Abstract
Palmoplantar pustulosis (PPP) is a rare chronic pustular disease. Psoriatic arthritis (PsA) is one of the common manifestations of arthritis in PPP associated with a high burden of disease. The treatment of PPP is difficult and still in the exploratory stage. Only a few cases show that PPP complicated with arthritis have been successfully treated with janus kinase inhibition, interleukin (IL)-6 inhibitors, IL-12/23 inhibitors and tumor necrosis factor inhibitors. Here we reported that two patients were diagnosed as PPP with PsA and initially treated with IL-17 inhibitors. One case was only partially relieved, and the other case had severe paradoxical reaction in the trunk. The joint and skin condition of two patients had been significantly improved without reported adverse reactions after 18 weeks treatment with upadacitinib, which support upadacitinib may be a potential option for patients with PPP combined PsA.
Introduction
Palmoplantar pustulosis (PPP) is a chronic inflammatory skin disease characterized by recurring sterile pustules, erythema, scale, and fissures on the palms and (or) soles. The prevalence of PPP is approximately 0.01–0.05% (Citation1). Female patients are more commonly involved. Nail and psoriatic arthritis (PsA) are common complications. It is reported that the prevalence of PsA in PPP is from 8.6 to 26% (Citation2). The pathogenesis of PPP is still unclear while the increased levels of inflammatory molecules are detected in vivo, such as interleukin (IL)-17, IL-1 and IL-23 (Citation3).
As serious bone-joint involvement in some patients of PPP may have a great impact on quality of life. There is no guideline or consensus for the treatment of PPP, nor to PPP combined with PsA. Only a few cases show that PPP complicated with arthritis have been successfully treated by with janus kinase (JAK) inhibitors (tofacitinib), IL-6 inhibitors (tocilizumab), IL-12/23 inhibitors (ustekinumab) and tumor necrosis factor inhibitors (TNFi, such as adalimumab) (Citation4,Citation5). Upadacitinib is a novel oral small molecule Janus kinase (JAK) inhibitor-1. It is FDA-approved to treat rheumatoid arthritis (2019), PsA (2021) and moderate-to-severe atopic dermatitis (2022). Only two cases reported efficient response of PPP to upadacitinib (Citation4,Citation6). Here we reported that successfully response of PPP with PsA to upadacitinib.
Case report
Patient 1 was a 47-year-old Chinese woman who presented to our clinic with painless pustules on her hands and feet in 2021. The patient developed swelling of some fingers in both hands and pain in the low back after 3 months. The detail characteristics were showed in . Laboratory tests showed elevated erythrocyte sedimentation rate (32 mm/h), hypersensitive C-reactive protein (CRP, 7.95 mg/L) and tuberculosis-interferon gamma release assay (TB-IGRA). Human leukocyte antigen-B27 (HLA-B27) was negative. Sagittal T2-weighted magnetic resonance images (MRI) revealed abnormal signals in L2-S1, right sacrum and right ilium, which indicated bone inflammation. The diagnosis of PPP with PsA was established based on the characteristic clinical and imaging examination.
Table 1. Characteristics of two patients with Palmoplantar pustulosis (PPP).
She had previously received treatment with ixekizumab, which was discontinued due to the pustule on the palmoplantar get worse and develop new red papules on the trunk after 6 weeks of therapy. Then, we decided to switch the patient to upadacitinib. After treatment with upadacitinib 15 mg daily for 4 weeks, the partial response was observed. After 20 weeks, the skin condition (), nail condition (Supplementary Figure 1) and pain of the low back showed significantly improvement (). The scores of skin and joint conditions were shown in .
Figure 1. Clinical features of the two patients. (A) Palm of patient 1 before using upadacitinib. (B) Sole of patient 1 before using upadacitinib. (C) Palm of patient 2 before using upadacitinib. (D) The sole of patient 2 before using upadacitinib. (E) Palm of patient 1 after using upadacitinib for 20 weeks. (F) Sole of patient 1 after using upadacitinib for 20 weeks. (G) Palm of patient 2 after using upadacitinib for 18 weeks. (H) Sole of patient 2 after using upadacitinib for 18 weeks.
