 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objectives:
We aimed to explore the potential role of omega-3 (ω-3) fatty acids on acne vulgaris by modulating gut microbiota.
Materials and Methods:
We randomly divided the untreated acne patients into two groups with or without ω-3 fatty acids intervention for 12 weeks. The Sprague Dawley (SD) rats with acne model were given isotretinoin, ω-3 fatty acids or their combination respectively. Then the colonic contents samples of the drug intervention SD rats were transferred to the pseudo sterile rats with acne model. The severity of the disease was assessed by the Global Acne Grading System (GAGS) score of the patients, and the swelling rate of auricle and the pathological section of the rat with acne model. The 16S rDNA gene sequencing was performed to detect the alteration of the gut microbiota.
Results:
ω-3 fatty acids could increase the diversity of the gut microbiota and regulate the flora structure positively both in the patients and rats, increase the abundance of butyric acid producing bacteria and GAGS score in the patients, and alleviate the inflammation and comedones of rats.
Conclusion:
Supplementation of ω-3 fatty acids could alleviate the inflammation of acne vulgaris by increasing the abundance of butyric acid producing bacteria.
Introduction
Acne Vulgaris (AV), a multifactorial inflammatory disease of the pilosebaceous follicles of the skin, is the eighth most common disease in the world affecting approximately up to 85% of people aged 12–25 years (Citation1). It often occurs in the face, and may induce post-inflammatory hyperpigmentation and post-inflammatory erythema, even scars, which can be long lasting and have negative impact on the physical and mental health of the patients (Citation2). Timely control of active lesions in moderate to severe cases will reduce the above acne sequelae (Citation3). In recent guidelines, oral antibiotics and isotretinoin are commonly recommended as the first-line treatment (Citation1,Citation4). However, concerning the antibiotic resistance, isotretinoin induced dry mucous membranes, elevated liver enzymes, dyslipidemia and fetal malformation, as well as the unexpected recurrence of the disease (Citation5), it is valuable to find a therapy which could both benefit the patients and even resist the side effects of the first line drugs. As we all known, dietary habits play a non-negligible role in the development, duration, and severity of acne vulgaris (Citation6,Citation7). Increasing intake of diets consisting of fish and healthy oils, which are rich in omega-3 (ω-3) fatty acids, could contribute to the improvement in inflammatory lesions of individuals with acne (Citation8,Citation9). However, the unveiled mechanism of ω-3 fatty acids on acne vulgaris limits its application.
In past decades, dietotherapy has been suggested to treat diseases such as tumors, metabolic and neuropsychiatric disorders through mediating gut microbiota (Citation10–13). While the supplement of ω-3 fatty acids had the vital role in the management of inflammatory, immune and metabolic diseases associated with western diet, through its prebiotic effects, which in turn have been implicated in a variety of immunological, metabolic, and hormonal effects (Citation14–16). Moreover, the gut microbiota in the acne is consistent with the intestinal type of western diet, and the disorder of gut microbiota is related to the occurrence of this disease (Citation17,Citation18). Especially, previous studies identified the decreased abundance of Bifidobacterium, Coprobacillus, Lactobacillus, Clostridia and Clostridiales in the gut of acne patients (Citation17,Citation18). Therefore, it is reasonable to speculate ω-3 fatty acids may treat acne vulgaris, recently considered as the metabolic syndrome of the pilosebaceous follicle, by regulating intestinal flora and its metabolites, which will be clarified in this study at both human and animal level.
Methods
Clinical trail design
From July 2020 to June 2021, 46 cases of patients with the presence of acne vulgaris and another 20 matched healthy controls (HC) from the outpatient and inpatient departments of the Affiliated Hospital of Southwest Medical University, were involved in our study. All patients were randomized (using a computational random grouping table made by IBM SPSS Statistics 21.0) to oral isotretinoin (Huabang Pharmaceutical Co., Ltd., Chongqing, China) in combination with (or without) omega-3 fatty acids (ω-3, Nature Made, LA, American) treatment for 12 weeks. The former was the AVW group, while the latter was the AVP group. The ω-3 fatty acids was given at the does of 2400 mg/d, in particular, the isotretinoin was started at the initial doses of 0.5–1.0 mg/(kg d), then adjusted the dose according to the drug side effects, and curative effect evaluated by the Global Acne Grading System (GAGS) (Citation19). When the GAGS was 1–30, the dose of isotretinoin was given by 0.5 mg/(kg d). If the GAGS > 30, to increase the dose of isotretinoin to 1.0 mg/(kg d). When the patient's primary skin lesions gradually improved without new skin lesions occurred, the dose of isotretinoin was reduced to 0.5 mg/(kg d) by degrees. Lifestyle changes also suggested during treatment, including less sweets, high-fat diet, fried food, dairy products, etc. In addition to meeting the inclusion and exclusion criteria mentioned in our previous study (Citation18), moreover, the participants who used isotretinoin or ω-3 fatty acids within 6 months, or known to be allergic to these drugs should also be excluded from this experiment.
The evaluation of GAGS and VISIA skin test, and the sample collection of blood and fresh fecal from the patients were arranged at baseline and after 12 weeks of treatment. The disease severity of acne vulgaris is divided into 4 stages by GAGS: mild (1–18), moderate (19–30), severe (31–38) and extremely severe (≥39). Notably, only moderate to severe cases can be included in our study. The percentage of red area and porphyrins (sclereythrin) on the front, left and right sides (the higher measured percentage means the lighter severity) was tested respectively by the VISIA-CR™ imaging system (Canfield Scientific Inc., Fairfield, NJ, USA), and took the average value for statistical analysis. The levels of blood glucose, cholesterol (TC), triglyceride (TG), low density lipoprotein (LDL) and high density lipoprotein (HDL) from the fasting venous blood were determined enzymatically with an automatic analyzer (Synchron LX-20, Beckman Coulter, CA, USA). Fasting insulin was measured according to the instructions of the ELISA kit (Promega, Madison, USA). Insulin resistant was applied by the homeostasis model assessment of insulin resistance (HOMA-IR). About 200–300 mg fresh fecal sample from each patient were collected for subsequent 16s rDNA sequence.
Written informed consent was obtained from all participants prior to their enrollment. Ethical approval for the involvement of human subjects in this study was granted by the Affiliated Hospital of Southwest Medical University Clinical Trial Ethics Committee, Reference number KY2020115, 06/07/2020. We certify all applicable institutional regulations concerning the ethical use of information and samples from human volunteers were followed during this research.
