Abstract
Background
Atopic dermatitis (AD) is a chronic eczematous disease with severe pruritus. Janus kinase (JAK) inhibitors, upadacitinib, baricitinib, and abrocitinib, are systemic treatments for AD. The outcomes of switching from one JAK inhibitor to another have not been examined.
Objectives
We assessed the outcomes of switching from baricitinib 4 mg to upadacitinib 30 mg in Japanese patients with moderate-to-severe AD.
Methods
Twenty patients treated with baricitinib 4 mg, showing insufficient response or adverse events, were switched to treatment with upadacitinib 30 mg. We evaluated total eczema area and severity index (EASI), EASI at head and neck, trunk, upper, or lower limbs, EASI of erythema, edema/papulation, excoriation, or lichenification, and peak pruritus numerical-rating scale (PP-NRS) at baseline (start of baricitinib), weeks 0 (time of switching), and 4 and 12 after switching.
Results
Total EASI, EASI at each anatomical site, EASI of each clinical sign, and PP-NRS were markedly reduced at weeks 4 or 12 compared to week 0. Achievement rates of more than 75% or 90% reduction of EASI from baseline significantly improved after switching.
Conclusions
Switching from baricitinib 4 mg to upadacitinib 30 mg effectively improved rash and pruritus.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by eczema and pruritus (Citation1,Citation2).The pathogenesis of AD involves cytokines, interleukin (IL)-4, IL-13, IL-22, thymic stromal lymphopoietin (TSLP), or IL-31 that transduce intracellular signals through Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (Citation3–6). Currently, three oral JAK inhibitors, upadacitinib, baricitinib, and abrocitinib, are approved in Japan as systemic treatments for AD. While upadacitinib and abrocitinib are JAK1 inhibitors, baricitinib is a JAK1/2 inhibitor. In previous clinical trials, upadacitinib demonstrated impressive efficacy for rash and pruritus in moderate-to-severe AD (Citation7–10). Previously, we reported the effectiveness and safety of upadacitinib 15 mg and baricitinib 4 mg in Japanese patients with moderate-to-severe AD (Citation11–16). However, in clinical practice, some patients who do not sufficiently respond to baricitinib, for whom switching to another JAK inhibitor can be considered as an alternative treatment. To date, the outcomes of switching among JAK inhibitors have not been examined. Especially, the effectiveness of switching from baricitinib 4 mg to upadacitinib 30 mg has not been investigated in either clinical trials or real-world clinical practice. In this study, we examined the therapeutic effectiveness of switching from baricitinib 4 mg to upadacitinib 30 mg in Japanese patients with AD in real-world clinical practice. We selected 30 mg dose of upadacitinib, expecting improvement of quality of life (QOL) in association with therapeutic effectiveness; network meta-analysis for AD has demonstrated higher efficacy of upadacitinib 30 mg compared to 15 mg (Citation17,Citation18).
Methods
Study design and data collection
Twenty Japanese patients with moderate-to-severe AD (aged ≥ 15 years) treated in our department between August 2021 and April 2023 were enrolled. The diagnosis of AD was made clinically based on the Japanese Atopic Dermatitis Guidelines 2021 (Citation19). The patients had moderate-to-severe AD. These patients received oral once daily baricitinib 4 mg plus twice daily topical corticosteroids of moderate-to-strongest classes for median 17.1 weeks (ranging 12.9 to 62.4 weeks), and were switched to treatment with oral once daily upadacitinib 30 mg without altering topical treatment, due to the ineffectiveness or adverse events (AEs) by baricitinib. The patients fulfilled at least one of the following conditions: eczema area and severity index (EASI) ≥ 16 or EASI at head and neck ≥ 2.4; peak pruritus-numerical rating score (PP-NRS) > 4; impaired QOL (defined as an AD control tool [ADCT] score of 7 or higher); AEs that led to discontinuation of baricitinib treatment. All the patients expressed the intention to switch from baricitinib to a different treatment.
