Abstract
For many patients including those with psoriasis, scientific manuscripts comprising clinical outcomes including psoriasis area severity index (PASI) and/or physician global assessment (PGA) may be difficult to understand. However, most patients can relate to images at baseline and follow-up, particularly for dermatological diseases. This study aimed to assess the proportion of shared clinical images in psoriasis trials. A systematic review adhering to the PRISMA guidelines was performed. The review was limited to randomized controlled trials, and among these, only investigations involving biological agents for treatment of psoriasis were included. The Embase, MEDLINE and Scopus databases were searched for eligible studies published from inception to October 26, 2021. In total, 152 studies were included. When combining these, 62,871 patients were randomized. Overall, 203 images were shared depicting 60 patients in the manuscripts yielding an overall sharing rate of 0.1%. Patient images are seldom incorporated in clinical trial manuscripts which impairs interpretation for patients. Inclusion of image material would strengthen the patients’ perspective and understanding on what treatment effects that can be expected. As such, this systematic review should be an invitation to the pharmaceutical industry, other sponsors, and editorial offices to improve easy transfer of information to patients using image data.
Introduction
Psoriasis is one of the most prevalent immune-mediated inflammatory diseases and is characterized by cutaneous plaques (Citation1). The disease may have significant impact on quality of life and is associated with several comorbidities including psoriatic arthritis, depression and cardiovascular disease (Citation2,Citation3). The treatment options for patients with psoriasis is rapidly expanding and during the past two decades several biologics have been introduced (Citation4,Citation5). Despite expiring patents and the introduction of biosimilars (Citation6–9), these drugs are costly and have a considerable impact on health care budgets worldwide.
In randomized prospective clinical trials involving patients with psoriasis, the most often used primary outcome is based on improvement of baseline psoriasis area severity index (PASI) and physician global assessment (PGA). PASI is an old scoring system introduced in the 1970s, and is a composite score for redness, scaling, induration and distribution in predefined body areas (Citation10). Thus, higher scores do not simply correlate with disease distribution, and PASI correlates poorly with quality of life impact in psoriasis (Citation11,Citation12). In a Delphi consensus meeting arranged by the International Psoriasis Council, the use of body surface area, along with disease involving special sites and failure to topical therapy was the preferred measures to define those patients that would require systemic treatment. The debate regarding what should be the preferred primary clinical outcome in psoriasis trials is ongoing (Citation13,Citation14).
Shared decision making is a collaborative process in which a healthcare professional works together with a patient to reach a decision about care (Citation15). In contemporary medicine, shared decision making should be the norm. For many patients, scientific manuscripts, and scores such as PASI are often hard to understand. Moreover, scientific manuscripts have traditionally not been tailored for patients. However, most patients can relate to images at baseline and during follow-up. Inclusion of image material along with the original manuscript certainly strengthens the patients’ perspective and understanding on what treatment effects that can be expected and could be an integral part of future patient decision aids.
The aim of this systematic review was to investigate at what proportion clinical images are shared in randomized prospective clinical trials including patient with psoriasis and biological treatments.
Materials and methods
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for all applicable items (Citation16). The PRISMA checklist is available in the supplementary material (Appendix S1). Prior the initiation, the review protocol (CRD42021286077) was published and registered at the PROSPERO platform (October 26, 2021) (Citation17).
Eligibility criteria
Population: no geographic restriction was imposed.
Psoriasis type: all psoriasis types except pustular subtypes.
Study design: randomized controlled trials published within the specified time frame, from the inception of selected databases until October 26, 2021.
Study drugs: biological treatment options.
Exclusion criteria
Investigations only in psoriatic arthritis (PsA).
Patients <18 years at inclusion (i.e. pediatric indication).
Follow-up, post-hoc or extension investigations.
Pustular palmoplantar psoriasis.
Non-English investigations.
Information sources and search strategy
The Embase (Ovid), MEDLINE (PubMed) and Scopus databases were searched for eligible studies published from inception to October 26, 2021. The search strings used (Appendix S2) were constructed in collaboration with medical librarians. A standard method was used to identify and remove duplicates (Citation18).
Selection and data collection process
Two reviewers (SP and FA) screened the titles and abstracts of all studies. In case of disagreement in the title/abstract screening the investigation was included in the full-text screening phase. All full texts were reviewed independently by both authors. Any disagreement about eligibility was resolved using a third reviewer (AE). The data obtained from the included investigations were verified by SP and FA.
Data items
The following items were extracted from included studies: first author; year of publication; journal; digital object identifier (DOI)-link (if available); primary outcome (if available); time to primary outcome (if available); number of randomized patients, number of patients depicted and number of available images in the manuscript (including all supplementary material). If an investigation included ≥2 stages, only the number of patients adding up to the primary outcome was included in the calculation. A data extraction work sheet was used to systematically address all data points above (Table S1).
Study risk of bias assessment
Due to the nature of this systematic review (i.e. dichotomous outcome) we did not use any quality assessments tools or tested for publication bias.
Effect measures and statistics
The measure for this review was binary (i.e. presence or absence of images). The proportion of shared images in each included investigation and for the complete dataset was calculated. Proportion rates was also analyzed for each represented medical journal. Three softwares; EndNote (Clarivate Analytics, Philadelphia, PA, USA), Rayyan (Rayyan Systems Inc., Cambridge, MA, USA) and Mendeley (Elsevier, Amsterdam, the Netherlands) were used throughout to compile and sort the records. All publications including data extraction were handled manually, and no automation software tools were used. The original EndNote libraries used for the review are available on request to the corresponding author. Microsoft Excel (Microsoft, Redmond WA, USA) was used for data tabulation and data extraction. Fisher’s exact test was used to analyze whether more recent publications (from 2017 and onwards) were more predisposed to sharing patient images. The year 2017 was selected since it aligns with the updated requirements for data sharing published by the International Committee of Medical Journal Editors (Citation19).
Results
Of the 1,918 records first identified, 219 investigations were reviewed in full text. Among these, 67 investigations were excluded (Table S2). After exclusions, 152 studies published in the time period of 2001 to 2021 were included in the analysis () (Citation20–171). Overall, 31 medical journals were represented (Table S3).
Figure 1. PRISMA flow chart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. #All excluded reports are available in Table S2. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71.doi: 10.1136/bmj.n71.
