Abstract
Langerhans cell histiocytosis (LCH) is a histiocytic neoplasm characterized by a mass of CD1a + CD207 + histiocytes, exhibiting a diverse range of clinical manifestations from a self-healing rash or single bone destruction to multi-organ disease with potentially fatal consequences. The identification of MAPK signaling pathway activation, particularly BRAFV600E mutations, has propelled targeted therapy into the forefront of therapeutic research for LCH. Several studies have demonstrated that Vemurafenib, a BRAF inhibitor, exhibits superior clinical efficacy and a more favorable safety profile in LCH. Herein, in this case report, we present a good response to vemurafenib in an infant diagnosed with multisystem Langerhans cell histiocytosis (LCH).
Introduction
Vemurafenib, a selective oral inhibitor of BRAFV600 kinase, has demonstrated significant survival benefits and an approximate 50% response rate in patients with metastatic melanoma harboring the BRAFV600E mutation (Citation1). BRAFV600 mutations also occur in a wide range of non-melanoma cancers. In some of these malignancies, the selective BRAF inhibitors, particularly vemurafenib, provided an early and prominent example of a lineage-dependent response to targeted therapy (Citation2). Several investigations have suggested that LCH may have a BRAFV600 mutation. As a result, target therapy has emerged as a new research focus for the current treatment. Vemurafenib could probably be used in treating LCH. We report that an infant with multisystem LCH achieved a good response to vemurafenib.
Case description
Male infant, six months, had crusted papules on the trunk, seborrheic eczema on the scalp, and axillary erosion for three months (). This exanthema comes with itching. There are no systemic symptoms like a fever, cough, or diarrhea. Antifungal creams, hormonal creams, and antimicrobial creams were taken continuously for more than a month without any relief. The rash keeps getting worse. This baby’s usual blood tests, liver function, renal function, serum myocardial enzymes, and blood fat levels revealed no notable deviations. A biopsy of the skin showed nodular proliferation of Langerhans cells at the skin’s epidermis-dermis interface. A positive immunohistochemical result was found for S100, CD1a, Langerin, LCA (Leucocyte common Antigen), CD68, and BRAFV600E (). Heart ultrasound imaging revealed a patent foramen ovale and minor tricuspid regurgitation. The ultrasound didn’t reveal any abnormalities in the pancreas, spleen, liver, and gallbladder. There were no visible abnormalities found in the pituitary gland, according to an MRI of the organ. According to a thoracic CT scan, the liver exhibited a low-density S2 shadow and both lungs showed distributed inflammation. The thymus also had several calcifications and a slightly enlarged heart shadow. The X-ray revealed that both lungs had a thicker texture, whereas the bones of the hand and wrists, thoracolumbar vertebrae, and head were normal.
Figure 1. Skin lesions including truncus, axilla, and scalp(A,B,C,E)before treatment. After two weeks of treatment, the truncus and scalp lesions basically subsided (D,F).
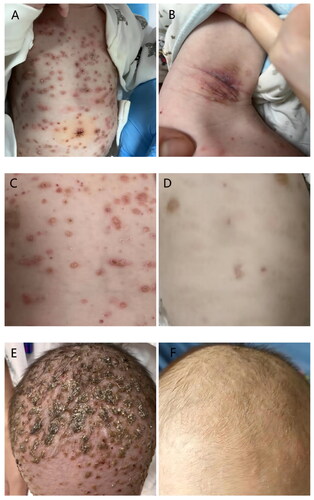
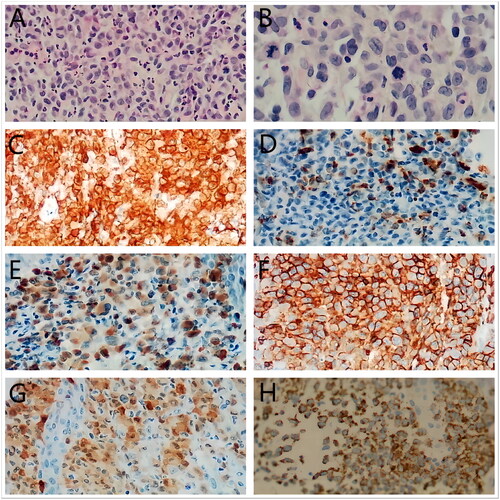
Figure 2. Histopathological features. (A,B) The characteristic infiltrate on hematoxylin and eosin (A) ×200, (B) ×400 was a large number of Langerhans cells with grooved or indented nuclei and abundant eosinophilic cytoplasms. Rare mitotic figures were seen and atypical form were presented. (C) CD1a(membranous), (D) CD68(cytoplasmic), (E) Langerin (cytoplasmic), (F) LCA(membranous), (G) S100 (cytoplasmic) staining showed positive results in the cell infiltrations(×200), (H) The mutant-specific antibody clone VE1 detects the BRAFV600E mutation by immunohistochemistry with 3+ strong cytoplasmic staining.
