Abstract
In dry skin (DS), skin-barrier function is easily disturbed and moisturizing factors in the stratum corneum are reduced. Despite being a common condition, DS is often overlooked in patients with advanced age or comorbid diseases. In September 2022, specialists in dermatology and skin care met to discuss unmet needs and management of patients with DS with existing medical conditions or DS induced by ongoing pharmacological treatments. There was consensus about the need to improve the current understanding and management of DS in patients with comorbidities, including type 2 diabetes, chronic kidney disease, radiodermatitis, and photodamaged skin. Clinical guidance related to optimal treatment of DS in patients with advanced age or comorbid diseases is needed. Dexpanthenol-containing emollients have been shown to provide rapid relief from the symptoms and clinical signs of skin inflammation and are well-tolerated and effective in terms of moisturizing and soothing DS and maintaining skin-barrier function. Thus, dexpanthenol-containing emollients may play an important role in future management of DS. Further research is needed to elucidate the efficacy of dexpanthenol across the spectrum of DS, irrespective of comorbidity status or age.
Introduction
Overview
Dry skin (DS) may severely affect more than one in four persons depending on the study setting (Citation1,Citation2). Despite this, DS is often overlooked, including in patients with preexisting medical conditions or advanced age.
In September 2022, specialists in dermatology and skin care met to discuss unmet needs and management of patients with DS owing to preexisting medical conditions, with or without advanced age. They then explored the existing evidence base regarding the use of dexpanthenol-containing treatments in these populations.
This review aims to provide an overview of current unmet needs for people with DS resulting from comorbidities or their associated treatments and advanced age, to provide recommendations for healthcare professionals to address these unmet needs, and to review the potential role of dexpanthenol in patients with DS with existing medical conditions.
DS signs and symptoms
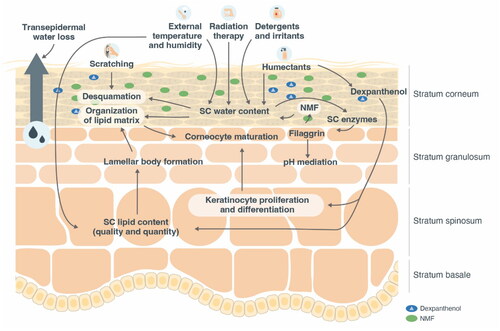
DS (also known as xerosis or xerosis cutis) is characterized by disturbed stratum corneum (SC) lipid composition and epidermal differentiation, which, when challenged, leads to disrupted skin-barrier function and a reduction of natural moisturizing factors (NMFs) within the skin () (Citation3,Citation4). Clinical signs of DS are well known and have previously been described (Citation4,Citation5). It is often associated with pruritus and may be a symptom of other skin conditions (such as asteatotic eczema, atopic dermatitis [AD], irritant contact dermatitis, ichthyosis, or psoriasis), or may be a separate condition (Citation5). Exogenous factors (environmental triggers, such as cold and low-humidity weather conditions, excessive skin cleansing/washing or washing with alkaline-containing soaps and cleansing agents, occupational factors, and hobbies) may lead to or aggravate DS (Citation4). Similarly, endogenous factors (aging; dermatological, internal, and psychiatric diseases; diet; excessive alcohol consumption; smoking; chronic stress; and pharmacological therapy) (Citation4,Citation6) may also be a cause of DS.
Figure 1. Adapted figure of schematic diagram showing a subset of key events affecting skin equilibrium. From Proksch E, et al. (Citation5) with kind permission from the Journal of Dermatological Treatment and Taylor & Francis Ltd (www.tandfonline.com). NMF, natural moisturizing factor; SC, stratum corneum.
DS pathophysiology
Filaggrin deficiency is observed in DS, including DS in AD regardless of filaggrin mutation status, and in ichthyosis vulgaris, suggesting that the absence of filaggrin is a key factor in the pathogenesis of this skin condition (Citation7,Citation8). DS has a weakened skin barrier including an elevated pH, which encourages the penetration of irritants, allergens, and bacteria into the skin (Citation9), which promotes the cyclic worsening of skin equilibrium ().
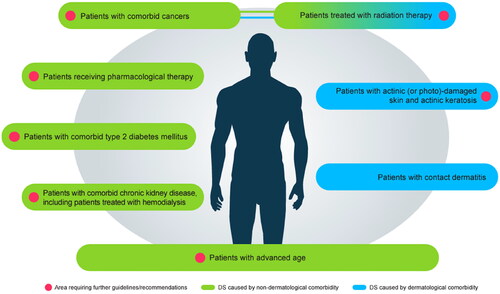
Expert opinions: DS associated with dermatologic conditions and diseases
There are numerous dermatological diseases associated with DS, including inflammatory skin disorders such as AD, contact dermatitis, genodermatoses, infectious dermatoses, and skin neoplasms (Citation4).