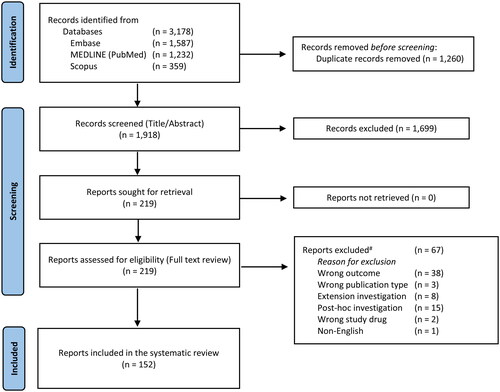
When combining the 152 investigations above, 62,871 patients were randomized. In total, 32 investigations included patients image material in the running manuscript and three additional investigations shared patient images in the supplementary material (). When combining these, 203 images were shared depicting 60 patients in the manuscripts (including all supplementary text and video material) yielding an overall sharing rate of 0.1% (range 0.1% to 25.0%; interquartile range 0.3,4.4%). Six investigations included video supplements (Citation26,Citation35,Citation110,Citation136,Citation148,Citation163), but only one of these included patient images (Citation26).
The majority of patients (n = 51,857) were randomized in trials that included PASI75, PASI90, or PASI100 (i.e. 75, 90, or 100% improvement of PASI compared to baseline) in the primary outcome. Among these individuals, 23 patients (0.04%) were depicted. The five medical journals with the highest number of randomized patients included 50,809 individuals which comprised 80.8% of all included individuals in the review. Overall, 32 patients (0.06%) of these patients were depicted ().
Investigations published from 2017 (n = 67) had a lower proportion of depicted patients compared to studies published prior to 2017 (n = 85) (0.065% vs. 0.12%; p < 0.05, Fisher’s exact test).
Table 1. List of investigations (n = 35) with included patient images in running manuscripts and supplements.
Table 2. Proportion of depicted patients in the five journals with most randomized patients.
Discussion
This systematic review highlights that image sharing in randomized controlled trials involving patient with psoriasis and biological treatment occurs very rarely. The majority (77.0%, n = 117) of all included investigations included no patient image material and only 0.1% (n = 60) of all randomized patients (n = 62,871) were depicted in the included manuscripts.
Over the past two decades, the treatment results for biologic treatment agents for patients with psoriasis have improved markedly and the number of treatment options has expanded significantly. However, the structure for reporting of randomized controlled trial data involving this group of patients has not changed much. Importantly, in this time span, digital dissemination of research data and online access has flourished. It is nowadays very easy to add supplementary material, including extensive image material, in the online version of the article. Particularly for clinical trials, data sharing of deidentified individual-patient data is nowadays the norm. In their first proposal, the International Committee of Medical Journal Editors (ICMJE) stated that there is an ethical obligation to responsibly share data generated by interventional clinical trials because participants have put themselves at risk (Citation172). Inclusion of data sharing statements in the clinical trials registration phase was listed as a requirement by the ICMJE in 2017 (Citation19). However, these requirements do not explicitly mention sharing image data. While the overall sharing rate of patient images was low, the proportion of images shared after the ratification of these guidelines was in fact lower compared to older investigations prior to 2017, albeit that most of these investigations were registered long before 2017.
We acknowledge that scientific manuscripts have traditionally not been tailored for our patients. However, in the evolving landscape of healthcare, patient empowerment and advocacy have become increasingly important. Patient advocates are now integral contributors to common care practices, with patient organizations frequently represented at the major dermatology conferences. As a result, there is a growing need to make scientific literature more accessible and patient friendly. This paradigm shift highlights the importance of bridging the gap between complex research findings and patients’ understanding, ensuring that our publications serve as valuable resources for both medical professionals and the patients they aim to help.
We see several important ancillary uses of sharing deidentified patient images. Firstly, they can be used as an external validation of the treatment effects. Secondly, the images have a high educational value for dermatologists including resident physicians and medical students. Thirdly, it increases the usefulness of the data to be used in other research settings, i.e. one recent and important interest in psoriasis research is to identify patient characteristics that may serve useful to predict treatment response. Baseline skin distribution of psoriasis might be one important factor for the selection of the appropriate treatment selection. Moreover, use of machine learning algorithms for evaluation and management of psoriasis is an idea currently pursued by several research groups (Citation173). Finally, these images could be an integral part in the patient decision aids when weighing several treatment options. Essentially, these investigations are all conducted to improve patient care and consequently researchers and trial sponsor have an ethical responsibility to promote shared decision making, which is the norm in contemporary medicine. In other words, we believe it is the research community’s responsibility to implement additional outcomes that can be easily used for patient education.
This review does not address the reason why the sharing rate is so low. We acknowledge that researchers and sponsors might be reluctant to share patient images due to privacy and medicolegal issues. Nonetheless, deidentified patient images of selected body parts illustrating treatment progress could most often be used without exposing patient anonymity. We acknowledge that editorial offices often have a restriction of how many tables and/or figures that are allowed in the running manuscript. Consequently, including a figure representing a curve of how many patients that meet the primary outcome, might be given higher priority compared to selected clinical images. However, it is nowadays very easy to add extensive supporting material, including image material, in online supplements or repositories. Moreover, supporting material that may help the reader to better understand the data presented is most often encouraged by medical journals. Some reviewers and readers in general might frown at representation of images of individual patients in manuscripts. Inclusion of a minority of patient images should of course raise important questions relating to selection bias (i.e. cherry picking). Of course, the mandatory inclusion of all available image material for all patients in supplements would help override this issue. Finally, most modern electronic case report forms enable uploading of images.
Participation in clinical trials is most often a solid commitment and time-consuming. Consequently, we believe that many patients would be interested in sharing selected and appropriately anonymized images of their skin to illustrate treatment effects. Patient consent forms would of course be mandatory, but since patients already signs multiple forms in a clinical trial, this should not be regarded as an obstacle. Since there is no standardized way of obtaining patient images for patients with psoriasis, we recognize that image material will be hard to use as a primary outcome measure. As such this review is an open invitation to such a debate, how to standardize patient images and at what visits images should be obtained. A consensus agreement how to enhance integration of image material would be a much-welcomed contribution to the dermatology community. Ideally whole-body photography should be used but this technique is far from broad implementation in routine healthcare. Nonetheless, the technical details (i.e. body part placement and photography set-up) are beyond the scope of this review. However, we advocate that use of images at baseline and at the timepoint for the primary outcome (most often 12 or 16 weeks) would be realistic and would be a supportive way of communicating the primary message of the manuscript to our patients. Most often patients can pinpoint a body region which has the highest impact on their daily lives. Preferably this body region could be selected. The minutes of extra time spent on photography and amending these images to an electronic clinical trial form stored at a safe server provided by the sponsor would be well invested. Obtaining clinical images is nowadays common practice at most dermatology clinics; it only takes a couple of minutes, and it is inexpensive since most departments already have a dedicated camera device for this purpose.
During the past years, plain language or capsule summaries have become an essential part of original reports. While there are many advantages in explaining the research in an easy way, the main purpose of these is to involve laypersons in the research. However, patient videos along with images illustrating the treatment effect of selected patients would probably convey a much stronger message to our patients. Overall, six investigations included video supplements (Citation26,Citation35,Citation110,Citation136,Citation148,Citation163), but only one included patient images (Citation26).
