Abstract
Introduction
We explored patient satisfaction with baricitinib, an oral Janus kinase inhibitor, in patients with atopic dermatitis (AD) treated in routine clinical practice.
Methods
Adults with moderate-to-severe AD treated with baricitinib in clinical practice for ≥4 weeks in France, Germany, and the UK completed a one-time online survey under market research methodologies. Treatment satisfaction was assessed using a Likert scale and abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9). Patients reported demographic, disease, and treatment information. Data were analyzed descriptively.
Results
The survey was completed by 170 patients with a mean age of 39.3 years (SD = 13.5), 59% (n = 101) were female. At baricitinib initiation, 79% rated their AD as “Severe”, yet 28% reported body surface area (BSA) involvement ≥10%. Most were “Satisfied” or “Very satisfied” (76%/18%) with baricitinib, with high rates reported for controlling itch (36%/56%). Itch improvements were noted by 97% of patients. Some tapered/stopped (50%/32%) topical corticosteroid use, aligned with reported improvements on the patient global assessment and BSA. Mean TSQM-9 convenience score was 78.0 (SD = 14.0).
Conclusions
Satisfaction with itch control was particularly high, reflected in rates of improvement in itch since starting baricitinib. On the TSQM-9, the convenience score was the highest. Many patients tapered/stopped concomitant topicals, indicating baricitinib’s effect in controlling AD symptoms.
Introduction
Atopic dermatitis (AD) is a chronic and relapsing inflammatory skin disease characterized by a high symptom burden for patients (Citation1–3). This burden is the highest among all skin diseases as measured by disability-adjusted life-years (DALYs) (Citation4) and is driven by the multifactorial nature of the disease. Itch is rated as the most burdensome symptom by adult patients (Citation5), contributing to lowered health-related quality of life (HRQoL) and relief from itch is a key treatment goal (Citation6). Additionally, skin pain and sleep disturbance are also bothersome symptoms for patients, with 33% to 87.1% of adults with AD affected by disturbed sleep (Citation7). Disease burden is evident, too, in how AD patients report that the disease limits their lifestyle, leads to reduced social interaction, and contributes to dissatisfaction with life (Citation8). AD is increasingly regarded as a systemic disease, and associations with comorbid conditions such as food allergy, asthma, allergic rhinitis, and allergic conjunctivitis are now well documented (Citation9,Citation10) Non-atopic comorbidities include autoimmune diseases such as alopecia areata and mental health disorders such as anxiety, depression, suicidal ideation, and cardiovascular disease (Citation11).
Until recently, treatment options for moderate-to-severe AD were limited to topical corticosteroids (TCS), topical calcineurin inhibitors (TCI), phototherapy, and conventional immunosuppressive agents like cyclosporine and systemic corticosteroids. Recently, new treatments for moderate-to-severe AD, such as novel biologics and oral Janus kinase (JAK) inhibitors have become available (Citation12). Baricitinib was the first oral selective JAK inhibitor approved by the European Medicines Agency in 2020 for the treatment of moderate-to-severe AD in adult patients who are candidates for systemic therapy (Citation13). Evidence from randomized controlled trials has shown baricitinib to be safe and effective in treating moderate-to-severe AD as both monotherapy and in combination with TCS (Citation14,Citation15), for as long as two years post initiation (Citation16). Rapid improvements in itch, sleep disturbance due to itch, and skin pain were also observed with baricitinib, with associated improvements in symptoms of anxiety, depression, quality of life, and work productivity (Citation14). The availability of treatments that can help achieve relief of these burdensome factors, especially itch, represents an important development in delivering patient-focused, holistic care as recommended in recently updated European guidelines (Citation12).
Although the benefit/risk profile of baricitinib has been well established in randomized controlled clinical trials, there is currently no data from routine clinical practice to understand treatment satisfaction in patients with moderate-to-severe AD following initiation of any oral JAK inhibitor. To address this gap, using data drawn from a patient survey, the aim of our study was to explore patients” experience with baricitinib in a routine clinical practice setting, by assessing the factors that contribute to treatment satisfaction as well as patient-reported disease outcomes in those who have initiated baricitinib for AD.
