Abstract
Purpose:
For individuals with atopic dermatitis (AD), interpreting scientific papers that present clinical outcomes including the Eczema Area and Severity Index (EASI) and Investigators Global Assessment may be difficult. When compared to tabulated data and graphs, images from before and after treatment are often far more meaningful to these patients that ultimately will be candidates for the treatment. This systematic review focused on determining the frequency of clinical image sharing in AD research.
Materials and methods:
Conducted in accordance with PRISMA guidelines, the review concentrated on randomized controlled trials that investigated predefined and available systemic treatments for AD. The search was performed in the MEDLINE database for studies published from the inception until 21 December 2023.
Results:
The review included 60 studies, encompassing 17,799 randomized patients. Across these studies, 16 images representing 6 patients were shared in the manuscripts, leading to a sharing rate of 0.3‰.
Conclusions:
The almost missing inclusion of patient images in clinical trial publications hinders patient understanding. Adding images to scientific manuscripts could significantly improve patients’ comprehension of potential treatment outcomes. This review highlights the need for authors, the pharmaceutical industry, study sponsors, and publishers to enhance and promote patient information through increased use of visual data.
Introduction
Atopic dermatitis (AD) is a common inflammatory skin condition characterized by recurring eczematous lesions and severe itching, affecting individuals across all age groups and ethnic backgrounds (Citation1,Citation2). AD not only adds to the global burden of skin diseases but also significantly impacts the psychosocial well-being of both patients and their families (Citation3,Citation4).
Historically, patients with psoriasis have had more available treatment options as compared to individuals with AD. However, the last decade has seen a significant expansion in novel systemic pharmacotherapy for AD, including the introduction of biologics and Janus kinase inhibitors (JAKi) (Citation5,Citation6). While often very effective, these novel drugs still have ongoing patents meaning that they have a considerable impact on healthcare budgets worldwide.
In randomized prospective clinical trials involving patients with AD, the most often used primary outcomes are based on improvement of baseline Eczema Area and Severity Index (EASI) and/or variations of the Investigators Global Assessment (IGA) scale. The EASI score, evaluates the intensity of symptoms (such as redness, swelling, crusting, and scaling) and the area affected by eczema on the body. This tool is commonly used by healthcare professionals to monitor the progression or improvement of eczema over time. The IGA score is a simple, overall clinical assessment tool used by investigators to rate the severity of AD, considering factors like redness, swelling, and the area of skin affected, at a specific point in time. The score ranges from 0, clear, to 5, severe.
Shared decision making is a collaborative process in which a healthcare professional works together with a patient to reach a decision about care (Citation7). It is generally agreed, and also stipulated in many legislations worldwide, that shared decision making should be the norm in contemporary medicine.
The majority of patients find scientific manuscripts, including scoring systems like EASI and IGA, challenging to comprehend. Furthermore, although the purpose of these manuscripts, particularly those that discuss pharmaceutical treatments, is to assess effectiveness with the goal of enhancing patient care, the format of scientific manuscripts has remained largely static for decades. Bearing this in mind, most patients and learners naturally resonate with visual aids.
The aim of this systematic review was to investigate at what proportion clinical images were shared in randomized prospective clinical trials of currently available systemic pharmacotherapy for AD.
Materials and methods
A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for all applicable items (Citation8). The PRISMA checklist is available in the supplementary material (Appendix S1). Prior the initiation, the review protocol was compiled by both authors. Our predefined list of systemic treatment options was developed in accordance with the American and European guidelines for the treatment of Atopic Dermatitis (AD) (Citation9–11).
Eligibility criteria
Population: no geographic restriction was imposed.
Atopic dermatitis: including all subtypes.
Study design: randomized controlled trials published within the specified time frame, from the inception of MEDLINE until 21 December 2023.
Outcome measure: Investigator-reported visual assessment of AD severity.
Study drugs: dupilumab, tralokinumab; abrocitinib, baricitinib, upadacitinib; azathioprine, cyclosporine, methotrexate, mycophenolate mofetil.