This study has some limitations. Firstly, for pragmatic reasons we limited the review to patients with psoriasis and biologic treatment options. Nonetheless this research question is of course equally relevant for other dermatological conditions as well as other treatment modalities. Although particularly applicable for dermatology in general, as long as disease severity clearly can be visualized in images, they can be used to illustrate the treatment progress. Secondly, small molecules such as apremilast, ciclosporin, deucravacitinib, dimethylfumarate, and methotrexate, were included only if they were used as comparative study drugs. Finally, we excluded non-English investigations.
To summarize, this review underlines that sharing patient images in clinical trials including patient with psoriasis and biological treatment options is a rare event. Moreover, sharing images has become increasingly rare during the past years despite the increased demand for data sharing in clinical trials. As such this review should be a wake-up call for researchers, sponsors, and other stakeholders to update our layout for clinical randomized trials to make them more useful for our patients.
Supplemental Material
Download PDF (714.2 KB)Acknowledgments
We thank the medical library of Sahlgrenska University Hospital and Linda Hammarbäck and Helen Sjöblom at the Biomedical Library at the University of Gothenburg for helping us generate the search strings used for this investigation.
Disclosure statement
SP: With no relation to the present manuscript, SP has received honoraria as consultant and/or speaker from AbbVie, Amgen, Astra Zeneca, Beiersdorf, Bristol Myers Squibb, Galderma, Janssen Pharmaceuticals, Leo Pharma, Novartis, and Sanofi.
FA: none.
AE: With no relation to the present manuscript, AE has received research funding from Pfizer, Eli Lilly, Novartis, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Samsung Bioepis Co., Ltd, Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Galapagos NV, Union Therapeutics, Mylan, Bristol Myers Squibb, and Janssen Pharmaceuticals.
References
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397(10281):1–12. doi: 10.1016/S0140-6736(20)32549-6.
- Andersen YMF, Wu JJ, Thyssen JP, et al. Chronologic order of appearance of immune-mediated inflammatory diseases relative to diagnosis of psoriasis. J Am Acad Dermatol. 2019;81(6):1283–1291. doi: 10.1016/j.jaad.2019.04.033.
- Egeberg A, Khalid U, Gislason GH, et al. Impact of depression on risk of myocardial infarction, stroke and cardiovascular death in patients with psoriasis: a danish nationwide study. Acta Derm Venereol. 2016;96(2):218–221. doi: 10.2340/00015555-2218.
- Ghoreschi K, Balato A, Enerback C, et al. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. 2021;397(10275):754–766. doi: 10.1016/S0140-6736(21)00184-7.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057.
- Reynolds KA, Pithadia DJ, Lee EB, et al. Safety and effectiveness of anti-Tumor necrosis Factor-Alpha biosimilar agents in the treatment of psoriasis. Am J Clin Dermatol. 2020;21(4):483–491. doi: 10.1007/s40257-020-00507-1.
- Ruiz-Villaverde R, Galan-Gutierrez M. Biosimilars in psoriasis: what should your positioning be? Expert Opin Biol Ther. 2021;21(1):81–86. doi: 10.1080/14712598.2020.1798924.
- Barker J, Girolomoni G, Egeberg A, et al. Anti-TNF biosimilars in psoriasis: from scientific evidence to real-world experience. J Dermatolog Treat. 2020;31(8):794–800. doi: 10.1080/09546634.2019.1610553.
- Cohen AD, Wu JJ, Puig L, et al. Biosimilars for psoriasis: worldwide overview of regulatory guidelines, uptake and implications for dermatology clinical practice. Br J Dermatol. 2017;177(6):1495–1502. doi: 10.1111/bjd.15756.
- Fredriksson T, Pettersson U. Severe psoriasis–oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–244. doi: 10.1159/000250839.
- Hesselvig JH, Egeberg A, Loft ND, et al. Correlation between dermatology life quality index and psoriasis area and severity index in patients with psoriasis treated with ustekinumab. Acta Derm Venereol. 2018;98(3):335–339. doi: 10.2340/00015555-2833.
- Silva MF, Fortes MR, Miot LD, et al. Psoriasis: correlation between severity index (PASI) and quality of life index (DLQI) in patients assessed before and after systemic treatment. An Bras Dermatol. 2013;88(5):760–763. doi: 10.1590/abd1806-4841.20132052.
- Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24 Suppl 2:10–16. doi: 10.1111/j.1468-3083.2009.03562.x.
- Robinson A, Kardos M, Kimball AB. Physician global assessment (PGA) and psoriasis area and severity index (PASI): why do both? A systematic analysis of randomized controlled trials of biologic agents for moderate to severe plaque psoriasis. J Am Acad Dermatol. 2012;66(3):369–375. doi: 10.1016/j.jaad.2011.01.022.
- NICE Evidence Reviews Collection. Evidence review for interventions to support effective shared decision making: shared decision making: evidence review B. London: national Institute for Health and Care Excellence (NICE) Copyright © NICE 2021. 2021.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Polesie S, Seyed-Alinaghi F, Egeberg A. A systematic review investigating the proportion of clinical images shared in prospective randomized controlled trials involving patients with psoriasis and biological agents. PROSPERO 2021 CRD42021286077 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021286077.
- Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc. 2016;104(3):240–243. doi: 10.3163/1536-5050.104.3.014.
- Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials: a requirement of the international committee of medical journal editors. Lancet. 2017;389(10086):e12–e14. doi: 10.1016/S0140-6736(17)31282-5.
- Almutairi N, Eassa B. Comparing the efficacy and safety of IL-17 inhibitors for treatment of moderate-to-severe psoriasis: a randomized double blind pilot study with a review of literature. Postepy Dermatol Alergol. 2021;38(2):281–288. doi: 10.5114/ada.2019.91496.
- AlMutairi N, Eassa BI. A randomized controlled ixekizumab Vs secukinumab trial to study the impact on sexual activity in adult patients with genital psoriasis. Expert Opin Biol Ther. 2021;21(2):297–298. doi: 10.1080/14712598.2021.1843629.
- Antoni C, Krueger GG, de Vlam K, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis. 2005;64(8):1150–1157. doi: 10.1136/ard.2004.032268.
- Asahina A, Nakagawa H, Etoh T, et al. Adalimumab in japanese patients with moderate to severe chronic plaque psoriasis: efficacy and safety results from a phase II/III randomized controlled study. J Dermatol. 2010;37(4):299–310. doi: 10.1111/j.1346-8138.2009.00748.x.