Animal study design
Three-week (90 ± 20 g) and six-week (200 ± 20 g) male Sprague–Dawley (SD) rats were purchased and raised in the Specific Pathogen Free (SPF) experimental animal center of Southwest Medical University (No. SYXK (Chuan) 2018-065). All of the rats had ad libitum access to the standard laboratory diet. The environment in the laboratory is constant temperature and humidity, and the 12 h light/dark cycle automatically alternates. All experiments were started at one week after adaptive feeding of SD rats. This research was conducted in accordance with the Regulations on the Management of Laboratory Animals, promulgated by the National Science and Technology Commission of the People's Republic of China, and have been approved by the animal experiment Ethics Management Committee of the Affiliated Hospital of Southwest Medical University. The ethical approval reference number is swmu20210427.
Stage one – acne model development and drug intervention
Seven-week old male SD rats were randomly divided into the following five groups (n = 6/group): (i) Con (blank control); (ii) Acne (pure acne model); (iii) Iso (acne model combined with isotretinoin (1 mg/kg) treatment); (iv) ω-3 (acne model combined with ω-3 fatty acids (1 g/kg) treatment) (Citation20); (v) ω-3 + Iso (acne model combined with ω-3 fatty acids and isotretinoin, does were equal to the Iso and the ω-3 groups respectively) treatment. Except for the Con, the model ear of other four groups were evenly coated with 0.3 ml oleic acid (purity ≥ 99.8%, Macklin Biochemical Co., Ltd., Shanghai, China) on the surface once a day for 14 continuous days, and injected intradermally P.acne suspension (1 × 107 cells/ml,) (ATCC6919, Zuoke Biotechnology Development Co., Ltd., Guangzhou, China) at the does of 50 µl/200 g once the next day (Citation21). At the same time, all intervention drugs were administered by gavage once a day according to the set drug dose, while the groups of Con and Acne were gavaged identical volume of soybean oil (menstruum, Aladdin Biochemical Technology Co., Ltd., Shanghai, China). Repeat the stage one completely three times to ensure the effectiveness of the data. The rats were humanely euthanized to collect samples for different analyses. The colonic contents were collected from half of the SD rats in each group which were randomly selected on day 0. The colonic contents from other half of SD rats and the modeled ear skin samples from all SD rats were collected on day 15.
Stage two – effects of fecal microbiota transplantation (FMT)
The four-week old male SD rats had antibiotics (Ampicillin, Neomycin Sulfate and Metronidazole, 1 g/L, SUOLAIBAO Technology Co., Ltd., Beijing, China) in their drinking water changed every 2 d for 14 continuous days (Citation22), the SD rats drank the antibiotic water ad libitum to establish a pseudo sterile model. Then the SD rats dosed with fecal bacterial suspension from relevant groups of stage one via oral gavage (2 ml/rat/d) one week prior to the start of the acne model. There were five intervention groups (n = 3/group) as follows: (i) Con-Tr; (ii) Acne-Tr; (iii) Iso-Tr; (iv) ω-3-Tr; (v) (ω-3 + Iso)-Tr. Except for the Con-Tr, the acne model was constructed again of other groups according to the stage one for 14 d, and the recipient rats received oral fecal bacterial suspension every other day for the whole duration of acne model. Repeat the stage two completely three times to ensure the effectiveness of the data. On the day 0, 8 and 15 of pseudo aseptic model, 2 g fresh feces were collected naturally discharged from SD rats. After acne model, the rats were humanely euthanized to collect samples such as the colonic contents and the modeled ear for different analyses.
Preparation of FMT
After 14 d of acne model in the stage one, the 10 g colonic contents collected from rats were dissolved in 100 ml sterile PBS (SUOLAIBAO Technology Co., Ltd., Beijing, China). Filtered the residue with a 30um filter, centrifuged the filtrate at 800 rpm for 10 min, and then discarded the supernatant. Finally, added 100 ml sterile PBS again for resuspension to obtain the fecal bacteria suspension with the final concentration of 100 mg/ml, and quickly store it in the refrigerator at −80 °C. Fecal bacterial suspension was thawed in room temperature or 37 °C water bath to prepare for the FMT experiment of stage two (Citation23).
Rats ear-swelling calculation
Visual vernier caliper was used to measure the thickness of SD rat modeled ear auricle which could evaluate the degree of acne formation. During measurement, the vernier caliper should be placed in the center of the auricle and perpendicular to the ear baseline. In order to avoid experimental errors, the measurement process is always carried out by the same researcher. The measured auricle thickness is quantified by the following formula for calculating the auricle swelling rate.
Histopathological analysis
After the acne model of animal experiment, SD rats were anesthetized by 4% pentobarbital sodium (RUIWODE Biochemical Co., Ltd., Shenzhen, China) and the modeled ear tissue was loaded into 10% formalin (Xilong Chemical Co., Ltd., Shantou, China) for subsequent HE staining to observe the histomorphological changes. The number of comedo in the epidermis and mononuclear cell in the dermis were counted under 40 times and 400 times microscope respectively. Three visual fields were randomly selected from each section, then took the average values as the final result.
DNA extraction of fecal samples, 16S rDNA amplification, and sequencing
Extracted the DNA of fecal bacteria samples according to the steps of Stool Genomic DNA Extraction Kit (Solebo Technology Co., Ltd., Beijing, China), and the quality of the DNA extraction was detected by 1% agarose gel electrophoresis (Aladdin Biochemical Technology Co., Ltd., Shanghai, China). The universal primers, 341 F 5′-CCTACGGGRSGCAGCAG-3′ and 806 R 5′-GGACTACVVGGGTATCTAATC-3′ for human fecal samples, 338 F 5′- ACTCCTACGGGAGGCAGCAG-3′ and 806 R 5′-GGACTACHVGGGTWTCTAA T-3′ for SD rat fecal samples, were used to amplify the V3–V4 hypervariable region of the 16S rDNA. The KAPA HiFi Hotstart ReadyMix PCR kit (Roche Diagnostics, Shanghai, China) was used for high-fidelity amplification. Then 2% agarose gel electrophoresis (Aladdin Biochemical Technology Co., Ltd., Shanghai, China) and QuantiFluor™ - St blue fluorescence quantitative system (Promega, Madison, USA) were used to evaluate and quantitatively detect the PCR products of the same sample. The Miseq library was constructed according to TruSeqTM DNA Sample Prep Kit (Axygen Biosciences, Union City, CA, USA), and the further paired-end sequencing was performed by Illumina MiSeq platform (Illumina, San Diego, California, USA).
Sequence-based microbiota analysis, metagenome prediction analysis and statistical analysis
According to whether there was overlapping relationship, the PE reads obtained were spliced into a sequence, at the same time, commanded and filtered the sequence quality and splicing effect, finally, the optimized reliable sequence with the average quality of value more than 20 and the number of N bases less than 3 was obtained. According to the 97% similarity, OTU clustering without non-repetitive sequences was carried out to obtain the representative sequence of OTU. All sample sequences are randomly selected to a unified data volume, so that each sample can be compared and analyzed at the same OTU sequence level.
