Abstract
Background
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease affecting approximately 1% of the population. The patient journey through the German health care system leads to high disease burden and substantial treatment costs. The EsmAiL study showed that an innovative, interprofessional, multimodal care-concept reduces disease activity and burden of HS compared to standard care. This paper examines the costs of treating HS in Germany and compares them with those of the innovative care concept implemented in EsmAiL.
Methods
EsmAiL was a two-arm, multicenter, prospective randomized controlled trial including 553 adults with HS. The study was registered in the German Clinical Trials Registry (DRKS00022135). The control group (CG) remained in standard care, whereas the intervention group (IG) was referred to specialized so-called ‘acne-inversa-centres (AiZ)’ where patients were treated with a structured, interdisciplinary approach. The present paper analyses the treatment costs for a subpopulation based on health insurance cost data from the two largest German health insurers. Quality-Adjusted Life Years (QALY) was assessed based on Dermatology Life Quality Index (DLQI).
Results
Total annual treatment costs per patient were €3,966.07 in standard care (n = 89) and €3,974.37 in the innovative care (n = 93). The costs per additional QALY amounted to €12,698.72 in the IG. Given the conventional and established threshold of €22,600 to €33,900 per QALY, the innovative treatment in AiZ proved to be cost-effective.
Conclusion
Treatment costs of HS are substantial and increase with disease severity. The new form of care is cost-effective and is expected to decrease costs in the long run.
KEY MESSAGE
A structured, multimodal form of care reduces costs in the treatment of Hidradenitis suppurativa compared to standard care.
1. Introduction
Hidradenitis suppurativa (HS) – also known as acne inversa (Ai) – is a chronic inflammatory skin condition with multiple associated comorbidities (Citation1, Citation2). This leads to an increased risk of early cardiovascular morbidity and mortality. Around 1% of the population suffers from HS (Citation3–5). On average it takes 7 years to get the proper diagnosis (Citation6). In addition to the limitations in personal life, the impact on work ability and on the use of the health care system is also substantial and consequently leads to high socioeconomic costs (Citation7–9).
Due to an insufficient ambulatory care network, early stages of the disease are frequently not treated effectively to halt its progression, and patients often receive adequate care only when the disease has advanced (Citation10).
The medical first-line treatments, according to the European guidelines, includes topical and oral antibiotics (Citation11, Citation12). For moderate to severe disease, continuous treatment with adalimumab or secukinumab as biologic therapy is applicable after failed systemic antibiotic treatment (Citation12). Even in the early stages of the disease, surgical interventions can be necessary. Both biologics and extensive surgeries impose substantial costs; thus expenditures rise with progression of the disease (Citation13).
The difficulties of HS patient care with the resulting burdens on those affected as well as the considerable costs for the health care system associated with the treatment of advanced disease stages are clearly addressed globally (Citation14). However, there is only limited literature on the specific magnitude of treatment costs of HS, separated by cost source.
EsmAiL was a two-arm, multicenter, prospective randomized controlled trial involving 553 adults with HS (Citation15, Citation16). The control group (CG) received standard care while the intervention group (IG) was treated according to a structured, interdisciplinary treatment plan based on current guidelines and innovative scientific evidence. Within this new form of care implemented in EsmAiL, outpatient offices, clinics and wound care centers of various specialties throughout Germany have been qualified as so-called ‘acne inversa centres (AiZ)’. In each AiZ, the intervention was provided by at least one trained physician (e.g., general practitioner, dermatologist or surgeon) and by at least two trained health care professionals (e.g., registered nurse, wound manager). The new form of care in AiZ placed a strong focus on patient education, improved pain management and lesion care. In addition, care in AiZ used a combination therapy of intense pulsed light and radiofrequency (LAight®-therapy, LENICURA, Germany) as a noninvasive, physical first-line treatment in addition to the recommendations of in the current guidelines. A preceding paper on EsmAiL has shown that the new care concept significantly outperforms standard care in reducing disease activity and burden (Citation16). This paper compares the health care costs of AiZ treatment with those of standard care.
2. Materials and methods
2.1. Trial design
EsmAiL was designed as a multicenter, randomized controlled trial (RCT) with blinded assessment. The CG remained in standard care while the intervention group IG was referred to care in AiZ. A detailed description of the intervention and the baseline characteristics of the included patients are given elsewhere (Citation15, Citation16). The study was approved by the ethics committees of all participating states and registered in the German Clinical Trials Registry (DRKS00022135).