Blood and bone marrow samples tested positive for BRAFV600E, with mutation loads of 0.105% and 0.137%, respectively. A bone marrow cytology showed that the bone marrow was hyperplastic. To sum up, this infant was diagnosed with multisystem LCH, which is accompanied by BRAFV600E mutation.
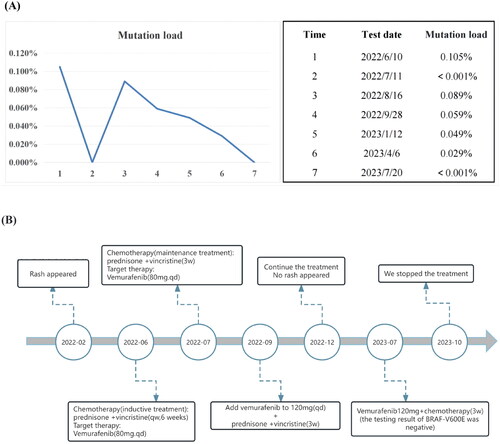
We originally utilized standard chemotherapy, which consisted of prednisone and vincristine once a week. Considering the BRAFV600E of the blood and bone marrow was positive and multiple systems were involved. The standard chemotherapy often had a limited therapeutic effect in multisystem LCH. With the parent’s approval, we added vemurafenib. This patient was underweight (about 6 kg) and had no weight gain for nearly three months. At the same time, we combined chemotherapy. So we used a conservative dose (vemurafenib 80 mg every day). The tablets (240 mg each) were split, crushed, and dissolved in water or milk to this small child. After two weeks, the rash greatly improved (). The load of BRAFV600E was less than 0.001%, as shown by retesting during the fifth chemotherapy. We began prednisone and vincristine as a maintenance treatment every three weeks after six weeks. We rechecked the BRAFV600E blood load after the first maintenance treatment, and the mutation load was 0.089%, which was somewhat increased. The rash then appears to have partially returned. We increased the vemurafenib dose to 120 mg/d in light of this circumstance. Chemotherapy was continued as a maintenance treatment. During the fourth maintenance therapy, the CT scan revealed that the low-density S2 shadow in the liver had diminished and that the dispersed inflammation in both lungs had vanished, while no rash had recently emerged. Subsequently, chemotherapy was maintained, combined with 120 mg of vemurafenib, and the load of BRAFV600E continued to decline. () Results indicate that vemurafenib paired with conventional chemotherapy effectively controls the illness. The last time we checked the mutation load of BRAF which indicated negative, the rash did not recur. Therefore, we stopped taking the medication in October. longer follow-up, including post-treatment discontinuation, will be necessary to assess the long-term efficacy.
Figure 3. (A) Mutation load of BRAFv600E during treatment. We use the droplet digital PCR(ddPCR) in ctDNA to monitor mutant BRAF. When the result is less than 0.001%, the mutation load is shown as ‘<0.001%’ and the test result is reported as ‘negative’; when the result is greater than 0.001% or less than 0.1%, the mutation load is shown as ‘specific value’ and the test result is reported as ‘positive (for reference)’; when the result is greater than or equal to 0.1%, the mutation load is reported as ‘specific value’, and the test result is reported as ‘positive’. (B) The figure show the types and duration of the various therapies applied to this patient.
Discussion
LCH is a histiocytic neoplasm characterized by abundant CD1a+/CD207+ cells (Citation3). It can occur at any age, with a higher incidence in children (5 cases per million annually, compared to 1 to 2 cases per million in adults) (Citation4). Depending on the extent of organ or system involvement, it is classified as either single-system LCH (SS-LCH) or multisystem LCH (MS-LCH). MS-LCH patients typically receive treatment regimens involving chemotherapy, targeted therapy, and hematopoietic stem cell transplantation.
Studies have shown that mutations in BRAFv600E mutations are associated with inferior clinical outcomes in multisystem LCH (Citation5). Therefore, it is imperative for clinicians to perform immunohistochemical and molecular analysis for gene detection when diagnosing MS-LCH, as this serves as a crucial foundation for tailored precision treatments.
In our study, the BRAFV600E mutation load exhibited a rapid decline following VMF initiation, accompanied by subsiding of the rash. However, after a few months, there was a recurrence of the rash concomitant with an increase in the mutation load. These findings are consistent with previous studies demonstrating decreased plasma levels of the BRAFV600E mutation load as an indicator of treatment efficacy. Conversely, rising indices suggest disease activity and potential relapse (Citation6). Thus, monitoring changes in BRAFV600E mutation load can be a valuable indicator for assessing treatment response.
Donadieu’s study (Citation7) demonstrated that the blood BRAFV600E mutation load remained positive during VMF treatment, which was different from our study and Awada’study (Citation6). It may be related to the test method or a combined treatment. We used the plasma BRAFV600E-mutant circulating tumor DNA(ctDNA) to monitor the blood BRAFV600E mutation load that turned negative during treatment, whereas Donadieu’s study used circulating cell-free DNA(cfDNA) to monitor the mutation load. Therefore, the study and comparison of BRAFV600E DNA detection methods are conducive to the regulation of disease surveillance. In addition, we initially utilized vemurafenib in combination with chemotherapy, while it is now extremely debatable, this combination may be an effective treatment.
