Abstract
Purpose: The efficacy of adjunctive ambrisentan treatment in patients with systemic sclerosis (SSc) suffering from digital ulcers (DUs) was investigated.
Material and methods: Patients (4 males, 7 females) diagnosed with SSc at our hospital between 2017 and 2022 were enrolled. Ten of them had diffuse SSc, while one had limited SSc. These patients received daily 5 mg doses of ambrisentan in addition to their regular SSc treatment for 16 weeks. Parameters including the total number and size of existing and new DUs, Visual Analog Score (VAS), frequency of Raynaud’s phenomenon (RP) attacks, and any adverse effects were assessed.
Results: At baseline, the median number and size of DUs was 3.0 (interquartile range (IQR): 2.0–4.0 cm) and 0.4 cm (IQR: 0.3–0.5 cm), respectively. Following the intervention, seven patients with a median of 2.0 DUs and a size of 0.35 cm (IQR: 0.15–0.45 cm) at baseline achieved complete healing. Significant improvements were also observed in other patients. VAS scores decreased from a baseline median of 5.0–0.0 (IQR: 0.0–1.0), and both the frequency and duration of RP attacks notably reduced.
Conclusion: Adjunctive ambrisentan therapy proved effective in promoting DU healing and preventing new DUs in SSc patients.
Introduction
Systemic sclerosis (SSc) is an autoimmune disease characterized by vasculopathy and fibrosis of connective tissues with various symptoms and unpredictable progression (Citation1–4). The complex mechanisms of endothelial progenitor cells are believed to be responsible for the vascular pathogenesis of SSc symptoms (Citation5). Raynaud’s phenomenon (RP) is an early symptom of reduced blood supply to the digits (Citation1–4,Citation6). Other common complications include painful digital ulcers (DUs) due to vascular injury, pulmonary arterial hypertension (PAH) from fibrotic tissue in the lungs, and secondary RP (Citation1–4,Citation7). DUs occur in about 50% of SSc patients (Citation7), representing digital vascular injury and internal organ involvement. DUs typically heal slowly, with an average healing time of 76.2 days and 93.6 days for calcinosis-related DUs (Citation8). Besides impacts on the life quality and hand function of patients, the presence of DUs also suggests SSc severity and mortality (Citation1–4,Citation7).
Currently, there are various pharmacological treatments for SSc-DU, and the oral bioavailability of endothelin receptor-1 antagonists (ETRAs) makes them the first choice (Citation7,Citation9). However, bosentan, the mainly used ETRA, is also associated with adverse effects such as an increased level of aspartate aminotransferase and/or alanine aminotransferase (Citation10). Ambrisentan, another ETRA approved by the US Food and Drug Administration in 2007 for treating PAH, was reported to treat DUs in small-scale studies in Europe and North America (Citation11,Citation12). Here, we reported the effects of ambrisentan on DUs in eleven Chinese patients diagnosed with SSc, further demonstrating the efficacy of ambrisentan and supporting its potential use as the first-line treatment for SSc-DUs.
Patients and methods
Patients diagnosed with SSc according to ACR criteria (Citation13) in our hospital between April 2017 and June 2022 were enrolled for this open-label study. Inclusion criteria were: (1) ANA level higher than 1:40; (2) follow-up in clinic longer than 2 years. The study protocol was approved by the Institution review board. Informed consent was obtained from all included patients. Besides regularly taking medications for SSc (i.e. prednisone, colchicine, hydroxychloroquine, mycophenolate mofetil, beraprost, tramadol, acetylcysteine, or tacrolimus), patients received additional oral-administered ambrisentan 5 mg daily for 16 weeks. All patients continued taking the abovementioned regular medicine for SSc after week 16.
Outcomes
Primary outcomes were the total number and maximal size of DUs (at the longest diameter), visual analog scale (VAS) for pain, RP attack frequency, and persisted duration for each time. All were evaluated at admission, week 16 of ambrisentan treatment, and 2 years after discontinuing ambrisentan. All follow-ups were conducted in clinic and the total number and maximal size of DUs were recorded. The secondary outcome was adverse effects during treatment. The follow-up time after discontinuing ambrisentan was 24 months.
Statistical analysis
Continuous data without normal distribution are presented as median, interquartile range (IQR) and performed by Wilcoxon signed rank test. Categorical data are presented as counts and percentages. All p-values were two-sided, and p < 0.05 was considered statistically significant. All statistical analyses were performed using the statistical software package SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 11 patients were included, 10 with diffuse SSc and 1 with limited SSc. The median age was 54.0 (35.0–63.0) years at diagnosis. All are Chinese, and 7 are females (63.6%). All patients were regularly taking medicines for SSc, including prednisone 5 mg qd (n = 6), colchicine 1 mg qd (n = 5), hydroxychloroquine 100 mg bid (n = 3), mycophenolate mofetil tablet 500 mg bid (n = 3), beraprost (n = 2), tramadol (n = 1), acetylcysteine (n = 1), and tacrolimus (n = 1). The total number and size of DUs were 3 (2.0–4.0) and 0.4 (0.3–0.5) cm at baseline, located at the tip and pulp of fingers.
