Abstract
Purpose: Low dose radiation has been reported as an effective treatment for rheumatoid arthritis via multiple dose exposures. The present study was designed to increase the therapeutic efficacy of low dose radiation with the minimum exposure level in arthritic rats by concurrent administration of resveratrol (RSV) as an adjunctive therapy with anti-inflammatory properties.
Materials and methods: Rats were rendered arthritic by sub-plantar injection of Freund’s complete adjuvant (FCA) and exposed to low dose radiation at a total exposure level of 0.5 Gy (2 × 0.25). During the exposure course, RSV (50 mg/kg) was orally administered once daily for two weeks. Diclofenac (3 mg/kg) was administered as a standard anti-inflammatory drug. Paw volume was measured every 4 days. After 28 days of induction, rats were sacrificed and serum was collected for estimation of tumor necrosis factor-alpha (TNF-α), interleukin-1beta (IL-1β), thiobarbituric acid reactive substances (TBARS), and total nitrate/nitrite (NOx). Furthermore, paws were dissected for histopathological examinations and immuno-histochemical estimation of nuclear factor-kappa B p65 (NF-κB p65) expression.
Results: Administration of RSV during the low dose radiation exposure course produced a significant decrease in the paw swelling and a potentiated inhibition in the serum levels of TNF-α, IL-1β, TBARs, and NOx. The dual treatment strategy alleviated the histopathological damage to a greater extent than that produced by each treatment. Moreover, a pronounced suppression of NF-κB p65 expression in the synovial tissue was observed in the combination group. The combination treatment showed a nearly similar potency to that observed in the diclofenac treated group.
Conclusion: Administration of RSV augmented the modulatory activity of low dose radiation with minimum exposure level on the disease progression.
Introduction
Rheumatoid arthritis (RA) is a systemic, chronic, autoimmune disease characterized by nonspecific inflammation of the peripheral joints and destruction of articular cartilage (Klareskog et al. Citation2009). About 1% of the world’s population is currently affected by RA (Gibofsky Citation2014). The pathogenesis of the disease is orchestrated by activated immune cells and pro-inflammatory mediators that cause cartilage and joint damage (Kinne et al. Citation2000; McInnes and Schett Citation2007). Current treatment options for RA include non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), disease modifying anti-rheumatic drugs (DMARDs) and biological drugs, but their clinical use has been limited due to their severe side effects (Agarwal Citation2011). Owing to the involvement of various pathways in the progression of RA, different therapeutic approaches have been developed to control the severity of the disease.
The beneficial effects of low dose radiation on living organisms have been widely discussed. Low dose radiation is capable of attenuating an established inflammatory process where it shifts the inflammatory condition to an anti-inflammatory one (Frey et al. Citation2015). The modulatory effect of low dose radiation to attenuate the progression of arthritis in different animal models was previously reported in several studies (El-Ghazaly et al. Citation1985; Calabrese and Calabrese Citation2013). Although, the underlying molecular mechanisms of low dose radiation were explored in several studies (Arenas et al. Citation2012; Rodel et al. Citation2012), yet they have not been completely elucidated. Previous experimental studies that reported the modulatory effect of low dose whole-body radiation on RA were based on multiple exposures (5 × 0.5 Gy) (Nakatsukasa et al. Citation2008; Frey et al. Citation2009; Nakatsukasa et al. Citation2010). Whole-body irradiation (typically at a dose of >1Gy) can cause damage to critically important organ systems involved the hematopoietic, gastrointestinal and neurovascular organ systems, respectively in a dose dependent manner (Lopez and Martin Citation2011). Therefore, enhancing the therapeutic activity of low dose radiation to minimize the radiation dose as well as the number of exposures was a target point of this study. Application of a dual treatment strategy by combining the low dose radiation exposure course with an anti-arthritic agent seems to be a safe approach to achieve the goal of this study.