Exclusion criteria
Follow-up, post-hoc or extension investigations.
Non-English investigations.
Information sources and search strategy
The MEDLINE (PubMed) database was searched for eligible studies published from inception to 21 December 2023. The search string employed is detailed in Appendix S2. We confined our search to MEDLINE, based on a previous review addressing a similar topic in psoriasis where all the papers ultimately selected after the screening phase were indexed in this database (Citation12).
Selection and data collection process
Both authors independently reviewed the titles and abstracts of all the studies. Any discrepancies during the title/abstract screening phase were settled through consensus. Subsequently, both authors independently assessed all the full texts. At this stage consensus was used to resolve any conflicting opinions regarding the eligibility of studies. Additionally, both authors confirmed the data extracted from the selected studies to ensure accuracy.
Data items
Data extracted from the included studies encompassed several key elements: the first author’s name, publication year, journal name, digital object identifier (DOI) link (where available), primary outcome (if specified), duration until the primary outcome (if specified), total number of patients randomized, and the count of patients and images presented in the manuscript, inclusive of all supplementary and video material. In cases where a study comprised more than one stage, only the patient numbers contributing to the primary outcome were included in the analysis. A customized data extraction worksheet was utilized to methodically collect all the aforementioned data points (Table SI).
Study risk of bias assessment
Given the dichotomous outcome focus of this review, we did not employ any quality assessment tools nor conduct tests for publication bias.
Effect measures and statistics
This review was based on a binary measure, specifically the presence or absence of clinical images. We calculated the proportion of images shared in each study and across the entire dataset. The compilation and organization of records were facilitated using three software tools: EndNote (Clarivate Analytics, Philadelphia, PA, USA), Rayyan (Rayyan Systems Inc., Cambridge, MA, USA), and Microsoft Excel (Microsoft, Redmond, WA, USA). The process of handling all publications and data extraction was conducted manually, without the use of any automation software. The original EndNote library utilized for this review can be made available upon request to the corresponding author.
Results
Of the 417 records first identified, 71 investigations were reviewed in full text. Among these, 11 investigations were excluded (Citation13–23) (Table SII). After exclusions, 60 studies published in the time period of 1991 to 2023 were included in the analysis (). Overall, 18 medical journals were represented (Table SIII).
Figure 1. PRISMA flow chart. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
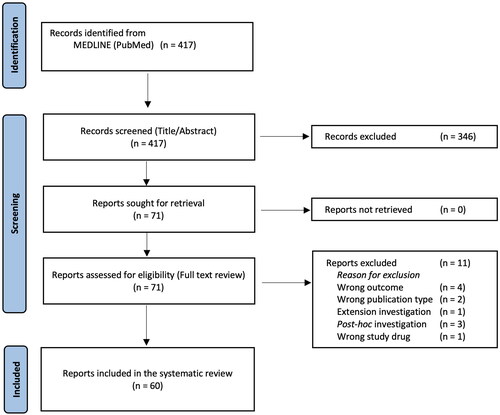
When combining the 60 investigations (Citation24–83), above, 17,799 patients were randomized. Overall, 3 investigations included patients image material in the running manuscript (Citation26,Citation40,Citation79), and one additional investigation (Citation37), shared patient images in the supplementary material (Table 1). When combining these four investigations, 16 images were shared depicting 6 patients in the manuscripts (including all supplementary text and video material) yielding an overall sharing rate of 0.3‰. One investigation included video supplement, however this recording did not include any patient images (Citation56). The majority of patients (n = 16,259) were randomized in trials that included EASI and/or variations of the IGA score as a primary outcome. Among these individuals, five patients (0.3‰) were depicted. The same proportion (i.e., 0.3‰) was observed when combining the five medical journals that included the highest number of patients ().
Table 1. List of investigations (n = 4) with included patient images in running manuscripts and supplements.