Contact dermatitis
At least 20% of the general population have skin sensitization to common environmental allergens (Citation10). Occupations that include sustained exposure to water, chemicals, machinery, sweat, or prolonged use of rubber gloves (with low permeation rate), are often associated with the development of contact dermatitis (Citation11,Citation12).
Based on our clinical experience, we recommend that patients with occupational dermatitis should clean their skin with a suitable mild and pH-adapted cleanser, dependent upon the specific area and whether soiling is present (). In areas where sticky substances are present and cleansing is needed, carers should be aware of subsequent symptoms of irritation, and if possible abrasive cleaners should be avoided. Continuous DS management is needed for employees working in occupations that have an increased DS risk. To meet this challenge, more employers should consider following the lead of those who are already supplying emollients to employees to improve self-care. Properties of the causal irritant (e.g., hydrophilic or hydrophobic) should be considered when selecting the appropriate treatment for contact dermatitis. Optimal treatment involves the supply and maintenance of water content in the epidermis, replenishment of NMFs, maintenance of skin-barrier lipids, and prevention of transepidermal water loss.
Table 1. Summary of author recommendations to improve the management of DS in patients with specific comorbidities.
Expert opinions: DS in patients with advanced age
DS is prevalent among patients with advanced age (often defined as persons aged ≥ 60 years) (Citation1,Citation2), is the most frequently diagnosed skin disease in nursing homes, and is often present in persons receiving home care (Citation13,Citation14). DS in patients with advanced age may be caused by intrinsic (decreased skin basal layer cell proliferation, reduced epidermal lipid synthesis, and downregulated cellular processes) and extrinsic (air pollution, smoking, poor nutrition, and sun exposure) factors (Citation15).
We believe that the application of emollients and eventually non-medical anti-inflammatory products and the monitoring of skin integrity are important aspects of preventive skin care that are often overlooked (). Patients may not be able to afford suitable skin care products (not provided to them free of cost), or the application of care may not be sufficiently provided by caregivers in busy nursing homes. There is room for improvement in the existing armamentarium of products in terms of efficacy to both cleanse and treat skin in patients with advanced age as well as the utilization of products in a nursing-home setting.
We believe that the challenges managing DS in patients with advanced age include care dependency, limited skin care in nursing homes, shortages of nursing staff, the high cost of skin care and limited resources of the retired population, and limited physical mobility of the patient. Further large-scale studies are needed to understand the needs of patients with advanced age and comorbidities ().
The development of treatment guidelines for patients with advanced age and comorbidities would improve the management of DS, and therefore baseline skin health, in this population.
Humectants are a key component of any formula developed to hydrate and treat DS (Citation5). These agents replicate the role of NMFs to aid moisturization of the skin and the depth at which a humectant exerts its effect is dependent on its molecular weight (Citation16). Hyaluronic acid moisturizes the uppermost layers of the SC, whereas glycerol and urea are able to penetrate the innermost layers of the SC (Citation5,Citation16). Considered both a humectant and an emollient, urea is a component of NMFs and is often present in emollients treating DS (Citation5). It is important to consider the concentration of urea in products intended to treat patients with DS and advanced age. In a recent study of patients with DS and advanced age, a 10% urea cream was well-tolerated and showed clinical improvement of DS (Citation17). At low doses (≤10% urea), urea-containing topical formulations act as skin moisturizers, while at higher concentrations (>10% urea), they exert keratolytic action (Citation18) and can facilitate the transport of molecules through the skin which may cause irritation (Citation19). Increased frequency of irritation is associated with higher concentrations of urea (Citation19). High concentrations of glycerol should also be avoided as this will exert an unwanted hygroscopic effect on skin (Citation5).
We opine that the intended treatment setting (nursing-home care and self-care) should be considered for any formulation, particularly when conditions such as eczema are present (). Products often vary in consistency (such as lotion, foam, cream, or ointment formulations) and may impact their ease of application to affected areas. Application of treatments to a patient with DS by a caregiver in a nursing home should be rapid, owing to limited time per patient, but may allow treatment of extremities such as the lower limbs, which patients may find difficult to reach. By contrast, patients with DS administering self-care may prefer treatments that take a little longer to apply but can be used on areas that they can reach without difficulty (such as the hands and face). Drying products are recommended when managing incontinence-associated dermatitis.
Expert opinions: DS associated with medical conditions or treatments
Type 2 diabetes mellitus
Type 2 diabetes mellitus is an endocrine disease that can lead to neuropathy and angiopathy, both of which can affect the skin and potentially lead to diseases such as pruritus () (Citation20). Fissures and cracks may act as entry sites for bacteria which, if left untreated, may cause complications such as diabetic foot ulceration, foot neuropathy, deformity, gangrene, and possible amputation (Citation21,Citation27).
Table 2. Symptoms and signs of DS in patients with specific comorbidities.