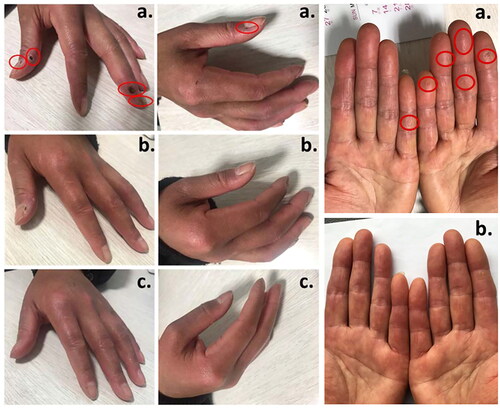
After adjunctive ambrisentan treatment for 16 weeks, DUs were completely healed in 7 patients (at baseline: 2.0 (1.0–3.0) DUs, 0.35 (0.15–0.45) cm), while in the other 4 patients, the number decreased to 1.0 (1.0–2.0) (from 3.5 (2.5–4.5)) DU with size to 0.2 (0.1–0.3) cm (from 0.55 (0.43–0.65) cm). Decreases in pain (VAS: from 5.0 (4.0–6.0) to 0 (0.0–1.0), p), RP attack frequency (from 38.5 (31.5–52.5) to 2.5 (1.5–3.5) times/week) and duration (from 10.0 (7.5–15.0) to 3.0 (1.5–4.5)) at week 16 compared to baseline were observed (all p < 0.05). No complications were reported. In the 2nd year of follow-up, no new DUs were found. None reported new DU onset during treatment and 2 years after discontinuing ambrisentan. The outcomes at baseline, week 16, and 2 years after discontinuing ambrisentan are summarized (). displays DUs measured prior to adjunctive ambrisentan treatment, at the 4th week, and at the 8th week following treatment.
Table 1. Comparisons of study population.
Figure 1. Digital ulcers (DUs) (a) before, (b) at 4th week, and (c) at 8th week after adjunctive ambrisentan treatment are indicated with the arrow. The appearance of DUs are round or oval, in different sizes of about 4 mm in diameter and about 2 mm in depth; flat edges: normal surrounding skin and pink fundus.
The patients were further stratified by seasons to determine the effect of weather on the DU healing and treatment (). In this study, the seasonal divisions were as follows: spring encompassed the period from March to May, summer spanned from June to August, autumn extended from September to November, and winter encompassed December through February of the subsequent year. There were 2, 1, 3, and 5 patients admitted to the facility in spring, summer, autumn, and winter, respectively. After discontinuing ambrisentan for 16 weeks, all patients experienced less DUs and lower frequency and duration of RP attacks.
Table 2. Comparison of study populations divided by seasons.
Discussion
This case series study reported the efficacy of ambrisentan adjunctive to regular medicine in treating DUs in SSc patients. Adjunctive ambrisentan treatment 16-week healed existing DUs, leading to decreased pain and RP attack. No new-onset DUs were reported during treatment and even 2 years after discontinuing ambrisentan. No adverse effects were reported during ambrisentan treatment. Adjunctive ambrisentan treatment shows favorable effects on heal and prevention of DUs in the present study. Neither new DU onset nor life quality deterioration was reported even in 2 years after drug discontinuation.
The present study shows that adjunctive ambrisentan treatment prevented the development of new DUs during treatment and even 2 years after discontinuation. This might result from good control of the disease because these patients received more intensive treatment after wound healing. Nevertheless, the safety and efficacy of adjunctive ambrisentan treatment provide an option to improve the life quality of SSc patients, although the underlying mechanism needs further investigation. Controversy remains about whether the ETRAs, bosentan mainly, are helpful in healing and preventing new DUs in SSc. No new DUs in 24-week treatment were also observed in bosentan-refractory SSc patients (Citation12), whereas the development of new DUs in 4 weeks after 24-week treatment was reported in 16 limited SSc patients (Citation11). SSc is an autoimmune disorder with unknown pathology and various symptoms related to connective tissues. Individual factors such as history, overall health status, and comorbidities may make essential patient differences that could affect outcomes.
VAS and RP attacks were improved after adjunctive ambrisentan treatment because of DU heal, which is consistent with the studies in bosentan-refractory SSc patients (Citation12) and limited SSc patients (Citation14), and those studies with bosentan treatment (Citation9,Citation10). Furthermore, no change in liver and lung function in SSc patients was reported during ambrisentan treatment compared to baseline (Citation12), while an increased level of aspartate aminotransferase and/or alanine aminotransferase in SSc patients was indicated during bosentan treatment (Citation10,Citation15). A meta-analysis comparing the adverse effects of ETRA revealed that bosentan increased the risk of abnormal liver function and anemia, whereas ambrisentan decreased the risk, although ambrisentan increased the risk of peripheral edema (Citation15). These findings suggest that adjunctive ambrisentan treatment may be preferable to bosentan in treating SSc-DUs mainly because of a lower incidence of liver dysfunction and convenient daily oral administration (Citation9–12). Given that ambrisentan has been indicated for treating PAH, its dual effects on treating DUs and PAH may have more advantages in treating patients with SSc.