Table 2. Proportion of depicted patients in the five journals with most randomized patients.
Discussion
This systematic review underlines the striking scarcity of image sharing in AD-related randomized controlled trials, pointing to an almost overlooked aspect in clinical research dissemination. The vast majority (93.3%, n = 56) of all included investigations included no patient image material and merely 0.3‰ (n = 6) of all randomized patients (n = 17,799) were depicted in the included manuscripts.
In recent years, the introduction of biologics and JAKi has greatly improved treatment outcomes for patients with AD, particularly those with moderate to severe disease. In contrast with the innovative development of new systemic pharmacotherapies, the way in which randomized controlled trial data is presented has largely remained the same. Digitalization has facilitated data collection including imaging, compilation, and dissemination to the potential benefit of patients, healthcare providers, and the pharmaceutical industry. This review pinpoints that these opportunities have largely been overlooked when it comes to presentation of clinical trial data that is accessible and comprehensible for patients.
In a proposal published in 2016, The International Committee of Medical Journal Editors (ICMJE) stated that there is an ethical obligation to responsibly share data generated by interventional clinical trials because participants have put themselves at risk (Citation84). In 2017, the ICMJE listed the inclusion of data sharing statements in the clinical trials registration phase as a requirement (Citation85). While sharing of deidentified individual-patient data from clinical trials is now considered the norm, the requirements unfortunately do not explicitly mention sharing image data.
Medical scientific articles in peer-reviewed journals are primarily aimed at healthcare providers and researchers, but as patients and patient advocacy groups are becoming integral contributors to standard care practices, we believe that they must be included among the target readers. One way to meet this demand has been to add plain language or capsule summaries for laypersons in original reports. Today, when the online open-access journal is the preferred medium for publication of original data, submission of supplementary material that supports the findings is often encouraged by the editorial offices. For randomized clinical trials, images illustrating disease distribution at the initial visit and the follow-up visits would be highly relevant as supplementary material. We are confident that visual amendments can play a crucial role in involving patients more closely. Enhancing patients’ comprehension of the potential treatment effects is expected to significantly improve their ability to make informed decisions together with their dermatologist.
Sharing deidentified clinical images of AD from interventional trials can also serve other important purposes. Visual representation, in addition to the scoring systems recommended for clinical trials by the Harmonizing Outcome Measures for Eczema (HOME) taskforce, aids external validation of the treatment effects (Citation86). Clinical images hold a predominant position in the field of dermatology education, serving as a fundamental tool for teaching and learning. Readily available sets of images depicting different AD phenotypes in different patient populations and the response to treatment can be used in the training of healthcare professionals, medical students as well as for patient education. It is important to consider that skin phototypes affects the visual manifestations of active AD as well as the post-inflammatory state (Citation87). For instance, hyperemia is not always perceived as red but rather as purple or brown in melanin-rich skin types. This limits the applicability of erythema as a scoring item in systems such as EASI or the SCORing of Atopic Dermatitis (SCORAD) index. Clinical trials with global recruitment could therefore provide valuable images of AD phenotypes in different skin phototypes, in active disease and remission, to improve healthcare providers’ clinical assessment.
Another potential ancillary use for large sets of images of atopic skin is in the development of machine learning (ML) algorithms to aid management of AD. It is highly conceivable that ML algorithms, which incorporate the baseline clinical phenotype together with patient metadata, could significantly aid physicians in pinpointing the most efficacious treatment options for the individual patient. Clearly, this approach has the potential to circumvent the traditional trial-and-error method, offering a more streamlined and precise pathway to optimal patient care.
We report an exceptionally low sharing rate of clinical images. Investigating the reasons for this was outside the scope of this review. One reason could be medicolegal regulations for clinical trial procedure and patient privacy. Another likely reason is a lack of clinical images to share. While standardized photography is an integrated part of patient follow-up in real-world dermatology it is currently not an established method for assessment and follow-up in clinical trials. Different aspects of this were previously discussed (Citation12). In addition, AD has a relapsing course with rapid fluctuations, which makes representative documentation with photography more challenging than for other skin diseases such as psoriasis.