- Atalay S, van den Reek J, den Broeder AA, et al. Comparison of tightly controlled dose reduction of biologics with usual care for patients with psoriasis: a randomized clinical trial. JAMA Dermatol. 2020;156(4):393–400. doi: 10.1001/jamadermatol.2019.4897.
- Bachelez H, van de Kerkhof PC, Strohal R, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386(9993):552–561. doi: 10.1016/S0140-6736(14)62113-9.
- Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77(4):667–674. doi: 10.1016/j.jaad.2017.05.033.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67(1):86–92. doi: 10.1016/j.jaad.2011.07.034.
- Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-Week CLARITY results). Dermatol Ther (Heidelb). 2018;8(4):571–579. doi: 10.1007/s13555-018-0265-y.
- Baranauskaite A, Raffayova H, Kungurov NV, et al. Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: the RESPOND study. Ann Rheum Dis. 2012;71(4):541–548. doi: 10.1136/ard.2011.152223.
- Barker J, Hoffmann M, Wozel G, et al. Efficacy and safety of infliximab vs. methotrexate in patients with moderate-to-severe plaque psoriasis: results of an open-label, active-controlled, randomized trial (RESTORE1). Br J Dermatol. 2011;165(5):1109–1117. doi: 10.1111/j.1365-2133.2011.10615.x.
- Bissonnette R, Poulin Y, Guenther L, et al. Treatment of palmoplantar psoriasis with infliximab: a randomized, double-blind placebo-controlled study. J Eur Acad Dermatol Venereol. 2011;25(12):1402–1408. doi: 10.1111/j.1468-3083.2011.03984.x.
- Blauvelt A, Gordon KB, Lee P, et al. Efficacy, safety, usability, and acceptability of risankizumab 150 mg formulation administered by prefilled syringe or by an autoinjector for moderate to severe plaque psoriasis. J Dermatolog Treat. 2022;33(4):2085–2093. doi: 10.1080/09546634.2021.1914812.
- Blauvelt A, Lacour JP, Fowler JF, Jr., et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179(3):623–631. doi: 10.1111/bjd.16890.
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(6):649–658. doi: 10.1001/jamadermatol.2020.0723.
- Blauvelt A, Papp K, Gottlieb A, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 12-week efficacy, safety and speed of response from a randomized, double-blinded trial. Br J Dermatol. 2020;182(6):1348–1358. doi: 10.1111/bjd.18851.
- Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–417. doi: 10.1016/j.jaad.2016.11.041.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015;172(2):484–493. doi: 10.1111/bjd.13348.
- Cai L, Gu J, Zheng J, et al. Efficacy and safety of adalimumab in chinese patients with moderate-to-severe plaque psoriasis: results from a phase 3, randomized, placebo-controlled, double-blind study. J Eur Acad Dermatol Venereol. 2017;31(1):89–95. doi: 10.1111/jdv.13746.
- Cai L, Zhang JZ, Yao X, et al. Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in chinese patients with moderate to severe plaque psoriasis. Chin Med J (Engl). 2020;133(22):2665–2673. doi: 10.1097/CM9.0000000000001163.
- Callis Duffin K, Bagel J, Bukhalo M, et al. Phase 3, open-label, randomized study of the pharmacokinetics, efficacy and safety of ixekizumab following subcutaneous administration using a prefilled syringe or an autoinjector in patients with moderate-to-severe plaque psoriasis (UNCOVER-A). J Eur Acad Dermatol Venereol. 2017;31(1):107–113. doi: 10.1111/jdv.13768.
- Caproni M, Antiga E, Melani L, et al. Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: a randomized-controlled trial. J Clin Immunol. 2009;29(2):210–214. doi: 10.1007/s10875-008-9233-0.
- Chaudhari U, Romano P, Mulcahy LD, et al. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet. 2001;357(9271):1842–1847. doi: 10.1016/s0140-6736(00)04954-0.
- de Vries AC, Thio HB, de Kort WJ, et al. A prospective randomized controlled trial comparing infliximab and etanercept in patients with moderate-to-severe chronic plaque-type psoriasis: the psoriasis infliximab vs. Etanercept comparison evaluation (PIECE) study. Br J Dermatol. 2017;176(3):624–633. doi: 10.1111/bjd.14867.
- Dubertret L, Sterry W, Bos JD, et al. CLinical experience acquired with the efalizumab (raptiva) (CLEAR) trial in patients with moderate-to-severe plaque psoriasis: results from a phase III international randomized, placebo-controlled trial. Br J Dermatol. 2006;155(1):170–181. doi: 10.1111/j.1365-2133.2006.07344.x.
- Elewski BE, Okun MM, Papp K, et al. Adalimumab for nail psoriasis: efficacy and safety from the first 26 weeks of a phase 3, randomized, placebo-controlled trial. J Am Acad Dermatol. 2018;78(1):90–99 e91. doi: 10.1016/j.jaad.2017.08.029.
- Feldman SR, Reznichenko N, Pulka G, et al. Efficacy, safety and immunogenicity of AVT02 versus originator adalimumab in subjects with moderate to severe chronic plaque psoriasis: a multicentre, Double-Blind, randomised, parallel group, active control, phase III study. BioDrugs, 2021;35(6):735–748. doi: 10.1007/s40259-021-00502-w.
- Ferris LK, Ott E, Jiang J, et al. Efficacy and safety of guselkumab, administered with a novel patient-controlled injector (One-Press), for moderate-to-severe psoriasis: results from the phase 3 ORION study. J Dermatolog Treat. 2020;31(2):152–159. doi: 10.1080/09546634.2019.1587145.
- Gambichler T, Tigges C, Scola N, et al. Etanercept plus narrowband ultraviolet B phototherapy of psoriasis is more effective than etanercept monotherapy at 6 weeks. Br J Dermatol. 2011;164(6):1383–1386. doi: 10.1111/j.1365-2133.2011.10358.x.
- Gisondi P, Del Giglio M, Cotena C, et al. Combining etanercept and acitretin in the therapy of chronic plaque psoriasis: a 24-week, randomized, controlled, investigator-blinded pilot trial. Br J Dermatol. 2008;158(6):1345–1349. doi: 10.1111/j.1365-2133.2008.08564.x.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83(5):991–1001. doi: 10.1111/bcp.13185.
- Goldminz AM, Suarez-Farinas M, Wang AC, et al. CCL20 and IL22 messenger RNA expression after adalimumab vs methotrexate treatment of psoriasis: a randomized clinical trial. JAMA Dermatol. 2015;151(8):837–846. doi: 10.1001/jamadermatol.2015.0452.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373(2):136–144. doi: 10.1056/NEJMoa1501646.
- Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–486. doi: 10.1016/S0140-6736(21)00126-4.