While digital ulcers (DU) can manifest at any time of the year, they frequently become more prominent in colder weather conditions due to the constriction of blood vessels (Citation16). Our study underscores this trend, with 63.6% (7 out of 11) of patients being admitted during the winter and spring seasons, and an additional 27.3% (3 out of 11) admitted during autumn. However, when we conducted follow-up assessments on all patients at the 16-week mark following the discontinuation of ambrisentan therapy, the weather conditions were entirely dissimilar from those present during their initial admissions. Remarkably, irrespective of the season at admission, all patients exhibited improved outcomes compared to their baseline status. This observation suggests that the therapeutic intervention under investigation yielded favorable results independent of seasonal influences.
This study has some limitations. First, this is a single-center study containing only subjects of Chinese ethnicity, limiting the generalizability of the findings. Second, the sample size is small. Third, this report had no comparable placebo control group and lacked blinding. The effect of ambrisentan therapy may be caused by unexpected placebo improvement. Finally, skin that has previously undergone DU can display discernible manifestations only in the short term, including the presence of scars or disparities in skin pigmentation relative to adjacent tissues. While the identification of DU indicators is typically observable, it is imperative to acknowledge the potential for underreporting.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Geyer M, Müller-Ladner U. The pathogenesis of systemic sclerosis revisited. Clin Rev Allergy Immunol. 2011;40(2):1–4. doi: 10.1007/s12016-009-8193-3.
- Varga J, Marangoni RG. Systemic sclerosis in 2016: dermal white adipose tissue implicated in SSc pathogenesis. Nat Rev Rheumatol. 2017;13(2):71–72. doi: 10.1038/nrrheum.2016.223.
- Matucci-Cerinic M, Steen VD, Furst DE, et al. Clinical trials in systemic sclerosis: lessons learned and outcomes. Arthritis Res Ther. 2007;9(Suppl 2):S7. doi: 10.1186/ar2191.
- Abraham DJ, Krieg T, Distler J, et al. Overview of pathogenesis of systemic sclerosis. Rheumatology (Oxford). 2009;48(suppl_3):iii3–7. doi: 10.1093/rheumatology/ken481.
- Manetti M, Guiducci S, Ibba-Manneschi L, et al. Mechanisms in the loss of capillaries in systemic sclerosis: angiogenesis versus vasculogenesis. J Cell Mol Med. 2010;14(6A):1241–1254. doi: 10.1111/j.1582-4934.2010.01027.x.
- Hughes M, Herrick AL. Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2016;30(1):112–132. doi: 10.1016/j.berh.2016.04.001.
- Hughes M, Herrick AL. Digital ulcers in systemic sclerosis. Rheumatology. 2017;56(1):14–25. doi: 10.1093/rheumatology/kew047.
- Amanzi L, Braschi F, Fiori G, et al. Digital ulcers in scleroderma: staging, characteristics and Sub-setting through observation of 1614 digital lesions. Rheumatology 2010;49(7):1374–1382. doi: 10.1093/rheumatology/keq097.
- Arefiev K, Fiorentino DF, Chung L. Endothelin receptor antagonis for the treatment of Raynaud’s phenomenon and digital ulcers in systemic sclerosis. Int J Rheumatol. 2011;2011:2017877. doi: 10.1155/2011/201787.
- Hamaguchi Y, Sumida T, Kawaguchi Y, et al. Safety and tolerability of Bosetan for digital ulcers in Japanese patients with systemic sclerosis: prospective multicenter, open-label study. J Dermatol. 2017;44(1):13–17. doi: 10.1111/1346-8138.13497.
- Chung L, Ball K, Yaqub A, et al. Effect of the endothelin type a selective endothelin receptor antagonist ambrisentan on digital ulcers in patients with systemic clerosis: results of a prospective pilot study. J Am Acad Dermatol. 2014;71(2):400–401. doi: 10.1016/j.jaad.2014.04.028.
- Parisi S, Peroni CL, Laganà A, et al. Efficacy of ambrisentan in the treatment of digital ulcers in patients with systemic sclerosis: a preliminaty study. Rheumatology. 2013;52(6):1142–1144. doi: 10.1093/rheumatology/ket019.
- Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510.
- Bose N, Bena J, Chatterjee S. Evaluation of the effect of ambrisentan on digital microvascular flow in patients with systemic sclerosis using laser Doppler perfusion imaging: a 12-week randomized double-blind placebo controlled trial. Arthritis Res Ther. 2015;17(1):44. doi: 10.1186/s13075-015-0558-9.
- Wei A, Gu Z, Li J, et al. Clinical adverse effects of endothelin receptor antagonists: insight from the meta-analysis of 4894 patients from 24 randomized double-blind placebo-controlled clinical trials. J Am Heart Assoc. 2016;5:e003896.
- Morrisroe K, Stevens W, Sahhar J, et al. Digital ulcers in systemic sclerosis: their epidemiology, clinical characteristics, and associated clinical and economic burden. Arthritis Res Ther. 2019;21(1):299. doi: 10.1186/s13075-019-2080-y.