The duration of maintenance treatment remains a critical concern. One study (Citation8) demonstrated that discontinuing the VMF after a total of 8 months of treatment resulted in disease relapse in LCH patients. Another study showed that VMF combined with chemotherapy (vincristine and prednisone) was discontinued after 12 months of MS-LCH treatment, with patients in complete remission with no recurrence after 12 months of follow-up (Citation9). Therefore, further clinical trials are needed to determine the optimal treatment duration, which may potentially exceed one year. Similarly, Goyal et al. (Citation10) suggested that cessation of BRAF inhibitors in adults would likely lead to disease reoccurrence, necessitating continuous treatment.
The potential adverse effects of BRAF inhibitors in LCH should not be disregarded. Commonly reported adverse events include skin rash, exacerbated skin photosensitivity, alopecia, panniculitis, hyperkeratosis, QT-interval prolongation, joint pain, liver damage, and hypertension (Citation7,Citation11). Although this patient did not experience these side effects, Clinicians should still enhance their awareness and promptly identify these adverse events.
Taken together, this study demonstrated that vemufenib and chemotherapy produced a rapid clinical response and high level of security in a baby with MS-LCH. More research is required to determine the ideal dosage, duration, combination, and safety of this therapy.
Ethical approval
Informed written patient consent was taken from the patient.
Immunohistochemistry(IHC) assay
Antibodies used for IHC are as follows: antibodies against CD1a(AQ medical, China, RTU-CD1a-235-QH, clone:MTB1, RTU), CD68(AQ medical, China, NCL-CD68, clone:KP1, 1:400), Langerin(Gene Tech, China, GT209002, clone:12D6, RTU), LCA(LANOU medical, China, LMO-1042, clone:2B11, RTU), S100(AQ medical, China, NCL-L-S100, clone:polyclone, 1:500), and BRAFV600E(Roche, CH, 07862270001, clone:VE1, RTU).
Acknowledgement
The wish to acknowledge Dr.Zhenglian Ha for her collaboration in working with the pathology of this case, as well as the assistance provided by the pediatrics department at Southern Hospital.
Disclosure statement
No potential conflict of interest was reported by the author.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the author.
Additional information
Funding
References
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):1–5. doi: 10.1056/NEJMoa1502309.
- Subbiah V, Puzanov I, Blay JY, et al. Pan-cancer efficacy of vemurafenib in BRAF (V600)-mutant non-melanoma cancers. Cancer Discov. 2020;10(5):657–663. doi: 10.1158/2159-8290.CD-19-1265.
- Rodriguez-Galindo C, Allen CE. Langerhans cell histiocytosis. Blood. 2020;135(16):1319–1331. doi: 10.1182/blood.2019000934.
- Allen CE, Ladisch S, Mcclain KL. How I treat langerhans cell histiocytosis. Blood. 2015;126(1):26–35. doi: 10.1182/blood-2014-12-569301.
- Kemps PG, Zondag TCE, Arnardóttir HB, et al. Clinicogenomic associations in childhood langerhans cell histiocytosis: an international cohort study. Blood Adv. 2023;7(4):664–679. doi: 10.1182/bloodadvances.2022007947.
- Awada G, Seremet T, Fostier K, et al. Long-term disease control of langerhans cell histiocytosis using combined BRAF and MEK inhibition. Blood Adv. 2018;2(16):2156–2158. doi: 10.1182/bloodadvances.2018021782.
- Donadieu J, Larabi IA, Tardieu M, et al. Vemurafenib for refractory multisystem langerhans cell histiocytosis in children: an international observational study. J Clin Oncol. 2019;37(31):2857–2865. doi: 10.1200/JCO.19.00456.
- Kolenová A, Schwentner R, Jug G, et al. Targeted inhibition of the MAPK pathway: emerging salvage option for progressive life-threatening multisystem LCH. Blood Adv. 2017;1(6):352–356. doi: 10.1182/bloodadvances.2016003533.
- Gaspari S, Di Ruscio V, Stocchi F, et al. Case report: early association of vemurafenib to standard chemotherapy in multisystem langerhans cell histiocytosis in a newborn: taking a chance for a better outcome?. Front Oncol. 2021;11:794498. (doi: 10.3389/fonc.2021.794498.
- Goyal G, Tazi A, Go RS, et al. International expert consensus recommendations for the diagnosis and treatment of langerhans cell histiocytosis in adults. Blood. 2022;139(17):2601–2621. doi: 10.1182/blood.2021014343.
- Diamond EL, Subbiah V, Lockhart AC, et al. Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and langerhans cell histiocytosis: analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. JAMA Oncol. 2018;4(3):384–388. doi: 10.1001/jamaoncol.2017.5029.