This study was conducted based on the Declaration of Helsinki (2004), and was approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital. Patients provided written informed consent. Total EASI, EASI scores at 4 anatomical sites, head and neck, upper limbs, lower limbs, or trunk, EASI scores of 4 clinical signs, erythema, edema/papulation, excoriation, or lichenification, and PP-NRS were analyzed at time of starting baricitinib treatment (baseline), time of switching (week 0), week 4, and 12 after switching. For each clinical sign on the EASI, the scores range from 0 to 3. Total eosinophil count (TEC), immunoglobulin E (IgE), thymus and activation-regulated chemokine (TARC), and lactate dehydrogenase (LDH) were measured at baseline, week 0, 4, and 12, and the values were statistically compared using their log-transformed values. The proportion of patients who achieved at least 75%, 90%, or 100% reduction of EASI from baseline (EASI 75, EASI 90, or EASI 100, respectively) was calculated at week 0, 4 and 12. The proportion of patients who achieved PP-NRS ≥ 4-point improvement (PP-NRS 4) was calculated at week 0, 4 and 12. The percent reductions from baseline in total EASI, EASI at four body sites, EASI of 4 clinical signs and PP-NRS were calculated at week 0, 4 and 12. In order to compare the treatment responses to upadacitinib 30 mg among different anatomical sites or among different clinical signs, we calculated the percent reductions from week 0 in EASI at 4 body sites or of 4 clinical signs at week 4 and 12.
Safety was assessed by the occurrence of treatment-emergent AEs (TEAEs) during baricitinib treatment and after switching to upadacitinib, until 30 days after the last dose of upadacitinib. A TEAE was defined as any AE that began or worsened after the initiation of treatment.
Patients with a history or at high risk of tuberculosis relapse, venous thromboembolism (VTE), major adverse cardiovascular events (MACE), and malignancies were excluded from this study. Moreover, this study did not include any patients aged ≧65 years.
Statistical analysis
Results are expressed as mean ± standard deviation for variables with a normal distribution, and median and interquartile range for variables with a non-parametric distribution. Differences in measurements among different points of time or different anatomical sites or different clinical signs were analyzed using Friedman’s test with Bonferroni post-hoc test. Differences in frequencies were analyzed by Fisher’s exact test. Statistical significance was set at p < 0.05. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical School).
Results
Patient demographics and baseline characteristics
The baseline characteristics of the patients are summarized in . The reasons for switching included high EASI in 6 patients (30%), high PP-NRS in 1 patient (5%), impaired QOL in 9 patients (45%), or AEs (renal impairment: estimated glomerular filtration rate < 60) in 4 patients (20%).
Table 1. Demographics and baseline characteristics of patients with atopic dermatitis (n = 20).
Improvement of total EASI and PP-NRS after switching from baricitinib to upadacitinib
Compared to baseline, total EASI () was partially but significantly reduced after treatment with baricitinib (week 0), and after switching to upadacitinib, further significantly reduced at week 4 and 12 compared to week 0. PP-NRS () partially but significantly reduced at week 0 compared to baseline, and further reduced at week 4 compared to week 0; PP-NRS at week 12 was significantly lower compared to baseline, but not significantly different from that at week 0. Total EASI and PP-NRS at week 12 were not significantly different from those at week 4.
Figure 1. The improvement of total EASI (a) and PP-NRS (b) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). (c) percent reductions of total EASI and PP-NRS from baseline. The data are shown as median [interquartile]. **p < 0.01 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.
![Figure 1. The improvement of total EASI (a) and PP-NRS (b) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). (c) percent reductions of total EASI and PP-NRS from baseline. The data are shown as median [interquartile]. **p < 0.01 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.](/cms/asset/11300065-b125-418b-8e4b-ad246b0c4cc1/ijdt_a_2276043_f0001_c.jpg)
After switching, the percent reductions from baseline in total EASI and PP-NRS significantly increased compared to week 0; median percent reduction of total EASI was 54.2%, 79.1%, 92.7%, and that of PP-NRS was 45%, 81.7%, 84.5%, at week 0, 4, 12, respectively (). Thus the reductions of total EASI and PP-NRS were improved after switching.
