Abstract
Purpose: Within the EU RENEB project, seven laboratories have taken part in training and harmonisation activities to strengthen triage gamma-H2AX-based radiation exposure assessment. This has culminated in a second triage biodosimetry exercise.
Materials and methods: Whole blood and separated lymphocyte samples were homogenously irradiated with 60Co gamma rays at 0.5, 2.5 (blind samples), 0 and 2 Gy (reference samples). Following post-exposure incubations of 4 and 24 h, 16 samples were shipped on ice packs to each partner. The samples were stained and scored for gamma-H2AX foci, using manual and/or automated fluorescence microscope scoring strategies. Dose estimates were obtained and used to assign triage categories to the samples.
Results: Average dose estimates across all the laboratories correlated well with true doses. The most accurate assignment of triage category was achieved by manual scoring of the 4-h blood and lymphocyte samples. Only three samples out of a total of 46 were miscategorized in a way that could have adversely effected the clinical management of a radiation casualty.
Conclusions: This inter-comparison exercise has demonstrated that following a recent acute radiation exposure, the gamma-H2AX assay could be a useful triage tool that can be successfully applied across a network of laboratories.
Introduction
In a mass casualty radiation incident the triage of potentially exposed individuals is a crucial first step in the medical management of casualties. However, given the non-specific nature of the clinical signs and symptoms used for triage, it is important to provide confirmation of a patient’s exposure status with the help of biological dosimetry tools (Voisin et al. Citation2001). Dosimetry information can be used to separate the ‘worried well’, who may exhibit some of the symptoms of acute radiation syndrome due to pre-existing conditions or stress reactions triggered by the event, from critically exposed individuals. In a large scale event, with potentially hundreds to thousands of casualties, a single biological dosimetry laboratory’s surge capacity would easily be exceeded and therefore assistance networks have been recognized as an important element of emergency response strategies (Roy et al. Citation2007; Blakely et al. Citation2009). The aim of the EU RENEB (Realizing the European Network of Biodosimetry) project was to establish a sustainable European network for existing laboratories and using six established biodosimetry assays – (1) the dicentric assay; (2) the FISH-translocation assay; (3) the micronucleus assay; (4) the premature chromosome condensation assay; (5) the gamma-H2AX assay; and 6) electron paramagnetic resonance/optically stimulated luminescence. The objective of this work was to further strengthen the basis of the assays for use in emergency scenarios by harmonising responses between the partners through standardization of methods, training and inter-comparison exercises (Kulka et al. Citation2012, Citation2015).
The gamma-H2AX foci assay is a sensitive surrogate marker of radiation induced DNA double strand breaks (Ivashkevich et al. Citation2012; Rothkamm et al. Citation2015) and may be a useful early biodosimetry tool from hours to about three days post-exposure (Roch-Lefèvre et al. Citation2010; Horn & Rothkamm Citation2011; Horn et al. Citation2011; Mandina et al. Citation2011). Several studies have shown that rapid processing time of the gamma-H2AX assay can produce dose estimates within a few hours of receiving a blood sample (Rothkamm et al. Citation2013a; Ainsbury et al. Citation2014). In addition, the potential for batch processing on finger prick blood samples, enabling high throughput (Moquet et al. Citation2014), makes the assay ideal for early triage categorization to separate the ‘worried well’ from critically exposed individuals by quantifying radiation-induced foci in peripheral blood lymphocytes. The first inter-comparison study of the RENEB partners was described by Barnard et al. Citation2015 and showed the potential for the gamma-H2AX assay, as a tool for rapid dose categorization in emergency response, to be established in a functional biodosimetry network within Europe.
During the first inter-comparison, isolated lymphocytes were shipped to all participants, because of intermittent problems with blood cell separation after prolonged incubation and storage of whole blood samples, e.g. haemolysis. However, in a real incident, it would be very difficult to undertake such a separation step prior to shipment of samples. For this reason, the second exercise aimed to compare the assay performance for shipped lymphocytes with that for whole blood as well as to further harmonise the assay between the RENEB partners.