Patients were eligible for study participation if they were diagnosed with HS of all Hurley stages, were of legal age and had the mental ability to understand the patient information, follow the study procedure and signed the written informed consent. Participation was voluntary and could be withdrawn at any time. Moreover, they must have had at least 3 inflammatory lesions at the time of inclusion and the disease had to have at least a moderate impact on quality of life as measured by the Dermatologic Quality of Life Index (DLQI, (Citation17)). Thus, the DLQI needed to be above 5. The two largest German health insurances participated in the project and contributed billing data of treatment costs of the intervention year. For this purpose, an additional patient consent was signed at inclusion into the trial, to allow evaluation of the cost data. No changes to endpoints or methods of data collection were made during the trial.
This paper analyses a subsample of n = 182 patients for whom treatment costs were available from data of the two largest German health insurances.
2.2. Methods and data
The analysis was carried out in the intention-to-treat (ITT) population and covered the 12 months after a patient’s individual inclusion into the trial. Patient characteristics of the subgroup were compared with the total sample using χ2-test and t-test, respectively. The statistical evaluation was carried out by the Martin Luther University Halle-Wittenberg, Germany as an independent institution.
The analysis used the following data:
The validated International Hidradenitis Suppurativa Severity Score System – IHS4 (Citation18) and Hurley Stage were assessed by blinded, trained physicians (‘screeners’) at baseline and after 12 months. The IHS4 was used for the stratified analysis of treatment costs and therapy allocation. These data were collected as part of the EsmAiL trial and were therefore available for the health-economic evaluation.
DLQI was assessed as patient reported outcome at baseline and after 12 months.
All treatment costs for standard care of HS were derived from patient data from the two largest health insurance companies in Germany. These treatment costs for standard care were calculated as the sum of the following components: Outpatient care, inpatient care and medication prescriptions.
Costs of inpatient and outpatient care were selected according to a list of ICD-10-codes that accounted for the primary code L73.2 as well as typical misdiagnoses (Supplement 1). LOCF imputation was applied to the outpatient care category to cover the entire study period, as these costs were only available until the end of December 2021 at the time of data extraction.
For medication prescriptions a list of ATC-codes (Anatomical Therapeutic Chemical Classification System) was defined including the suggestions of the guidelines as well as other relevant medications (e.g., topical antibiotics, systemic antibiotics, biologics and analgesics, Supplement 2). The source was again the patient data from the health insurance companies. To represent costs of medication, the average of the analyzed and the preceding quarter was calculated. The reason for this was that that costs of medications were presented in billing date once they were prescribed. However, medication use often extends into the next quarter, especially in HS, where treatment cycles often last up to 10–12 weeks and longer, or are continuous treatment (e.g., with biologics).
The costs for the new care concept were calculated according to the real utilization in the project. This data was extracted from the treatment file recorded for each individual patient during the EsmAiL trial. The new care concept for the IG included the following components (see Supplement 3): Initial assessment, patient education, follow-up assessment, final assessment, wound and lesion care and LAight therapy. For the CG, we recorded the number of LAight therapies that patients accessed independently and outside the study intervention (self-paid). Number of LAight therapies for CG was based on self-reported data from the patient questionnaire and the final study assessment.
All costs were presented as averages for both standard care and AiZ treatment (new form of care), stratified by quarter and also summed over the whole study year. Moreover, the proportion of patients receiving a certain medical cost category (inpatient care, outpatient care, medication, new care concept, no care) in each group was reported. Differences in relative frequencies were tested for statistical significance using the χ2 test. Analyses were stratified according to the three IHS4 severity categories (IHS4-score: mild ≤ 3, moderate 4 to 10 and severe >10; (Citation18)).
To compare the cost-effectiveness of AiZ-care to that of standard care, we applied two analyses:
Isolated cost-utility evaluation of project year:
As a conservative case, the delta in direct costs between IG and CG of the intervention year was compared to the difference in QALY. The result was then assessed with respect to the standard values proposed by the National Institute for Health and Care Excellence (Citation19) to evaluate cost-effectiveness. The cost-utility analysis was based on the changes in DLQI from baseline to the final screener assessment. Based on the mapping procedure described by Ali et al. the changes in DLQI were transformed into corresponding EuroQoL Five-Dimension Three-Level questionnaire (EQ-5D-3L) values (Citation20). Making use of the preference weights determined by Greiner et al. for Central Europe, these estimated EQ-5D-3L utility values were converted into the VAS-based weighted health status index from which Quality-Adjusted Life Years (QALY) gains could be derived (Citation21).