We believe that optimal patient management requires effective type 2 diabetes and DS treatment. We find that patients with DS and comorbid type 2 diabetes mellitus typically present to dermatologists with chronic DS, rather than in the early stages of DS progression—highlighting a potential communication gap between other specialists and dermatologists. Patients and physicians would benefit from additional education on managing DS in the setting of type 2 diabetes mellitus ().
Chronic kidney disease
Chronic kidney disease together with hemodialysis is associated with dermatological conditions including DS and pruritus (Citation28,Citation29). Similarly, a substantial proportion of patients with non-dialysis chronic kidney disease develop pruritus (Citation30). Chronic kidney disease-associated pruritus is often not associated with a single body part or region and may be generalized in up to 50% of patients. Areas that are affected include the face, chest, and limbs (). Up to 80% of patients with chronic kidney disease-associated pruritus also have coexisting DS (Citation23).
It is our opinion that treating patients with chronic kidney disease and DS is challenging, as optimal therapies have not yet been identified, owing to limited research. Even with appropriate DS symptom management, patients with chronic kidney disease may still experience underlying pruritus. For patients receiving treatment by hemodialysis who develop chronic kidney disease-associated pruritus, the potential effectiveness of dermatological management during patient clinical visits to provide additional routine treatment needs further research (). Hemodialysis appointments present opportunities for patients to receive dermatological assessment and treatment while hemodialysis is being performed ().
Radiation therapy-induced DS
An important mechanism of radiation therapy is that it increases reactive oxygen species, which damage tumor cells (Citation31). Reactive oxygen species also exacerbate skin aging and inflammation, contributing to radiation therapy-induced DS (Citation32). Short-term effects of radiation therapy on skin may include erythema and flaky skin, while long-term effects may include altered pigmentation, alopecia, and ulcerations () (Citation24). Topical corticosteroids, silver sulfadiazine, washing with water and soap, and use of deodorant on affected areas may be recommended for the management of radiodermatitis (RD) (Citation33). However, we believe that these agents have little effect on improving skin health of patients with RD. There is minimal consensus on the optimal dressings or protective creams for RD prophylaxis or management (Citation33).
We believe that identifying patients at increased risk of RD, and assigning adequate preventive measures, are clinical challenges in radiation oncology. Approaches to pharmaceutical prevention and management of RD are heterogeneous across different institutions. A survey of radiation oncologists based in Germany sought to understand the surveillance, nonpharmacological management, and pharmacological prevention and treatment approaches of RD and found that consensus exists on several risk factors and nonpharmaceutical prevention recommendations. However, identification of radiation therapy-dependent risk factors, such as the fractionation scheme, or hygienic measures like deodorant use, remain controversial. Of the 244 radiation oncologists who responded to the survey, 59.0% reported RD at least partially arbitrarily, and 83.7% were unaware of patient-reported outcome assessments (Citation34). Broad variations exist in the utilization of pharmacological prevention and treatment approaches (Citation35).
We feel that the relevant scientific societies should intensify their efforts to raise awareness of baseline RD management to overcome these present shortcomings. In addition, the radiation oncology community needs to examine current practice to ensure that a baseline level of care for RD is ubiquitously achieved. Further research on treatments and prevention for RD related to radiation therapy is required as well as a multidisciplinary approach to randomized controlled trials to improve future RD treatments ().
Pharmacological therapy-induced DS
Therapies such as retinoids, topical corticosteroids of high potency, diuretics, lipid-lowering agents, calcium antagonists, beta blockers, antirheumatic drugs, and cytostatic agents may directly cause, or contribute to, the presence of DS (Citation2,Citation4). We encourage dermatologists to proactively consider whether their patients’ ongoing drug therapies may be inducing DS and to take steps to treat it adequately ().
Actinic (or photo)-damaged skin and actinic keratosis
Actinic keratoses are rough scaly patches on skin caused by exposure to ultraviolet rays, which, if exposure persists, may progress to keratinocyte carcinoma () (Citation25). Although working outdoors is associated with an increased risk of actinic keratosis (Citation36), there are several mechanisms besides sun exposure that underlie the formation of actinic keratoses, including oxidative stress, inflammation, and altered cell proliferation (Citation26). Topically applied creams, gels, and solutions for the management of actinic keratosis commonly include 5-fluorouracil, imiquimod, and several other ingredients such as diclofenac gel and ingenol mebutate (Citation25). From our experience, there may be spontaneous resolution of actinic keratosis which possibly is supported by treatment with emollients, which many patients apply themselves. However, studies to verify reasons for resolution of actinic keratosis are lacking.