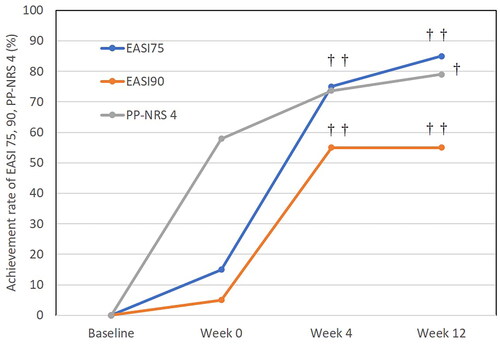
The achievement rates of EASI 75, EASI 90, and PP-NRS 4 after switching from baricitinib to upadacitinib
After switching, the achievement rates of total EASI 75 and EASI 90 significantly increased compared to week 0; that of EASI 75 was 15%, 75%, 85% () and that of EASI 90 was 5%, 55%, 55% () at week 0, 4, 12, respectively. After switching, the achievement rate of PP-NRS 4 significantly increased compared to week 0; 57.9%, 73.7%, 78.9% at week 0, 4, 12, respectively (). The difference between week 12 versus week 0 in achievement rate of PP-NRS 4 was significant while that between week 4 versus week 0 was not significant. Thus the achievement rate of EASI 90 appeared maximized at week 4 while those of EASI 75 and PP-NRS 4 continued to increase slightly until week 12.
Improvement of EASI at different anatomical sites after switching from baricitinib to upadacitinib
EASI at head and neck () did not reduce significantly at week 0 compared to baseline, while those at the other body sites (–d) significantly reduced at week 0 compared to baseline, indicating that head and neck rash might be rather resistant to baricitinib treatment relative to the other body sites. After switching to upadacitinib, EASI at four anatomical sites all significantly reduced at week 4 and 12 compared to week 0 and baseline (–d). The EASI scores at four anatomical sites at week 12 were not significantly different from those at week 4.
Figure 3. The improvement of EASI scores at head and neck (a), upper limbs (b), lower limbs (c), and trunk (d) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). the data are shown as median [interquartile range]. *p < 0.05, **p < 0.01 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.
![Figure 3. The improvement of EASI scores at head and neck (a), upper limbs (b), lower limbs (c), and trunk (d) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). the data are shown as median [interquartile range]. *p < 0.05, **p < 0.01 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.](/cms/asset/ab9441b9-9eea-4f03-8cc2-57cff81f48bd/ijdt_a_2276043_f0003_c.jpg)
Percent reductions from baseline in EASI at four anatomical sites all significantly increased at week 4 and 12 compared to week 0 ().
Figure 4. Percent reductions from baseline in EASI scores on head and neck, upper limbs, lower limbs, and trunk at week 0, 4 and 12 after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). data are presented as median [interquartile range]. †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.
![Figure 4. Percent reductions from baseline in EASI scores on head and neck, upper limbs, lower limbs, and trunk at week 0, 4 and 12 after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). data are presented as median [interquartile range]. †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.](/cms/asset/ad203c87-23a1-4c27-8abf-38561d6a770e/ijdt_a_2276043_f0004_c.jpg)
We then tried to know if the treatment responses to upadacitinib 30 mg might differ among different anatomical sites, by comparing the percent reductions from week 0 in EASI among different anatomical sites (Supplemental Figure S1). There were no significant differences among different anatomical sites at week 4 and 12; percent reduction of EASI at head and neck at week 4 appeared lower compared to the other body sites, however, the differences were not significant (p > 0.05, by Friedman’s test with Boferonni post-hoc test). The results indicate that upadacitinib might improve rash at all body sites similarly.
Improvement of EASI sign scores after switching from baricitinib to upadacitinib
The severity of erythema () and of edema/papulation () at week 0 appeared lower than those of baseline, however, the differences were not statistically significant (p > 0.05, by Friedman’s test with Boferonni post-hoc test), while severity of excoriation () and of lichenification () significantly reduced at week 0 compared to baseline, indicating that erythema and edema/papulation might be rather resistant to baricitinib treatment relative to excoriation and lichenification. After switching to upadacitinib, severity of erythema (), excoriation (), and lichenification () significantly reduced at week 4 and 12 compared to week 0 and baseline. Severity of edema/papulation () significantly reduced at week 4 and 12 compared to baseline, and the difference between week 12 versus week 0 was significant while that between week 4 versus week 0 was not significant. EASI scores of four signs at week 12 were not significantly different from those at week 4.
Figure 5. The improvement of EASI scores of erythema (a), edema/papulation (b), excoriation (c), or lichenification (d) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). the data are shown as median [interquartile range]. *, p < 0.05, **p < 0.01 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.