One of the possibilities for such agent is resveratrol (3,5,4′-trihydroxystilbene). Resveratrol (RSV) is a non-flavonoid polyphenolic compound found in grapes, peanuts and other foods that are commonly consumed as part of human diet. The compound was first isolated from the root of Polygonum cuspidatum, a plant used in traditional Chinese and Japanese medicine (Vastano et al. Citation2000). Furthermore, the anti-inflammatory activity of RSV in RA has been reported in several studies by suppression of pro-inflammatory cytokines (Yang et al. Citation2018), down-regulation of prostaglandin E2 (PGE2) synthesis (Chen et al. Citation2014), inhibition of cellular infiltration (Riveiro-Naveira et al. Citation2016). In addition, the suppression of nuclear factor-kappa B (NF-κB) expression by RSV has been reported in several in vitro studies (Lei et al. Citation2012; Valcarcel-Ares et al. Citation2014).
Based on the aforementioned data, our study was constructed to study the effect of administration of RSV as an adjunctive therapy on the therapeutic activity of low dose radiation. Moreover, the efficacy of the combination treatment was compared to diclofenac, a well-known anti-inflammatory NSAIDs drug, conventionally used in the treatment of RA and employed as a reference standard in several studies (Choudhary et al. Citation2014; Pan et al. 2015; Lan et al. Citation2016; Wenjin and Jianwei Citation2017).
Materials and methods
Animals
Male Wistar albino rats, weighing 120–150 g, were obtained from the animal breeding house of the National Center for Radiation Research and Technology (NCRRT). Rats were acclimatized in the animal facility of NCRRT for at least one week before subjecting them to experimentation. They were fed standard laboratory chow and water ad libitum. The study was conducted in accordance with the guidelines set by the Environment European Commission (EEC) regulations (revised Directive 86/609/EEC) and was approved by the Ethics Committee at the Faculty of Pharmacy, Cairo University, Egypt (Permit number: PT1417).
Drugs and chemicals
Freund’s complete adjuvant (FCA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Resveratrol and polyethylene glycol (mwt 400) were purchased from Carl-Roth (Karlsruhe, Germany). Diclofenac sodium was obtained from Novartis (Cairo, Egypt). Enzyme-linked immunosorbent assay (ELISA) rat specific kits for the determination of TNF-α was purchased from Komabiotech (Yeongdeungpo-gu, Seoul, Korea) and that for IL-1β was purchased from Abcam (Cambridge, MA, USA). Rabbit anti-NF-κB/p65 (Rel A) Ab-1 polyclonal antibody (Cat. #RB-1638) was purchased from thermo fisher scientific (Waltham, MA, USA). Horseradish peroxidase (HRP) was purchased from DAKO (Glostrup, Denmark). All other chemicals and reagents used in this study were of analytical grade.
Resveratrol preparation and administration
RSV was dissolved in a vehicle of PEG-400-Saline (40:60 v/v). RSV was administered orally by using an oral needle at a dose of 50 mg/kg (Kubota et al. Citation2009; Chen et al. Citation2014). This dose is equivalent to 7 mg/kg/day in humans (Approximately 500 mg RSV in a person with a mass of 70 kg) according to the human equivalent dose formula.
Freund’s complete adjuvant-induced arthritis in rats
Adjuvant arthritis was induced in rats according to the method previously described by Pearson (Citation1964). On day zero, rats were rendered arthritic by sub-plantar injection of 0.1 mL of FCA consisting of heat killed and dried Mycobacterium tuberculosis emulsified in mineral oil (each mL contains 1 mg Mycobacterium tuberculosis, 0.85 mL mineral oil and 0.15 mL mannide monooleate) into the right hind paw.