Collecting clinical images would be an additional task for investigators but could prove rewarding. Images of selected body parts illustrating treatment progress could most often be used in publications without exposing the patient’s identity. As a service to readers, editorial offices have restrictions on the amount of data that is presented in the running manuscript, but including online supplementary files is usually encouraged. To maintain an overview for readers, only a minority of patient outcomes in trials can be presented with clinical images even in such supplementary files. This in turn introduces a risk for “cherry picking” (i.e., selection bias). Accessibility to all available image material for all patients could partly help resolve this issue.
While the comprehensive design of a system for managing clinical images collected in clinical trials falls outside the scope of this review, we envision such a system as an open-access, unified, authenticated, and secure database. Ideally this platform would garner universal support from the pharmaceutical industry, healthcare providers, academic institutions, and publishers. An interface with intuitive design would be essential for such a system. Image data should be collected in a standardized manner, specified in the trial protocol, to protect patient integrity. Moreover, the online platform should feature a user-friendly navigation system to ensure that patients, healthcare providers, and other stakeholders can maximize its utility.
The limitations of this study include that the search was confined to MEDLINE. The reason for this is explained in the Materials and Methods section. Investigations published in other languages than English were excluded and could potentially differ in the sharing rate of clinical images although we considered this unlikely. Also, it is likely that all pivotal clinical trials for new systemic pharmacotherapy for AD are published in English. It is possible that there are other subsets of publications on AD (apart from case reports) that contain more clinical images.
In the era of new systemic pharmacotherapy, online image-based communication, and increased demand for patient participation in clinical decision-making, we report an almost non-existent inclusion of patient images in published clinical AD trials. Patients who commit to participation in clinical trials devote considerable time and effort to improve future care. We believe that they would like to share appropriately anonymized images to illustrate treatment effects. We welcome a discussion on how clinical images can be used in the execution and communication of clinical trials to the benefit of patients, healthcare providers, sponsors, and healthcare authorities.
Supplemental Material
Download PDF (228 KB)Disclosure statement
SP: With no relation to the present manuscript, Sam Polesie has received honoraria as consultant and/or speaker from AbbVie, Amgen, Astra Zeneca, Beiersdorf, Bristol Myers Squibb, Galderma, Janssen Pharmaceuticals, Leo Pharma, Novartis, and Sanofi.
MA: With no relation to the present manuscript, Mikael Alsterholm has received speaker honoraria and/or been in advisory boards for AbbVie, Almirall, Eli-Lilly, Essity, LEO Pharma, Pfizer, and Sanofi-Genzyme, and is/has been an investigator for AbbVie, LEO Pharma, and Sanofi-Genzyme.
Additional information
Funding
References
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):1–9. doi: 10.1016/S0140-6736(20)31286-1.
- Ständer S. Atopic dermatitis. N Engl J Med. 2021;384(12):1136–1143. doi: 10.1056/NEJMra2023911.
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123(2):144–151. doi: 10.1016/j.anai.2019.04.020.
- Yang EJ, Beck KM, Sekhon S, et al. The impact of pediatric atopic dermatitis on families: a review. Pediatr Dermatol. 2019;36(1):66–71. doi: 10.1111/pde.13727.
- Baghoomian W, Na C, Simpson EL. New and emerging biologics for atopic dermatitis. Am J Clin Dermatol. 2020;21(4):457–465. doi: 10.1007/s40257-020-00515-1.
- Huang IH, Chung WH, Wu PC, et al. JAK-STAT signaling pathway in the pathogenesis of atopic dermatitis: an updated review. Front Immunol. 2022;13:1068260. doi: 10.3389/fimmu.2022.1068260.
- NICE Evidence Reviews Collection. Evidence review for interventions to support effective shared decision making: shared decision making: evidence review B. London: National Institute for Health and Care Excellence (NICE); 2021.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71.