Materials and methods
Blood sampling, irradiation and shipment
The blood sampling and the irradiations occurred on two consecutive days in order to allow the 24 h and 4 h ex vivo incubated samples to be shipped to the seven participating laboratories at the same time. Heparinised venous whole blood was taken with written informed consent in accordance with local ethics procedures from two healthy volunteers on day 1 and three donors on day 2. The volume of blood required for the inter-comparison and the limits prescribed for ethics approval meant that more than one blood donor had to be used. However, each laboratory received blood and separated lymphocytes from the same donor for a given time-point and all doses. Two or three laboratories received samples from the same donor. Based on previous experience using the five donors and on published studies (Roch-Lefèvre et al. Citation2010; Chua et al. Citation2011), any variability between the donors would be expected to make only a small contribution to overall uncertainties.
The freshly drawn blood was separated on histopaque 1077 (Sigma-Aldrich, Dorset, UK) and the isolated lymphocytes suspended in Minimal Essential Medium (MEM), supplemented with 10% heat-inactivated foetal bovine serum (FBS), 100 units/ml penicillin plus 100 μg/ml streptomycin and 2 mM L-glutamine (all from Invitrogen, Paisley, UK). In addition, whole blood was diluted 1:1 with MEM in an effort to keep the samples in good condition, as previous work during the MULTIBIODOSE project (Rothkamm et al. 2013b) had shown that incubating blood at 37 °C, even for short periods, to simulate in vivo repair resulted in poor sample processing following shipment. Diluting blood would not be required in an actual event. Irradiation of the separated lymphocytes and diluted blood samples was performed at 37 °C with a 60Co gamma source, at a dose rate of 0.67 Gy/min. Doses of 0.5, 2 and 2.5 Gy were used and a zero dose control sample was also transported to the irradiation facility for sham exposure. Following irradiation, samples were incubated at 37 °C for 4 or 24 h, to simulate in vivo repair, then cooled to 4 °C. Lymphocyte samples were spun down and resuspended in 100% cold FBS at approximately 0.5 x 106 cells/ml and aliquoted into cryovials. Four ml diluted blood per dose point per laboratory was aliquoted into Falcon® tubes. The samples were then wrapped and shipped on frozen cold packs, together with a temperature logger, to the participating laboratories. Reference samples of 0 and 2 Gy were included in the shipment, together with two coded samples X and Y (0.5 and 2.5 Gy, respectively). All seven laboratories received the 4-h ex vivo incubated blood and isolated lymphocyte samples. Five participants volunteered to analyse the 24-h ex vivo incubated samples.
Gamma-H2AX immunofluorescence staining and microscope analysis
Following receipt of the samples, they were processed in each laboratory according to the standard protocol based on the method described in Rothkamm et al. (2013b). In brief, the lymphocyte samples were spun down and washed in phosphate buffered saline (PBS). The diluted whole blood was layered onto histopaque 1077 in order to isolate the lymphocytes. Cell suspensions were then spotted onto adhesive microscope slides, fixed in formaldehyde (e.g. Polysciences Inc. Warrington, PA), permeabilised and extracted with Triton X (e.g. Sigma-Aldrich, Dorset, UK), blocked with bovine serum albumin (e.g. Fisher Scientific, Loughborough, UK) and immunostained for gamma-H2AX using fluorophore-conjugated secondary antibodies (e.g. AlexaFluor 488 goat anti-mouse, Invitrogen, Paisley, UK). All participants scored foci manually (Horn et al. Citation2011) in a total of 50 cells per sample and in addition three laboratories scored a total of 200 cells per sample automatically using Cellprofiler (www.cellprofiler.org) or MetaCyte (Metasystems, Altlussheim, Germany; Vandersicklel et al. Citation2010). Also, participants used the 2 Gy reference samples to test the validity of their calibration curves and the unirradiated samples gave an indication of the level of background staining achieved on the day of the exercise, as suggested in Rothkamm et al. (2013b). A standardized scoring sheet was used by the participants to report foci numbers and dose estimates for 10, 20, 30, 40 and 50 cells scored manually or 20, 50, 100, 150 and 200 cells scored automatically. All the participants’ dose estimates were returned to the coordinating laboratory for statistical analysis.