Irradiation of animals
Irradiation of rats was carried out at the NCRRT, using Gamma Cell-40 biological irradiator with a Cesium137 (Cs137) source (Atomic Energy of Canada Limited; Sheridan Science and Technology Park, Mississauga, Ontario, Canada). In the current study, whole body irradiation was carried out based on the previous experimental studies that concerned with whole body irradiation (Nakatsukasa et al. Citation2008; Frey et al. Citation2009; Nakatsukasa et al. Citation2010; Weng et al. Citation2010). Rats were exposed to a total dose level of 0.5 Gy either single (1 × 0.5 Gy) or split (2 × 0.25 Gy) separated by 7 days interval and delivered at a dose rate of 0.46 Gy/min. For each required irradiation exposure, non-anaesthetized rats were placed in the plastic sample tray of the Gamma cell-40 and left for an appropriate interval of time to reach the exposure level.
Experimental design
The study was designed in two steps:
The first step involved the estimation of the optimum irradiation regimen in adjuvant-induced arthritis model. Rats were randomly classified into five groups (n = 8): Group I: normal control rats. Group II: FCA induced-arthritic rats. Group III: arthritic rats exposed to a single radiation dose level of 0.5 Gy at day 14. Group IV: arthritic rats exposed to irradiation dose of 2 × 0.25 Gy at days 14&21. Group V: arthritic rats exposed to irradiation dose of 2 × 0.25 Gy at days 21&28. Selection of the optimum schedule based only on the paw volume measurements and extended to the biochemical parameters. As a result, the irradiation regimen of 2×0.25 Gy at days 14&21 showed to be effective and was selected to be combined with RSV (see the results section).
The second step was designed to estimate the effect of the selected low dose radiation regimen in combination with RSV in adjuvant-induced arthritis model; rats were randomly classified into six groups (n = 8); Group I: normal control rats. Group II: FCA-induced arthritic rats. Group III: arthritic rats exposed to irradiation (2x0.25 Gy at days 14&21). Group IV: arthritic rats treated with RSV (50 mg/kg). Group V: arthritic rats exposed to irradiation and treated with RSV concurrently. Group VI: arthritic rats treated with diclofenac (3 mg/kg) as a reference drug. All treatments were administered orally once daily for two weeks starting from day 14.
On day 29 after FCA immunization, rats were sacrificed by decapitation and the blood samples were collected for estimation of biochemical parameters from each of the eight rats in all groups and the paws were dissected for the histopathalogical and immnuno-histochemical examinations from the same rats after blood withdrawal.
Evaluation of paw edema
The volume (in mL) of the right hind paw of each rat was measured at day zero before FCA induction and then at 4-day intervals up to day 28 using a water plethysmometer (Panlab Harvard, Barcelona, Spain). The volume of edema was calculated by subtracting the initial paw volume (basal) from the volume measured at each time point. While, The percentage of edema was calculated according to the following equation % of edema = [(Vf – Vi)/Vi] × 100 where Vi: basal paw volume at day zero and Vf: volume at each time point (Pearson Citation1964).
Biochemical estimation
The blood samples were collected after decapitation in centrifuge tubes and serum was separated by centrifugation using a cooling centrifuge (Hettich Mikro 22 R, Tuttlingen, Germany) at 3000 rpm for 10 min at 4 °C and then sub-divided in eppendorf tubes to be stored at –20 °C for later measurement of TNF-α and IL-1β using ELISA kits and ELISA plate reader (Dynatech® MR5000, Guernsey, UK). The serum levels of TBARS and NOx were determined colorimetrically using a spectrophotometer (Unicam 8625, Cambridge, UK) according to the method of Uchiyama and Mihara (Citation1978) and Miranda et al. (Citation2001), respectively.