Alpha diversity can reflect the species abundance and diversity in each sample, including Shannon index and Simpson index (the higher Shannon index means the higher diversity; the higher Simpson index means the lower diversity), which was calculated by QIIME (v1.9.1) software. The non-parametric rank sum test was used to compare the alpha diversity index differences among the groups. Beta diversity can reflect the difference of species composition among samples. The unweighted UniFrac phylogenetic distance calculated by QIIME (v1.9.1) software was used as measurement index, which was displayed by principal coordinates analysis (PCoA) diagram. Non-parametric rank sum test was used to compare the species among groups at the level of genus.
PICRUSt is a bioinformatics tool for high-throughput computational prediction of functional content from 16S rDNA sequencing data. The OTU abundance is standardized through PICRUSt, and then annotated with KEGG function (http://picrust.github.io/picrust/) to obtain the annotation information of OTU at each functional level of KEGG and the abundance information of each functional level in different samples.
All statistical analyses were performed by SPSS 19.0 software. Quantitative data are expressed as mean ± standard deviation. Kruskal–Wallis rank sum test was used to analyze the data between multiple groups. Mann–Whitney U test was used to analyze the data between and within the two groups. p < .05 considered the difference statistically significant. Graphpad prism 8.0 software was used for drawing.
Results
Synergistic effect of ω-3 fatty acids in the treatment of the AV patients
Forty six patients with moderate and severe acne vulgaris and 20 healthy controls were recruited in our research. Among them, 3 patients in AVP group and AVW group were lost to follow-up due to the coronavirus pandemic, and the final analysis data of two groups were 20 cases respectively. Demographic and clinical baseline data are shown in below. Although there was a difference in age among the three groups (p < .001), the ages of all subjects were in the range of 18–30 years old. There was no difference in baseline indexes such as GAGS score, red area, sclererythrin, TC, TG, LDL, HDL, fasting insulin and HOMA- IR (p > .05) between AVP and AVW group. After 12 weeks of treatment, the GAGS score in AVW group were lower than that in AVP group (p = .02), although the GAGS, the percentages of red area and sclererythrin could be improved by isotretinoin intervention with or without ω-3 fatty acids (p < .001, p = .008, p < .001). Additionally, the TG (p = .01) was lower and the HDL (p = .03) was higher in the AVW group than that in the AVP after treatment.
Table 1. Clinical and laboratory findings of moderate to severe acne patients and healthy controls.
Effects of ω-3 fatty acids on gut microbiota in the AV patients
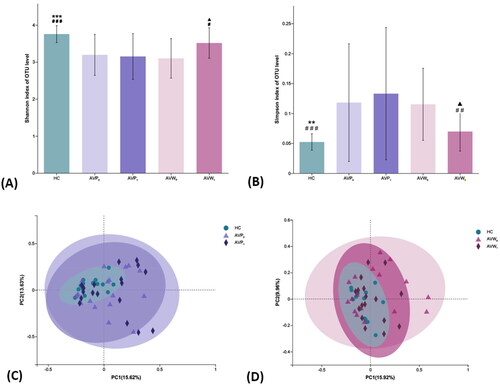
To characterize the effects of ω-3 fatty acids on the gut microbiome, we performed 16s rDNA sequencing of 100 fecal samples. There were 2, 3 and 5 people in AVP, AVW and control group respectively, who were removed by the failure of fecal bacteria DNA extraction. On average, we obtained 28,218 paired-end reads for each sample. The residual 85 samples clustered a total of 1100 OTUs at a 3% dissimilarity cutoff, with 109–421 OTUs per sample. The alpha diversity analysis showed decreased Shannon index (AVP: p < .001, AVW: p < .001) and increased Simpson diversity index (AVP: p = .002, AVW: p < .001), when compared with them in control group, suggesting the reduced diversity of gut microbiota in Acne Vulgaris patients (). There was no difference of microbial diversity between AVP and AVW at baseline (Shannon: p = .61, Simpson: p = .54). After treatment, the diversity of gut microbiota in the AVW group enhanced by ω-3 fatty acids contained treatment (Shannon: p = .01, Simpson: p = .008), but there were no change of bacterial diversity by isotretinoin intervention alone in AVP group (Shannon: p = .83; Simpson: p = .45). The composition of gut microbiota was analyzed by beta diversity analysis which found the PCoA plots of both the AVP and AVW group were obviously separated from that in HC group at baseline (), while after treatment, only the composition of gut microbiota in the AVW group showed a trend close to that in HC group ().
Figure 1. Comparisons of alpha and beta diversity indices of gut microbiota among the samples. (A,B) Comparison of Shannon and Simpson indexes among the baseline HC group, the AVP and AVW group before and after treatment. (C) Differences among the HC group and the AVP group before and after treatment were assessed by PCoA (R2 = 0.06, p = .03). (D) Differences among the HC group and the AVW group before and after treatment were assessed by PCoA (R2 = 0.05, p = .18). 0, the group before treatment; 1, the group after treatment; * (AVP0 vs.), *p < .05, **p < .01, ***p < .001; # (AVW0 vs.), #p < .05, ##p < .01, ###p < .001; ▲ (AVP1 vs AVW1), ▲p < .05, ▲▲p < .01, ▲▲▲p < .001; AVP: the AV patients with treatment of isotretinoin; AVW: the AV patients with treatment of ω-3 fatty acids; HC: healthy control group.
