkhof PCM, Kamsteeg M. Use of oral antidepressants in patients with chronic pruritus: a systematic review. J Am Acad Dermatol. 2017;77(6):1068–1073 e7. doi:10.1016/j.jaad.2017.08.025.
- Sreekantaswamy SA, Mollanazar N, Butler DC. Gabapentinoids for pruritus in older adults: a narrative review. Dermatol Ther (Heidelb). 2021;11(3):669–679. doi:10.1007/s13555-021-00513-z.
- Roh YS, Khanna R, Patel SP, et al. Circulating blood eosinophils as a biomarker for variable clinical presentation and therapeutic response in patients with chronic pruritus of unknown origin. J Allergy Clin Immunol Pract. 2021;9(6):2513–2516 e2. doi:10.1016/j.jaip.2021.01.034.