Histopathological examination
After sacrifice, the ankle joints were dissected and fixed in 10% formol saline and decalcified in EDTA until complete decalcification. Specimens were cleared in xylene and embedded in paraffin at 56 degrees in hot air oven for twenty four hours. Paraffin bees wax tissue blocks were prepared for sectioning at 5 microns thickness by sledge microtome. The obtained tissue sections were collected on glass slides, deparaffinized, and stained by hematoxylin & eosin (H&E) stain for examination through the light electric microscope (Bancroft et al. Citation1996). Tissue sections were scored for infiltration of inflammatory cells, synovial proliferation, pannus formation and cartilage damage on a scale 0–3 (0 = none, 1 = mild, 2 = moderate, and 3 = severe) and the mean score was calculated (Wei et al. Citation2013). In addition, the number of inflammatory cells per microscopic field was counted and average number was obtained for each group. Evaluation of the joint pathology of the tissue specimen was carried out by two independent observers who were blinded to the experimental protocol.
Immunohistochemical analysis of NF-κB p65 in synovial tissues
5 μ thick tissue sections from all groups were cut by rotatory microtome and processed for immuno-histochemical staining with rabbit anti-NF-κB/p65 polyclonal antibody (dilution 1:100) followed by washing and then 30 min incubation with HRP kit. The expression NF-κB p65 was observed with the development of brown color under light microscopy. Mean expression area percentage of NF-κB was obtained from 8 random fields by using a Leica application suite for slide analysis (Leica biosystems- Germany).
Statistical analysis
All data were expressed as mean values ± standard error of the mean (SEM). Data normality was verified by Kolmogorov-Smirnov (KS, p > .10) test and normal distribution was found for all data. Statistical comparisons between different groups were carried out by one-way analysis of variance (ANOVA) followed by Tukey-Kramer multiple comparison test. Statistical analysis was carried out using Graph Pad prism 5 (Graph Pad Software Inc, San Diego, California, USA). A p-value < .05 was considered significant. Microsoft Excel program was used for figure representation.
Results
Estimation of the optimum irradiation regimen
Irradiation at a dose level of 0.5 Gy either single (1 × 0.5 Gy) or split dose (2 × 0.25 Gy) failed to produce any significant change in the volume of edema as compared to the FCA group (, ). Accordingly, the selection of the optimum irradiation regimen cannot be based on the paw volume results only and the pilot study was extended for estimation of biochemical parameters. Concerning the biochemical parameters, as shown in , the group exposed to irradiation (2 × 0.25 Gy) at days 14&21 was the only group that exhibited a significant reduction in the serum levels of TNF-α, IL-1β, TBARS and NOx reaching 28%, 29%, 21% and 41%, respectively, as compared to the FCA group (p < .05).
Figure 1. Effect of different low dose radiation exposure regimens on the paw volume in FCA-induced arthritic rats. Rats were exposed to a total exposure level of 0.5 Gy applied either single (1 × 0.5 Gy at day 14) or split (2 × 0.25 Gy at days 14&21 or at days 21&28). Results are expressed as mean volume of edema ± SEM (n = 8/group).
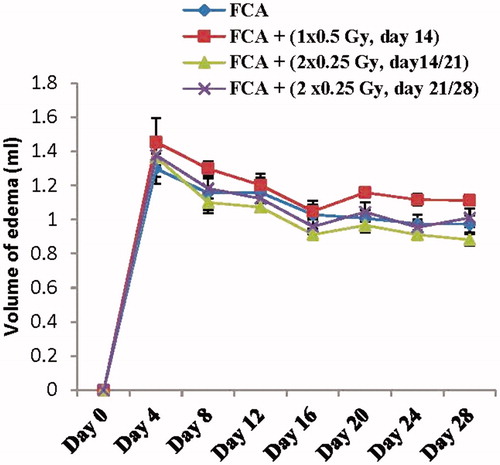
Table 1. Effect of different low dose radiation exposure regimens on the paw volume in in the Freund’s complete adjuvant-induced arthritic rats.
Table 2. Effect of different low dose radiation exposure regimens on the serum levels of pro-inflammatory cytokines and oxidative stress markers in the Freund’s complete adjuvant-induced arthritic rats.