Scenario analysis considering consecutive years, changes in severity and treated patients only:
Projected costs for a subsequent year were calculated for both study groups. These projected costs were derived from the costs of the regukar care components by IHS4 disease severity, weighted by the severity distribution in the IG and CG at the end of the 12-month intervention. Only enrolled patients who received at least one treatment for HS during the study period were included in the analysis to account for the higher proportion of untreated patients within standard care.
To compare the allocation of therapies between IG and CG, we plotted the percentages of patients receiving topical antibiotics, systemic antibiotics, biologics, outpatient care, and inpatient care during the intervention period. We also investigated the proportions of patients receiving none of these therapies during the intervention year (‘without care’). Differences in relative frequencies were tested for statistical significance using the χ2 test.
3. Results
3.1. Patient characteristics
Patients were recruited throughout Germany from September 9th, 2020, until July 31st, 2021, by 15 trained, blinded screeners and allocated to either the CG (n = 279) or IG (n = 274). Billing data on HS-associated costs were available for 182 patients. Of those 89 patients belonged to the CG and 93 to the IG. Both groups were comparable on baseline characteristics (Supplement 4). The characteristics of the sub-group () mimicked the whole sample of the 553 patients participating in the EsmAiL study, except for the Hurley stage distribution (p = 0.013), a lower IHS4 (p = 0.007) and a lower DLQI (p = 0.011).
Table 1. Comparison of baseline characteristics between patients with and without available billing data for the economic evaluation.
3.2. Therapy allocation
In line with the suggestions of the guidelines, antibiotics were the most utilized treatment modality in standard care ( and ). Comparing this distribution with the self-reported data from the patient questionnaires of the EsmAiL total sample () the results show a high accordance in tendencies. Both distributions coincide in the finding that about one fourth of the CG did not receive any treatment. The EsmAiL project only enrolled patients with a certain disease activity and burden, indicating that treatment was necessary for the CG but not provided.
Figure 1. Allocated therapies during the intervention period for the patients in the economic analysis based on the health insurance data.
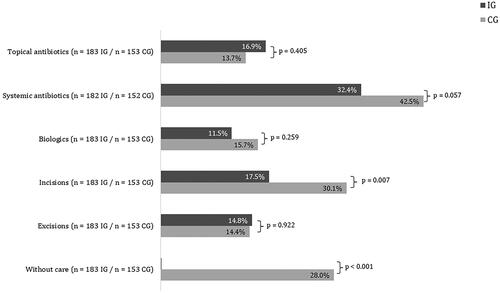
Figure 2. Comparison of medical distribution with the self-reported data from the patient questionnaires of the EsmAiL total sample (note the categories ‘incision’ and ‘excision’ in cannot be clearly assigned to the outpatient and inpatient care services from and ).
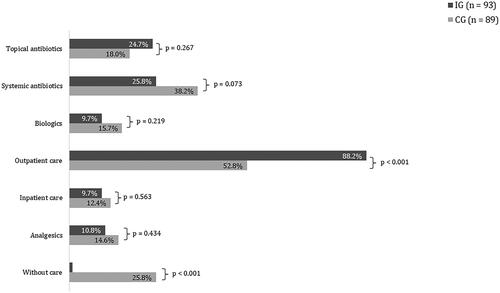
Table 2. Allocation of therapies during the intervention period for treated patients in the economic analysis based on health insurance data, broken down by IHS4 severity category.
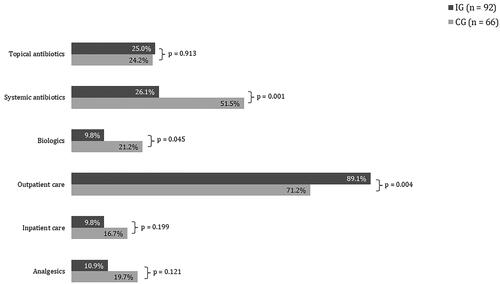
As compared to the CG, a lower proportion of patients in the IG received systemic antibiotics, biologics, analgesics, and inpatient hospital services. These differences were not statistically significant. A higher proportion of patients in the IG received topical antibiotics. Also, only 1.1% of the IG did not receive any treatment with respect to HS during the intervention period. To better understand the allocation of treatment modalities in the new care concept vs. standard care, shows the corresponding proportions only for those patients who received at least one medical intervention during the 12-month study. This analysis shows a significantly higher proportion of prescribed systemic antibiotics and biologicals within the CG compared to the IG (51.5% vs. 26.1% and 21.2% vs. 9.8%, ), as well as a tendency toward more inpatient hospital services and prescriptions of analgesics (9.8% vs. 16.7% and 10.9% vs. 19.7%, ). As expected, a higher proportion of the IG received outpatient care services (89.1% vs. 71.2%) while topical antibiotics were used in an almost identical proportion of patients in both the IG and CG (25.0% vs. 24.2%). stratifies this subgroup analysis with respect to the IHS4 severity stages at baseline. Particularly in the mild and moderate severity stages, the patients of the IG show a significantly lower rate of prescribed biologics and systemic antibiotics.