Dexpanthenol and emollient-plus-containing products: exploring the evidence base
Emollient-plus products
The EuroGuiDerm Guidelines describe basic emollient therapy as topical formulations that contain vehicle-type substances without active ingredients (Citation37). The same clinical guidelines describe emollients-plus products as containing additional active, non-medicated substances (Citation37). The guidelines distinguish between emollients and next-generation emollient plus, which aim to improve lesions by preserving barrier lipid content, prevent skin dehydration, and maintain skin moisture (Citation37,Citation38). In addition, the biological effects of emollients plus may include improved skin-barrier function, reduced inflammation, maintenance of a healthy skin microbiome, provision of essential lipids, and the relief of pruritus (Citation37,Citation38).
A definitive definition of this next-generation emollient class is lacking, but guidelines mention numerous examples of ingredients that may be included in an emollient-plus product. This may include flavonoids, saponins, riboflavins from protein-free oat plantlet extracts, synthetic derivatives of menthol, and bacterial lysates from Aquaphilus dolomiae and Vitreoscilla filiformis (Citation37).
In a real-world, prospective, observational, multicenter study based in Europe, an emollient-plus treatment containing A. dolomiae was investigated for efficacy in patients with DS induced by anticancer therapy (other ingredients in this treatment included omega fatty acids, ceramide, and sterols). Based on daily application of the emollient plus in 319 eligible patients, the treatment was effective at reducing the severity and symptoms of DS irrespective of anticancer therapy received (Citation39). Similarly, an observational study aimed to evaluate the efficacy of an emollient-plus treatment containing nonpathogenic V. filiformis in adult patients with mild-to-severe AD or skin disorders associated with DS. The results from this study of 1,339 showed that 85.6% of patients with at least mild DS at baseline had improved symptoms by at least one grade of disease severity. Moreover, overall satisfaction, including quality-of-life parameters, for the treatment was positive by both patient and dermatologists alike (Citation40).
V. filiformis lysates-containing emollient-plus products have demonstrated benefit in terms of reducing symptoms of pruritus. In a randomized, controlled, multicenter study of patients with moderate-to-severe AD (n = 57), the V. filiformis emollient-plus product provided a significantly greater reduction in existing pruritus (p = 0.0277) compared with control (preexisting emollient and cleanser) after 10 weeks of application (Citation41). The same ingredient has also demonstrated benefit in reducing corticosteroid consumption. In a randomized control trial (n = 119) of patients with mild-to-moderate AD, mean amount of corticosteroid usage was lower in the emollient-plus group versus control (6.03 vs 9.16 g; p = 0.041), was applied on fewer days (37.5% vs 46.9% of days; p = 0.0256), with fewer applications per day (0.55 vs 0.71 applications per day; p = 0.0203) (Citation42).
Utilizing dexpanthenol across the spectrum of DS: an emollient-plus ingredient?
We believe that skin-care agents containing dexpanthenol could be utilized across the spectrum of DS (). Dexpanthenol maintains and moisturizes the skin barrier, reduces transepidermal water loss (Citation43), has antipruritic properties, and increases elasticity in the SC (Citation16). Dexpanthenol may also be considered a cosmetic ingredient. Unlike traditional humectants, it also preserves and improves skin-barrier function (Citation43,Citation44).
Dexpanthenol-containing skin-care agents have been explored in several settings (). They have been shown to be well tolerated (Citation45) and effective in terms of moisturizing, soothing, and maintaining the integrity of the skin barrier when applied as a wash product and as part of a sun-care cream (Citation46). Other studies have suggested that these agents provide rapid relief from the signs and symptoms of skin inflammation, are pleasant to use, and do not affect microbiome diversity (Citation56,Citation57). Post-operative application of dexpanthenol-containing ointment together with background petroleum jelly following carbon dioxide laser therapy in patients with photodamaged skin has been shown to increase wound closure compared with petroleum jelly alone (Citation54). Significantly improved skin tension and reduced itching and dryness were associated with two dexpanthenol-containing lotions when patients with diabetes applied them to their feet and leg areas which had exhibited DS symptoms for 4 weeks (Citation58). The use of dexpanthenol-containing emollients as skin care for adult patients with diabetes was associated with significant improvements in skin hydration and skin-barrier function, and significantly reduced pruritic severity when applied once daily for 14 days (Citation44).
Table 3. Summary of selected studies and trials exploring the efficacy of dexpanthenol-containing agents in the setting of DS.
These studies demonstrate the versatility of dexpanthenol as an active ingredient in effective therapies in multiple treatment settings (). Although dexpanthenol is not currently listed as an example of an emollient-plus ingredient, published evidence aligns with the description of the biological effects of emollients plus, such as improved skin-barrier function, reduced inflammation, maintenance of a healthy skin microbiome, provision of essential lipids, and the relief of pruritus (Citation37,Citation38) as described previously.
Although not a direct comparison study with an emollient-plus product, previous research concluded that a topical panthenol-containing emollient was at least as efficacious as an established reference emollient in improving signs of AD and may be integrated as a skin-care regimen that is free of corticosteroid use for maintenance and management of patients with mild AD. After an initial stabilization phase (baseline) using a corticosteroid-free topical medical device, persons were randomized to either receive a topical panthenol-containing emollient (n = 52) or reference emollient (n = 56). During the maintenance phase, local SCORing AD scores reduced from baseline in both groups by the end of the study (−1.2 ± 1.3 [panthenol-containing emollient] and −1.0 ± 1.9 [reference emollient]) (Citation50).