Methods
Survey design
This study represents a protocol-driven analysis of data collected in a multi-country, cross-sectional online survey conducted using a market research methodology. It included patients from France, Germany, and the United Kingdom (UK). Data were collected via a one-time online structured questionnaires with closed-ended questions designed to meet the study objectives. Surveys were completed between June 6th, 2022 and 16 January, 2023. More detail is provided in the methodological appendix.
Participants and recruitment
Participants were recruited to the survey through their managing healthcare practitioner (HCP). To be eligible to partake in the study, patients had to be ≥18 years old, be prescribed baricitinib for moderate-to-severe AD in a routine clinical practice setting, and be receiving the drug for ≥4 weeks when completing the survey. Participants were recruited under a market research code of conduct. The research was carried out in compliance with all national laws and relevant national and international codes of conduct for healthcare market research, including from the European Society for Opinion and Market Research and the European Pharmaceutical Marketing Research Association. Study objectives and survey measures
The primary objective of the study was to describe the proportion of patients overall “Very satisfied” and “Satisfied” with baricitinib treatment for moderate-to-severe AD in routine clinical practice. To achieve this objective, the survey posed a series of questions related to patient satisfaction with baricitinib (overall satisfaction, perception of speed of treatment effect, skin clearance, itch control, skin pain control, and sleep improvement) which were measured on a 4-point Likert scale adapted from the work of Steinke and Colleagues (2014) (Citation17). Potential responses to each dimension of satisfaction were, “Very satisfied”, “Satisfied”, “Dissatisfied”, and “Very dissatisfied”. Patients could also indicate if their AD did not cause a problem in any given dimension.
Global patient satisfaction with baricitinib, together with perspectives on effectiveness and convenience were also measured using the medication-generic Abbreviated Treatment Satisfaction Questionnaire for Medication-9 (TSQM-9) (Citation18). The TSQM-9 ranges from 0 to 100, with higher scores indicating higher satisfaction.
Information collected in the survey also included patient demographics (age, sex, education, employment status), disease diagnosis and co-morbidities (age at AD diagnosis, family history of AD, diagnosed comorbidities), treatment information (duration of baricitinib treatment, baricitinib dosing, concomitant and prior treatment use, reason for changing prior treatment), assessment of disease (body surface area [BSA] involvementFootnote1, location of AD, Patient Global Assessment [PGA]), and symptom severity (Itch Numerical Rating Scale [Itch NRS], Sleep Disturbance Numerical Rating Scale [SD NRS]). Disease and symptom severity assessments were performed at both the time of baricitinib initiation and at the time of survey completion. Additionally, data were collected on patient-reported disease characteristics both overall and regarding specific symptoms since starting baricitinib using the Patient’s Global Impression of Change (PGI-C) (Citation19).
Statistical analysis
Descritptive statistics were reported using observed data. No formal statistical testing was performed. Analyses were conducted using IBM Survey Reporter, version 7.5. Subgroups were described by dosage (2 mg and 4 mg) and duration of baricitinib treatment (determined by the median time on baricitinib observed in the sample). Additional information is included in the methodological appendix.
Informed consent
Prior to beginning the survey, patients were provided with study information and electronic informed consent forms to indicate agreement with how their data would be used. Participation in the survey was voluntary. As part of providing consent, participants were informed of their right to withdraw consent and stop participation at any time.
Results
Demographics and clinical characteristics of survey patients treated with baricitinib
The survey was completed by 170 patients treated with baricitinib for their moderate-to-severe AD (France = 48, Germany = 53, UK = 69). At baricitinib initiation, the mean patient age was 39.3 years (standard deviation [SD] = 13.5), 59% were female, and the mean time since AD diagnosis was 20.9 years (SD = 14.0). 47% reported a family history of AD (). In all, 71% of patients reported an atopic comorbidity with 38% stating that they had more than one. The most frequently reported comorbidity () was allergic rhinitis. A concomitant psychiatric condition (e.g., depression or anxiety) was reported by 30% of patients and 7% reported suffering from concomitant alopecia areata (AA) ().
Table 1. Patient and clinical characteristics at the start of baricitinib treatment.