![Figure 5. The improvement of EASI scores of erythema (a), edema/papulation (b), excoriation (c), or lichenification (d) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). the data are shown as median [interquartile range]. *, p < 0.05, **p < 0.01 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0, by friedman’s test with Bonferroni post-hoc test.](/cms/asset/049544d1-35f6-48fe-8c69-a2908069565c/ijdt_a_2276043_f0005_c.jpg)
Figure 6. Percent reductions from baseline in EASI scores of erythema, edema/papulation, excoriation, and lichenification at week 0, 4 and 12 after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). data are presented as median [interquartile range]. †p < 0.05, ††p < 0.01 versus values at week 0; §p < 0.05, versus values at week 4, by friedman’s test with Bonferroni post-hoc test.
![Figure 6. Percent reductions from baseline in EASI scores of erythema, edema/papulation, excoriation, and lichenification at week 0, 4 and 12 after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). data are presented as median [interquartile range]. †p < 0.05, ††p < 0.01 versus values at week 0; §p < 0.05, versus values at week 4, by friedman’s test with Bonferroni post-hoc test.](/cms/asset/ed943014-656a-43f5-a3ca-b835342d12da/ijdt_a_2276043_f0006_c.jpg)
Percent reductions from baseline in EASI of 4 clinical signs all significantly increased at week 4 and 12 compared to week 0 (). Percent reduction from baseline in severity of lichenification was higher at week 12 compared to week 4, indicating the continuous improvement of lichenification by upadacitinib 30 mg until week 12.
We then tried to know if the treatment responses to upadacitinib 30 mg might differ among 4 clinical signs, by comparing the percent reductions from week 0 in EASI among 4 different signs (Supplemental Figure S2). There were no significant differences among 4 signs at week 4 and 12. The results indicate that upadacitinib 30 mg might improve all clinical signs similarly.
Transition of laboratory parameters after switching from baricitinib to upadacitinib
TEC () and LDH () decreased significantly at week 4 compared to week 0 and baseline. TARC decreased significantly at week 4 compared to week 0 (). In contrast, IgE significantly increased at week 12 compared to week 4 ().
Figure 7. The transition of total eosinophil count (TEC) (a), LDH (b), TARC (c) and IgE (d) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). the data are shown as median [interquartile range]. *p < 0.05 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0; §p < 0.05, versus values at week 4, by Friedman’s test with Bonferroni post-hoc test.
![Figure 7. The transition of total eosinophil count (TEC) (a), LDH (b), TARC (c) and IgE (d) after switching from baricitinib 4 mg to upadacitinib 30 mg in patients with atopic dermatitis (n = 20). the data are shown as median [interquartile range]. *p < 0.05 versus values at baseline; †p < 0.05, ††p < 0.01 versus values at week 0; §p < 0.05, versus values at week 4, by Friedman’s test with Bonferroni post-hoc test.](/cms/asset/acd48284-1c67-42d6-ab2d-1e42bb9280a4/ijdt_a_2276043_f0007_c.jpg)
Safety outcomes
During treatment with baricitinib, TEAEs occurred in 16 patients (80%), and AEs leading to discontinuation of baricitinib occurred in 4 patients (20%) (), which was mild renal impairment, and resolved spontaneously after switching to upadacitinib. After switching, TEAEs occurred in 10 patients (50%) without serious AEs or AEs leading to treatment discontinuation or death. Elevation in serum creatine phosphokinase level was observed in 4 patients (20%), which was mild and resolved spontaneously. Cellulitis occurred in 1 patient (5%), herpes labialis occurred in 2 patients (10%), and herpes zoster occurred in 3 patients (15%). These infections were mild and improved with appropriate medication. In our study, it was observed that the same patient did not experience the same AEs for both baricitinib and upadacitinib.
Table 2. Treatment-emergent adverse events (TEAEs) during treatment with baricitinib 4 mg or after switching to upadacitinib 30 mg in patients with atopic dermatitis (n = 20).
Discussion
The present study evaluated the effectiveness of switching from baricitinib 4 mg to upadacitinib 30 mg in patients with AD. Our results showed that upadacitinib 30 mg improved rash and pruritus in AD patients with insufficient response to baricitinib or AEs which discontinued baricitinib treatment. Upadacitinib 30 mg improved the symptoms resistant to baricitinib treatment, head and neck rash, erythema, and edema/papulation.
