Abstract
The purpose of the article
Pyoderma gangrenosum (PG) is an ulcerating neutrophilic dermatosis with an incidence of 3-10 patients per million. PG equally affects patients of both sexes and of any age. Of these patients, 50-75% are associated with auto-immune disease. The lower extremities are the most commonly affected body parts. Minor trauma to the skin may result in the development of new lesions. Patients complain of chronic, nonhealing ulcers with associated pain. Treatment starts with systemic or intralesional corticosteroids, however, no official treatment protocol currently exists. Recent success has been found with biologic agents such as TNF-a inhibitor, although the treatment efficacy in these reports is limited. As for the pregnant patient, the drug selection is difficult. In this report, we want to assess the efficiency of certolizumab in the pregnant patient.
Results
We report a case of a patient with PG, who responded well to certolizumab, 400 mg as a booster dose, followed by 200 mg biweekly for 8 weeks. The lesions gradually resolved and followed up for 5months without side effect. In addition, we reviewed the literature and compared the current treatment efficiency in the treatment of PG.
Conclusion
Certolizumab may be a promising therapeutic option for patients with severe PG.
Pyoderma gangrenosum (PG) is an ulcerating neutrophilic dermatosis with an incidence of 3–10 patients per million (Citation1). PG equally affects patients of both sexes and of any age (Citation2). Of these patients, 50–75% are associated with auto-immune disease, the most common being inflammatory bowel disease and arthritis (Citation3). The lower extremities are the most commonly affected body parts. Minor trauma to the skin may result in the development of new lesions, which is known as pathergy (Citation3). Patients complain of chronic, nonhealing ulcers with associated pain. Treatment starts with systemic or intralesional corticosteroids; however, no official treatment protocol currently exists. The course of PG can vary greatly, from relatively indolent to aggressive or fulminant (Citation4). Herein we report a pregnant patient with PG responsive to certolizumab.
Case report
A 32-year-old pregnant Chinese female presented with a painful ulcer with rapid evolution on the right lower leg () as well as swelling on the back of the left hand () for 2 weeks. The patient recalled a minor trauma that had occurred 1 month earlier and recalled having had the same lesions on the lower left leg 3 years previously. A skin biopsy confirmed the diagnosis of pyoderma gangrenosum 3 years ago (), which completely resolved without recurrence on treatment with tapering doses of glucocorticoids. When 23 weeks pregnant, the patient was scared to use glucocorticoids.
Figure 1. Ulcerated plaque on the lower extremity and left hand. (a) The ulcer on the right lower leg. (b) Swelling on the back of left hand. Figure 1(c,d) 2 Months after treatment with certolizumab. (c) The right lower leg. (d) The back of left hand.
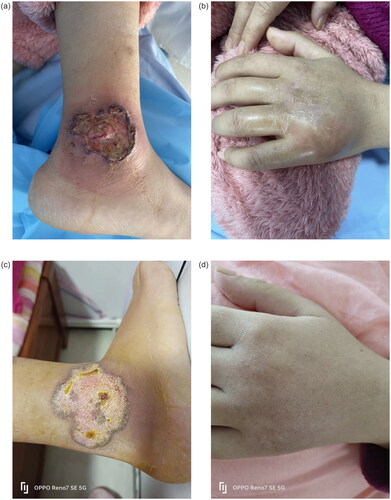
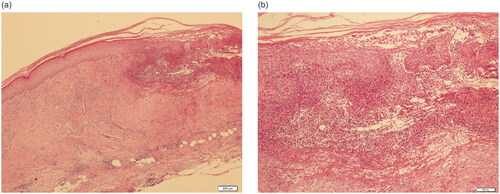
Figure 2. The biopsy of the skin lesion. Neutrophil infiltration, fibrous tissue proliferation below and around the ulcer, fibrinoid degeneration of some vascular walls, and a few lymphocytes and eosinophils around the blood vessels. (a) 40×, (b) 200×.