3.3. Direct costs
The average yearly total costs per patient for standard care components (computed as the sum of outpatient care, inpatient care, medication prescriptions) amounted to €2,281.81 for the IG and to €2,679.71 for the CG (). Of note is the lower cost for medication prescriptions in the IG compared to the CG (€1,447.38 vs €1,837.92), even though 25.8% of patients in the CG did not receive any care during the intervention period. This difference is mainly due to the comparatively less frequent prescriptions of biologicals in the IG (the respective costs per medication category are shown in Supplement 5). When stratifying with respect to IHS4 severity categories (), the average total annual costs of standard care components per patient range from €174.43 (mild HS) to €2,760.06 (severe HS) in the IG and from €626.15 (mild HS) to €2,358.46 (severe HS) in the CG. Once limiting the analysis to patients, who received treatment (, lower three rows), mild and moderate cases in the IG resulted in considerably lower costs while for severe cases the costs were comparable for both groups.
Table 3. Average total annual cost of standard care components and new care concept per total patient and treated patients after baseline and broken down by IHS4 severity category.
The average total costs per patient over 12 months in an AiZ amounted to €1,649.83 (Supplement 3). 95.7% of the patients in the IG used at least one of the innovative components (4.3% of IG patients did not enter AiZ-care but were kept in the analysis due to the ITT procedure). The LAight treatment accounted for the majority of costs, averaging to €1,354.20 per IG patient. Based on self-reporting, 26.4% of patients in the CG received at least one LAight treatment during the 12 months of EsmAiL outside the intervention as self-payer, which resulted in estimated average costs per patient of €261.42 (Supplement 3).
The total costs per patient amounted to €3,931.63 in the IG and to €2,941.13 in the CG (). When considering the high proportion of patients in the CG who did not receive any treatment the differences in the average total of annual costs between IG and CG leveled out to a difference of €8.30 (€3,974.37 to €3,966.07).
3.4. Cost-utility-analysis
Isolated cost-utility evaluation of project year:
Total annual treatment costs (including both standard care and new care components) per included patient were €2,941.13 in CG and €3,931.63 in IG (). For the IG, the 12-month-treatment in an AiZ resulted in a decrease in DLQI of 6.9 ± 6.22 points and an estimated gain of 0.145 QALYs while the CG achieved a decrease in DLQI of 3.7 ± 6.41 points and an estimated gain of 0.067 QALYs (Supplement 6).
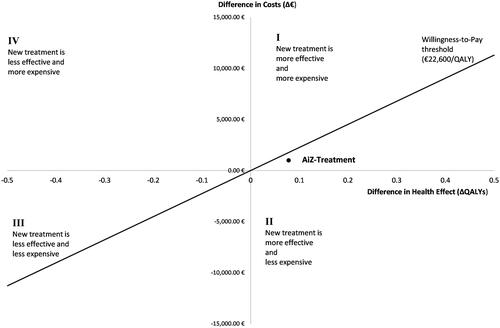
Focusing on the single intervention year, care in an AiZ resulted in additional total costs of €990.50 compared to standard care and differed from standard care by a gain of 0.078 additional QALYs. This results in costs of €12,698.72 per gained QALY. The National Institute for Health and Care Excellence (NICE) currently estimates a threshold of €22,600 to €33,900 (£20,000 to £30,000, €1.13 per £) per QALY in the implementation of new therapies (Citation19). As shown in the corresponding cost-effectiveness plane (), the new care concept is cost-effective compared to standard care in the context of this threshold for the single intervention year.
Scenario analysis considering consecutive years, changes in severity and treated patients only:
When only considering treated patients, the cost difference between IG and CG during the first year of intervention decreases to €8.31 per patient (, lower rows). Under the assumption of the shifted disease IHS4 severity distributions, the continuation of the new care concept leads to cost savings of €889.33 for projected subsequent years (Supplement 7).
4. Discussion
Up to date, there is only limited literature that addresses treatment costs of HS even though those are estimated to be substantial. However, studies imply that higher health care costs do not necessarily correlate with better treatment outcomes (Citation22,Citation23). There is consensus that inpatient care and biologic therapy are the prominent cost drivers (Citation9,Citation22,Citation24). Medical costs have been shown to be up to 2.4 times higher in more severe HS cases (Citation25) which is also confirmed by the analysis at hand (). The recent approval of additional monoclonal antibodies for the treatment of HS such as secukinumab (Citation26) and the expected approval of bimekizumab (Citation27) are likely to reinforce this trend (Citation10).