Non-medicated treatments that contain dexpanthenol and do not fulfill the definition of, nor need a license as, a topical drug, could be considered an emollient plus (Citation37) and have been described this way in the published literature (Citation38).
Existing evidence suggests that the use of dexpanthenol-containing emollients can be beneficial in restoring or maintaining skin integrity, but the potential role of these agents should be investigated further across a wider range of underlying comorbidities (). Dexpanthenol has been, and is currently being, investigated for use on DS in patients with erythema (Citation52,Citation53), AD (Citation50), and in the processes of wound healing (Citation54,Citation59) and skin recovery () (Citation48,Citation49).
The use of dexpanthenol across the spectrum of DS has so far been limited, yet the potential roles and applications of dexpanthenol-containing formulations are numerous. As described earlier, a standard treatment for DS in patients with chronic kidney disease has yet to be identified. The potential efficacy of dexpanthenol in patients with DS induced by renal impairment requires further research.
Trials are needed to explore the potential efficacy of dexpanthenol in patients with RD. Current guidelines in Germany for prophylaxis of acute RD do not recommend the use of dexpanthenol (Citation60), possibly owing to a lack of supporting clinical data. However, in routine clinical practice most German-speaking radiation oncologists prescribe dexpanthenol-containing agents as topical RD prevention (Citation35). Should evidence suggest that dexpanthenol-containing emollients are efficacious in patients with RD, clinical management approaches will need to be consistent to ensure that patients receive optimal treatment ().
From our narrative review of literature, the efficacy of dexpanthenol in treating patients with DS and comorbid disease or advanced age has only been explored in small trials. Large-scale randomized controlled trials assessing the efficacy and safety of dexpanthenol-containing agents in patients with DS across the spectrum of age and comorbid disease in different care settings (nursing homes and self-care) may add to the growing body of evidence supporting their use to address unmet needs in DS treatment ().
Conclusions
DS is associated with significant burden of disease in patients with advanced age or who have common comorbid medical conditions such as chronic kidney disease or type 2 diabetes mellitus.
To summarize the authors’ viewpoints, we believe that there are unmet needs that require addressing, and potential areas of DS management that require improvement, irrespective of age and existing medical conditions ().
Certain drug therapies or radiation therapy can lead to additional burden of disease associated with DS in patients who are already experiencing the burdens associated with serious illnesses such as cancer. Development of specific DS clinical management guidelines for persons of advanced age might help address unmet needs in that population. As part of these guidelines, a focus on treatment management in nursing homes would be advisable.
Promoting a multidisciplinary approach to DS management may allow treatment of DS earlier in the pathophysiological cycle and reduce the burden of illness for those needing care. Utilizing routine clinical appointments for comorbidities (such as those for hemodialysis or management of type 2 diabetes mellitus) to simultaneously manage skin health may promote prevention of chronic DS and avoid future skin-related complications.
The impact of comorbid conditions and certain treatments on DS pathology requires further study. For example, cytokine suppression could be explored to alleviate symptoms and signs of DS. Cytokine modulation research would require a holistic approach given the potential systemic organ impact.
Dexpanthenol-containing emollients have already shown promise in terms of their ability to maintain skin-barrier integrity and moisture as well as reducing the symptoms and clinical signs of DS in specific populations. The value that dexpanthenol may provide in patients with comorbidities, irrespective of age, requires additional research across the spectrum of age and comorbid disease.
Author contributions
All authors were involved in the conception and design, or analysis and interpretation of the data; the drafting of the paper; revising it critically for intellectual content; and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to thank Bayer Consumer Care AG for supporting and funding the advisory board that led to the development of this publication. Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments, was provided by Thomas Carvell, MSc, and editorial, formatting, proofreading, and submission support was provided by Melissa Ward, BA, both of Scion (a division of Prime, London, UK), supported by Bayer Consumer Care AG, Germany, according to Good Publication Practice guidelines (Link). The sponsor was involved in the interpretation of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Disclosure statement
EP has received funding from Bayer Consumer Care AG. MA has served as a consultant, lecturer, researcher, and/or has received research grants from AbbVie, Almirall, Bayer Consumer Care AG, Beiersdorf, Galderma, Heliocare, and LEO Pharma. UBP has served as an advisor, consultant, and lecturer for AbbVie, Boots Healthcare, CeraVe, Dermocosmétique Vichy, Galderma Laboratorium GmbH, Eli Lilly, Pfizer, and Sanofi Regeneron, and has been involved in clinical studies for Amryt, Bayer, Cassiopeia, Concert Pharmaceuticals/Sun Pharma, Pierre Fabre, Novartis, Legacy, LEO Pharma, and Mayne Pharma. EB, UB-P, PE, and DS have all received funding from Bayer Consumer Care AG. EP, MA, EB, UB-P, PE, and DS have all participated in specialist panels organized by Bayer Consumer Care AG. DS is an investigator of a Bayer-sponsored clinical trial investigating Bepanthen. LCS has no disclosures to declare.