Table 2. Comorbidities reported by patients with AD on baricitinib.
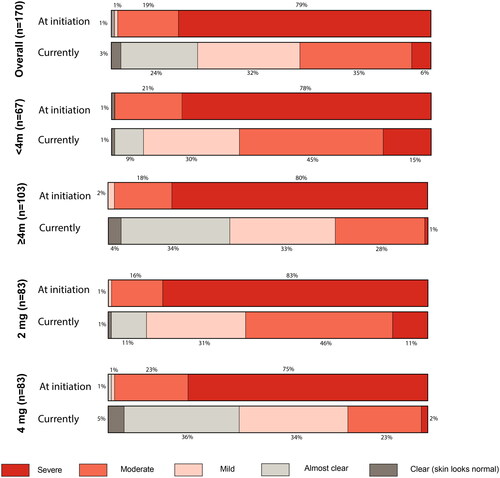
At baricitinib initiation, the majority of patients rated their disease as either “severe” (79%) or “moderate” (19%) (). Mean BSA was 8.1% (SD = 8.3%) and 28% of patients reported experiencing ≥10% BSA. Patients experienced high levels of itch, with a mean Itch NRS of 6.8 (SD = 2.0) at treatment initiation, and rated the extent of AD-related sleep loss as 5.1 (SD = 2.4), with ten being sleep entirely prevented ().
Treatment history of survey patients treated with baricitinib
The most common reason provided by patients for discontinuing, or switching from, their prior treatment to baricitinib was insufficient skin clearance (61%) ().
Table 3. Reason for stopping prior treatmentTable Footnotea and treatments received prior to baricitinib inititation.
Table 4. Change in concomitant topical treatment use.
The most frequently used treatments prior to initiating baricitinib were TCS (76%) and emollients (73%). Two-thirds of patients (65%) had previously received systemic AD treatments including systemic corticosteroids, immunosuppressants, and biologics (34%). Just over one third (38%) of those receiving systemic immunosuppressants and a quarter (25%) of those on a biologic prior to baricitinib were on this treatment for in excess of one year. Over half of those on oral corticosteroids prior to baricitinib received these for more than a year ().
Baricitinib treatment information
Median time on baricitinib was four months (inter-quartile range 2.3-7.0 months). Patients on baricitinib for less than four months, were on the drug for a median time of two months, while those receiving it for four months or more had a median time on baricitinib of six months and a maximum time of 24 months. Most of the patient sample (56%) were prescribed 2 mg as their starting dose of baricitinib, while 30% received the recommended 4 mg dose; 14% could not say what their starting dose was. Between baricitinib initiation and survey completion, 62% remained on the same dose, 19% had their dose increased and 4% saw their dose reduced. By the time of survey completion, 49% were on 2 mg, a further 49% were on the 4 mg dose and the dose was unknown for 2%.
Patient satisfaction with baricitinib treatment
Based on the Likert scale satisfaction measures (), most patients currently on baricitinib were overall either “Satisfied” (76%) or “Very satisfied” (18%) with their treatment. High rates for “Very satisfied” and “Satisfied” were reported for controlling itch (36%/56%) and for satisfaction in relation to the speed of effect (21%/69%), with the highest overall rate for “very satisfied” related to itch control (36%). Amongst those who experienced AD-linked sleep disturbance, 16% and 55% reported that they were “very satisfied” and “satisfied” respectively and these figures were 26%/51% with respect to those experiencing AD-related skin pain. For skin clearnace, 20% and 63% respectively of patients stated that they were “satisfied” or “very satisfied” with baricitinib.
Figure 1. Patient global assessment of disease severity at baricitinib initiation and at survey completion (currently), including by subgroups for time on treatment and current dosage.
Using the medication-generic short form TSQM-9, patients reported a mean global satisfaction score of 62.7 (SD = 20.5) and an effectiveness score of 68.1 (SD = 15.3). Mean TSQM-9 convenience score was 78.0 (SD = 14.0), with 57% and 64% of patients describing baricitinib as “Very/extremely convenient” and “Very/extremely easy” to use.