- Davis DMR, Drucker AM, Alikhan A, et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J Am Acad Dermatol. 2024;90(2):e43–e56. doi: 10.1016/j.jaad.2023.08.102.
- Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. doi: 10.1111/jdv.18345.
- Wollenberg A, Kinberger M, Arents B, et al. First update of the living European guideline (EuroGuiDerm) on atopic eczema. J Eur Acad Dermatol Venereol. 2023;37(11):e1283–e1287. doi: 10.1111/jdv.19269.
- Polesie S, Alinaghi F, Egeberg A. A systematic review investigating at what proportion clinical images are shared in prospective randomized controlled trials involving patients with psoriasis and biological agents. J Dermatolog Treat. 2023;34(1):2281261. doi: 10.1080/09546634.2023.2281261.
- Bangert C, Loesche C, Skvara H, et al. IgE depletion with ligelizumab does not significantly improve clinical symptoms in patients with moderate-to-severe atopic dermatitis. J Invest Dermatol. 2023;143(10):1896 e1898–1905 e1898. doi: 10.1016/j.jid.2023.01.040.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: results from a phase IIa open-label trial and subsequent phase III open-label extension. Br J Dermatol. 2020;182(1):85–96. doi: 10.1111/bjd.18476.
- Cork MJ, Thaçi D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged >/= 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184(5):857–870. doi: 10.1111/bjd.19460.
- Granlund H, Erkko P, Reitamo S. Comparison of the influence of cyclosporine and topical betamethasone-17,21-dipropionate treatment on quality of life in chronic hand eczema. Acta Derm Venereol. 1997;77(1):54–58. doi: 10.2340/00015555775458.
- Guttman-Yassky E, Silverberg JI, Thaci D, et al. Upadacitinib treatment withdrawal and retreatment in patients with moderate-to-severe atopic dermatitis: results from a phase 2b, randomized, controlled trial. J Eur Acad Dermatol Venereol. 2023;37(12):2558–2568. doi: 10.1111/jdv.19391.
- Irvine AD, Jones AP, Beattie P, et al. A randomized controlled trial protocol assessing the effectiveness, safety and cost-effectiveness of methotrexate vs. ciclosporin in the treatment of severe atopic eczema in children: the TREatment of severe atopic eczema trial (TREAT). Br J Dermatol. 2018;179(6):1297–1306. doi: 10.1111/bjd.16717.
- Katoh N, Ohya Y, Murota H, et al. A phase 3 randomized, multicenter, double-blind study to evaluate the safety of upadacitinib in combination with topical corticosteroids in adolescent and adult patients with moderate-to-severe atopic dermatitis in Japan (rising up): an interim 24-week analysis. JAAD Int. 2022;6:27–36. doi: 10.1016/j.jdin.2021.11.001.
- Rossi M, Rovati C, Arisi M, et al. A short cycle of narrow-band UVB phototherapy in the early phase of dupilumab therapy can provide a quicker improvement of severe atopic dermatitis. Dermatology. 2021;237(3):407–415. doi: 10.1159/000512456.
- Siegfried EC, Simpson EL, Cork MJ, et al. Dupilumab treatment leads to rapid and consistent improvement of atopic dermatitis in all anatomical regions in patients aged 6 months to 5 years. Dermatol Ther. 2023;13(9):1987–2000. doi: 10.1007/s13555-023-00960-w.
- Silverberg JI, de Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD up study results. J Allergy Clin Immunol. 2022;149(3):977–987 e914. doi: 10.1016/j.jaci.2021.07.036.
- Thaçi D, L Simpson E, Deleuran M, et al. Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci. 2019;94(2):266–275. doi: 10.1016/j.jdermsci.2019.02.002.