Data analysis
Standard errors were calculated according to Poisson statistics, which are assumed to dominate the random error (IAEA, Citation2011; Barnard et al. Citation2015). Foci counts were converted to dose estimates by the participants using their own calibration curves and the software package Dose Estimate_v5.1 (Ainsbury & Lloyd Citation2010); although one laboratory used R (www.r-project.org). The calibration data were fitted to a linear model using iteratively reweighted least squares fitting in Dose Estimate according to standard practice (Barnard et al. Citation2015). Alpha is the linear coefficient and C the intercept of the fit. χ2 testing was used to compare the observed and expected foci yields for the reference samples. The general linear model analysis of variance (ANOVA) and post-hoc testing (Tukey’s pairwise comparisons) were performed for the dose estimates using Minitab® 15. The following experimental factors were tested against the dose estimates: post-exposure time (4 or 24 h); scoring method (manual or automatic); temperature during transit; donor nested in post-exposure time (1–5); laboratory (1–8; omitting lab 2 as they were unable to take part in the second exercise); number of cells scored nested in scoring method (10–50 for manual and 50–200 for automated scoring); and sample type (whole blood or lymphocytes). In addition, a z-score of the dose was calculated for each dose estimate in order to determine a laboratory’s results as satisfactory (|z| ≤ 2), questionable (2 < |z| < 3), and unsatisfactory (|z| > 3) (Romm et al. Citation2014). It should be noted that, throughout the paper, the statistical power of the data is in some cases fairly weak due to the small numbers of participants. However, in all cases there were sufficient laboratories for statistical significance of the results.
Results
Seven laboratories took part in the second inter-comparison and for comparison the allocated laboratory number corresponds to that used in the first exercise (Barnard et al. Citation2015). Six participants received the samples within 23 h (range 17–23 h), but for one laboratory (lab 8) the shipment was delayed at the airport and arrived at the institute after about 44 h in transit. As a consequence this participant was only able to analyse the 4-h ex vivo incubated blood and lymphocyte samples. During shipment the temperature of the sample containers remained between 6 and 15 °C, only rising to above 20 °C on reaching the laboratory. The temperature of the delayed sample only rose above 16 °C to a maximum of 19.5 °C for about the last 1.5 h of transit. Each laboratory processed samples immediately upon receipt. A few of the laboratories had difficulty processing the diluted whole blood samples, especially the 24 h ex vivo samples, due to haemolysis. This made lymphocyte separation and subsequent staining problematic or impossible.
shows the foci yields ± Poisson standard errors and the calibration coefficients used to convert foci counts into dose estimates for each laboratory. All the partner laboratories have appropriate calibration curves for gamma radiation for 4 and 24 h incubation periods plus overnight shipment on cold packs. Four laboratories (labs 1, 3, 5 and 6) used calibration curves that had been produced during the first inter-comparison (Barnard et al. Citation2015). Labs 4, 7 and 8 used more recently prepared curves. Three participants chose to score foci manually as well as automatically. The data from the reference samples were grouped into 0 Gy and 2 Gy and χ2 testing revealed there was no significant difference between the observed and expected foci yields, calculated from each laboratory’s original calibration curve, p = 1.0 and 0.46, respectively. At 2 Gy, most of the χ2 value came from just a few of the data points and labs 6 and 8 used the 2 Gy reference sample to adjust their calibration curves, before producing a dose estimate. The calibration curve produced by lab 7 used automated scoring mode; therefore the manual dose estimate was also produced by adjusting the curve with the 2 Gy manually scored reference sample. Full details of the reference sample procedure are given in Ainsbury et al. Citation2016.
Table 1. Reported foci yields ± Poisson standard errors (SE) obtained by the laboratories using manual and automated scoring. Also shown are the constant (C) and linear calibration coefficients (α) and associated standard errors used to convert foci counts into dose estimates.