Laboratory tests including urea, electrolytes, liver function, blood glucose, HbA1c level, and blood cell count were normal. A rapid plasma reagin (RPR) or Venereal Disease Research Laboratory (VDRL) test for syphilis, perinuclear antineutrophil cytoplasmic antibody (pANCA) or cytoplasmic antineutrophil cytoplasmic antibody (cANCA) tests for autoimmune diseases (in particular vasculitis), anti-Saccharomyces cerevisiae test for Crohn’s disease, antibodies against mutated citrullinated vimentin (anti-MCV), dsDNA, ANA, SSA, SSB for autoimmune diseases were all in negative. Female tumor markers (AFP was elevated, which was considered to be pregnant-related) and abdominal ultrasound showed no signs of underlying systemic disease.
Following screening the appropriate biological treatment, with subcutaneous certolizumab 400 mg as a booster dose, followed by 200 mg biweekly. The lesions improving greatly within 2 weeks. The patient took certolizumab for a total of 8 weeks, during which time the skin lesions gradually resolved (). The patient delivered a healthy baby and in the following 8 weeks has discontinued certolizumab. She has been followed up for 5 months with no discomfort and her baby is healthy.
Discussion
PG proposed criteria include the fulfillment of two major and two minor criteria, as listed in the Afsaneh Alavi’s paper (Citation2). PG is challenging with respect to diagnosis, management, and treatment, which is directed toward reducing the associated inflammation that leads to ulceration. Optimal management may include the clinically relevant aspects of PG: avoidance of triggers, appropriate wound care, adequate pain management, and topical, systemic and targeted immunomodulatory therapies. The overall mortality rate of PG during an 8-year retrospective study was reported to be 16% (Citation5). Systemic corticosteroids and cyclosporine are considered first-line options based on the results of the randomized clinical STOP GAP trial (NCT02821013) (Citation6).
The pathophysiology of PG is not fully understood, but a prominent role of genetics facilitating immune dysregulation has been proposed. The Moura’ group (Citation7) recently performed variant enrichment analysis and a bioinformatic pipeline applicable for Whole Exome Sequencing data in unrelated PG patients and found genetically altered pathways involved in immune system biology and wound repair appear to be pathogenic drivers in PG pathogenesis. Researches have also confirmed the proinflammatory cytokines such as TNFα, interleukin-1 (IL-1), IL-6, IL-8, IL-17, and IL-23 are upregulated in the skin with PG (Citation8). In recent decades, evidence exists resulting from the off-label use of these drugs, such as biologics in the form of TNFα, IL-1β, IL-17, and IL-23 inhibitors et al. and a semi-systematic review has demonstrated the effectiveness of TNFα inhibitors in the treatment of PG (Citation9,Citation10). Recent reports confirmed that the Janus kinase (JAK) inhibitors are also effective in treatment of PG (Citation10).
However, certain drugs and class of medications have been reported to trigger or exacerbate PG, which was categorized as ‘drug induced PG.’ The Romagnuolo’ group (Citation11) reported a patient with chronic recurrent multifocal osteomyelitis who presented with PG lesions on the lower leg after the administration of adalimumab. This type of PG is an ideal model to better understand PG pathogenesis. Until now, the only three reports discussed the efficacy of certolizumab in PG are in the context of disseminated PG and associated Crohn’s disease or rheumatoid arthritis (Citation12). Gawdzik reported (Citation13) a case with ankylosing spondylitis developed paradoxical skin reactions to certolizumab.