- Agarwal US, Besarwal RK. Topical clobetasol propionate 0.05% cream alone and in combination with azathioprine in patients with chronic hand eczema: an observer blinded randomized comparative trial. Indian J Dermatol Venereol Leprol. 2013;79(1):101–103. doi: 10.4103/0378-6323.104679.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–139. doi: 10.1056/NEJMoa1314768.
- Bemanian MH, Movahedi M, Farhoudi A, et al. High doses intravenous immunoglobulin versus oral cyclosporine in the treatment of severe atopic dermatitis. Iran J Allergy Asthma Immunol. 2005;4:139–143.
- Berth-Jones J, Takwale A, Tan E, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol. 2002;147(2):324–330. doi: 10.1046/j.1365-2133.2002.04989.x.
- Bieber T, Reich K, Paul C, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in patients with moderate-to-severe atopic dermatitis with inadequate response, intolerance or contraindication to ciclosporin: results from a randomized, placebo-controlled, phase III clinical trial (BREEZE-AD4). Br J Dermatol. 2022;187(3):338–352. doi: 10.1111/bjd.21630.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi: 10.1056/NEJMoa2019380.
- Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi: 10.1016/S0140-6736(17)31191-1.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023.
- Czech W, Bräutigam M, Weidinger G, et al. A body-weight-independent dosing regimen of cyclosporine microemulsion is effective in severe atopic dermatitis and improves the quality of life. J Am Acad Dermatol. 2000;42:653–659.
- de Bruin-Weller M, Thaci D, Smith CH, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin a or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018;178(5):1083–1101. doi: 10.1111/bjd.16156.
- Eichenfield LF, Flohr C, Sidbury R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. 2021;157(10):1165–1173. doi: 10.1001/jamadermatol.2021.2830.
- El-Khalawany MA, Hassan H, Shaaban D, et al. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr. 2013;172(3):351–356. doi: 10.1007/s00431-012-1893-3.
- Flohr C, Rosala-Hallas A, Jones AP, et al. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): a multicentre parallel group assessor-blinded clinical trial. Br J Dermatol. 2023;189(6):674–684. doi: 10.1093/bjd/ljad281.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi: 10.1001/jamadermatol.2019.2855.
- Goujon C, Viguier M, Staumont-Salle D, et al. Methotrexate versus cyclosporine in adults with moderate-to-severe atopic dermatitis: a phase III randomized noninferiority trial. J Allergy Clin Immunol Pract. 2018;6(2):562 e563–569 e563. doi: 10.1016/j.jaip.2017.07.007.
- Granlund H, Erkko P, Eriksson E, et al. Comparison of cyclosporine and topical betamethasone-17,21-dipropionate in the treatment of severe chronic hand eczema. Acta Derm Venereol. 1996;76(5):371–376. doi: 10.2340/0001555576371376.
- Gutermuth J, Pink AE, Worm M, et al. Tralokinumab plus topical corticosteroids in adults with severe atopic dermatitis and inadequate response to or intolerance of ciclosporin A: a placebo-controlled, randomized, phase III clinical trial (ECZTRA 7). Br J Dermatol. 2022;186(3):440–452. doi: 10.1111/bjd.20832.
- Guttman-Yassky E, Silverberg JI, Nemoto O, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol. 2019;80(4):913–921 e919. doi: 10.1016/j.jaad.2018.01.018.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (measure up 1 and measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi: 10.1016/S0140-6736(21)00588-2.
- Guttman-Yassky E, Thaci D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi: 10.1016/j.jaci.2019.11.025.
- Haeck IM, Knol MJ, Ten Berge O, et al. Enteric-coated mycophenolate sodium versus cyclosporin a as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol. 2011;64(6):1074–1084. doi: 10.1016/j.jaad.2010.04.027.
- Harper JI, Ahmed I, Barclay G, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol. 2000;142(1):52–58. doi: 10.1046/j.1365-2133.2000.03241.x.
- Huang D, Lu J, Tan F. Improvement of pruritus and efficacy in the early stage of therapy with upadacitinib, abrocitinib, or dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: prospective, cohort, observational study. Dermatitis. 2024;35(1):77–83. doi: 10.1089/derm.2023.0132.