Additional information
Funding
References
- Augustin M, Kirsten N, Körber A, et al. Prevalence, predictors and comorbidity of dry skin in the general population. J Eur Acad Dermatol Venereol. 2019;33(1):1–9. doi:10.1111/jdv.15157.
- Jiang Q, Wang Y, Liu Y, et al. Prevalence and associated factors of dry skin among older inpatients in hospitals and nursing homes: a multicenter cross-sectional study. Int J Nurs Stud. 2022;135:104358. doi:10.1016/j.ijnurstu.2022.104358.
- Yosipovitch G, Misery L, Proksch E, et al. Skin barrier damage and itch: review of mechanisms, topical management and future directions. Acta Derm Venereol. 2019;99(13):1201–1209. doi:10.2340/00015555-3296.
- Augustin M, Wilsmann-Theis D, Körber A, et al. Diagnosis and treatment of xerosis cutis - a position paper. J Dtsch Dermatol Ges. 2019;17(S7):3–33. doi:10.1111/ddg.13906.
- Proksch E, Berardesca E, Misery L, et al. Dry skin management: practical approach in light of latest research on skin structure and function. J Dermatolog Treat. 2020;31(7):716–722. doi:10.1080/09546634.2019.1607024.
- Zhang J, Loman L, Oldhoff M, et al. Association between moderate to severe atopic dermatitis and lifestyle factors in the dutch general population. Clin Exp Dermatol. 2022;47(8):1523–1535. doi:10.1111/ced.15212.
- Smith FJ, Irvine AD, Terron-Kwiatkowski A, et al. Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet. 2006;38(3):337–342. doi:10.1038/ng1743.
- Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134(4):792–799. doi:10.1016/j.jaci.2014.06.014.
- Thyssen JP, Rinnov MR, Vestergaard C. Disease mechanisms in atopic dermatitis: a review of aetiological factors. Acta Derm Venereol. 2020;100(12):adv00162. doi:10.2340/00015555-3512.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80(2):77–85. doi:10.1111/cod.13119.
- Bauer A, Pesonen M, Brans R, et al. Occupational contact allergy: the european perspective - analysis of patch test data from ESSCA between 2011 and 2020. Contact Dermatitis. 2023;88(4):263–274. doi:10.1111/cod.14280.
- Thyssen JP, Schuttelaar MLA, Alfonso JH, et al. Guidelines for diagnosis, prevention, and treatment of hand eczema. Contact Dermatitis. 2022;86(5):357–378. doi:10.1111/cod.14035.
- Hahnel E, Blume-Peytavi U, Trojahn C, et al. Prevalence and associated factors of skin diseases in aged nursing home residents: a multicentre prevalence study. BMJ Open. 2017;7(9):e018283. doi:10.1136/bmjopen-2017-018283.
- Lichterfeld-Kottner A, Lahmann N, Blume-Peytavi U, et al. Dry skin in home care: a representative prevalence study. J Tissue Viability. 2018;27(4):226–231. doi:10.1016/j.jtv.2018.07.001.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738. doi:10.1177/0963689717725755.
- Kruglova LS, Lvov AN, Araviyskaya ER, et al. Practical issues of use of emollients in management of patients with xerosis: resolution of the panel of experts from russia, Kazakhstan and the republic of Belarus. Kremline Med. 2021;4:103–115.
- Lacarrubba F, Verzì AE, Dinotta F, et al. 10% Urea cream in senile xerosis: clinical and instrumental evaluation. J Cosmet Dermatol. 2021;20(Suppl 1):5–8. doi:10.1111/jocd.14093.
- Celleno L. Topical urea in skincare: a review. Dermatol Ther. 2018;31(6):e12690. doi:10.1111/dth.12690.
- Piquero-Casals J, Morgado-Carrasco D, Granger C, et al. Urea in dermatology: a review of its emollient, moisturizing, keratolytic, skin barrier enhancing and antimicrobial properties. Dermatol Ther (Heidelb). 2021;11(6):1905–1915. doi:10.1007/s13555-021-00611-y.
- Lima AL, Illing T, Schliemann S, et al. Cutaneous manifestations of diabetes mellitus: a review. Am J Clin Dermatol. 2017;18(4):541–553. doi:10.1007/s40257-017-0275-z.
- Pavicic T, Korting HC. Xerosis and callus formation as a key to the diabetic foot syndrome: dermatologic view of the problem and its management. J Dtsch Dermatol Ges. 2006;4(11):935–941. doi:10.1111/j.1610-0387.2006.06123.x.