There may be some reasons why upadacitinib 30 mg improved the rash and pruritus resistant to baricitinib 4 mg. One possible reason is the superior JAK inhibition by upadacitinib compared to baricitinib in magnitude and duration. Upadacitinib in vitro shows lower half maximum inhibitory concentration (IC50) for STAT phosphorylation by various cytokines (IL-2, IL-4, IL-15, IL-21, IL-3, granulocyte monocyte-colony stimulating factor, interferon-α) linked to JAK1/3, JAK2/2, JAK2/Tyk2, or JAK1/2/Tyk2 in human leukocytes, and longer time above IC50 per day, and resultant higher average daily percent STAT inhibition compared to baricitinib (Citation20,Citation21). The second reason is the selection of higher upadacitinib dose 30 mg rather than 15 mg since the intake of 30 mg might provide higher local concentrations of upadacitinib at sites of inflammation, and provide longer time above IC50 and higher average daily percent STAT inhibition, compared to 15 mg (Citation20,Citation21).
In the present study, PP-NRS significantly reduced 4 weeks after switching to upadacitinib 30 mg. The results suggest that upadacitinib 30 mg can rapidly relieve the pruritus resistant to baricitinib. Upadacitinib might suppress JAK1, downstream of cytokine receptors at sensory nerve endings, and block the onset of itch sensation mediated by IL-4, IL-13, IL-31, or TSLP transducing JAK1/STAT signaling pathway (Citation22,Citation23), JAK1/2 inhibitor baricitinib may inhibit JAK1 activation mediated by these cytokines at sensory nerve endings, however, the inhibition may be incomplete based on the higher IC50 values for STAT phosphorylation and shorter time above IC50 compared to upadacitinib (Citation20,Citation21).
In the present study, EASI score at head and neck was not significantly reduced by baricitinib. We also previously detected lower percent reduction of EASI at head and neck compared to lower limbs at week 12 of treatments with upadacitinib 15 mg and baricitinib 4 mg (Citation12,Citation13). Further, the treatment response to dupilumab of head and neck rash was lower compared to that of other anatomical sites (Citation24). These suggest that head and neck rash may be rather resistant to treatments independently on types of treatment. Several reasons may exist for the treatment resistance of head and neck rash. Firstly, the head and neck site is more exposed to external stimuli, such as UV, aeroallergens or cosmetics compared to the other body sites (Citation25). Secondly, Malassezia furfur or Demodex colonized on head and neck may promote the inflammation or disruption of stratum corneal barrier in AD patients (Citation26–28). Meanwhile, it is reported that upadacitinib 15 mg or 30 mg improved head and neck rash resistant to dupilumab treatment (Citation29), possibly because upadacitinib may suppress the effects of JAK-dependent cytokines other than IL-4 and IL-13.
In the present study, severity of erythema was not observed to decrease significantly by baricitinib. The erythema of AD might reflect the abundant infiltration and activation of Th2 cells, type 2 innate lymphoid cells (ILC2), eosinophils, mast cells, basophils, or dendritic cells (DCs), and might be mediated by type 2 cytokines, IL-4, IL-13, IL-31 or TSLP transducing JAK1/STAT signals (Citation30–34). JAK1/2 inhibitor baricitinib may inhibit the effects of above JAK1-activating cytokines, however, the inhibition may be incomplete based on the higher IC50 values for STAT phosphorylation and shorter time above IC50 among JAK inhibitors (Citation20,Citation21), and also due to the high levels of cytokines in AD lesions exceeding the inhibitory effects of baricitinib, which may be related to the insufficient improvement of erythema by baricitinib.
In the present study, severity of erythema was not significantly reduced by baricitinib. The erythema of AD might reflect the abundant infiltration and activation of Th2 cells, type 2 innate lymphoid cells (ILC2), eosinophils, mast cells, basophils, or dendritic cells (DCs), and might be mediated by type 2 cytokines, IL-4, IL-13, IL-31 or TSLP transducing JAK1/STAT signals (Citation30–34). JAK1/2 inhibitor baricitinib might inhibit the effects of above JAK1-activating cytokines, however, the inhibition may be incomplete based on the higher IC50 values for STAT phosphorylation and shorter time above IC50 among JAK inhibitors (Citation20,Citation21), and also due to the high levels of cytokines in AD lesions exceeding the inhibitory effects of baricitinib, which may be related to the insufficient improvement of erythema by baricitinib.