Disease assessment since starting baricitinib treatment
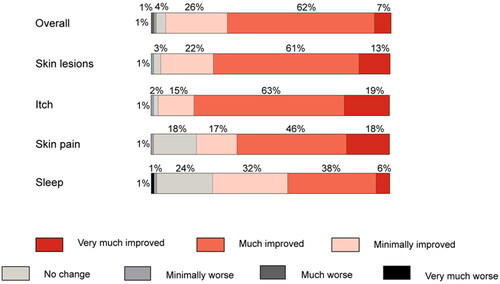
Since starting baricitinib, patients reported that their overall condition was either “very much improved” or “much improved” in 7% and 62% of cases, as assessed by the PGI-C (). A further 26% noted that their overall condition had “minimally improved”. The stable condition was reported for 4% of patients.
In line with reported improvements, 32% rated their disease severity as “Mild”, with 24% rating it as “almost clear” and 3% as “Clear” at survey completion. While most rated their disease as either “severe” (79%) or “moderate” (19%) at baricitinib initiation, only 6% and 35% respectively rated it so at survey completion. Nearly all (96%) patients reported some improvement in their skin lesions with about three-quarters saying that they were “Much improved” or “Very much improved” (). Reported improvements in skin lesions were reflected in reported BSA involvement, which dropped from a mean rating of 8.1 (SD = 8.3) at baricitinib initiation to 3.2 (SD = 2.5) at the time of survey completion, with only 4% of patients noting a disease extent of ≥10% BSA (28% at baricitinib initiation). Controlled skin inflammation with baricitinib also led to reduced co-application of topical AD treatments. For TCS, 50% and 32% of patients respectively reported that they had reduced its use or discontinued it entirely since initiating baricitinib. For TCI, 22% reported reduced usage and 31% ceased TCI completely since baricitinib initiation. Nearly half (44%) of those using emollients reported using less since baricitinib initiation ().
On the PGI-C, across all domains, the highest rates for “very much improved” (19%) and “much improved” (63%) were reported for improvements in itch, while smaller proportions reported “minimal improvement” (15%) or “no change” (2%). Mean Itch NRS had decreased from 6.8 (SD = 2.0) at baricitinib initiation to 2.7 (SD = 1.9) by the time of survey completion. For other symptoms assessed, such as skin pain, and sleep disturbance, improvements were seen on the PGI-C between baricitinib initiation and survey completion (). For sleep, this was also reflected in an improvement in mean NRS from 5.1 at baricitinib initiation to 2.3 at survey completion).
Patient satisfaction and disease assessment by dose and duration on baricitinib treatment
Subgroup analyses were performed by a current dose of baricitinib (2 mg or 4 mg), and by the duration of baricitinib treatment at the time of the survey (less than 4 months and 4 months or more). While patients on baricitinib 2 mg reported a shorter treatment duration with a median of 2.8 months (IQR= 2.0–6.0 months), those on the 4 mg dose had been receiving their treatment for a median of 5.0 months (IQR 4.0–8.0 months). Accordingly, amongst those who were on baricitinib for less than four months at the time of the survey, 73% were on 2 mg and 25% were on 4 mg. For those who had been receiving baricitinib for four months or more when completing the survey, 33% were taking 2 mg and 64% were receiving the 4 mg dose. This indicates that those on treatment for shorter durations mostly received the 2 mg dose, while those on baricitinib for longer durations were mostly receiving 4 mg.
At treatment initiation, for both dosage groups, most patients rated their disease as either “Moderate” (16% and 23%, respectively) or “Severe” (83%/75%) (). By the time of survey completion, the number reporting severe disease fell in both groups (11% for the 2 mg group and 2% for the 4 mg group), while more patients now rated their disease as “Clear” (5%) or “Almost clear” (36%) in the 4 mg group versus 1% and 11% in the 2 mg group. High proportions of patients reporting a BSA ≥10% were found starting on the 4 mg dose (42%) and also in those who were on baricitinib for four months or more (36%).
Figure 2. Patient satisfaction with baricitinib overall and by symptom dimension on a 4-point Likert scale.