Effect of low dose radiation and resveratrol on the paw volume in FCA-induced arthritis
FCA immunization into the right hind paw induced a marked increase in the paw volume with a peak value of 170% at day 4 and extends to day 28 with a gradual decrease to reach a value of 145% in the FCA group. Irradiation of rats (2 × 0.25 Gy at days 14&21) did not significantly affect the edema volume. Also, oral administration of RSV at a dose of 50 mg/kg failed to produce any significant inhibition in the volume of edema. Whereas, the application of the dual treatment strategy produced a significant inhibition in the paw volume observed at days 24 and 28 by 23% and 25%, respectively, as compared to the FCA group (p < .05). A similar extent of inhibition was observed in the diclofenac treated group (p < .05) (, ).
Figure 2. Effect of low dose radiation exposure (2 × 0.25 Gy, days 14&21) and RSV (50 mg/kg) administration on the paw volume in FCA-induced arthritic rats. RSV and diclofenac were given orally for two weeks started from day 14. Results are expressed as mean volume of edema ± SEM (n = 8/group). @p < .05 compared to FCA group.
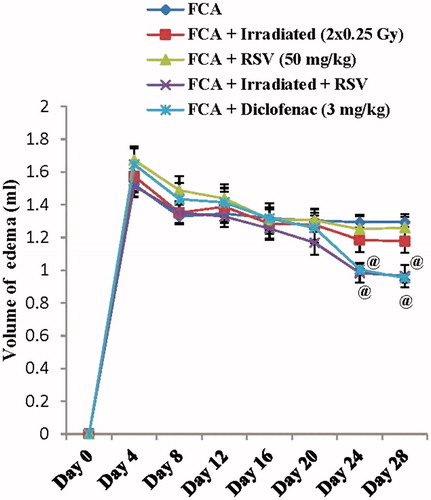
Table 3. Effect of low dose radiation exposure and resveratrol administration on the paw volume in the Freund’s complete adjuvant-induced arthritic rats.
Effect of low dose radiation and resveratrol on the serum level of TNF-α and IL-1β in FCA-induced arthritis
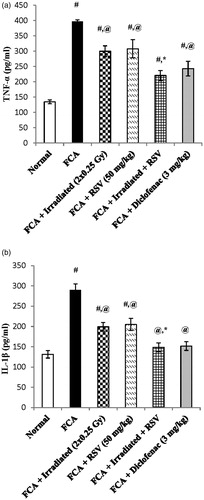
Serum levels of TNF-α and IL-1β were induced after FCA immunization by nearly three-fold and two-fold reaching values of 396.9 ± 5.68 and 289.8 ± 15.28, respectively, as compared to the normal group (p < .05). Irradiation of rats (2 × 0.25 Gy at days 14&21) significantly decreased both TNF-α and IL-1β serum levels by 24% and 31%, respectively, as compared to the FCA group (p < .05). Similarly, oral treatment with RSV (50 mg/kg) significantly decreased both TNF-α and IL-1β serum levels by 22% and 29%, respectively, as compared to the FCA group (p < .05). However, oral treatment of RSV as an adjunctive therapy during the radiation exposure regimen largely suppressed TNF-α and IL-1β serum level by 44% and 49%, respectively, where it almost reaches the normal level and showed a significant difference as compared to the irradiated group (p < .05). Diclofenac (3 mg/kg) significantly decreased the serum levels of TNF-α and IL-1β by 38% and 47%, respectively, as compared to the FCA group (p < .05). ().
Figure 3. Effect of low dose radiation exposure (2 × 0.25 Gy, days 14&21) and RSV (50 mg/kg) administration on the serum levels of TNF-α (a) and IL-1β (b) in FCA-induced arthritic rats. RSV and diclofenac were given orally for two weeks started from day 14. Results are expressed as mean ± SEM (n = 8/group). #p < .05 compared to Normal group. @p < .05 compared to FCA group. *p < .05 compared to irradiated group.