- Gordon KB, Langley RG, Gottlieb AB, et al. A phase III, randomized, controlled trial of the fully human IL-12/23 mAb briakinumab in moderate-to-severe psoriasis. J Invest Dermatol. 2012;132(2):304–314. doi: 10.1038/jid.2011.304.
- Gordon KB, Langley RG, Leonardi C, et al. Clinical response to adalimumab treatment in patients with moderate to severe psoriasis: double-blind, randomized controlled trial and open-label extension study. J Am Acad Dermatol. 2006;55(4):598–606. doi: 10.1016/j.jaad.2006.05.027.
- Gordon KB, Papp KA, Hamilton TK, et al. Efalizumab for patients with moderate to severe plaque psoriasis: a randomized controlled trial. JAMA. 2003;290(23):3073–3080. doi: 10.1001/jama.290.23.3073.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392(10148):650–661. doi: 10.1016/S0140-6736(18)31713-6.
- Gottlieb A, Sullivan J, van Doorn M, et al. Secukinumab shows significant efficacy in palmoplantar psoriasis: results from GESTURE, a randomized controlled trial. J Am Acad Dermatol. 2017;76(1):70–80. doi: 10.1016/j.jaad.2016.07.058.
- Gottlieb AB, Blauvelt A, Thaci D, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks from 2 phase 3, multicenter, randomized, double-blinded, placebo-controlled studies (CIMPASI-1 and CIMPASI-2). J Am Acad Dermatol. 2018;79(2):302–314 e306. doi: 10.1016/j.jaad.2018.04.012.
- Gottlieb AB, Chaudhari U, Mulcahy LD, et al. Infliximab monotherapy provides rapid and sustained benefit for plaque-type psoriasis. J Am Acad Dermatol. 2003;48(6):829–835. doi: 10.1067/mjd.2003.307.
- Gottlieb AB, Evans R, Li S, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51(4):534–542. doi: 10.1016/j.jaad.2004.02.021.
- Gottlieb AB, Langley RG, Strober BE, et al. A randomized, double-blind, placebo-controlled study to evaluate the addition of methotrexate to etanercept in patients with moderate to severe plaque psoriasis. Br J Dermatol. 2012;167(3):649–657. doi: 10.1111/j.1365-2133.2012.11015.x.
- Gottlieb AB, Leonardi C, Kerdel F, et al. Efficacy and safety of briakinumab vs. etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165(3):652–660. doi: 10.1111/j.1365-2133.2011.10418.x.
- Gottlieb AB, Matheson RT, Lowe N, et al. A randomized trial of etanercept as monotherapy for psoriasis. Arch Dermatol. 2003;139(12):1627–1632; discussion 1632. doi: 10.1001/archderm.139.12.1627.
- Gottlieb AB, Merola JF, Reich K, et al. Efficacy of secukinumab and adalimumab in patients with psoriatic arthritis and concomitant moderate-to-severe plaque psoriasis: results from EXCEED, a randomized, double-blind head-to-head monotherapy study. Br J Dermatol. 2021;185(6):1124–1134. doi: 10.1111/bjd.20413.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386(9993):541–551. doi: 10.1016/S0140-6736(15)60125-8.
- Griffiths CE, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362(2):118–128. doi: 10.1056/NEJMoa0810652.
- Griffiths CEM, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomized, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, vs. the originator product in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2017;176(4):928–938. doi: 10.1111/bjd.15152.
- Harris KM, Smilek DE, Byron M, et al. Effect of costimulatory blockade with abatacept after ustekinumab withdrawal in patients with moderate to severe plaque psoriasis: the PAUSE randomized clinical trial. JAMA Dermatol. 2021;157(11):1306–1315. doi: 10.1001/jamadermatol.2021.3492.
- Hercogova J, Papp KA, Chyrok V, et al. AURIEL-PsO: a randomized, double-blind phase III equivalence trial to demonstrate the clinical similarity of the proposed biosimilar MSB11022 to reference adalimumab in patients with moderate-to-severe chronic plaque-type psoriasis. Br J Dermatol. 2020;182(2):316–326. doi: 10.1111/bjd.18220.
- Igarashi A, Kato T, Kato M, et al. Efficacy and safety of ustekinumab in japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39(3):242–252. doi: 10.1111/j.1346-8138.2011.01347.x.
- Karakawa M, Komine M, Kishimoto M, et al. Effects of maxacalcitol ointment on skin lesions in patients with psoriasis receiving treatment with adalimumab. J Dermatol. 2016;43(11):1354–1357. doi: 10.1111/1346-8138.13515.
- Kaul M, Jarvis P, Rozenberg I, et al. First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2021;35(5):1143–1151. doi: 10.1111/jdv.17071.
- Khattri S, Goldblum O, Solotkin K, et al. Early onset of clinical improvement with ixekizumab in a randomized, open-label study of patients with moderate-to-severe plaque psoriasis. J Clin Aesthet Dermatol. 2018;11:33–37.
- Kopp T, Riedl E, Bangert C, et al. Clinical improvement in psoriasis with specific targeting of interleukin-23. Nature. 2015;521(7551):222–226. doi: 10.1038/nature14175.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136(1):116–124 e117. doi: 10.1016/j.jaci.2015.01.018.
- Krueger JG, Wharton KA, Jr., Schlitt T, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750–763. doi: 10.1016/j.jaci.2019.04.029.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258.
- Langley RG, Papp K, Gooderham M, et al. Efficacy and safety of continuous every-2-week dosing of ixekizumab over 52 weeks in patients with moderate-to-severe plaque psoriasis in a randomized phase III trial (IXORA-P). Br J Dermatol. 2018;178(6):1315–1323. doi: 10.1111/bjd.16426.
- Langley RG, Tsai TF, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178(1):114–123. doi: 10.1111/bjd.15750.
- Lebwohl M, Blauvelt A, Paul C, et al. Certolizumab pegol for the treatment of chronic plaque psoriasis: results through 48 weeks of a phase 3, multicenter, randomized, double-blind, etanercept- and placebo-controlled study (CIMPACT). J Am Acad Dermatol. 2018;79(2):266–276 e265. doi: 10.1016/j.jaad.2018.04.013.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373(14):1318–1328. doi: 10.1056/NEJMoa1503824.
- Lebwohl M, Tyring SK, Hamilton TK, et al. A novel targeted T-cell modulator, efalizumab, for plaque psoriasis. N Engl J Med. 2003;349(21):2004–2013. doi: 10.1056/NEJMoa030002.
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69(3):385–392. doi: 10.1016/j.jaad.2013.03.031.
- Lee JH, Youn JI, Kim TY, et al. A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis. BMC Dermatol. 2016;16(1):11. doi: 10.1186/s12895-016-0048-z.