Certolizumab pegol is an Fc-free, PEGylated, anti-TNFα monoclonal antibody, which differs from the other TNFα-inhibitors in its lack of an Fc region, which minimizes potential Fc-mediated effects such as complement-dependent cytotoxicity or antibody dependent cell-mediated cytotoxicity. The lack of an Fc region may also be a factor in the prevention of active transfer of certolizumab pegol across the placenta during pregnancy (Citation14). Reproduction studies were performed in rats at doses up to 100 mg/kg and revealed no evidence of impaired fertility or harm to the fetus due to cTN3 PF (Citation15). Our patient was pregnant and we chose certolizumab, which is safer than other biologics and JAK inhibitors. Fortunately, this patient responds well for certolizumab.
Researches for PG and other neutrophilic dermatoses seems to be promising as more and more specific and targeted therapies are currently being developed.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data sharing is not applicable to this article.
Additional information
Funding
References
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73(4):1–4. doi:10.1016/j.jaad.2015.06.021.
- Alavi A, French LE, Davis MD, et al. Pyoderma gangrenosum: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2017;18(3):355–372. doi:10.1007/s40257-017-0251-7.
- Ashchyan HJ, Nelson CA, Stephen S, et al. Neutrophilic dermatoses: pyoderma gangrenosum and other bowel- and arthritis-associated neutrophilic dermatoses. J Am Acad Dermatol. 2018;79(6):1009–1022. doi:10.1016/j.jaad.2017.11.063.
- Patel F, Fitzmaurice S, Duong C, et al. Effectice strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95(5):525–531. doi:10.2340/00015555-2008.
- Binus AM, Qureshi AA, Li V, et al. Pyoderma gangrenosum: a retrospective review of patients characteristics, cormorbidities and therapy in 103 patients. Br J Dermatol. 2011;165(6):1244–1250. doi:10.1111/j.1365-2133.2011.10565.x.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350(jun12 3):h2958–h2958. doi:10.1136/bmj.h2958.
- Moura RR, Brandão L, Moltrasio C, et al. Different molecular pathways are disrupted in pyoderma gangrenosum patients and are associated with the severity of the disease. Sci Rep. 2023;13(1):4919. doi:10.1038/s41598-023-31914-z.
- Ben Abdallah H, Fogh K, Vestergaard C, et al. Pyoderma gangrenosum and interleukin inhibitors: a semi-systematic review. Dermatology. 2022;238(4):785–792. doi:10.1159/000519320.
- Ben Abdallah H, Fogh K, Bech R. Pyoderma gangrenosum and tumour necrosis factor alpha inhibitors: a semi-systematic review. Int Wound J. 2019;16(2):511–521. doi:10.1111/iwj.13067.
- Maronese CA, Pimentel MA, Li MM, et al. Pyoderma gangrenosum: an updated literature review on established and emerging pharmacological treatments. Am J Clin Dermatol. 2022;23(5):615–634. doi:10.1007/s40257-022-00699-8.
- Romagnuolo M, Moltrasio C, Iannone C, et al. Pyoderma gangrenosum following anti-TNF therapy in chronic recurrent multifocal osteomyelitis: drug reaction or cutaneous manifestation of the disease? A critical review on the topic with an emblematic case report. Front Med. 2023;10:1197273. doi:10.3389/fmed.2023.1197273.
- Pender TM, Ayandibu G, Van VA. Certolizumab for the treatment of localized pyoderma gangrenosum associated with Crohn’s disease: a case report. Dermatol Ther. 2020;33(6):e14352.
- Gawdzik A, Ponikowska M, Jankowska-Konsur A, et al. Paradoxical skin reaction to certolizumab, an overlap of pyoderma gangrenosum and psoriasis in a young woman treated for ankylosing spondylitis: case report with literature review. Dermatol Ther. 2020;10(4):869–879. doi:10.1007/s13555-020-00398-4.
- Goel N, Stephens S. Certolizumab pegol. MAbs. 2010;2(2):137–147. doi:10.4161/mabs.2.2.11271.
- Stephens S, Brown D, Nesbitt A, et al. Lack of placental transfer and accumulation in milk of an anti-TNF PEGylated fab’ fragment in rats. J Crohn’s Colitis. 2007;1:43.