In the present study, severity of edema/papulation was not significantly reduced by baricitinib. The pathogenesis of edema/papulation in AD might involve vascular hyperpermeability due to the disruption of endothelial integrity (Citation35). Interleukin-33 and IL-4 reduce the expression of occludin and vascular endothelial-cadherin (VE-cadherin) in endothelial cells (Citation35). Interleukin-4 induces apoptosis of endothelial cells, promoting the leakage of plasma components or leukocytes from the damaged endothelial barrier (Citation35). Interleukin-4 and IL-13 promote the efflux of plasma proteins, such as fibrinogens and fibronectin (Citation36). Further, vascular endothelial growth factor (VEGF) disrupts the alignment of VE-cadherin around the endothelial cell borders, leading to tissue edema and leakage of plasma components and leukocytes (Citation37–39). The recovery from the disrupted endothelial integrity might take rather long time, which may be related to the insufficient improvement of edema/papulation by baricitinib.
Our present results suggest that the treatment response to upadacitinib 30 mg is consistent across all anatomical sites and clinical signs. This implies that upadacitinib 30 mg may provide consistently strong and universal therapeutic effects on symptoms across various anatomical sites and clinical signs.
After switching from baricitinib to upadacitinib 30 mg, there was a significant decrease of TEC at week 4. The results are consistent with previous studies on upadacitinib 15 mg, showing that percent reduction of TEC was correlated with that of EASI at week 4 of treatment (Citation11). The previous and present results totally suggest that TEC might be a biomarker reflecting treatment responses to upadacitinib in AD, and that eosinophils may be the treatment target for upadacitinib. After switching from baricitinib to upadacitinib, there was also a significant decrease of LDH at week 4. LDH is a tetrameric oxidoreductase and is present in the cytoplasm of almost all cells in all tissues (Citation40). The increase of LDH in AD patients reflects the cell breakdown in skin lesions due to the inflammation or scratch while its decrease reflects the restoration of skin and vascular barrier and reduction of inflammation. Our results indicate that LDH can be used as an index reflecting responsiveness to treatment with upadacitinib 30 mg as well as TEC. In our present study, IgE levels increased at 4 and 12 weeks of treatment with upadacitinib. Since IL-21 suppresses IgE class switch recombination in human B cells dependently on JAK1/STAT pathway (Citation41), JAK1 inhibitor upadacitinib might suppress this effect of IL-21, which may result in the increase of IgE levels during treatment with upadacitinib.
The present study revealed a favorable safety profile for switching to upadacitinib 30 mg; no serious AEs or AEs leading to treatment discontinuation or death were observed, supporting tolerability of upadacitinib 30 mg. However, a safety clinical trial conducted by the U.S. Food and Drug Administration (FDA) on tofacitinib, another JAK inhibitor, observed an increased risk for death, major adverse cardiovascular events, malignancies, and thrombosis compared to TNF-a inhibitors in patients with rheumatoid arthritis aged >50 years and more than 1 risk of cardiovascular diseases (Citation42). Similar risks could be considered for other JAK inhibitors including upadacitinib. Japanese Dermatological Association also promoted clinicians’ attention to perform screening and regular monitoring for those risks in patients treated with JAK inhibitors (jak_statment-AD2.pdf (dermatol.or.jp)). Clinicians should balance the risk and benefit in deciding dose and duration of upadacitinib treatment based on the background of patients. In the usage of upadacitinib 30 mg, it is advisable to reduce the dose to 15 mg after achieving remission, such as achieving investigator’s global assessment = 0 (clear) or 1 (almost clear). Encouraging patients to improve adherence to topical treatments and skin care is also essential for the maintenance of remission.
This study has some limitations. Firstly, the sample size was small, which may account for the lack of significant differences of the sample size was small. The lack of significant differences in several statistical analyses might reflect the small sample size, and future large-scale studies are warranted. Secondly, we only used 30 mg upadacitinib. The outcomes of switching to 15 mg upadacitinib from baricitinib 4 mg should further be examined, and should be compared with those of upadacitinib 30 mg. Thirdly, this study evaluated the short-term effectiveness and safety after switching from baricitinib to upadacitinib, and long-term outcomes such as 1-year should further be investigated.
Conclusion
In conclusion, the present results suggest that upadacitinib 30 mg might improve the rash and pruritus resistant to baricitinib 4 mg, and that switching to upadacitinib 30 mg may be considerable treatment option for AD patients showing insufficient responsiveness or AEs by baricitinib.