- Leonardi C, Langley RG, Papp K, et al. Adalimumab for treatment of moderate to severe chronic plaque psoriasis of the hands and feet: efficacy and safety results from REACH, a randomized, placebo-controlled, double-blind trial. Arch Dermatol. 2011;147(4):429–436. doi: 10.1001/archdermatol.2010.384.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366(13):1190–1199. doi: 10.1056/NEJMoa1109997.
- Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet. 2008;371(9625):1665–1674. doi: 10.1016/S0140-6736(08)60725-4.
- Leonardi CL, Papp KA, Gordon KB, et al. Extended efalizumab therapy improves chronic plaque psoriasis: results from a randomized phase III trial. J Am Acad Dermatol. 2005;52(3 Pt 1):425–433. doi: 10.1016/j.jaad.2004.09.029.
- Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349(21):2014–2022. doi: 10.1056/NEJMoa030409.
- Liu LF, Chen JS, Gu J, et al. Etanercept biosimilar (recombinant human tumor necrosis factor-alpha receptor II: igG Fc fusion protein) and methotrexate combination therapy in chinese patients with moderate-to-severe plaque psoriasis: a multicentre, randomized, double-blind, placebo-controlled trial. Arch Dermatol Res. 2020;312(6):437–445. doi: 10.1007/s00403-019-02024-6.
- Lynde CW, Gupta AK, Guenther L, et al. A randomized study comparing the combination of nbUVB and etanercept to etanercept monotherapy in patients with psoriasis who do not exhibit an excellent response after 12 weeks of etanercept. J Dermatolog Treat. 2012;23(4):261–267. doi: 10.3109/09546634.2011.607795.
- Mease PJ, Smolen JS, Behrens F, et al. A head-to-head comparison of the efficacy and safety of ixekizumab and adalimumab in biological-naive patients with active psoriatic arthritis: 24-week results of a randomised, open-label, blinded-assessor trial. Ann Rheum Dis. 2020;79(1):123–131. doi: 10.1136/annrheumdis-2019-215386.
- Menter A, Arenberger P, Balser S, et al. Similar efficacy, safety, and immunogenicity of the biosimilar BI 695501 and adalimumab reference product in patients with moderate-to-severe chronic plaque psoriasis: results from the randomized phase III VOLTAIRE-PSO study. Expert Opin Biol Ther. 2021;21(1):87–96. doi: 10.1080/14712598.2021.1851362.
- Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31 e31–15.
- Menter A, Gordon K, Carey W, et al. Efficacy and safety observed during 24 weeks of efalizumab therapy in patients with moderate to severe plaque psoriasis. Arch Dermatol. 2005;141(1):31–38. doi: 10.1001/archderm.141.1.31.
- Menter A, Tyring SK, Gordon K, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010.
- Micali G, Wilsmann-Theis D, Mallbris L, et al. Etanercept reduces symptoms and severity of psoriasis after cessation of cyclosporine therapy: results of the SCORE study. Acta Derm Venereol. 2015;95(1):57–61. doi: 10.2340/00015555-1845.
- Moore A, Gordon KB, Kang S, et al. A randomized, open-label trial of continuous versus interrupted etanercept therapy in the treatment of psoriasis. J Am Acad Dermatol. 2007;56(4):598–603. doi: 10.1016/j.jaad.2006.09.002.
- Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73(1):27–36 e21. doi: 10.1016/j.jaad.2015.04.011.
- Nakagawa H, Niiro H, Ootaki K. Brodalumab, a human anti-interleukin-17-receptor antibody in the treatment of japanese patients with moderate-to-severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81(1):44–52. doi: 10.1016/j.jdermsci.2015.10.009.
- Nemoto O, Hirose K, Shibata S, et al. Safety and efficacy of guselkumab in japanese patients with moderate-to-severe plaque psoriasis: a randomized, placebo-controlled, ascending-dose study. Br J Dermatol. 2018;178(3):689–696. doi: 10.1111/bjd.16236.
- Ohtsuki M, Fujita H, Watanabe M, et al. Efficacy and safety of risankizumab in japanese patients with moderate to severe plaque psoriasis: results from the SustaIMM phase 2/3 trial. J Dermatol. 2019;46(8):686–694. doi: 10.1111/1346-8138.14941.
- Ohtsuki M, Kubo H, Morishima H, et al. Guselkumab, an anti-interleukin-23 monoclonal antibody, for the treatment of moderate to severe plaque-type psoriasis in japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo-controlled study. J Dermatol. 2018;45(9):1053–1062. doi: 10.1111/1346-8138.14504.
- Oliver R, Krueger JG, Glatt S, et al. Bimekizumab for the treatment of moderate-to-severe plaque psoriasis: efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase IIa, randomized, double-blind multicentre study. Br J Dermatol. 2022;186(4):652–663. doi: 10.1111/bjd.20827.
- Ortonne JP, Paul C, Berardesca E, et al. A 24-week randomized clinical trial investigating the efficacy and safety of two doses of etanercept in nail psoriasis. Br J Dermatol. 2013;168(5):1080–1087. doi: 10.1111/bjd.12060.
- Papp K, Bachelez H, Costanzo A, et al. Clinical similarity of biosimilar ABP 501 to adalimumab in the treatment of patients with moderate to severe plaque psoriasis: a randomized, double-blind, multicenter, phase III study. J Am Acad Dermatol. 2017;76(6):1093–1102. doi: 10.1016/j.jaad.2016.12.014.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173(4):930–939. doi: 10.1111/bjd.13932.
- Papp KA, Barber K, Bissonnette R, et al. A randomized, blinded assessor study to evaluate the efFIcacy and safety of etanercept 50 mg once weekly plus as needed topical agent vs. Etanercept 50 mg twice weekly in patients with moderate to severe plaque psoriasis (REFINE). J Eur Acad Dermatol Venereol. 2015;29(2):361–366. doi: 10.1111/jdv.12555.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-Severe plaque psoriasis. N Engl J Med. 2017;376(16):1551–1560. doi: 10.1056/NEJMoa1607017.
- Papp KA, Bressinck R, Fretzin S, et al. Safety of efalizumab in adults with chronic moderate to severe plaque psoriasis: a phase IIIb, randomized, controlled trial. Int J Dermatol. 2006;45(5):605–614. doi: 10.1111/j.1365-4632.2006.02777.x.
- Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet. 2008;371(9625):1675–1684. doi: 10.1016/S0140-6736(08)60726-6.
- Papp KA, Langley RG, Sigurgeirsson B, et al. Efficacy and safety of secukinumab in the treatment of moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled phase II dose-ranging study. Br J Dermatol. 2013;168(2):412–421. doi: 10.1111/bjd.12110.
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366(13):1181–1189. doi: 10.1056/NEJMoa1109017.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of both interleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79(2):277–286 e210. doi: 10.1016/j.jaad.2018.03.037.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175(2):273–286. doi: 10.1111/bjd.14493.