- Jin SY, Lim WS, Sung NH, et al. Combination of glucosamine and low-dose cyclosporine for atopic dermatitis treatment: a randomized, placebo-controlled, double-blind, parallel clinical trial. Dermatol Ther. 2015;28(1):44–51. doi: 10.1111/dth.12163.
- Kim JE, Shin JM, Ko JY, et al. Importance of concomitant topical therapy in moderate-to-severe atopic dermatitis treated with cyclosporine. Dermatol Ther. 2016;29(2):120–125. doi: 10.1111/dth.12333.
- Koppelhus U, Poulsen J, Grunnet N, et al. Cyclosporine and extracorporeal photopheresis are equipotent in treating severe atopic dermatitis: a randomized cross-over study comparing two efficient treatment modalities. Front Med. 2014;1:33. doi: 10.3389/fmed.2014.00033.
- Kwon HB, Ahn BJ, Choi Y, et al. Combination of glucosamine improved therapeutic effect of low-dose cyclosporin a in patients with atopic dermatitis: a pilot study. J Dermatol. 2013;40(3):207–210. doi: 10.1111/1346-8138.12003.
- Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet. 2006;367(9513):839–846. doi: 10.1016/S0140-6736(06)68340-2.
- Merola JF, Chiou AS, During E, et al. Dupilumab significantly improves sleep in adults with atopic dermatitis: results from the 12-week placebo-controlled period of the 24-week phase IV randomized double-blinded placebo-controlled DUPISTAD study. Br J Dermatol. 2023;189(6):685–694. doi: 10.1093/bjd/ljad284.
- Munro CS, Levell NJ, Shuster S, et al. Maintenance treatment with cyclosporin in atopic eczema. Br J Dermatol. 1994;130(3):376–380. doi: 10.1111/j.1365-2133.1994.tb02936.x.
- Pacor ML, Di Lorenzo G, Martinelli N, et al. Comparing tacrolimus ointment and oral cyclosporine in adult patients affected by atopic dermatitis: a randomized study. Clin Exp Allergy. 2004;34(4):639–645. doi: 10.1111/j.1365-2222.2004.1907.x.
- Paller AS, Flohr C, Cork M, et al. Efficacy and safety of tralokinumab in adolescents with moderate to severe atopic dermatitis: the phase 3 ECZTRA 6 randomized clinical trial. JAMA Dermatol. 2023;159(6):596–605. doi: 10.1001/jamadermatol.2023.0627.
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282–1293. doi: 10.1016/j.jaad.2020.06.054.
- Paller AS, Simpson EL, Siegfried EC, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2022;400(10356):908–919. doi: 10.1016/S0140-6736(22)01539-2.
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(12):1333–1343. doi: 10.1001/jamadermatol.2020.3260.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/S0140-6736(21)00589-4.
- Reich K, Thyssen JP, Blauvelt A, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–282. doi: 10.1016/S0140-6736(22)01199-0.
- Salek MS, Finlay AY, Luscombe DK, et al. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double-blind, placebo-controlled trial. Br J Dermatol. 1993;129(4):422–430. doi: 10.1111/j.1365-2133.1993.tb03170.x.
- Schmitt J, Schäkel K, Fölster-Holst R, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol. 2010;162(3):661–668. doi: 10.1111/j.1365-2133.2009.09561.x.
- Schram ME, Roekevisch E, Leeflang MM, et al. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol. 2011;128(2):353–359. doi: 10.1016/j.jaci.2011.03.024.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406.
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184(3):450–463. doi: 10.1111/bjd.19573.
- Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020.
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85(1):62–70. doi: 10.1016/j.jaad.2021.02.028.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255. doi: 10.1111/bjd.18898.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi: 10.1001/jamadermatol.2019.3336.
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE Mono-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/S0140-6736(20)30732-7.