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Nippon Medical School Chiba Hokusoh Hospital (protocol code H-2022-945 and 10 February 2022 of approval).
Author contributions
Teppei Hagino conceptualized the study, and mainly organized the manuscript. Mai Yoshida and Risa Hamada performed the statistical analyses. Naoko Kanda supervised the study. Hidehisa Saeki and Eita Fujimoto revised the manuscript.
Supplemental Material
Download PDF (229.9 KB)Disclosure statement
Hidehisa Saeki received a lecture fee and research cost from AbbVie GK. Teppei Hagino, Hidehisa Saeki and Naoko Kanda received lecture fees from AbbVie GK and Eli Lilly.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):1–9. doi:10.1016/j.jaad.2013.10.010.
- Carroll CL, Balkrishnan R, Feldman SR, et al. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi:10.1111/j.1525-1470.2005.22303.x.
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(4s):S65–S76. doi:10.1016/j.jaci.2017.01.011.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4(1):1. doi:10.1038/s41572-018-0001-z.
- Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi:10.3389/fimmu.2019.02342.
- Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol. 2020;21(6):783–798. doi:10.1007/s40257-020-00548-6.
- Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. Mardoi:10.1016/j.jaci.2019.11.025.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-Severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi:10.1001/jamadermatol.2021.3023.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi:10.1016/S0140-6736(21)00588-2.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi:10.1016/S0140-6736(21)00589-4.
- Hagino T, Saeki H, Kanda N. The efficacy and safety of upadacitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2022;49(11):1158–1167. doi:10.1111/1346-8138.16549.
- Hagino T, Saeki H, Fujimoto E, et al. Efficacy and safety of baricitinib treatment for moderate to severe atopic dermatitis in real-world practice in Japan. J Dermatol. 2023;50(7):869–879. Juldoi:10.1111/1346-8138.16763.
- Hagino T, Saeki H, Fujimoto E, et al. The differential effects of upadacitinib treatment on skin rashes of four anatomical sites in patients with atopic dermatitis. J Dermatolog Treat. 2023;34(1):2212095.
- Hagino T, Saeki H, Fujimoto E, et al. Background factors predicting the occurrence of herpes zoster in atopic dermatitis patients treated with upadacitinib. J Dermatol. 2023;50(10):1301–1312. doi:10.1111/1346-8138.16879.
- Hagino T, Yoshida M, Hamada R, et al. Therapeutic effectiveness of upadacitinib on individual types of rash in japanese patients with moderate-to-severe atopic dermatitis. J Dermatol. 2023;2023:16950. doi:10.1111/1346-8138.16950.
- Hagino T, Saeki H, Fujimoto E, et al. The eosinophil-to-Lymphocyte ratio acts as an indicator for improvement of clinical signs and itch by upadacitinib treatment in atopic dermatitis. J Clin Med. 2023;12(6):2201. doi:10.3390/jcm12062201.
- Drucker AM, Morra DE, Prieto-Merino D, et al. Systemic immunomodulatory treatments for atopic dermatitis: update of a living systematic review and network meta-analysis. JAMA Dermatol. 2022;158(5):523–532. doi:10.1001/jamadermatol.2022.0455.
- Wan H, Jia H, Xia T, et al. Comparative efficacy and safety of abrocitinib, baricitinib, and upadacitinib for moderate-to-severe atopic dermatitis: a network meta-analysis. Dermatol Ther. 2022;35(9):e15636.
- Saeki H, Ohya Y, Furuta J, et al. Executive summary: Japanese guidelines for atopic dermatitis (ADGL) 2021. Allergol Int. 2022;71(4):448–458. doi:10.1016/j.alit.2022.06.009.
- Traves PG, Murray B, Campigotto F, et al. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann Rheum Dis. 2021;80(7):865–875. doi:10.1136/annrheumdis-2020-219012.
- McInnes IB, Byers NL, Higgs RE, et al. Comparison of baricitinib, upadacitinib, and tofacitinib mediated regulation of cytokine signaling in human leukocyte subpopulations. Arthritis Res Ther. 2019;21(1):183. doi:10.1186/s13075-019-1964-1.
- Meng J, Li Y, Fischer MJM, et al. Th2 modulation of transient receptor potential channels: an unmet therapeutic intervention for atopic dermatitis. Front Immunol. 2021;12:696784. doi:10.3389/fimmu.2021.696784.