In the analyzed sample, 25.4% of patients in the CG did not receive any therapy at all in standard care (). Due to the diagnostic gap (Citation28) and currently often dysfunctional patient journeys through ambulatory care in Germany many patients get access to specialists only after they have experienced a more severe manifestation of their disease, which makes measures such as surgery or treatment with biologics necessary interventions that significantly increase costs (Citation29). These may be prevented by early adequate care hindering progression.
A preceding publication of the EsmAiL project has shown that the establishment of specialized AiZs in ambulatory care outperforms standard care in decreasing the severity and burden of HS and significantly increases patient satisfaction (Citation16). The present analysis is the first of its kind to estimate the costs of treating HS in standard care in Germany and shows that these costs can be reduced by structured, multimodal care. The present analyses did not aim to evaluate the cost-effectiveness of individual components of the new care model. Instead, the aim of the present analyses was to compare the cost of the new care approach – including the interactions between all its individual components with the cost of standard care. In contrast to the CG, 99% of the included IG patients were treated with respect to their HS. Given the progressive disease course of HS, this is an important finding. Our results reveal that especially in the mild and moderate IHS4 severity categories patients in the IG needed less biologics, systemic antibiotics, and inpatient care. These findings suggest that the treatment algorithm within the new care concept was able to achieve remission in early steps of the algorithm, preventing the allocation of burdensome and costly medication therapies and surgery. However, the present analysis implies that – even though prescribed more often – the treatment with antibiotics imposes lower costs in the CG than in the IG (Table S1a). Yet, it should be emphasized that the guidelines suggest long-term antibiotic therapy of up to 16 weeks (Citation12). Short-term treatment cycles are certainly cheaper but are not recommended. Thus, the presented cost analysis also implies that CG patients may not have been treated according to current HS therapy recommendations.
The new form of care proved to be cost-effective and is even expected to decrease cost of care in the long run (Supplement 7): Within the conservative model based on the solitary intervention year, the additional total annual cost of the IG of €990.50 per patient are justified by the additional gain of 0.078 QALYs over the CG. Considering that missing treatment will not lead to any improvement and could result in higher costs when untreated patients seek medical care in the future, the scenario analysis based on a projected subsequent year shows that the additional costs of the first year of the new care concept decrease to a marginal €8.31, while for the projected subsequent years cost savings per patient amount to €889.33 (Supplement 7).
Our study has limitations. We acknowledge that the economic evaluation could not be applied to all patients in the EsmAiL cohort. The studied subgroup appears to be somewhat less affected considering disease activity than the total EsmAiL-cohort (). This may underestimate the costs of care presented. However, since the analysis distinguishes between IHS4 severity categories, the present results can be directly transferred to the general EsmAiL-cohort. Another limitation of our study is that we did not obtain generic quality of life questionnaire data, such as the EQ-5D-3L, during the trial. Instead, we transformed the DLQI to EQ-5D-3L values post-trial, which may introduce biases in the assessment of health-related quality of life.
It should be noted that medication costs have been identified with respect to a list of typical HS-relevant substances. However, the German system does not allow for a direct association of medication prescriptions with diagnoses. Thus, a certain bias can be arising from the fact that medication might have been prescribed for other reasons. However, those effects are assumed to be mitigated due to the randomization of the study population.
It is described that even up to 50% of patients diagnosed with HS do not receive any treatment (Citation30). So, the proportion of 25.2% of patients not treated in standard care is rather low. This might be because patients of the CG were usually contacted by the study team if they missed a timely completion of the questionnaires; it thus appears likely that the additional attention motivated CG patients to also seek care for their disease.
In the context of our study, it is worth noting that health economic evaluations have also been conducted for other inflammatory skin diseases, such as psoriasis or atopic dermatitis (Citation31, Citation32). Their findings suggest that, similar to ours in HS, the increased use of biologic treatments has had a significant impact on the economic burden of treatment. The introduction of biologics in psoriasis and atopic dermatitis has led to a notable increase in direct costs, reflecting a trend that is expected to be fostered for the treatment of HS. Our analysis implies that structured care concepts can have a substantial impact on treatment costs in various dermatoses, not only HS.