- Papp KA, Reid C, Foley P, et al. Anti-IL-17 receptor antibody AMG 827 leads to rapid clinical response in subjects with moderate to severe psoriasis: results from a phase I, randomized, placebo-controlled trial. J Invest Dermatol. 2012;132(10):2466–2469. doi: 10.1038/jid.2012.163.
- Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152(6):1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x.
- Papp KA, Weinberg MA, Morris A, et al. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: a multicentre, randomised, placebo-controlled, phase 2b study. Lancet. 2021;397(10284):1564–1575. doi: 10.1016/S0140-6736(21)00440-2.
- Park KK, Wu JJ, Koo J. A randomized, ‘head-to-head’ pilot study comparing the effects of etanercept monotherapy vs. etanercept and narrowband ultraviolet B (NB-UVB) phototherapy in obese psoriasis patients. J Eur Acad Dermatol Venereol. 2013;27(7):899–906. doi: 10.1111/j.1468-3083.2012.04611.x.
- Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE). J Eur Acad Dermatol Venereol. 2015;29(6):1082–1090. doi: 10.1111/jdv.12751.
- Paul C, Puig L, Kragballe K, et al. Transition to ustekinumab in patients with moderate-to-severe psoriasis and inadequate response to methotrexate: a randomized clinical trial (TRANSIT). Br J Dermatol. 2014;170(2):425–434. doi: 10.1111/bjd.12646.
- Paul C, Stalder JF, Thaci D, et al. Patient satisfaction with injection devices: a randomized controlled study comparing two different etanercept delivery systems in moderate to severe psoriasis. J Eur Acad Dermatol Venereol. 2012;26(4):448–455. doi: 10.1111/j.1468-3083.2011.04093.x.
- Pinter A, Hoffmann M, Reich K, et al. A phase 4, randomized, head-to-head trial comparing the efficacy of subcutaneous injections of brodalumab to oral administrations of fumaric acid esters in adults with moderate-to-severe plaque psoriasis (CHANGE). J Eur Acad Dermatol Venereol. 2021;35(3):701–711. doi: 10.1111/jdv.16932.
- Reich K, Armstrong AW, Foley P, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double-blind, placebo- and active comparator-controlled VOYAGE 2 trial. J Am Acad Dermatol. 2017;76(3):418–431. doi: 10.1016/j.jaad.2016.11.042.
- Reich K, Armstrong AW, Langley RG, et al. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–839. doi: 10.1016/S0140-6736(19)31773-8.
- Reich K, Augustin M, Thaci D, et al. A 24-week multicentre, randomized, open-label, parallel-group study comparing the efficacy and safety of ixekizumab vs. fumaric acid esters and methotrexate in patients with moderate-to-severe plaque psoriasis naive to systemic treatment. Br J Dermatol. 2020;182(4):869–879. doi: 10.1111/bjd.18384.
- Reich K, Gooderham M, Green L, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017;31(3):507–517. doi: 10.1111/jdv.14015.
- Reich K, Gooderham M, Thaci D, et al. Risankizumab compared with adalimumab in patients with moderate-to-severe plaque psoriasis (IMMvent): a randomised, double-blind, active-comparator-controlled phase 3 trial. Lancet. 2019;394(10198):576–586. doi: 10.1016/S0140-6736(19)30952-3.
- Reich K, Korber A, Mrowietz U, et al. Secukinumab 2-weekly vs. 4-weekly dosing in patients with plaque-type psoriasis: results from the randomized GAIN study. Br J Dermatol. 2021;184(5):849–856. doi: 10.1111/bjd.19398.
- Reich K, Langley RG, Papp KA, et al. A 52-week trial comparing briakinumab with methotrexate in patients with psoriasis. N Engl J Med. 2011;365(17):1586–1596. doi: 10.1056/NEJMoa1010858.
- Reich K, Nestle FO, Papp K, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366(9494):1367–1374. doi: 10.1016/S0140-6736(05)67566-6.
- Reich K, Ortonne JP, Gottlieb AB, et al. Successful treatment of moderate to severe plaque psoriasis with the PEGylated fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167(1):180–190. doi: 10.1111/j.1365-2133.2012.10941.x.
- Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498. doi: 10.1016/S0140-6736(21)00125-2.
- Reich K, Papp KA, Blauvelt A, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390(10091):276–288. doi: 10.1016/S0140-6736(17)31279-5.
- Reich K, Pinter A, Lacour JP, et al. Comparison of ixekizumab with ustekinumab in moderate-to-severe psoriasis: 24-week results from IXORA-S, a phase III study. Br J Dermatol. 2017;177(4):1014–1023. doi: 10.1111/bjd.15666.
- Reich K, Puig L, Szepietowski JC, et al. Secukinumab dosing optimization in patients with moderate-to-severe plaque psoriasis: results from the randomized, open-label OPTIMISE study. Br J Dermatol. 2020;182(2):304–315. doi: 10.1111/bjd.18143.
- Reich K, Sullivan J, Arenberger P, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2019;181(5):954–966. doi: 10.1111/bjd.17351.
- Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–152. doi: 10.1056/NEJMoa2102383.
- Rich P, Sigurgeirsson B, Thaci D, et al. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br J Dermatol. 2013;168:402–411.
- Ryan C, Menter A, Guenther L, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol. 2018;179(4):844–852. doi: 10.1111/bjd.16736.
- Saraceno R, Pietroleonardo L, Mazzotta A, et al. TNF-alpha antagonists and nail psoriasis: an open, 24-week, prospective cohort study in adult patients with psoriasis. Expert Opin Biol Ther. 2013;13(4):469–473. doi: 10.1517/14712598.2013.736960.
- Saurat JH, Stingl G, Dubertret L, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol. 2008;158(3):558–566. doi: 10.1111/j.1365-2133.2007.08315.x.
- Seo SJ, Shin BS, Lee JH, et al. Efficacy and safety of brodalumab in the korean population for the treatment of moderate to severe plaque psoriasis: a randomized, phase III, double-blind, placebo-controlled study. J Dermatol. 2021;48(6):807–817. doi: 10.1111/1346-8138.15733.
- Sigurgeirsson B, Schakel K, Hong CH, et al. Efficacy, tolerability, patient usability, and satisfaction with a 2 mL pre-filled syringe containing secukinumab 300 mg in patients with moderate to severe plaque psoriasis: results from the phase 3 randomized, double-blind, placebo-controlled ALLURE study. J Dermatolog Treat. 2022;33(3):1718–1726. doi: 10.1080/09546634.2021.1902925.
- Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–1040. doi: 10.1016/j.jaci.2014.01.025.