Z-score analysis showed inter-laboratory variation across the different samples and that most laboratories overestimated the dose of the 0.5 Gy samples and underestimated the 2.5 Gy samples. Thirteen out of the 23 dose estimates for the 0.5 Gy samples were classed as satisfactory from the z-score. Six were classified questionable. These were both samples from lab 5, the lymphocyte samples from lab 6 (24 h; manual scoring) and lab 7 (4 h; manual and automatic scoring) and the blood sample from lab 8 (4 h; automatic scoring). Four were classed as unsatisfactory. The dose estimates based on the lymphocyte samples for lab 1 were both unsatisfactory (4 and 24 h; manual scoring). In addition, dose estimates based on lymphocyte samples from lab 3 (24 h; manual scoring) and lab 6 (4 h; automated scoring) were also unsatisfactory. However, in all cases the estimate of dose for the corresponding whole blood samples from these laboratories had satisfactory z-scores. Fourteen of the 23 dose estimates were satisfactory for the 2.5 Gy samples, when the z-score was taken into account. One dose estimate from lab 6 (blood; 4 h; automatic scoring) was classed as questionable. Eight dose estimates were unsatisfactory. These were the 4 h lymphocyte samples scored manually by lab 1 and 7 and the 24 h lymphocyte samples analysed by lab 3, 4 and 6. The 24 h blood samples scored manually by labs 1 and 3 produced unsatisfactory dose estimates, as well as the 4 h lymphocyte samples scored automatically by lab 7. There are additional, alternative, methods such as precision analysis that could be applied to assess the accuracy of results. However, as the z-test is recommended to be applied by the appropriate ISO standards for rapid biodosimetry categorization (ISO 5725-5:1998 and ISO 13528:2015) and moreover, the classification technique applied here (satisfactory, questionable or unsatisfactory) is also becoming the standard in biodosimetry and has been applied in many previous inter-comparisons, the z-test has been judged to be the most appropriate method to compare the results.
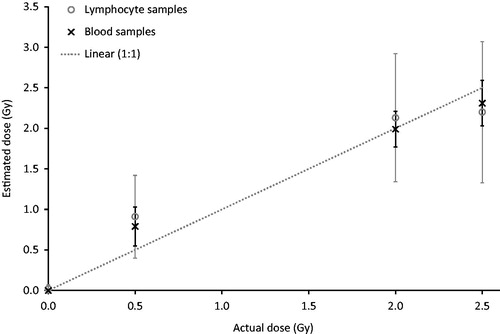
General linear model ANOVA was carried out on the data set in order to investigate the contribution of experimental factors (number of cells scored; post-exposure time; scoring method; temperature; donor; laboratory; sample type) on the dose estimates. No significant effect was seen for the number of cells scored (p = 0.964), post-exposure time (p = 0.143), scoring method (p = 0.873) and temperature during transit (p = 0.057). A significant difference was observed, however, between the donors (p = 0.008), laboratories (p < 0.001) and sample type (p < 0.001). Pairwise comparisons demonstrated that the difference between the donors was due to a variation between donors 2 and 4 (p = 0.026). Donor 2 was used at the 24 h post exposure time-point and donor 4 at 4 h. When the data for the 24 h blood samples, which were problematic to separate, stain and score, were excluded from the analysis no significant effect of donor was seen (p = 0.109). The inter-laboratory difference was due to the variation between lab 7 and all the other laboratories (p < 0.001). Post-hoc testing of sample type revealed that there was no significant difference between blood and lymphocytes at 4 h post irradiation (p = 0.999), but there was a significant difference between the sample types at 24 h (p < 0.001). Average dose estimates across all laboratories for both the reference and the unknown samples correlated well with the true dose, as shown in , although the 24 h blood estimates had to be omitted due to the technical difficulties encountered with these samples. ANOVA and pairwise analysis revealed significant differences between the doses (p ≤ 0.006), except for 2 and 2.5 Gy (p = 0.253).