In summary, the analysis shows that cost for the care of HS are substantial and increase with disease severity with inpatient surgical care and biologic therapy as main cost drivers. Antibiotics are still the most frequently prescribed intervention. The new form of care has a positive impact on the course of the disease, is cost-effective and is expected to decrease direct cost of care in the long run. It is known that HS is also associated with high secondary costs, such as comorbidities and impairment of work ability (Citation13,Citation33). It is hardly possible to measure the indirect costs of the HS. However, it can be assumed that the indirect costs of disease will also decrease as a result of the increased ability to work due to the new form of care.
Due to the specifics of the German health care system, in which the costs for biologics and other components of standard care are reimbursed directly by the health insurance funds, and the LAight therapy is available nationwide, results are not fully transferable to other countries with different health care systems.
Supplemental Material
Download Zip (123.2 KB)Acknowledgements
We would like to thank the company LAUER-FISCHER GmbH, who kindly provided us with free access to LAUER-TAXE®. On this basis, pharmaceutical indices from the routine data of the health insurance funds could be assigned to the respective therapy allocations.
We would like to thank Dr Faraz Mahmood Ali MBBCh PhD MRCP(Derm) PGCert(Med Ed) who kindly provided us the mapping algorithm ‘DLQI to EQ5D Utility Values OLR Model Program’.
Disclosure statement
Michael Schultheis: Grants or contracts from any entity: LENICURA GmbH – auditor activity on the implementation of the contract ‘AOK-Priomed Acne inversa’ | Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Pfizer AbbVie – honoraria for lectures | Support for attending meetings and/or travel: AbbVie, Pfizer, Almirall – funding of travel, congress, and hotel fees
Petra Staubach: Grants or contracts from any entity: Novartis; Allmirall | Consulting fees: AbbVie, Allergika, Almirall-Hermal, Amgen, Beiersdorf, Biocryst, BMS, Boehringer-Ingelheim, Celgene, CSL-Behring, Eli-Lilly, Falk, Galderma, Hexal, Janssen, Klinge, Klosterfrau, LEO-Pharma, LETI-Pharma, L’Oreal, Novartis, Octapharma, Pfizer, Pflüger, Pharming, Regeneron, Shire, Takeda, Sanofi-Genzyme, UCB Pharma | Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid unrelated to current work presented here: Society of dermopharmazie
Georgios Nikolakis: Consulting fees – Dessau Medical Center received a consulting fee from Mölnlycke Health Care GmbH, for which I served as a consulting physician |Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Speaker for the EADV HS Course 28-30.11.2022, Porto, Portugal | Support for attending meetings and/or travel: Elli Lilly Scholarship for attending EADV 2021 | Participation in a data protection monitoring board or advisory board – Dessau Medical Center received a consulting fee from Mölnlycke Health Care GmbH, for which I served as a consulting physician
Lukas Schollenberger: None
Melanie Mauch: None
Marion Burckhardt: None
Marcus Heise: None
Marina Zamsheva: None
Alexandra Strobel: None
Gero Langer: None
Falk Bechara: Consulting fees: AbbVie Int., Incyte Corporation, AbbVie Deutschland GmbH und Co., MoonLake, Novartis Pharma GmbH, Janssen Cilag GmbH, UCB Pharma | Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational event:
AbbVie Int., Incyte Corporation, AbbVie Deutschland Gmbh und Co, Novartis Pharma GmbH, Janssen Cilag GmbH, UCB Pharma | Support for attending meetings and/or travel: AbbVie Int., Incyte Corporation, AbbVie Deutschland GmbH und Co., Novartis Pharma GmbH, Janssen Cilag GmbH, UCB Pharma | Participation on a data protection monitoring board or advisory board: AbbVie Int., Incyte Corporation, AbbVie Deutschland Gmbh und Co, Novartis Pharma GmbH, Janssen Cilag GmbH, UCB Pharma, Boehringer Ingelheim Pharma
Katharina Hennig: Patents planned, issued or pending: DE102015000150B4 | Stocks or stock options LENICURA GmbH – CEO and stockholder of the company
Christian Kunte: None
Matthias Goebeler: Grants or contracts from any entity: Clinical studies on Hidradenitis suppurativa; my role: Deputy-PI; sponsors: Novartis, Janssen, UCB; clinical studies on pemphigus vulgaris and bullous pemphigoid; my role: PI; sponsor: Argenx; clinical study on Prurigo nodularis; my role: PI; Sponsor: Galderma – contract with my institution; no personal payment; consulting fees: Allmirall – personal payment; Argenx – payment to institution | Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: GSK, Lilly, JANSSEN – personal payment | Support for attending meetings and/or travel: UCB – travel support | Participation in a data protection monitoring board or advisory board: UCB, GSK, LEO – personal payment | Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid unrelated to current work presented here: Member of the Board of Directors and Treasurer of the German Society of Dermatology (‘Deutsche Dermatologische Gesellschaft’), Section Editor ‘Journal der Dt. Dermatologischen Gesellschaft’, Member of the Board of Directors of the University Hospital Würzburg – all unpaid
Maurizio Podda: Maurizio Podda: Consulting fees: AbbVie, CSL, Galderma, Novartis, Janssen Cilag, UCB | Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: AbbVie, Beiersdorf, BMS, Eli Lilly, Galderma, Janssen Cilag, Leo Pharma, L’Oreal, Novartis, MSD, UCB | Support for attending meetings and/or travel: AbbVie, Beiersdorf, BMS, Eli Lilly, Galderma, Janssen-Cilag, Leo Pharma, L’Oreal, Novartis, MSD, UCB | Participation on a Data Safety Monitoring Board or Advisory Board: AbbVie, Boehringer Ingelheim, CSL, Galderma, Janssen-Cilag, MoonLake, Novartis, L’Oreal, UCB | Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: HiSNet Rhein Main e.V., President.