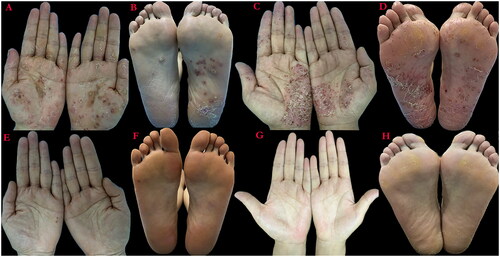
Table 2. The palmoplantar and joint conditions of the two patients.
Patient 2 was a 54-year-old Chinese female with erythema and pain pustules on her palmoplantar region for one year. After one month, the patient developed lumbar and sacroiliac joint pain. Laboratory tests showed elevated CRP (37.2 mg/L), TB-IGRA (61.87 pg/ml) and antinuclear antibodies (ANA, suspicious positive). Rheumatoid factor (RF) and HLA-B27 were all negative. MRI and Computed Tomography (CT) revealed bilateral sacroiliitis. The diagnosis was PPP with PsA. The detail data were showed in .
Secukinumab was initiated to apply. The patient showed a partial response on skin and joint pain after 6 weeks of the therapy, while after 14 weeks her symptoms had not improved further, which resulted in switching to upadacitinib. After treatment with upadacitinib 15 mg daily for 4 weeks, the partial response was observed. After 18 weeks, the skin condition () and pain of the low back showed completely improvement (). The scores of skin and joint conditions were shown in .
Discussion
Here, we reported two cases of upadacitinib successfully treating PPP with PsA. To our knowledge, the upadacitinib in the treatment of PPP with PsA had not been reported in English literatures.
It is difficult to diagnose PPP with PsA from with SAPHO sydrome in clinic. PsA is one of the common manifestations of arthritis in PPP associated with a high burden of disease (Citation7). Although some observational studies found that patient with PPP with co-occurring psoriasis vulgaris have a higher prevalence of PsA (Citation8–10), there was a lack of precise data about the association of PPP with PsA. Usually, the SAPHO syndrome (synovitis, acne, pustulosis, hyperostosis, osteitis) and pustulotic arthro-osteitis (PAO) are considered as the most common PPP-associated disorders that often associated with bone-joint involvement. The SAPHO sydrome is often to be considered an umbrella containing many diseases, including PAO, PsA and spondyloarthritis, etc. (Citation11). In 1997, Kahn firstly proposed the criteria for SAPHO syndrome and revised in 2003 (Citation12). Inflammatory lesions at the sternoclavicular is a classic feature of SAPHO syndrome. However, the understanding of SAPHO syndrome is still insufficient. In this case, Patient 1 had a history of psoriasis lesions in the palm and sole, nail involvement and finger joint swelling. Patient 2 had psoriasis lesions in the palm and sole, and her father suffered from psoriasis, and the RA test is negative. According to classification critetia for psoriatic arthritis (CASPAR) diagnostic criteria, the diagnosis of PsA in the two patients was established.
There is no guideline or consensus for the treatment of PPP, nor to PPP combined with PsA. Usually, patients with PPP with PsA can use antirheumatic drugs, followed by biological agent therapy such as IL-12/23 inhibitors, TNFi and IL-17 inhibitors, or other targeted therapies such as JAK inhibition (upadacitinib) and phosphodiesterase-4 inhibition (apremilast) (Citation13). Therefore, we initially choose IL-17 inhibitors as the initial treatment, and their joint condition had partially improved. However, the skin condition had not improved obviously, and one of patients even had serious paradoxical reaction on her trunk. So, we decided to stop using IL-17 inhibitors. Considering that upadacitinib is a novel selective oral systemic JAK inhibitors that has approval for PsA, and might work on PPP (Citation14–16), we decided to switch to upadacitinib for treatment. After one month of treatment, the skin and joint of the two patients were obviously improved. The joint and skin condition of two patients had been significantly continuously improved without other adverse reactions in the next three months. Thus, our case report showed upadacitinib may be a potential treatment option for refractory PPP with PsA. However, more clinical studies were still needed to confirm it.