Effects of ω-3 fatty acids on gut microbiota at genus level in the AV patients
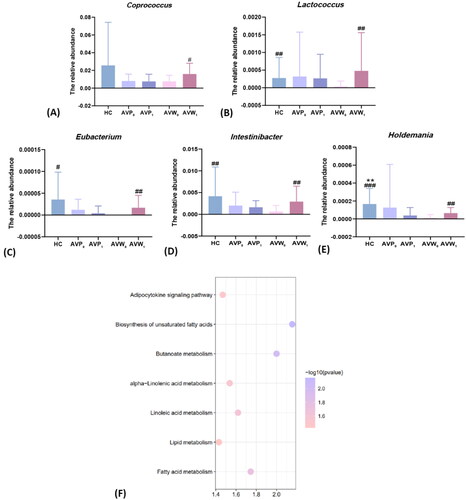
Besides, the gut microbiota of participants were analyzed at the genus level to identify the changed intestinal bacteria with different intervention. As the showed, the abundance of Lactococcus (3.8× 10−5± 1.5× 10−4, p = .006), Eubacterium (0, p = .03), Intestinibacter (6.2× 10−4± 1.4× 10−3, p = .008) and Holdemania (1.3× 10−5± 3.5× 10−5, p < .001) decreased in the acne patients of AVW when compared with that of healthy controls. While after 12 weeks of treatment with ω-3 fatty acids, the abundance of Coprococcus (1.6 × 10−2± 1.2× 10−2, p = .04), Lactococcus (4.8× 10−4± 1.1× 10−3, p = .004), Eubacterium (1.7 × 10−5± 2.8× 10−5, p = .008), Intestinibacter (2.9 × 10−3± 3.5× 10−3, p = .001) and Holdemania (6.5× 10−5± 6.2× 10−5, p = .004) were elevated when compared with that before treatment. In addition, though the abundance of Holdemania was decreased in the AVP (1.3× 10−4± 4.8× 10−4) when compared with the HC group (1.7 × 10−4 ± 1.7 × 10−4, p = .001), all bacteria were not different in the AVP group before and after isotretinoin treatment alone (p > .05). Meanwhile, to explore the functional feature of the gut microbiota in the patients treated by ω-3 fatty acids, we annotated the gene catalogue by KEGG. As the showed, 7 differently abundant pathways at 3 level were identified, including a decrease in adipocytokine signaling pathway and a relatively enrich in biosynthesis of unsaturated fatty acids, butanoate metabolism, alpha-linolenic acid metabolism, linoleic acid metabolism, lipid metabolism and fatty acid metabolism.
Figure 2. Comparisons of gut differential bacteria in genus level among the samples before and after different intervention, and distribution of KEGG functional categories on intestinal microbiota in the ω-3 fatty acids intervention group. (A–E) Comparison of the relative contents of Coprococcus, Lactococcus, Eubacterium, Intestinibacter and Holdemania before and after the intervention in each group. (F) Comparison of KEGG functional enrichment pathways on intestinal microbiota on level 3 between acne patients before and after ω-3 fatty acids treatment. The abscissa is the enrichment significance -log10 (p-value), and only the pathways with p < .05 which is considered statistically significant are listed in the figure; the ordinate is the name of KEGG pathway. The size of bubble in the figure represents the amount of metabolites enriched in the metabolic concentration in this pathway; 0, the group before treatment; 1, the group after treatment; * (AVP0 vs.), *p < .05, **p < .01, ***p < .001; # (AVW0 vs.), #p < .05, ##p < .01, ###p < .001; AVP, the AV patients with treatment of isotretinoin; AVW, the AV patients with treatment with ω-3 fatty acids; HC, healthy control group.
Effect of ω-3 fatty acids on the acne modeled auricle tissue in SD rats
In order to validate the effect of ω-3 fatty acids on the skin lesion and histopathology of acne vulgaris, acne model of SD rats were employed. As shown in , after modeled, in the Acne group, the auricle skin was obviously reddened, swollen, and hardened, epidermis became rough and thickened, accompanied by an increased skin temperature and a small amount of desquamation visible, while other treatment groups showed lighter redness, swelling and lower auricle swelling rate, when compared with that of the Acne group (p < .05). Although there was no difference of the auricle swelling rate between the Iso (1.8 ± 0.2) and the ω-3 group (1.6 ± 0.2) (p = .15), the auricle swelling rate in the ω-3 + Iso (1.6 ± 0.2) was lower than that in the Iso (p = .02).
Figure 3. Effects of different intervention methods on the severity and histopathology of auricle lesions in SD rats of stage one. (A) The changes of auricle lesions of SD rats in each group after acne modeled. (B) The comparison of auricle swelling rate of SD rats in each group after acne modeled (n = 9). (C) The pathological changes of auricle tissue of SD rats in each group after acne modeled (40×, 400×). (D) The comparison of the number of Comedo in the rats' auricle pathological tissue in each group after modeled (n = 9) (HE staining, 40×). (E) The comparison of the number of inflammatory cells in the rats' auricle pathological tissue in each group after modeled (n = 9) (HE staining, 400×). * (acne vs.), *p < .05, **p < .01, ***p < .001; ▲ (Iso vs.), ▲p < .05, ▲▲p < .01, ▲▲▲p < .001; ♦ (ω-3 vs ω-3 + Iso), ♦p < .05, ♦♦p < .01, ♦♦♦p < .001; acne, the pure model group; Con, the blank control group; Iso, acne model combined with the isotretinoin treatment group; ω-3, acne model combined with the ω-3 fatty acids treatment group; ω-3 + Iso, acne model combined with the ω-3 fatty acids plus isotretinoin treatment group.
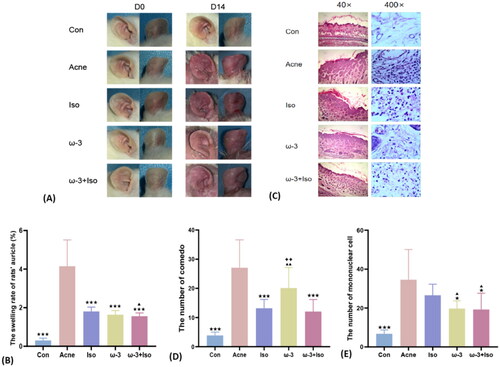
Meanwhile, the average number of comedo and mononuclear cell per field of vision at 40 and 400 magnification respectively under microscope were evaluated by the histological examination from the modeled auricle tissues with H&E staining. As shown in , the number of comedo was lower in the Iso (13.2 ± 3.0, p < .001) and the ω-3 + Iso (12.1 ± 4.1, p < .001), when compared with the Acne (27.1 ± 9.6). The number of mononuclear cell in the Iso group (26.6 ± 5.7) was even similar to that in the Acne group (34.5 ± 15.5) (p = .31). However, the number of mononuclear cells in the ω-3 (19.7 ± 4.0, p = .02) and the ω-3 + Iso (19.2 ± 8.3, p = .03) supplemented with ω-3 fatty acids were lower than that in the Acne group.
Effects of ω-3 fatty acids on gut microbiota in acne model of SD rats
The colonic contents were collected for 16S analysis in order to evaluate whether the ω-3 fatty acids have an effect on the gut microbiota of acne modeled SD rats. As shown in , there was no difference of the gut microbiota diversity in each group at the baseline level (p > .05). Interestingly, the Shannon diversity index increased (ω-32 vs ω-31, p = .008; (ω-3 + Iso)2 vs (ω-3 + Iso)1, p = .004) and Simpson indexes decreased (ω-32 vs ω-31, p = .04; (ω-3 + Iso)2 vs (ω-3 + Iso)1, p = .04) in the ω-3 fatty acids contained groups after treatment when compared with them at baseline. But the results revealed no apparent changes of both Shannon (p = .92) and Simpson (p = .42) only by isotretinoin treatment. The above results were consistent with the clinical trial.