Figure 1. Average gamma-H2AX-based dose estimates versus the true doses for blood and lymphocyte ex vivo incubated samples; excluding 24-h blood samples. Error bars show the standard deviation between 5 and 7 (blood) and 11–14 (lymphocyte) measurements for each sample taken from . The line is not a fit but indicates the ideal 1:1 relationship.
The dose estimates from all the laboratories were then used to assign the unknown samples to triage categories of either (1) low exposure <1 Gy; (2) medium exposure 1–2 Gy, or (2) high exposure >2 Gy. The triage categories used here were those chosen for the MULTIBIODOSE project and implemented in the MULTIBIODOSE emergency triage categorization software (Jaworska et al. Citation2015). shows the triage categories for the foci-based dose estimates of coded blood and lymphocyte samples for 50 cells scored manually and 200 cells assessed automatically.
Table 2. Reported foci-based dose estimates ± standard error obtained by the laboratories for the coded blood and lymphocyte samples shipped from lab1 to the participants following 60Co gamma irradiation and 4-/24-h ex vivo incubation at 37 °C. Triage categories of <1 Gy, 1–2 Gy and >2 Gy are highlighted in the table in white, light grey and dark grey, respectively.
Discussion
The calibration curves used to convert foci yields to dose were prepared during the MULTIBIODOSE or RENEB projects. Each laboratory constructed their curves as described in Barnard et al. Citation2015. In brief, separated lymphocytes were homogeneously exposed to 0, 1, 2, 3 and 4 Gy of Co60 gamma-rays and then held at 37 °C for 4 h and 24 h. As expected from previous work (Rothkamm et al. Citation2013b; Barnard et al. Citation2015), the 4 h and 24 h calibration curves in show a good fit to the linear dose response although some individual data showed a degree of saturation between 3 and 4 Gy. The Dose Estimate software, used to fit the dose response curves, gives p-values, based on the z-test, for each of the calibration coefficients and these were always found to be satisfactory. The observed foci numbers varied between laboratories, despite using a standard protocol, because small variations in sample handling and processing, as well as the reagents used, can affect the staining and subsequent scoring of foci (Rothkamm & Horn Citation2009), so no common calibration curve can be used by all the laboratories. The inclusion of reference samples also aimed to control for any variation in staining between the day of the inter-comparison and setting up the calibration curves in each laboratory, as suggested by Rothkamm et al. (Citation2013b). In addition, the calibration curves demonstrate the significant effect of post-exposure time on foci loss, with the average foci yields at 4 h approximately two times higher than at 24 h. Therefore, when using foci numbers to convert to a dose estimate in a real event, it will be important to know the exact time of irradiation so that an appropriate calibration curve can be used (Roch-Lefèvre et al. Citation2010; Beels et al. Citation2010).
The present inter-comparison was not conducted in real time; however, all laboratories reported their results within the deadline set by the coordinating laboratory. Timed inter-comparisons have been described by Rothkamm et al. (Citation2013a) and Ainsbury et al. (Citation2014) with triage dose estimates, based on foci counts in 50 cells, reported within 7 h (10 samples) and 3 h (8 samples), respectively. With a more rapid processing technique and scoring just 20 cells it may be possible to produce dose estimates for 96 samples in 7–11 h (Moquet et al. Citation2014). Past experience (Rothkamm et al. Citation2013a; Ainsbury et al. Citation2014) and the first inter-comparison (Barnard et al. Citation2015) have shown that for rapid triage purposes, i.e. detection of moderate to high doses, manual scoring of 50 cells or 200 cells scored automatically, is fully sufficient and even scoring 20 cells manually or 50 automatically, may be sufficiently accurate in a triage setting. The present inter-comparison has confirmed that scoring such low numbers of cells per sample is possible without drastically changing the dose estimate. The number of cells scored can have a huge impact on the precision of the dose estimate where an assay is robust but events scarce, e.g. the dicentric assay (Romm et al. Citation2014). However, this is much less of an issue for the gamma-H2AX assay where, in comparison, there are many events (foci) per cell and many other factors contributing to dose uncertainty (Rothkamm & Horn Citation2009; Rothkamm et al. Citation2015). In addition, no significant effect of post-exposure time on the dose estimate was seen, as found in the first inter-comparison. In the first exercise manual and automatic scoring had produced significantly different results (Barnard et al. Citation2015), but this was not the case in the second exercise.