- Sterry W, Ortonne JP, Kirkham B, et al. Comparison of two etanercept regimens for treatment of psoriasis and psoriatic arthritis: PRESTA randomised double blind multicentre trial. BMJ. 2010;340(feb02 2):c147–c147. doi: 10.1136/bmj.c147.
- Sticherling M, Mrowietz U, Augustin M, et al. Secukinumab is superior to fumaric acid esters in treating patients with moderate-to-severe plaque psoriasis who are naive to systemic treatments: results from the randomized controlled PRIME trial. Br J Dermatol. 2017;177(4):1024–1032. doi: 10.1111/bjd.15707.
- Strober BE, Crowley JJ, Yamauchi PS, et al. Efficacy and safety results from a phase III, randomized controlled trial comparing the safety and efficacy of briakinumab with etanercept and placebo in patients with moderate to severe chronic plaque psoriasis. Br J Dermatol. 2011;165(3):661–668. doi: 10.1111/j.1365-2133.2011.10419.x.
- Strohal R, Puig L, Chouela E, et al. The efficacy and safety of etanercept when used with as-needed adjunctive topical therapy in a randomised, double-blind study in subjects with moderate-to-severe psoriasis (the PRISTINE trial). J Dermatolog Treat. 2013;24(3):169–178. doi: 10.3109/09546634.2012.658015.
- Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013.
- Thaci D, Eyerich K, Pinter A, et al. Direct comparison of risankizumab and fumaric acid esters in systemic therapy-naive patients with moderate-to-severe plaque psoriasis: a randomized controlled trial. Br J Dermatol. 2022;186(1):30–39. doi: 10.1111/bjd.20481.
- Thaci D, Humeniuk J, Frambach Y, et al. Secukinumab in psoriasis: randomized, controlled phase 3 trial results assessing the potential to improve treatment response in partial responders (STATURE). Br J Dermatol. 2015;173(3):777–787. doi: 10.1111/bjd.13814.
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163(2):402–411. doi: 10.1111/j.1365-2133.2010.09791.x.
- Thaci D, Pinter A, Sebastian M, et al. Guselkumab is superior to fumaric acid esters in patients with moderate-to-severe plaque psoriasis who are naive to systemic treatment: results from a randomized, active-comparator-controlled phase IIIb trial (POLARIS). Br J Dermatol. 2020;183(2):265–275. doi: 10.1111/bjd.18696.
- Torii H, Nakagawa H, Japanese Infliximab Study i. Infliximab monotherapy in japanese patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. A randomized, double-blind, placebo-controlled multicenter trial. J Dermatol Sci. 2010;59(1):40–49. doi: 10.1016/j.jdermsci.2010.04.014.
- Tsai TF, Ho JC, Song M, et al. Efficacy and safety of ustekinumab for the treatment of moderate-to-severe psoriasis: a phase III, randomized, placebo-controlled trial in taiwanese and korean patients (PEARL). J Dermatol Sci. 2011;63(3):154–163. doi: 10.1016/j.jdermsci.2011.05.005.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X.
- Umezawa Y, Sakurai S, Hoshii N, et al. Certolizumab pegol for the treatment of moderate to severe plaque psoriasis: 16-Week results from a phase 2/3 japanese study. Dermatol Ther (Heidelb). 2021;11(2):513–528. doi: 10.1007/s13555-021-00494-z.
- van Bezooijen JS, Balak DM, van Doorn MB, et al. Combination therapy of etanercept and fumarates versus etanercept monotherapy in psoriasis: a randomized exploratory study. Dermatology. 2016;232(4):407–414. doi: 10.1159/000448135.
- van de Kerkhof PC, Segaert S, Lahfa M, et al. Once weekly administration of etanercept 50 mg is efficacious and well tolerated in patients with moderate-to-severe plaque psoriasis: a randomized controlled trial with open-label extension. Br J Dermatol. 2008;159(5):1177–1185. doi: 10.1111/j.1365-2133.2008.08771.x.
- Warren RB, Barker J, Finlay AY, et al. Secukinumab for patients failing previous tumour necrosis factor-alpha inhibitor therapy: results of a randomized open-label study (SIGNATURE). Br J Dermatol. 2020;183(1):60–70. doi: 10.1111/bjd.18623.
- Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–141. doi: 10.1056/NEJMoa2102388.
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184(1):50–59. doi: 10.1111/bjd.19341.
- Wolf P, Hofer A, Legat FJ, et al. Treatment with 311-nm ultraviolet B accelerates and improves the clearance of psoriatic lesions in patients treated with etanercept. Br J Dermatol. 2009;160(1):186–189. doi: 10.1111/j.1365-2133.2008.08926.x.
- Wolf P, Hofer A, Weger W, et al. 311 nm ultraviolet B-accelerated response of psoriatic lesions in adalimumab-treated patients. Photodermatol Photoimmunol Photomed. 2011;27(4):186–189. doi: 10.1111/j.1600-0781.2011.00594.x.
- Wolf P, Weger W, Legat FJ, et al. Treatment with 311-nm ultraviolet B enhanced response of psoriatic lesions in ustekinumab-treated patients: a randomized intraindividual trial. Br J Dermatol. 2012;166(1):147–153. doi: 10.1111/j.1365-2133.2011.10616.x.
- Yang HZ, Wang K, Jin HZ, et al. Infliximab monotherapy for chinese patients with moderate to severe plaque psoriasis: a randomized, double-blind, placebo-controlled multicenter trial. Chin Med J (Engl). 2012;125(11):1845–1851.
- Yu Q, Tong Y, Cui L, et al. Efficacy and safety of etanercept combined plus methotrexate and comparison of expression of pro-inflammatory factors expression for the treatment of moderate-to-severe plaque psoriasis. Int Immunopharmacol. 2019;73:442–450. doi: 10.1016/j.intimp.2019.05.042.
- Zachariae C, Mork NJ, Reunala T, et al. The combination of etanercept and methotrexate increases the effectiveness of treatment in active psoriasis despite inadequate effect of methotrexate therapy. Acta Derm Venereol. 2008;88(5):495–501. doi: 10.2340/00015555-0511.
- Zhu X, Zheng M, Song M, et al. Efficacy and safety of ustekinumab in chinese patients with moderate to severe plaque-type psoriasis: results from a phase 3 clinical trial (LOTUS). J Drugs Dermatol. 2013;12:166–174.
- Taichman DB, Backus J, Baethge C, et al. Sharing clinical trial data: a proposal from the international committee of medical journal editors. Lancet. 2016;387(10016):e9–e11. doi: 10.1016/S0140-6736(15)01279-9.
- Yu K, Syed MN, Bernardis E, et al. Machine learning applications in the evaluation and management of psoriasis: a systematic review. J Psoriasis Psoriatic Arthritis. 2020;5(4):147–159. doi: 10.1177/2475530320950267.