- Lechner A, Akdeniz M, Tomova-Simitchieva T, et al. Comparing skin characteristics and molecular markers of xerotic foot skin between diabetic and non-diabetic subjects: an exploratory study. J Tissue Viability. 2019;28(4):200–209. doi:10.1016/j.jtv.2019.09.004.
- Swarna SS, Aziz K, Zubair T, et al. Pruritus associated with chronic kidney disease: a comprehensive literature review. Cureus. 2019;11(7):e5256. doi:10.7759/cureus.5256.
- Simões FV, Santos VO, Silva RND, et al. Effectiveness of skin protectors and calendula officinalis for prevention and treatment of radiodermatitis: an integrative review. Rev Bras Enferm. 2020;73(suppl 5):e20190815. doi:10.1590/0034-7167-2019-0815.
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85(4):e209–e233. doi:10.1016/j.jaad.2021.02.082.
- de Oliveira ECV, da Motta VRV, Pantoja PC, et al. Actinic keratosis - review for clinical practice. Int J Dermatol. 2019;58(4):400–407. doi:10.1111/ijd.14147.
- Mishra SC, Chhatbar KC, Kashikar A, et al. Diabetic foot. BMJ. 2017;359:j5064. doi:10.1136/bmj.j5064.
- Tajalli F, Mirahmadi SM, Mozafarpoor S, et al. Mucocutaneous manifestations of patients with chronic kidney disease under hemodialysis: a cross-sectional study of 49 patients. Dermatol Ther. 2021;34(4):e15015. doi:10.1111/dth.15015.
- Galperin TA, Cronin AJ, Leslie KS. Cutaneous manifestations of ESRD. Clin J Am Soc Nephrol. 2014;9(1):201–218. doi:10.2215/CJN.05900513.
- Wulczyn KE, Rhee EP, Myint L, et al. Incidence and risk factors for pruritus in patients with nondialysis CKD. Clin J Am Soc Nephrol. 2023;18(2):193–203. doi:10.2215/CJN.09480822.
- Perillo B, Di Donato M, Pezone A, et al. ROS in cancer therapy: the bright side of the moon. Exp Mol Med. 2020;52(2):192–203. doi:10.1038/s12276-020-0384-2.
- Nakai K, Tsuruta D. What are reactive oxygen species, free radicals, and oxidative stress in skin diseases? Int J Mol Sci. 2021;22(19):10799. doi:10.3390/ijms221910799.
- Finkelstein S, Kanee L, Behroozian T, et al. Comparison of clinical practice guidelines on radiation dermatitis: a narrative review. Support Care Cancer. 2022;30(6):4663–4674. doi:10.1007/s00520-022-06829-6.
- Layer K, Layer JP, Glasmacher AR, et al. Risk assessment, surveillance, and nonpharmaceutical prevention of acute radiation dermatitis: results of a multicentric survey among the german-speaking radiation oncology community. Strahlenther Onkol. 2023;199(10):891–900. doi:10.1007/s00066-023-02074-w.
- Layer JP, Layer K, Glasmacher AR, et al. Pharmaceutical management of acute radiation dermatitis in the german speaking radiation oncology community. J Dtsch Dermatol Ges. 2024;22(2):198–207. doi:10.1111/ddg.15279.
- Grandahl K, Olsen J, Friis KBE, et al. Photoaging and actinic keratosis in danish outdoor and indoor workers. Photodermatol Photoimmunol Photomed. 2019;35(4):201–207. doi:10.1111/phpp.12451.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema – part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022;36(11):1904–1926. doi:10.1111/jdv.18429.
- Araviiskaia E, Pincelli C, Sparavigna A, et al. The role of a novel generation of emollients, ‘emollients plus’, in atopic dermatitis. Clin Cosmet Investig Dermatol. 2022;15:2705–2719. doi:10.2147/CCID.S389697.
- Vendrely V, Mayor-Ibarguren A, Stennevin A, et al. An emollient plus balm is useful for the management of xerosis in patients treated for cancer: a real-world, prospective, observational, multicenter study. Dermatol Ther (Heidelb). 2022;12(3):683–699. doi:10.1007/s13555-022-00685-2.
- Patsatsi A, Vakirlis E, Kanelleas A, et al. Effect of a novel "emollient plus" formulation on mild-to-severe atopic dermatitis and other dry skin-related diseases as monotherapy or adjunctive therapy: an observational study on efficacy, tolerance and quality of life in adult patients. Eur J Dermatol. 2023;33(2):137–146. doi:10.1684/ejd.2023.4449.
- Magnolo N, Jaenicke T, Tsianakas A, et al. Comparison of different skin care regimens in patients with moderate to severe atopic dermatitis receiving systemic treatment: a randomized controlled trial. J Eur Acad Dermatol Venereol. 2023;37 Suppl 5:18–26. doi:10.1111/jdv.18949.