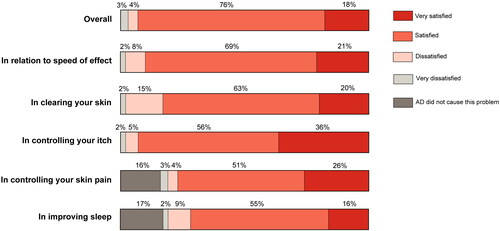
Figure 3. Patient global impression of change overall and by treatment dimension with baricitinib treatment (n = 170).
Across all facets of treatment satisfaction, a higher percentage of patients receiving the 4 mg dose reported themselves as “Very satisfied” than those on 2 mg (Supplemental Table 2). For example in itch, 42% of those on 4 mg were “very satisfied” versus 31% on 2 mg. For those on baricitinib for four months or more, 44% expressed themselves to be “Very satisfied” with how baricitinib reduced their itch versus 25% of those on 2 mg . TSQM scores tended to be higher for the 4 mg subgroup vs the 2 mg subgroup and those on baricitinib for four months or more.
Discussion
In this cross-sectional survey of patients treated with baricitinib for their AD in clinical practice, we found that among disease dimensions, satisfaction with control of itch, the most burdensome symptom for patients, was the highest. This was also reflected in particularly high rates in patients” perception of improvement in itch since starting baricitinib. Our findings regarding the medication-generic satisfaction measure, the TSQM-9, also found scores from the upper range of that measure with the highest scores seen in the convenience dimension. These findings help to provide more information about patient’s experiences in the light of new treatment options for moderate-to-severe AD, and provide important insights into the factors that contribute to patients” satisfaction with baricitinib in routine care.
One striking finding in this study was the perceived discrepancy between patient-assessed disease severity and the extent of skin lesions. At baricitinib initiation, eight out of ten patients on bariticitinib treatment rated their disease as “severe”, which was consistent across dosage and time on treatment. While only about a quarter of the patients in the sample reported a BSA of ≥10%, consistent with moderate-severe AD, patients reported a high degree of itch burden, along with sleep disturbance in the majority of patients. This itch burden was also evinced by the comparatively high rate of anti-histamine use seen in our sample prior to baricitinib initiation. This discrepancy is congruent with emerging evidence that disease severity assessment based on skin involvement alone might not reflect how patients perceive AD severity themselves and how AD impacts their quality of life (Citation20). These characteristics indicate that the patients who maintain baricitinib and are satisfied with this treatment in clinical practice are those with a significant itch burden and more restrained skin involvement. This is in contrast to the baricitinib clinical trial program in which patients were required to have a minimum of 10% BSA involvement, and accordingly, mean BSA was as high as 48–52% in patients enrolled in BREEZE-AD7 (Citation14). Our results, however, align with the findings of a recent post-hoc analysis that reported patients with moderate-to-severe AD and a BSA affecting 10–40% and Itch NRS ≥ 7 were likely to benefit most from baricitinib 4 mg TCS combination therapy (Citation21).
Patients participating in our survey exhibited high levels of systemic pretreatment, with nearly two-thirds reporting prior systemic treatment use and long disease duration, indicating a quite refractory patient population, consistent with high perceived severity at baricitinib initiation. Common reasons provided by patients for discontinuing their prior treatment were insufficient skin clearance, the effects of treatment not lasting, or having failed in reducing symptoms. These are factors where there is evidence from both the clinical trials (Citation14,Citation15) and from our study that baricitinib can provide improvements. Interestingly, of those receiving systemic corticosteroids prior to baricitinib initiation, over half were in receipt of this treatment for >1 year. However, current AD treatment guidelines recommend the use of systemic corticosteroids only as rescue therapy for acute flares (Citation12). Conversely, biologics were used for >1 year in only a quarter of patients, with most receiving biologics between four and twelve months. This indicates that many patients receiving biologics might not maintain treatment control or realize sufficient symptom reduction, underlining the importance of the growing treatment armamentarium for AD management, with biologics and oral JAK inhibitors recommended for patients who are candidates for systemic treatment, though we await further evidence confirming the longer term performance of JAK inhibitors in real-world practice (Citation12).