Figure 4. Comparisons of alpha diversity index and PCoA in each SD rat treatment group (n = 6) of stage one. (A,B) Comparison of Shannon and Simpson indies among each group before and after acne modeled. (C–E) Differences of gut microbiota structure between the Iso group (R = 0.04, p = .33), ω-3 group (R = 0.44, p = .003) and the ω-3 + Iso group (R = 0.68, p = 0.004) before and after acne modeled were assessed by PCoA. 1, before acne modeled; 2, after acne modeled; * (Acne2 vs.), *p < .05, **p < .01, ***p < .001; ▲ (Iso2 vs.), ▲p < .05, ▲▲p < .01, ▲▲▲p < .001; ● (ω-32 vs ω-31), *p < .05, **p < .01, ***p < .001; # [(ω-3 + Iso)2 vs (ω-3 + Iso)1], #p < .05, ##p < .01, ###p < .001; acne, the pure model group; Con, the blank control group; Iso, acne model combined with the isotretinoin treatment group; ω-3, acne model combined with the ω-3 fatty acids treatment group; ω-3 + Iso, acne model combined with the ω-3 fatty acids plus isotretinoin treatment group.
![Figure 4. Comparisons of alpha diversity index and PCoA in each SD rat treatment group (n = 6) of stage one. (A,B) Comparison of Shannon and Simpson indies among each group before and after acne modeled. (C–E) Differences of gut microbiota structure between the Iso group (R = 0.04, p = .33), ω-3 group (R = 0.44, p = .003) and the ω-3 + Iso group (R = 0.68, p = 0.004) before and after acne modeled were assessed by PCoA. 1, before acne modeled; 2, after acne modeled; * (Acne2 vs.), *p < .05, **p < .01, ***p < .001; ▲ (Iso2 vs.), ▲p < .05, ▲▲p < .01, ▲▲▲p < .001; ● (ω-32 vs ω-31), *p < .05, **p < .01, ***p < .001; # [(ω-3 + Iso)2 vs (ω-3 + Iso)1], #p < .05, ##p < .01, ###p < .001; acne, the pure model group; Con, the blank control group; Iso, acne model combined with the isotretinoin treatment group; ω-3, acne model combined with the ω-3 fatty acids treatment group; ω-3 + Iso, acne model combined with the ω-3 fatty acids plus isotretinoin treatment group.](/cms/asset/9e50caf9-ee88-47b5-81de-eef0784f3c9f/ijdt_a_2299107_f0004_c.jpg)
Additionally, the composition of gut microbiota in the groups before and after intervention was conducted by PCoA analysis of Beta diversity. As shown in Figure S1, after treatment, the Con group, Acne group, and Iso group samples were close to each other and mainly clustered on the left side, while the samples of ω-3 group and ω-3 + Iso group overlapped with each other, mainly clustered in the middle, and showed a clear separation trend from the other three groups (p = .001). Specific to the treatment groups, as the showed, the ω-3 (p = .003) and ω-3 + Iso (p = .004) plots before and after treatment obviously separated, but Iso plots after treatment were not well separated from it at baseline (p = .33).
The influence of FMT on the phenotype of acne lesions in SD rats
Not isotretinoin, ω-3 fatty acids do have an impact on gut microbiota, which was proved by clinical and animal experiments. We firstly conducted the pseudo aseptic model of SD rats, then transplanted the gut microbiota from the first-stage donors as intervention into the second-stage pseudo aseptic recipients to verify that, the therapeutic effect of ω-3 fatty acids on acne is based on the regulation of gut microbiota. The pseudo aseptic model and FMT was successfully conducted and shown in Figure S2.
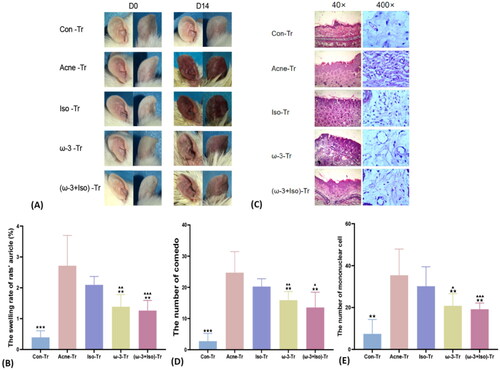
After FMT both the Acne-Tr and Iso-Tr group showed obviously redness swelling and rough auricle (). Also, the auricle swelling rate in Acne-Tr group (2.7 ± 1.0) was not different from it in Iso-Tr (2.1 ± 0.3) (p = 0.12). However, the auricle swelling rate of the ω-3-Tr (1.4 ± 0.4, p = .007) and the (ω-3 + Iso)-Tr group (1.3 ± 0.3, p = .005) with ω-3 induced microbiota was lower, when compared with Acne-Tr group (). The number of comedo and mononuclear cell were also evaluated in tissue sets among FMT groups. The showed there was no difference of the number of both comedo and mononuclear cell between the Acne-Tr and the Iso-Tr groups simultaneously (p > .05). However, the number of comedo (ω-3-Tr: 15.9 ± 2.7, (ω-3 + Iso)-Tr: 13.5 ± 4.9), and infiltration of mononuclear cells (ω-3-Tr: 20.8 ± 5.8, (ω-3 + Iso)-Tr: 19.2 ± 3.0) in the pathological tissue of the ω-3-Tr and the (ω-3 + Iso)-Tr, which were transplanted the gut microbiota altered by ω-3 fatty acids, were lighter than that of the Acne-Tr group (comedo: p = .005, p = .003; mononuclear cell: p = .009, p = .005).
Figure 5. The severity and histopathology of auricle lesions in SD rats after FMT of stage two. (A) The changes of auricle lesions of SD rats in each group after acne modeled combined with FMT. (B) The comparison of auricle swelling rate of SD rats in each group after acne modeled combined with FMT (n = 9). (C) The pathological changes of auricle tissue of SD rats in each group after acne modeled combined with FMT (40×, 400×). (D) The comparison of the number of comedo in the rats' auricle pathological tissue in each group after modeled combined with FMT (n = 9) (HE staining, 40×). (E) The comparison of the number of inflammatory cells in the rats' auricle pathological tissue in each group after modeled combined with FMT (n = 9) (HE staining, 400×). * (acne-Tr vs.), *p < .05, **p < .01, ***p < .001; ▲ (Iso-Tr vs.), ▲p < .05, ▲▲p < .01, ▲▲▲p < .001; acne-Tr, FMT of the acne group combined with acne model; Con-Tr, FMT of the Con group; Iso-Tr, FMT of the Iso group combined with acne model; ω-3-Tr, FMT of the ω-3 group combined with acne model; (ω-3 + Iso)-Tr, FMT of the ω-3 + Iso group combined acne with model.