The purpose of triage biodosimetry is to rapidly estimate dose and although this initial estimation may not be very accurate the aim is to place a casualty into the correct triage category (IAEA Citation2011). As shows, manual scoring of the 4 h blood samples achieved the most accurate assignment of triage category for both the 0.5 Gy samples (4 out of 5) and the 2.5 Gy samples (5 out of 5). The 4 h lymphocyte samples also provided good triage categorization with five out of seven samples correctly assigned at each dose. Automated scoring of the 4 h samples for both blood and lymphocytes showed greater deviation than the manual scoring; with two out of four blood and four out of six lymphocytes samples were correctly assigned to a triage category at both doses. Dose estimates for the 24 h blood samples irradiated with 0.5 Gy gave good triage categorization (2 out of 2), but the 2.5 Gy samples were poor (0 out of 2). The reverse was seen with the 24 h lymphocyte samples, where one out of four of the 0.5 Gy samples were correctly categorized compared to three out of four at 2.5 Gy.
It is important to note that in total only three of the 46 blind samples, i.e. <7% were miscategorized such that this could have had an adverse effect on the clinical management of a radiation casualty. The other 12 miscategoriztions merely placed the samples one category up or down into the medium exposure group, which would have no clinical implications as both high and the medium exposure groups would have been double-checked using the ‘gold standard’ cytogenetic assays for follow-up biodosimetry. Of the three 2.5 Gy samples that fell into the <1 Gy triage category, which the z-score analysis had also shown to give unsatisfactory dose estimates, one was a 4 h lymphocyte sample scored automatically in lab 7. The results of the 2 Gy reference sample from lab 7 also showed very low foci numbers compared to that expected from their calibration curve. In addition, the foci numbers observed with manual scoring were higher than those found with automated scoring for all samples from this lab. Lab 7 is not as experienced in using automatic scoring as partners 6 and 8 and may require more training in using an automated system. The other two samples in the <1 Gy triage category came from the 24 h blood (manual scoring) samples. The need for an ex vivo incubation at 37 °C caused the problem of haemolysis during lymphocyte separation, which affected the subsequent staining and analysis of the whole blood samples, especially at the 24 h time-point. However, in a real event there would be no need to simulate in vivo repair, so the issue of haemolysis would be much less of a problem.
The aim of the work reported in this manuscript was to assess the usefulness of the gamma-H2AX assay for rapid dose categorization to aid in emergency response. As such, the assay has been applied according to the standardized methodologies developed during MULTIBIODOSE and RENEB (Horn et al. Citation2011; Rothkamm et al. Citation2013b; Barnard et al. Citation2015). To improve dose estimates, however, there are a number of additional points that will need to be addressed going forward, before all the influencing factors are fully understood and the assay can be categorized as fully operational for routine dose estimation. This includes the variability between individual slides (staining reproducibility) and variability between images (image/microscope setting reproducibility) (Rothkamm & Horn Citation2009; Rothkamm et al. Citation2015).
Conclusions
The aim of the RENEB project has been to establish a sustainable European network that can provide rapid triage biodosimetry, using existing biological and retrospective techniques, in the event of a large scale radiological emergency. Unlike the more established biodosimetry methods, such as the dicentric and micronucleus techniques, the gamma-H2AX assay is a more recent method for radiation dose assessment. The RENEB partners were able to categorize the majority of the samples in such a way that there would be no adverse effect on the clinical management of a radiation casualty. Overall, the results of the second RENEB inter-comparison suggest that the gamma-H2AX assay could be a very useful triage tool following a recent acute radiation exposure. Severely exposed individuals within a large cohort can be identified in a very short space of time and then given priority for further follow-up, including more accurate traditional chromosome dosimetry. Furthermore, with standardization of methodology, application across a network of laboratories can be very successful. However, it is recommended that any new partner wishing to join the network must have laboratory-specific calibration curves and a program of continuous training/quality assurance, e.g. inter-comparisons, is maintained for existing and new partners.