Conclusions
PPP with PsA is a rare condition and still at the exploratory stage for treatment. Based on our case report, upadacitinib seems to be a potential and promising option for PPP with PsA.
Ethical approval
The patients in this manuscript has given written informed consent to publication of their case details.
Author contributions
ZYO-Z collected samples and write the paper. WY-M draft the figures and charts. YY-W analyzed the data and revised this manuscript. W-L performed clinical evaluation, sample collection, treatment and planned experimental design. All authors read and approved the final manuscript.
Supplemental Material
Download PNG Image (645 KB)Acknowledgements
We thank the patients for their participation in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mrowietz U, van de Kerkhof PC. Management of palmoplantar pustulosis: do we need to change? Br J Dermatol. 2011;164(5):1–4. doi: 10.1111/j.1365-2133.2011.10233.x.
- Brunasso AMG, Massone C. Recent advances in palmoplantar pustulosis. Fac Rev. 2021;10:62. doi: 10.12703/r/10-62.
- Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21(3):355–370. doi: 10.1007/s40257-020-00503-5.
- Ma M, Lu S, Hou X, et al. Novel JAK-1 inhibitor upadacitinib as a possible treatment for refractory SAPHO syndrome: a case report. Int J Rheum Dis. 2023;26(11):2335–2337. doi: 10.1111/1756-185X.14774.
- Koga T, Sato T, Umeda M, et al. Successful treatment of palmoplantar pustulosis with rheumatoid arthritis, with tofacitinib: impact of this JAK inhibitor on T-cell differentiation. Clin Immunol. 2016;173:147–148. doi: 10.1016/j.clim.2016.10.003.
- Mohr J, Rahbar Kooybaran N, Schon MP, et al. Response of palmoplantar pustulosis to upadacitinib. J Dtsch Dermatol Ges. 2023;21(3):280–282. doi: 10.1111/ddg.14969.
- Coates LC, Soriano ER, Corp N, et al. Group for research and assessment of psoriasis and psoriatic arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–479. doi: 10.1038/s41584-022-00798-0.
- Andersen YMF, Augustin M, Petersen J, et al. Characteristics and prevalence of plaque psoriasis in patients with palmoplantar pustulosis. Br J Dermatol. 2019;181(5):976–982. doi: 10.1111/bjd.17832.
- Trattner H, Bluml S, Steiner I, et al. Quality of life and comorbidities in palmoplantar pustulosis - a cross-sectional study on 102 patients. J Eur Acad Dermatol Venereol. 2017;31(10):1681–1685. doi: 10.1111/jdv.14187.
- Wang HM, Xu JM, Jin HZ. Characteristics and burdens of disease in patients from Beijing with generalized pustular psoriasis and palmoplantar pustulosis: multicenter retrospective cohort study using a regional database. Am J Clin Dermatol. 2023;24(6):991–1002. doi: 10.1007/s40257-023-00807-2.
- Furer V, Kishimoto M, Tsuji S, et al. The diagnosis and treatment of adult patients with SAPHO syndrome: controversies revealed in a multidisciplinary international survey of physicians. Rheumatol Ther. 2020;7(4):883–891. doi: 10.1007/s40744-020-00235-2.
- Hayem G. SAPHO syndrome. Rev Prat. 2004;54(15):1635–1636.
- Coates LC, Kavanaugh A, Mease PJ, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573.
- McInnes IB, Anderson JK, Magrey M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227–1239. doi: 10.1056/NEJMoa2022516.
- Burmester GR, Cohen SB, Winthrop KL, et al. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023;9(1):e002735. doi: 10.1136/rmdopen-2022-002735.
- McInnes IB, Kato K, Magrey M, et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7(3):e001838. doi: 10.1136/rmdopen-2021-001838.