In our study, the reduction in symptom burden, particularly itch, in conjunction with improvements in other signs and symptoms (e.g., skin lesions, itch, skin pain, and sleep) is likely behind the high levels of satisfaction that patients reported regarding their treatment. For all aspects of burden assessed in our study, at least three-quarters of patients reported some improvement in these factors with the majority of these patients reporting their symptom was “Very much” or “Much” improved. This is particularly true for itch, where 82% of patients reported that they were “Very much” or “Much” improved since initiating baricitinib. Indeed, evidence from a recent study has shown that approximately half of the QoL improvement seen in patients treated for their AD with baricitinib is related to relief from itch (Citation22). This underlines the importance of itch as the primary treatment goal for patients, and provides routine care evidence to support the findings of the clinical trials (Citation14–16) that have shown the ability of baricitinib to relieve itch rapidly. Moreover, likely arising from improvements in skin clearance, many patients reported that they felt able to taper or even discontinue their TCS and TCI use. This indicates how baricitinib can foster steroid-sparing and can reduce the burden on patients accruing from the known long-term use side effects of TCS.
On the medication-generic satisfaction measure, the TSQM-9 we also saw scores in the upper range of the spectrum, with the highest scores seen for the convenience score, and rates comparable to or even higher compared to that seen for other systemic treatments. Evidence from the BIODAY registry (Citation23) found that dupilumab scored 78.9 on the global satisfaction score, 72.8 on the effectiveness score, and 73.4 on the convenience score. In our study, we found that baricitinib scored 62.7, 68.1, and 78.0 on the global satisfaction, effectiveness, and convenience subscales respectively. When considered against the previous generation of systemic therapies, before the advent of JAK inhibitors, we can see that across all domains of the TSQM-9, baricitinib scored higher in this study than those systemic therapies evaluated by Wei and colleagues in their 2019 paper (Citation24). Cross-study heterogeneity in populations, sampling, and methodologies, though, limits the ability to make direct comparisons, even when considering results from the same standardized measure. Overall, TSQM-9 results showed that convenience scores were the highest within that measure, which may be linked to the once-daily oral method of administration for baricitinib.
Based on the clinical trial evidence, 4 mg is the recommended dose for moderate-to-severe AD with the 2 mg dose indicated only for special patient populations and those at risk for adverse events of special interest. Our study shows that in a real-world setting, baricitinib provides the option of dose adaptability to step up or down therapy to best meet disease control needs. While many were prescribed the lower 2 mg dose initially, a sizeable proportion moved from 2 mg to 4 mg (∼20%) over the course of the study. In general, across measures, higher levels of satisfaction, greater levels of reduction in disease burden and higher skin clearance rates were seen in the 4 mg group which bears out the results of the clinical trials and underlines the opportunity for patients to achieve better disease control on the higher 4 mg dose (Citation14–16).
Strengths and limitations
Our study represents the first attempt to provide data on treatment satisfaction for patients receiving an oral JAKi for their AD in a routine clinical care setting. Such information is of particular importance as European clinical practice in this treatment space moves toward a more patient-centric model in keeping with recent guidelines (Citation12).
However, the study inclusion criteria may have biased the sample toward those patients who were more engaged and/or satisfied with their treatment, as patients who were unsatisfied may have stopped taking the treatment and were consequently not eligible for inclusion in the survey. There may be bias toward patients who were more likely to complete a questionnaire, i.e., potentially an inherently more engaged patient population. Additionally, while our study was adequately powered to allow statistical precision around rates of satisfaction, the sample size may limit the generalizability of our findings. As some data were gathered based on the retrospective observations of those participating in the study, recall bias is also an issue that can be encountered with survey data based on such observations. Finally, data for this study was gathered at one survey interaction rather than tracked across several survey time points.
Future research in this area could usefully examine several questions that were outside the scope of our research, including examining the relationship, if any, between patient-reported outcomes and treatment satisfaction, allowing for sub-analyses of treatment satisfaction by topographic site, and examining what drove discontinuation of prior lines of therapy before the initiation of baricitinib.