Acknowledgements
The authors wish to thank R. Sokolowski of the Medical Research Council, Harwell, UK, for performing the 60Co gamma irradiation and Mark Hill and James Thompson, of the Gray Institute for Radiation Oncology and Biology, Oxford, UK, for the dosimetry.
Disclosure statement
The authors report no conflicts of interest.
References
- Ainsbury E, Lloyd D. 2010. Dose estimation software for radiation biodosimetry. Health Phys. 98:290–295.
- Ainsbury EA, Al-hafidh J, Bajinskis A, Barnard S, Barquinero JF, Beinke C, de Gelder V, Gregoire E, Jaworska A, Lindholm C, et al. 2014. Inter- and intra-laboratory comparison of a multibiodosimetric approach to triage in a simulated, large scale radiation emergency. Int J Radiat Biol. 90:193–202.
- Ainsbury EA, Higueras M, Puig P, Einbeck J, Samaga D, Barquinero JF, Barrios L, Brzozowska B, Fattibene P, Gregoire E, et al. 2016. Uncertainty of fast biological dose assessment for emergency response scenarios. Int J Radiat Biol, in this issue.
- Barnard S, Bouffler S, Rothkamm K. 2013. The shape of the radiation dose response for DNA double strand break induction and repair. Genome Integrity. 4:1–8.
- Barnard S, Ainsbury EA, Al-hafidh J, Hadjidekova V, Hristova R, Lindholm C, Monteiro Gil O, Moquet J, Moreno M, Rößler U, et al. 2015. The first gamma-H2AX biodosimetry inter-comparison exercise of the developing European biodosimetry network RENEB. Radiat Prot Dosimetry. 164:265–270.
- Beels L, Werbrouck J, Thierens H. 2010. Dose response and repair kinetics of gamma-H2AX foci induced by in vitro irradiation of whole blood and T-lymphocytes with x- and gamma-radiation. Int J Radiat Biol. 86:760–768.
- Blakely WF, Carr Z, Chu MC, Dayal-Drager R, Fujimoto K, Hopmeir M, Kulka U, Lillis-Hearne P, Livingston GK, Lloyd DC, et al. 2009. WHO 1st consultation on the development of a global biodosimetry network for radiation emergencies (BioDoseNet). Radiat Res. 171:127–139.
- Chua ML, Somaiah N, Bourne S, A'Hern R, Nuta O, Davies S, Herskind C, Pearson A, Warrington J, Helyer S, et al. 2011. Inter-individual and inter-cell type variation in residual DNA damage after in vivo irradiation of human skin. Radiother Oncol. 99:225–230.
- Horn S, Rothkamm K 2011. Candidate protein biomarkers as rapid indicators of radiation exposure. Radiat Meas. 46:903–906.
- Horn S, Barnard S, Rothkamm K. 2011. Gamma-H2AX-based dose estimation for whole and partial body radiation exposure. PLoS One. 6:e25113.
- International Atomic Energy Agency. 2011. Cytogenetic Dosimetry: applications in preparedness for and response to radiation emergencies. EPR-Biodosimetry. Vienna, IAEA.
- International Organization for Standardization. 1998. Accuracy (trueness and precision) of measurement methods and results – Part 5: alternative methods for the determination of the precision of a standard measurement method. ISO 5725-5:1998. Geneva: ISO.
- International Organization for Standadization. 2015. Statistical methods for use in proficiency testing by inter-laboratory comparisons. ISO 13528:2015. Geneva: ISO.
- Ivashkevich A, Redon C. Nakamura A, Martin R, Martin O. 2012. Use of the gamma-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 327:123–133.