Stephan Grabbe: Grants or contracts from any entity: Novartis, Pierre Fabre | Consulting fees: AbbVie, BMS, MSD, Genzyme, Klinge Pharma, Sun Pharma, Kyowa-Kirin, Novartis, Pierre Fabre | Participation on a Data Safety Monitoring Board or Advisory Board: Alcedis | Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid unrelated to current work presented here: DeCOG, German dermatological cooperative oncology group – unrelated to current work presented here.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/09546634.2024.2302710)
Additional information
Funding
References
- Cartron A, Driscoll MS. Comorbidities of hidradenitis suppurativa: a review of the literature. Int J Womens Dermatol. 2019;5(5):1–10. doi: 10.1016/j.ijwd.2019.06.026.
- Zouboulis CC, Bechara FG, Fritz K, et al. S1 guideline for the treatment of hidradenitis suppurativa/acne inversa * (number ICD-10 L73.2). JDDG. 2012;10(Suppl 5):s 1–31.
- Jemec GB. Hidradenitis suppurativa. J Cutan Med Surg. 2003;7(1):47–56. doi: 10.1177/120347540300700109.
- Cosmatos I, Matcho A, Weinstein R, et al. Analysis of patient claims data to determine the prevalence of hidradenitis suppurativa in the United States. J Am Acad Dermatol. 2013;69(5):819. doi: 10.1016/j.jaad.2013.06.042.
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178(3):709–714. doi: 10.1111/bjd.15939.
- Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173(6):1546–1549. doi: 10.1111/bjd.14038.
- Yao Y, Jørgensen AR, Thomsen SF. Work productivity and activity impairment in patients with hidradenitis suppurativa: a cross-sectional study. Int J Dermatol. 2020;59(3):333–340. doi: 10.1111/ijd.14706.
- Egeberg A, Gislason GH, Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. 2016;152(4):429–434. doi: 10.1001/jamadermatol.2015.6264.
- Kirsten N, Frings V, Nikolakis GD, et al. Epidemiology, patient quality of life, and treatment costs of hidradenitis suppurativa/acne inversa. Hautarzt. 2021;72(8):651–657. doi: 10.1007/s00105-021-04851-z.
- Garg A, Neuren E, Cha D, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the global survey of impact and healthcare needs (VOICE) project. J Am Acad Dermatol. 2020;82(2):366–376. doi: 10.1016/j.jaad.2019.06.1301.
- Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. doi: 10.1111/jdv.12966.
- Gulliver W, Zouboulis CC, Prens E, et al. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the european guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17(3):343–351. doi: 10.1007/s11154-016-9328-5.
- Frings VG, Schöffski O, Goebeler M, et al. Correction: economic analysis of the costs associated with hidradenitis suppurativa at a german university hospital. PLoS One. 2021;16(9):e0257481. doi: 10.1371/journal.pone.0257481.
- Gulliver W, Landells IDR, Morgan D, et al. Hidradenitis suppurativa: a novel model of care and an integrative strategy to adopt an orphan disease. J Cutan Med Surg. 2018;22(1):71–77. doi: 10.1177/1203475417736290.
- Schultheis M, Grabbe S, Staubach P, et al. Clinical features of persons suffering from acne inversa. Dtsch Arztebl Int. 2023;120(19):345–346.