Conclusions
Our study sought to assess which factors contribute to treatment satisfaction for patients receiving baricitinib for their AD in a routine care setting. We found that while skin involvement was comparatively limited for our survey of patients receiving baricitinib in clinical practice, itch burden was high. We found that satisfaction with control of itch, the most burdensome symptom for patients, was particularly high. This was also reflected in high rates in patients” perception of improvement in itch since starting baricitinib. Results from the TSQM-9 showed especially high scores in the convenience dimension. Many patients were able to stop or reduce concomitant topical medication, also indicating baricitinibs’s effect in controlling AD symptoms. These findings will aid HCPs and their patients in informing treatment choices that better incorporate patient perspectives.
Author contributions
All authors contributed to the interpretation of the data, the drafting of the manuscript, and provided critical revisions to the work for important intellectual content.
Medical writing and editorial assistance
Mr. Alan Ó Céilleachair, an employee of Eli Lilly and Company, provided scientific writing and editorial support on the manuscript. This support was funded by Eli Lilly and Company.
Supplemental Material
Download Zip (2.3 MB)Acknowledgements
The authors would like to thank the participants of the study for their time and contribution to the work. Without their participation, this study would not have been possible.
Disclosure statement
MA has received grants from AbbVie, Almirall, Beiersdorf, Eli Lilly, Galderma, Incyte, LEO, Menlo, MSD, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, Trevi; consulting fees from AbbVie, Almirall, Beiersdorf, Eli Lilly, Galderma, Incyte, LEO, Menlo, MSD, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, Trevi; payments/honoraria from AbbVie, Almirall, Beiersdorf, Eli Lilly, Galderma, Incyte, LEO, Menlo, MSD, Novartis, Pfizer, Regeneron, Sanofi-Genzyme, Trevi.
AN has received grants from Sanofi, Regeneron, Pierre Fabre, Janssen Cilag, Celgene, Eli Lilly, Leo Pharma, Galderma, AbbVie; consulting fees from Sanofi Regeneron, Pierre Fabre, Novartis, Galderma, Pfizer, AbbVie, Eli Lilly, Leo Pharma, Medac, Almirall; payment/honoraria from ALK, Sanofi Regeneron, Novartis, AbbVie, Pierre Fabre, Eli Lilly, Medac, Pfizer, L’Oréal; meetings/travel support from Sanofi Regeneron, AbbVie, Janssen. She has participated on Advisory Boards for Sanofi Regeneron, AbbVie, Eli Lilly and Company, Pfizer, Leo Pharma.
TW has received payment for presentations from Eli Lilly, Sanofi-Regeneron, Abbvie, Pfizer, Galderma, Leo; participated on Advisory Boards for Eli Lilly, Sanofi-Regeneron, Abbvie, Pfizer, Galderma, Leo, holds office with German Societies for Dermatoloy (DDG)/Allergology (DGAKI).
ADI has received consulting fees from AbbVie, Eli Lilly, Pfizer, Benevolent AI, Arena, Novartis, Regeneron, Sanofi, Leo Pharma; payments/honoraria from Regeneron, Sanofi, AbbVie, Eli Lilly, Leo Pharma, Janssen; has a patent issued with Johnson and Johnson. He has participated on Advisory Boards for Novartis, OM Pharma. President Elect of the International Eczema Council.
SG, AL, CR, and NT are employees/minor shareholders of Eli Lilly.
ER, a former Eli Lilly employee, has received consulting fees from Eli Lilly, payments/honoraria from Eli Lilly, Pelpharma; and has been issued patents. The study, analysis and all costs relating to the publication of this manuscript, were funded by Eli Lilly and Company.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available as consent was not received from participants for the sharing of their data with third parties.
Notes
1 BSA involvement was determined based on the number of palms required to cover those parts of the patient’s body that were affected by their AD, with one palm = 1% body area coverage.
References
- Akdis CA, Akdis M, Bieber T, et al. Diagnosis and treatment of atopic dermatitis in children and adults: european academy of allergology and clinical immunology/American Academy of allergy, asthma and immunology/PRACTALL consensus report. J Allergy Clin Immunol. 2006;118(1):1–8. doi: 10.1016/j.jaci.2006.03.045.
- Lee SY, Lee E, Park YM, et al. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol Res. 2018;10(4):354–362. doi: 10.4168/aair.2018.10.4.354.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023.