Discussion
In previous studies, the disorder of lipid metabolism for AV patients has long been confirmed (Citation24). Acne patients were reported to be accompanied by the higher levels of TC, TG and LDL-C and even metabolic syndrome (MetS) (Citation25–27). Although we did not find differences in serum lipidomics between patients and healthy control, the serum concentration of TG in AV patients treated with ω-3 fatty acids was lower and the HDL concentration was higher than those of isotretinoin treatment obviously, which could be explained by the effect of ω-3 fatty acids on the biosynthesis of TG (Citation28). Furthermore, the duration of sustained action in ω-3 fatty acids is linearly related to TG content and HDL status (Citation29,Citation30), several systematic reviews and meta-analyses even suggested, ω-3 fatty acids could reduce the serum content of total cholesterol, as well as effectively improve the blood pressure in MetS patients and abnormal blood lipids in young children (Citation29,Citation30). This kind of lipid-lowering function of ω-3 fatty acids has been widely concerned, its long term use could reduce the risk of cardiovascular diseases such as carotid atherosclerosis (Citation31), and even may be a safe and effective substitutes for the Bates and Niacin in the treatment and management of hypertriglyceridemia (Citation32). More powerful evidence reported the supplement of ω-3 fatty acids during acne treatment as a useful auxiliary means to control dyslipidemia induced by isotretinoin (Citation9). Anyway, both literatures and this study supported the supplementation of ω-3 fatty acids during the treatment of acne vulgaris not only contribute to the therapeutic effect, but also benefit to correcting the lipid metabolism disorder of acne vulgaris.
Gut microbiota is a complex and dynamic community deeply affected by dietary patterns, and alterations in microbiota composition and function are related to the occurrence of different intestinal and extra-intestinal diseases (Citation33). As opposed to other inflammatory facial dermatoses, acne is regarded as a disease of western civilization like obesity and coronary heart disease (Citation34), and the effect of western diet on the development and clinical severity of acne has been clearly demonstrated in previous studies (Citation35–37). The western diet (dairy products, refined carbohydrates, complex fat mixture) was thought to fundamentally change the gut microbiota by reducing the diversity of gut flora and increasing the concentration of lipopolysaccharide, and eventually results in the development of acne vulgaris and metabolic diseases (Citation18,Citation38). Our results consistent with literature that, the patients with moderate and severe acne showed lower diversity of gut microbiota than that in healthy controls, and the flora composition showed a typical intestinal characteristics of western diet (Citation18). After isotretinoin treatment, there was no change of both diversity and composition of gut microbiota, which could be confirmed by Becker et al. (Citation39). Expectably, after supplement of ω-3 fatty acids, not only the diversity of gut microbiota of the acne vulgaris was increased, but also the composition after intervention showed a trend toward that of healthy population. In previous studies, ω-3 fatty acids has been reported to improve the condition of the patients with inflammatory bowel disease by restoring the gut microbiota to healthier components (Citation40), and rebuild the changes of gut microbiota caused by environmental stress to normal in mice (Citation41). Moreover, ω-3 fatty acids also could increase the diversity of gut microbiota and correct the disordered flora composition (Citation42), and play a role in the protection of the related metabolic disorders such as type 2 diabetes (Citation43) and obesity (Citation44) associated with unhealthy dietary habits (Citation45), as well as decrease the level of proinflammatory mediators to reduce the occurrence of chronic low-grade inflammation (Citation46). In this study, fecal transfer to pseudo germ-free mice was finally conducted and showed both inflammatory phenotype and comedones of acne could be improved in recipients rats of ω-3 fatty acids-altered microbiota from donors, which verified the beneficial effects of ω-3 fatty acids on acne vulgaris through modulating gut microbiota.
So far, the positive changes in the composition of intestinal flora after the supplement of ω-3 fatty acids into the diet has been confirmed (Citation41,Citation47). Our study consistently showed the increased abundance of Lactococcus, as well as Coprococcus and Eubacterium (butyric acid producing bacteria) by ω-3 supplementation, and their positive correlation has been stressed in previous studies (Citation48). The decreased abundance of Lactococcus and metabolic diseases such as obesity, diabetes, cardiovascular disease and others has been reported (Citation49). The increased abundance of Lactococcus in the intestinal of diabetic mouse model induced by high-fat diet was attribute to the ω-3 intake (Citation50), while the Lactococcus could prevent the harmful effects of TNF-α and IFN-γ on epithelial permeability and ion transport (Citation51), and then results in reducing the occurrence of intestinal inflammation (Citation52). Interestingly, the clinical symptoms of acne could be improved after drinking fermented beverages of Lactococcus (Citation53). In addition, decreased abundance of butyric acid producing bacteria has also been observed in patients with other inflammatory skin diseases such as psoriasis, atopic dermatitis and Behcet's disease (Citation54,Citation55). As an important short chain fatty acid (SCFA), the maintenance of intestinal barrier integrity and healthy intestinal microenvironment are affected by the butyrate, that could exert anti-inflammatory function by inhibiting histone deacetylase in regulatory T cells through G protein coupled receptor in blood circulation, and decrease the proliferation, migration, adhesion and cytokine production of inflammatory cells in the intestinal cavity to inhibit the immune response (Citation56,Citation57).
Furthermore, negative associations of Coprococcus with triglyceride-rich lipoproteins such as very low density lipoprotein (VLDL) and VLDL-TG has been reported (Citation15), acne patients showed decreased TG and enhanced HDL with ω-3 fatty acids intervention. These were also confirmed in the KEGG analysis of gut microbiota. After ω-3 fatty acids treatment, we not only observed an increase in fatty acids related pathways but also a decrease in adipocytokine signaling pathway. Specifically, the disorder of intestinal microbiota structure and function in patients with hyperlipidemia could induce pro-inflammatory local immune response (Citation58), which is accompanied by the production of adipokines (Citation59). Meanwhile, researchers also found the low levels of fecal acetate, butyrate and propionate in the hyperlipidemia was related to the decreased SCFA producing bacteria (Citation60). The SCFA could act as a regulatory signal of energy metabolism through the gut-brain axis and adenosine monophosphate activated protein kinase (AMPK) pathway to improve dyslipidemia (Citation61). In addition, the absorption of SCFA by the liver can also activate AMPK pathway and inhibit the synthesis of TC and TG (Citation62). Therefore, we speculated the therapeutic effect of ω-3 fatty acids on the acne vulgaris due to the reduction of the inflammatory response by increasing the abundance of butyric acid producing bacteria and modulating lipid metabolism.