- Zelenkova H, Kerob D, Salah S, et al. Impact of daily use of emollient ‘plus’ on corticosteroid consumption in patients with atopic dermatitis: an open, randomized controlled study. J Eur Acad Dermatol Venereol. 2023;37 Suppl 5:27–34. doi:10.1111/jdv.18947.
- Stettler H, de Salvo R, Brandt M, et al. Performance and acceptability of a new dexpanthenol-containing hand cream in subjects with sensitive and very dry skin: a randomized controlled study. Cosmetics. 2022;9(3):44. doi:10.3390/cosmetics9030044.
- Stettler H, Doarika A, Reber F. Performance, acceptability and tolerability of a new dexpanthenol-containing emollient in the care of dry skin in adult diabetics. EADV. Virtual Conference. 2020.
- Stettler H, de Salvo R, Olsavszky R, et al. Performance and tolerability of a new topical dexpanthenol-containing emollient line in subjects with dry skin: results from three randomized studies. Cosmetics. 2021;8(1):18. doi:10.3390/cosmetics8010018.
- Schmid DA, Domingues MP, Nanu A, et al. Exploratory evaluation of tolerability, performance, and cosmetic acceptance of dexpanthenol-containing dermo-cosmetic wash and sun-care products for tattoo aftercare. Health Sci Rep. 2022;5(4):e635. doi:10.1002/hsr2.635.
- ClinicalTrials.gov. A clinical study of adherence to methylprednisolone aceponate in different carriers (AD-HERE) [Internet]. https://clinicaltrials.gov/ct2/show/NCT04016025.
- ClinicalTrials.gov. A study to gain information how well dexpanthenol dermal spray helps the skin to recover after a peeling in the external genital area of women [Internet]. https://clinicaltrials.gov/ct2/show/NCT03853512.
- ClinicalTrials.gov. A study to gain information how well dexpanthenol dermal spray helps the skin to recover after laser hair removal in the groin and intimate area [Internet]. https://clinicaltrials.gov/ct2/show/NCT03853525.
- Stettler H, Kurka P, Kandzora J, et al. A new topical panthenol-containing emollient for maintenance treatment of childhood atopic dermatitis: results from a multicenter prospective study. J Dermatolog Treat. 2017;28(8):774–779. doi:10.1080/09546634.2017.1328938.
- Heise R, Skazik C, Marquardt Y, et al. Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol. 2012;25(5):241–248. doi:10.1159/000341144.
- ClinicalTrials.gov. A study to gain information how well dexpanthenol dermal cream helps the face skin to recover after cosmetic lasering [Internet]. https://clinicaltrials.gov/ct2/show/NCT03852563.
- ClinicalTrials.gov. A study to gain information how well dexpanthenol dermal spray helps the face skin to recover after cosmetic lasering [Internet]. https://clinicaltrials.gov/ct2/show/NCT03853538.
- Heise R, Schmitt L, Huth L, et al. Accelerated wound healing with a dexpanthenol-containing ointment after fractional ablative CO2 laser resurfacing of photo-damaged skin in a randomized prospective clinical trial. Cutan Ocul Toxicol. 2019;38(3):274–278. doi:10.1080/15569527.2019.1597879.
- ClinicalTrials.gov. Comparison of the effect of Vaseline and Bepanthen® "Wund- und Heilsalbe" on the wound healing following laser therapy (CO2) [Internet]. https://clinicaltrials.gov/ct2/show/NCT03095872.
- Peltier E, de Salvo R, Ehret A, et al. Evaluation of a 5% dexpanthenol-containing ointment for the treatment of infant irritant diaper dermatitis through the lens of the caregiver-A real-world data observational study. Health Sci Rep. 2023;6(1):e1033. doi:10.1002/hsr2.1033.
- Peltier E, Trapp S, de Salvo R, et al. A new dexpanthenol-containing liquid cleanser for atopic-prone skin: results from two prospective clinical studies evaluating cutaneous tolerability, moisturization potential, and effects on barrier function. J Cosmet Dermatol. 2022;21(9):3859–3866. doi:10.1111/jocd.15252.
- Proksch E, de Bony R, Trapp S, et al. Topical use of dexpanthenol: a 70th anniversary article. J Dermatolog Treat. 2017;28(8):766–773. doi:10.1080/09546634.2017.1325310.
- Gorski J, Proksch E, Baron JM, et al. Dexpanthenol in wound healing after medical and cosmetic interventions (postprocedure wound healing). Pharmaceuticals (Basel). 2020;13(7):138. doi:10.3390/ph13070138.
- Reinartz G, Eich HT, Pohl F, German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD). DEGRO practical guidelines for the radiotherapy of non-malignant disorders - part IV: symptomatic functional disorders. Strahlenther Onkol. 2015;191(4):295–302. doi:10.1007/s00066-014-0789-8.