- Jaworska A, Ainsbury E, Fattibene P, Lindholm C, Oestreicher U, Rothkamm K, Romm H, Thierens H, Trompier F, Voisin P, et al. 2015. Operational guidance for radiation emergency response organizations in Europe for using biodosimetric tools developed in EU MULTIBIODOSE project. Radiat Prot Dosimetry. 164:165–169.
- Kulka U, Ainsbury L, Atkinson M, Barquinero JF, Barrios L, Beinke C, Bognar G, Cucu A, Darroudi F, Fattibene P, et al. 2012. Realising the European Network of Biodosimetry (RENEB). Radiat Prot Dosimetry. 151:621–625.
- Kulka U, Ainsbury L, Atkinson M, Barnard S, Smith R, Barquinero JF, Barrios L, Bassinet C, Beinke C, Cucu A, et al. 2015. Realising the European network of biodosimetry: RENEB-status quo. Radiat Prot Dosimetry. 164:42–45.
- Mandina T, Roch-Lefèvre S, Voisin P, González J, Lamadrid A, Romero I, Garcia O, Voisin, P, Roy L. 2011. Dose-response relationship of gamma-H2AX foci induction in human lymphocytes after X-rays exposure. Radiat Meas. 46:997–999.
- Moquet J, Barnard S, Rothkamm K. 2014. Gamma-H2AX biodosimetry for use in large scale radiation incidents: comparison of a rapid '96 well lyse/fix' protocol with a routine method. Peer J. 6;2. peerj.282
- Roch-Lefèvre S, Mandina T, Voisin P, Gaetan G, Mesa J, Valente M, Bonnesoeur P, Garcia O, Voisin P, Roy, L. 2010. Quantification of gamma-H2AX foci in human lymphocytes: a method for biological dosimetry after ionizing radiation exposure. Radiat Res. 174:185–194.
- Romm H, Ainsbury E, Barnard S, Barrios L, Barquinero JF, Beinke C, Deperas M, Gregoire E, Koivistoinen A, Lindholm C, et al. 2014. Validation of semi-automatic scoring of dicentric chromosomes after simulation of three different irradiation scenarios. Health Phys. 106:764–771.
- Rothkamm K, Horn S. 2009. γ-H2AX as protein biomarker for radiation exposure. Ann. 1st Super Sanità. 45:265–271
- Rothkamm K, Horn H, Scherthan H, Rößler U, De Amicis A, Barnard S, Kulka U, Lista L, Meineke V, Braselmann H, ET AL. 2013a. Laboratory inter-comparison on the gamma-H2AX foci assay. Radiat Res. 180:149–155.
- Rothman K, Barnard S, Ainsbury E, Al-hafidh J, Barquinero J-F, Lindholm C, Moquet J, Perälä M, Roch-Lefèvre S, Scherthan H, et al. 2013b. Manual versus automated γ-H2AX foci analysis across five European laboratories: can this assay be used for rapid biodosimetry in a large scale radiation accident? Mutat Res. 756:170–173.
- Rothkamm K, Barnard S, Moquet J, Ellender M, Rana Z, Burdak-Rothkamm S. 2015. DNA damage meaning and significance. Environ Mol Mutagen. 56:491–504.
- Roy L, Roch-Lefèvre S, Vaurijoux A, Voisin PA, Voisin P. 2007. Optimization of cytogenetic procedures for population triage in case of a radiological emergency. Radiat Meas. 42:1143–1146.
- Vandersicklel V, Depuydt J, van Bockstaele B, Perletti G, Philippe J, Thierens H, Vral A. 2010. Early increase of radiation-induced γH2AX foci in a human Ku70/80 knockdown cell line characterized by an enhanced radiosensitivity. J Radiat Res. 51:633–641.
- Voisin P, Benderitter M, Claraz M, Chamrette V, Sorokine-Durm L, Delbos M, Durand V, Leroy A, Paillole N. 2001. The cytogenetic dosimetry of recent accidental overexposure. Cell Mol Biol. 47:557–564.