In summary, our work showed supplementation of ω-3 fatty acids in diet could alleviate both the inflammation and comedones of moderate to severe acne by regulating gut microbiota. Although KEGG based functional analysis has indirectly revealed the potential mechanism of ω-3 fatty acids in treating this disease through modulating gut microbiota, further investigations could be conducted to uncover the molecular mechanisms how the microbial metabolites or proteins mediated by ω-3 fatty acids interact with the host targets in improving acne vulgaris.
Supplemental Material
Download PDF (587.1 KB)Acknowledgement
We are sincerely grateful to all those who participated in this study.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
Data described in the manuscript will be made available upon request.
Additional information
Funding
References
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):1–12. doi: 10.1016/j.jaad.2015.12.037.
- Gieler U, Gieler T, Kupfer JP. Acne and quality of life - impact and management. J Eur Acad Dermatol Venereol. 2015;29(S4):12–14. doi: 10.1111/jdv.13191.
- Layton AM. Optimal management of acne to prevent scarring and psychological sequelae. Am J Clin Dermatol. 2001;2(3):135–141. doi: 10.2165/00128071-200102030-00002.
- A, Dréno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(s1):1–29. doi: 10.1111/j.1468-3083.2011.04374.x.
- Oliveira JM, Sobreira G, Velosa J, et al. Association of isotretinoin with depression and suicide: a review of current literature. J Cutan Med Surg. 2018;22(1):58–64. doi: 10.1177/1203475417719052.
- Dai R, Hua W, Chen W, et al. The effect of milk consumption on acne: a meta-analysis of observational studies. J Eur Acad Dermatol Venereol. 2018;32(12):2244–2253. doi: 10.1111/jdv.15204.
- Matsui MS. Update on diet and acne cutis. Cutis. 2019;104(1):11–13.
- Rubin MG, Kim K, Logan AC. Acne vulgaris, mental health and omega-3 fatty acids: a report of cases. Lipids Health Dis. 2008;7(1):36. doi: 10.1186/1476-511X-7-36.
- Krishna S, Okhovat JP, Kim J, et al. Influence of ω-3 fatty acids on triglyceride levels in patients using isotretinoin. JAMA Dermatol. 2015;151(1):101–102. doi: 10.1001/jamadermatol.2014.2402.
- Matsushita M, Fujita K, Hayashi T, et al. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 2021;81(15):4014–4026. doi: 10.1158/0008-5472.CAN-20-4090.
- Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, et al. Ketogenic diet and epilepsy. Nutrients. 2019;11(10):2510. doi: 10.3390/nu11102510.
- Li H, Wang Q, Chen P, et al. Ursodeoxycholic acid treatment restores gut microbiota and alleviates liver inflammation in non-alcoholic steatohepatitic mouse model. Front Pharmacol. 2021;12:788558. doi: 10.3389/fphar.2021.788558.
- Ye J, Zhao Y, Chen X, et al. Pu-erh tea ameliorates obesity and modulates gut microbiota in high fat diet fed mice. Food Res Int. 2021;144:110360. doi: 10.1016/j.foodres.2021.110360.
- Rousseau G. Microbiota, a new playground for the omega-3 polyunsaturated fatty acids in cardiovascular diseases. Mar Drugs. 2021;19(2):19. doi: 10.3390/md19020054.
- Vijay A, Astbury S, Le Roy C, et al. The prebiotic effects of omega-3 fatty acid supplementation: a six-week randomised intervention trial. Gut Microbes. 2021;13(1):1–11. doi: 10.1080/19490976.2020.1863133.
- Fu Y, Wang Y, Gao H, et al. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediators Inflamm. 2021;2021:8879227–8879211. doi: 10.1155/2021/8879227.
- Yan HM, Zhao HJ, Guo DY, et al. Gut microbiota alterations in moderate to severe acne vulgaris patients. J Dermatol. 2018;45(10):1166–1171. doi: 10.1111/1346-8138.14586.
- Deng Y, Wang H, Zhou J, et al. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venerol. 2018;98(8):783–790. doi: 10.2340/00015555-2968.
- Doshi A, Zaheer A, Stiller MJ. A comparison of current acne grading systems and proposal of a novel system. Int J Dermatol. 1997;36(6):416–418. doi: 10.1046/j.1365-4362.1997.00099.x.
- de Andrade AM, Fernandes MC, de Fraga LS, et al. Omega-3 fatty acids revert high-fat diet-induced neuroinflammation but not recognition memory impairment in rats. Metab Brain Dis. 2017;32(6):1871–1881. doi: 10.1007/s11011-017-0080-7.
- Strauss JS, Jackson EM. American academy of dermatology invitational symposium on comedogenicity. J Am Acad Dermatol. 1989;20:272–277.
- Zhan G, Yang N, Li S, et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in SAMP8 mice. Aging. 2018;10(6):1257–1267. doi: 10.18632/aging.101464.
- He Y, Li X, Yu H, et al. The functional role of fecal microbiota transplantation on dextran sulfate Sodium-Induced colitis in mice. Front Cell Infect Microbiol. 2019;9:393. doi: 10.3389/fcimb.2019.00393.
- Vergani C, Finzi AF, Pigatto PD, et al. Low levels of HDL in severe cystic acne. N Engl J Med. 1982;307(18):1151–1152. doi: 10.1056/NEJM198210283071817.
- Gayen R, Podder I, Chakraborty I, et al. Sex hormones, metabolic status, and obesity in female patients with acne vulgaris along with clinical correlation: an observational cross-sectional study. Indian J Dermatol. 2021;66(1):60–66. doi: 10.4103/ijd.IJD_82_20.
- Nagpal M, De D, Handa S, et al. Insulin resistance and metabolic syndrome in young men with acne. JAMA Dermatol. 2016;152(4):399–404. doi: 10.1001/jamadermatol.2015.4499.
- Gocka K, Woźniak M, Kaczmarek-Skamira E, et al. Abnormal plasma lipids profile in women with post-adolescent acne. Postepy Dermatol Alergol. 2018;35(6):605–608. doi: 10.5114/ada.2018.77612.
- Ruiz-López N, Sayanova O, Napier JA, et al. Metabolic engineering of the omega-3 long chain polyunsaturated fatty acid biosynthetic pathway into transgenic plants. J Exp Bot. 2012;63(7):2397–2410. doi: 10.1093/jxb/err454.