- Kunimura K, Fukui Y. The molecular basis for IL-31 production and IL-31-mediated itch transmission: from biology to drug development. Int Immunol. 2021;33(12):731–736. doi:10.1093/intimm/dxab065.
- Vittrup I, Krogh NS, Larsen HHP, et al. A nationwide 104 weeks real-world study of dupilumab in adults with atopic dermatitis: ineffectiveness in head-and-neck dermatitis. J Eur Acad Dermatol Venereol. 2023;37(5):1046–1055. doi:10.1111/jdv.18849.
- Maarouf M, Saberian C, Lio PA, et al. Head-and-neck dermatitis: diagnostic difficulties and management pearls. Pediatr Dermatol. 2018;35(6):748–753. doi:10.1111/pde.13642.
- Darabi K, Hostetler SG, Bechtel MA, et al. The role of malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol. 2009;60(1):125–136. Jandoi:10.1016/j.jaad.2008.07.058.
- Chu H, Kim SM, Zhang K, et al. Head and neck dermatitis is exacerbated by malassezia furfur colonization, skin barrier disruption, and immune dysregulation. Front Immunol. 2023;14:1114321. doi:10.3389/fimmu.2023.1114321.
- Uchida H, Kamata M, Egawa S, et al. Newly developed erythema and red papules in the face and neck with detection of demodex during dupilumab treatment for atopic dermatitis improved by discontinuation of dupilumab, switching to upadacitinib or treatment with oral ivermectin: a report of two cases. J Eur Acad Dermatol Venereol. 2023;37(3):e300–e302. doi:10.1111/jdv.18743.
- Gori N, Ippoliti E, Antonelli F, et al. Successful response to upadacitinib in the treatment of atopic dermatitis lesions involving sensitive and visible areas resistant to dupilumab treatment. Clin Exp Dermatol. 2023;48(5):558–559. doi:10.1093/ced/llad040.
- Fujita H, Asahina A, Sugaya M, et al. Differential production of Th1- and Th2-type chemokines by mouse langerhans cells and splenic dendritic cells. J Invest Dermatol. 2005;124(2):343–350. doi:10.1111/j.0022-202X.2004.23607.x.
- Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra16. doi:10.1126/scitranslmed.3005374.
- Luo J, Zhu Z, Zhai Y, et al. The role of TSLP in atopic dermatitis: from pathogenetic molecule to therapeutical target. Mediators Inflamm. 2023;2023:7697699–7697698.
- Kunsleben N, Rüdrich U, Gehring M, et al. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J Invest Dermatol. 2015;135(7):1908–1911. doi:10.1038/jid.2015.106.
- Nakashima C, Otsuka A, Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp Dermatol. 2018;27(4):327–331. doi:10.1111/exd.13533.
- Chalubinski M, Wojdan K, Luczak E, et al. IL-33 and IL-4 impair barrier functions of human vascular endothelium via different mechanisms. Vascul Pharmacol. 2015;73:57–63. doi:10.1016/j.vph.2015.07.012.
- Leung DYM, Bissonnette R, Kreimer S, et al. Dupilumab inhibits vascular leakage of blood proteins into atopic dermatitis skin. J Allergy Clin Immunol Pract. 2023;11(5):1421–1428. doi:10.1016/j.jaip.2023.03.020.
- Amarbayasgalan T, Takahashi H, Dekio I, et al. Content of vascular endothelial growth factor in stratum corneum well correlates to local severity of acute inflammation in patients with atopic dermatitis. Int Arch Allergy Immunol. 2012;157(3):251–258. doi:10.1159/000327556.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi:10.1038/nm0603-669.
- Ashina K, Tsubosaka Y, Kobayashi K, et al. VEGF-induced blood flow increase causes vascular hyper-permeability in vivo. Biochem Biophys Res Commun. 2015;464(2):590–595. doi:10.1016/j.bbrc.2015.07.014.
- Livesey A, Garty F, Shipman AR, et al. Lactate dehydrogenase in dermatology practice. Clin Exp Dermatol. 2020;45(5):539–543. doi:10.1111/ced.14134.
- Yang Z, Wu CM, Targ S, et al. IL-21 is a broad negative regulator of IgE class switch recombination in mouse and human B cells. J Exp Med. 2020;217(5):e20190472.
- U.S. Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions; [cited 2021 Sep 1. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death.