- Sowden JM, Berth-Jones J, Ross JS, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet. 1991;338(8760):137–140. doi: 10.1016/0140-6736(91)90134-b.
- Thaçi D, Simpson EL, Beck LA, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387(10013):40–52. doi: 10.1016/S0140-6736(15)00388-8.
- Torrelo A, Rewerska B, Galimberti M, et al. Efficacy and safety of baricitinib in combination with topical corticosteroids in paediatric patients with moderate-to-severe atopic dermatitis with an inadequate response to topical corticosteroids: results from a phase III, randomized, double-blind, placebo-controlled study (BREEZE-AD PEDS). Br J Dermatol. 2023;189(1):23–32. doi: 10.1093/bjd/ljad096.
- Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial. Br J Dermatol. 2018;178(2):406–414. doi: 10.1111/bjd.15905.
- van Joost T, Heule F, Korstanje M, et al. Cyclosporin in atopic dermatitis: a multicentre placebo-controlled study. Br J Dermatol. 1994;130(5):634–640. doi: 10.1111/j.1365-2133.1994.tb13111.x.
- Voorberg AN, Kamphuis E, Christoffers WA, et al. Efficacy and safety of oral alitretinoin versus oral azathioprine in patients with severe chronic hand eczema: results from a prematurely discontinued randomized controlled trial. Contact Dermatitis. 2022;87(4):366–368. doi: 10.1111/cod.14161.
- Voorberg AN, Kamphuis E, Christoffers WA, et al. Efficacy and safety of dupilumab in patients with severe chronic hand eczema with inadequate response or intolerance to alitretinoin: a randomized, double-blind, placebo-controlled phase IIb proof-of-concept study. Br J Dermatol. 2023;189(4):400–409. doi: 10.1093/bjd/ljad156.
- Wahlgren CF, Scheynius A, Hägermark O. Antipruritic effect of oral cyclosporin a in atopic dermatitis. Acta Derm Venereol. 1990;70(4):323–329.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–449. doi: 10.1111/bjd.19574.
- Wollenberg A, Howell MD, Guttman-Yassky E, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol. 2019;143(1):135–141. doi: 10.1016/j.jaci.2018.05.029.
- Zhao Y, Wu L, Lu Q, et al. The efficacy and safety of dupilumab in Chinese patients with moderate-to-severe atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2022;186(4):633–641. doi: 10.1111/bjd.20690.
- Zonneveld IM, De Rie MA, Beljaards RC, et al. The long-term safety and efficacy of cyclosporin in severe refractory atopic dermatitis: a comparison of two dosage regimens. Br J Dermatol. 1996;135 Suppl 48:15–20. doi: 10.1111/j.1365-2133.1996.tb00704.x.
- Zurbriggen B, Wüthrich B, Cachelin AB, et al. Comparison of two formulations of cyclosporin a in the treatment of severe atopic dermatitis. A double-blind, single-centre, cross-over pilot study. Dermatology. 1999;198(1):56–60. doi: 10.1159/000018065.
- Taichman DB, Backus J, Baethge C, et al. Sharing clinical trial data: a proposal from the international committee of medical journal editors. Lancet. 2016;387(10016):e9–e11. doi: 10.1016/S0140-6736(15)01279-9.
- Taichman DB, Sahni P, Pinborg A, et al. Data sharing statements for clinical trials: a requirement of the international committee of medical journal editors. Lancet. 2017;389(10086):e12–e14. doi: 10.1016/S0140-6736(17)31282-5.
- Williams HC, Schmitt J, Thomas KS, et al. The HOME core outcome set for clinical trials of atopic dermatitis. J Allergy Clin Immunol. 2022;149(6):1899–1911. doi: 10.1016/j.jaci.2022.03.017.
- Silverberg JI, Horeczko J, Alexis A. Development of an eczema area and severity index atlas for diverse skin types. Dermatitis. 2024;35(2):173–177. doi: 10.1089/derm.2023.0051.