- Wang Y, Wang Y, Shehzad Q, et al. Does omega-3 PUFAs supplementation improve metabolic syndrome and related cardiovascular diseases? A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2023 May 24:1–28. doi: 10.1080/10408398.2023.2212817.
- Khorshidi M, Hazaveh ZS, Alimohammadi-Kamalabadi M, et al. Effect of omega-3 supplementation on lipid profile in children and adolescents: a systematic review and meta-analysis of randomized clinical trials. Nutr J. 2023;22(1):9. doi: 10.1186/s12937-022-00826-5.
- Shahidi F, Ambigaipalan P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu Rev Food Sci Technol. 2018;9(1):345–381. doi: 10.1146/annurev-food-111317-095850.
- Ito MK. Long-chain omega-3 fatty acids, fibrates and niacin as therapeutic options in the treatment of hypertriglyceridemia: a review of the literature. Atherosclerosis. 2015;242(2):647–656. doi: 10.1016/j.atherosclerosis.2015.06.012.
- Tojo R, Suárez A, Clemente MG, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20(41):15163–15176. doi: 10.3748/wjg.v20.i41.15163.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36(1):29–40. doi: 10.1016/j.clindermatol.2017.09.006.
- Dall Oglio F, Nasca MR, Fiorentini F, et al. Diet and acne: review of the evidence from 2009 to 2020. Int J Dermatol. 2021;60(6):672–685. doi: 10.1111/ijd.15390.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic–load diet versus a conventional, high glycemic–load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57(2):247–256. doi: 10.1016/j.jaad.2007.01.046.
- Maarouf M, Platto JF, Shi Vivian Y. The role of nutrition in inflammatory pilosebaceous disorders: implication of the skin-gut axis. Australas J Dermatol. 2019;60(2):e90-8–e98. doi: 10.1111/ajd.12909.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes. 2014;5(2):185–199. doi: 10.3920/BM2012.0060.
- Becker E, Schmidt TSB, Bengs S, et al. Effects of oral antibiotics and isotretinoin on the murine gut microbiota. Int J Antimicrob Agents. 2017;50(3):342–351. doi: 10.1016/j.ijantimicag.2017.03.017.
- Santoru ML, Piras C, Murgia A, et al. Cross sectional evaluation of the gut-microbiome metabolome axis in an Italian cohort of IBD patients. Sci Rep. 2017;7(1):9523. doi: 10.1038/s41598-017-10034-5.
- Davis DJ, Hecht PM, Jasarevic E, et al. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain Behav Immun. 2017;59:38–48. doi: 10.1016/j.bbi.2016.09.003.
- Prossomariti A, Scaioli E, Piazzi G, et al. Short-term treatment with eicosapentaenoic acid improves inflammation and affects colonic differentiation markers and microbiota in patients with ulcerative colitis. Sci Rep. 2017;7(1):7458. doi: 10.1038/s41598-017-07992-1.
- Zhu L, Sha L, Li K, et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 2020;19(1):20. doi: 10.1186/s12944-019-1167-4.
- Bellenger J, Bellenger S, Escoula Q, et al. N-3 polyunsaturated fatty acids: an innovative strategy against obesity and related metabolic disorders, intestinal alteration and gut microbiota dysbiosis. Biochimie. 2019;159:66–71. doi: 10.1016/j.biochi.2019.01.017.
- Bidu C, Escoula Q, Bellenger S, et al. The transplantation of ω3 PUFA-altered gut microbiota of fat-1 mice to wild-type littermates prevents obesity and associated metabolic disorders. Diabetes. 2018;67(8):1512–1523. doi: 10.2337/db17-1488.
- Telle-Hansen VH, Holven KB, Ulven SM. Impact of a healthy dietary pattern on gut microbiota and systemic inflammation in humans. Nutrients. 2018;10(11):1783. doi: 10.3390/nu10111783.
- Kaliannan K, Wang B, Li XY, et al. A host-microbiome interaction mediates the opposing effects of omega-6 and omega-3 fatty acids on metabolic endotoxemia. Sci Rep. 2015;5(1):11276. doi: 10.1038/srep11276.
- Menni C, Zierer J, Pallister T, et al. Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep. 2017;7(1):11079. doi: 10.1038/s41598-017-10382-2.
- Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017;317(4):355–356. doi: 10.1001/jama.2016.20099.
- Mujico JR, Baccan GC, Gheorghe A, et al. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110(4):711–720. doi: 10.1017/S0007114512005612.
- Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-gamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130(3):731–746. doi: 10.1053/j.gastro.2005.12.015.
- David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. doi: 10.1038/nature12820.
- Kim J, Ko Y, Park YK, et al. Dietary effect of lactoferrin-enriched fermented milk on skin surface lipid and clinical improvement of acne vulgaris. Nutrition. 2010;26(9):902–909. doi: 10.1016/j.nut.2010.05.011.
- Hidalgo-Cantabrana C, Gómez J, Delgado S, et al. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br J Dermatol. 2019;181(6):1287–1295. doi: 10.1111/bjd.17931.
- Petersen EBM, Skov L, Thyssen JP, et al. Role of the gut microbiota in atopic dermatitis: a systematic review. Acta Derm Venereol. 2019;99(1):5–11. doi: 10.2340/00015555-3008.
- Sgambato A, Puglisi MA, Errico F, et al. Post-translational modulation of CD133 expression during sodium butyrate-induced differentiation of HT29 human colon cancer cells: implications for its detection. J Cell Physiol. 2010;224(1):234–241. doi: 10.1002/jcp.22124.
- Hamer HM, Jonkers DM, Bast A, et al. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28(1):88–93. doi: 10.1016/j.clnu.2008.11.002.
- Sanz Y, Moya PA. Microbiota, inflammation and obesity. Adv Exp Med Biol. 2014;817:291–317. doi: 10.1007/978-1-4939-0897-4_14.
- Kanneganti TD, Dixit VD. Immunological complications of obesity. Nat Immunol. 2012;13(8):707–712. doi: 10.1038/ni.2343.
- Gargari G, Deon V, Taverniti V, et al. Evidence of dysbiosis in the intestinal microbial ecosystem of children and adolescents with primary hyperlipidemia and the potential role of regular hazelnut intake. FEMS Microbiol Ecol. 2018;94(5):94.
- Dalile B, Van Oudenhove L, Vervliet B, et al. The role of Short-Chain fatty acids in microbiota-gut-Brain communication. Nat Rev Gastroenterol Hepatol. 2019;16(8):461–478. doi: 10.1038/s41575-019-0157-3.
- Lin HV, Frassetto A, Kowalik EJ, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-Independent mechanisms. PLoS One. 2012;7(4):e35240. doi: 10.1371/journal.pone.0035240.