- Schultheis M, Staubach P, Nikolakis G, et al. A centre-based ambulatory care concept for hidradenitis suppurativa improves disease activity, disease burden and patient satisfaction: results from the randomized controlled EsmAiL trial. Br J Dermatol. 2023;189(2):170–179. doi: 10.1093/bjd/ljad135.
- Basra MKA, Fenech R, Gatt RM, et al. The dermatology life quality index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035. doi: 10.1111/j.1365-2133.2008.08832.x.
- Zouboulis CC, Tzellos T, Kyrgidis A, European Hidradenitis Suppurativa Foundation Investigator Group., et al. Development and validation of the international hidradenitis suppurativa severity score system (IHS4), a novel dynamic scoring system to assess HS severity. Br J Dermatol. 2017;177(5):1401–1409. doi: 10.1111/bjd.15748.
- National Institute for H, Care E. NICE Process and Methods Guides. Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence (NICE). Copyright © 2013 National Institute for Health and Clinical Excellence, unless otherwise stated. All rights reserved; 2013.
- Ali FM, Kay R, Finlay AY, et al. Mapping of the DLQI scores to EQ-5D utility values using ordinal logistic regression. Qual Life Res. 2017;26(11):3025–3034. doi: 10.1007/s11136-017-1607-4.
- Greiner W, Weijnen T, Nieuwenhuizen M, et al. A single European currency for EQ-5D health states. Results from a six-country study. Eur J Health Econ. 2003;4(3):222–231. doi: 10.1007/s10198-003-0182-5.
- Kirby JS, Miller JJ, Adams DR, et al. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150(9):937–944. doi: 10.1001/jamadermatol.2014.691.
- Bodenheimer T, Fernandez A. High and rising health care costs. Part 4: can costs be controlled while preserving quality? Ann Intern Med. 2005;143(1):26–31. doi: 10.7326/0003-4819-143-1-200507050-00007.
- Gáspár K, Hunor Gergely L, Jenei B, et al. Resource utilization, work productivity and costs in patients with hidradenitis suppurativa: a cost-of-illness study. Expert Rev Pharmacoecon Outcomes Res. 2022;22(3):399–408. doi: 10.1080/14737167.2021.1895753.
- Kosunen MP, Ranta M, Hirvonen M, et al. Pss22 – economic burden of disease of hidradenitis suppurativa IN Finland: results FROM THE hi-fi study. Value in Health. 2018;21:S426. doi: 10.1016/j.jval.2018.09.2522.
- Kimball AB, Jemec GBE, Alavi A, et al. Secukinumab in moderate-to-severe hidradenitis suppurativa (SUNSHINE and SUNRISE): week 16 and week 52 results of two identical, multicentre, randomised, placebo-controlled, double-blind phase 3 trials. Lancet. 2023;401(10378):747–761. doi: 10.1016/S0140-6736(23)00022-3.
- Glatt S, Jemec GBE, Forman S, et al. Efficacy and safety of bimekizumab in moderate to severe hidradenitis suppurativa: a phase 2, double-blind, placebo-controlled randomized clinical trial. JAMA Dermatol. 2021;157(11):1279–1288. doi: 10.1001/jamadermatol.2021.2905.
- Kirsten N, Petersen J, Hagenström K, et al. Epidemiology of hidradenitis suppurativa in Germany – an observational cohort study based on a multisource approach. J Eur Acad Dermatol Venereol. 2020;34(1):174–179. doi: 10.1111/jdv.15940.
- Marvel J, Vlahiotis A, Sainski-Nguyen A, et al. Disease burden and cost of hidradenitis suppurativa: a retrospective examination of US administrative claims data. BMJ Open. 2019;9(9):e030579. doi: 10.1136/bmjopen-2019-030579.
- Calao M, Wilson JL, Spelman L, et al. Hidradenitis suppurativa (HS) prevalence, demographics and management pathways in Australia: a population-based cross-sectional study. PLoS One. 2018;13(7):e0200683. doi: 10.1371/journal.pone.0200683.
- Burgos-Pol R, Martínez-Sesmero JM, Ventura-Cerdá JM, et al. The cost of psoriasis and psoriatic arthritis in 5 European countries: a systematic review. Actas Dermosifiliogr. 2016;107(7):577–590. doi: 10.1016/j.ad.2016.04.018.
- Igarashi A, Yuasa A, Yonemoto N, et al. A systematic literature review of economic evaluations and cost studies of the treatment of psoriasis, atopic dermatitis, and chronic urticaria. Dermatol Ther (Heidelb). 2022;12(8):1729–1751. doi: 10.1007/s13555-022-00774-2.
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181(1):147–154. doi: 10.1111/bjd.17101.