- Laughter M, Maymone M, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the global burden of disease study 1990–2017. Br J Dermatol. 2021;184(2):304–309. doi: 10.1111/bjd.19580.
- Legat FJ. Itch in atopic dermatitis - What is new? Front Med . 2021;8:644760. doi: 10.3389/fmed.2021.644760.
- Augustin M, Langenbruch A, Blome C, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol. 2020;34(1):142–152. Jandoi: 10.1111/jdv.15919.
- Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol. 2018; 142(4):1033–1040. doi: 10.1016/j.jaci.2018.08.005.
- Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006.
- Brunner PM, Silverberg JI, Guttman-Yassky E, et al. Increasing comorbidities suggest that atopic dermatitis is a systemic disorder. J Invest Dermatol. 2017;137(1):18–25. doi: 10.1016/j.jid.2016.08.022.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019; 29(3):250–258. doi: 10.1684/ejd.2019.3557.
- Paller A, Jaworski JC, Simpson EL, et al. Major comorbidities of atopic dermatitis: beyond allergic disorders. Am J Clin Dermatol. 2018;19(6):821–838. doi: 10.1007/s40257-018-0383-4.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. doi: 10.1111/jdv.18345.
- European Medicines A. EMA/505843/2020: an overview of olumiant and why it is authorised in the EU. Amsterdam: European Medicines Agency; 2017.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343. doi: 10.1001/jamadermatol.2020.3260.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255. doi: 10.1111/bjd.18898.
- Bieber T, Reich K, Paul C, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis with inadequate response, intolerance or contraindication to ciclosporin: results from a randomized, placebo-controlled, phase III clinical trial (BREEZE-AD4). Br J Dermatol. 2022;187(3):338–352. Sepdoi: 10.1111/bjd.21630.
- Steinke S, Langenbruch A, Ständer S, et al. Therapeutic benefits in atopic dermatitis care from the patients” perspective: results of the German national health care study “atopic health. Dermatology. 2014;228(4):350–359. doi: 10.1159/000358587.
- Bharmal M, Payne K, Atkinson MJ, et al. Validation of an abbreviated treatment satisfaction questionnaire for medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7(1):36. doi: 10.1186/1477-7525-7-36.
- Hall R, Lebwohl MG, Bushmakin AG, et al. Development and content validation of pruritus and symptoms assessment for atopic dermatitis (PSAAD) in adolescents and adults with moderate-to-severe AD. Dermatol Ther . 2021;11(1):221–233. doi: 10.1007/s13555-020-00474-9.
- Paul C, Griffiths CEM, Costanzo A, et al. Factors predicting quality of life impairment in adult patients with atopic dermatitis: results from a patient survey and machine learning analysis. Dermatol Ther . 2023;13(4):981–995. doi: 10.1007/s13555-023-00897-0.
- Thyssen JP, de Bruin-Weller M, Costanzo A, et al. Baseline body surface area and itch severity define response to baricitinib in patients with moderate-to-severe atopic dermatitis at week 16. Adv Ther. 2023;40(8):3574–3587. In Press. doi: 10.1007/s12325-023-02528-8.
- Yosipovitch G, Papp K, Forman S, et al. The contribution of itch and skin severity improvements to the dermatology life quality index in patients with atopic dermatitis in baricitinib phase III trials. Br J Dermatol. 2022;186(6):1047–1049. doi: 10.1111/bjd.21015.
- Oosterhaven JAF, Spekhorst LS, Zhang J, et al. Eczema control and treatment satisfaction in atopic dermatitis patients treated with dupilumab - a cross-sectional study from the BioDay registry. J Dermatolog Treat. 2022;33(4):1986–1989. doi: 10.1080/09546634.2021.1937485.
- Wei W, Ghorayeb E, Andria M, et al. A real-world study evaluating adeQUacy of existing systemic treatments for patients with moderate-to-severe atopic dermatitis (QUEST-AD): baseline treatment patterns and unmet needs assessment. Ann Allergy Asthma Immunol. 2019;123(4):381–388 e2. doi: 10.1016/j.anai.2019.07.008.