Effect of low dose radiation and resveratrol on the serum level of TBARS and NOx in FCA-induced arthritis
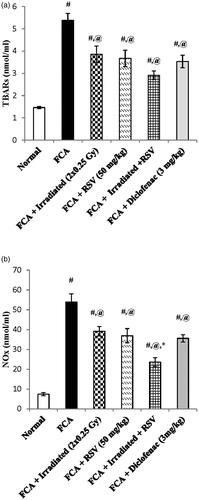
Results of the present study showed that FCA immunization significantly increased the serum levels of TBARS and NOx nearly by four-fold and seven-fold reaching values of 5.38 ± 0.31 and 53.91 ± 4.18, respectively, as compared to the normal group (p < .05). Furthermore, low dose radiation exposure (2 × 0.25 Gy at days 14&21) resulted in an significant decrease in the serum level of TBARs and NOx by 28% and 27%, respectively, as compared to the FCA group (p < .05). On the same line, oral administration of RSV significantly decreased the serum level of TBARS and NOx by 31% and 31%, respectively, as compared to the FCA group (p < .05). Irradiation of rats in combination with RSV treatment increased the percentage of inhibition of NOx serum level to 56%, which is statistically significant from the irradiated group (p < .05). Although the combination treatment decreased the TBARS serum level by 46%, it did not show a significant difference from the irradiated group. Diclofenac (3 mg/kg) significantly decreased the serum levels of TBARS and NOx by 34% and 34%, respectively, as compared to the FCA group (p < .05) ().
Figure 4. Effect of low dose radiation exposure (2 × 0.25 Gy, days 14&21) and RSV (50 mg/kg) administration on the serum levels of TBARs (a) and NOx (b) in FCA-induced arthritic rats. RSV and diclofenac were given orally for two weeks started from day 14. Results are expressed as mean ± SEM (n = 8/group). #p < .05 compared to Normal group. @p < .05 compared to FCA group. *p < .05 compared to irradiated group.
Effect of low dose radiation and resveratrol on the histopathological changes of ankle joint in FCA-induced arthritis
Histological features of paw joints sections were stained with H&E. In normal control rats group, the joint structures were clear, synoviocytes were monolayer and inflammatory cells had not infiltrated the synovial membrane. The articular surface appeared smooth with prominent nucleated chondrocytes inside the lacunae and clear basophilic cartilaginous matrix (). The FCA group showed obvious joint destruction with inflammatory cells infiltration mainly lymphocytes and plasma cells predominated, but polymorphonuclear leukocytes, histiocytes, and fibroblasts were also present. Hyperplasia of synovial epithelial lining, pannus formation and degeneration of articular surface were seen (). These histopathological changes were partially ameliorated in each of irradiated (2 × 0.25 Gy) () and RSV () groups, showed declined of inflammatory cells infiltration and pannus formation. Whereas in the combination () and diclofenac (3 mg/kg) () groups, mild morphological changes in the ankle joint were observed with normal joint space, few inflammatory cells infiltration, and no obvious cartilage destruction.
Figure 5. Representative sections of ankle joint stained with hematoxylin and eosin (H&E magnification x200) of (a) Normal group, (b) FCA group, (c) FCA + Irradiated (2 × 0.25 Gy, days 14&21) group, (d) FCA + RSV (50 mg/kg) group, (e) FCA + Irradiated + RSV group and (f) FCA + diclofenac (3 mg/kg) group. (g) Tissue sections were scored individually for infiltration of inflammatory cells (large arrow), synovial proliferation, pannus formation (small arrow) and cartilage damage (arrow head) on a scale 0–3. (h) Total histological scoring of joints was evaluated and the sum of all mean scores was calculated. (i) Number of inflammatory cells/microscopic field. Results are expressed as mean ± SEM (n = 8/group). #p < .05 compared to Normal group. @p < .05 compared to FCA group. *p < .05 compared to irradiated group.
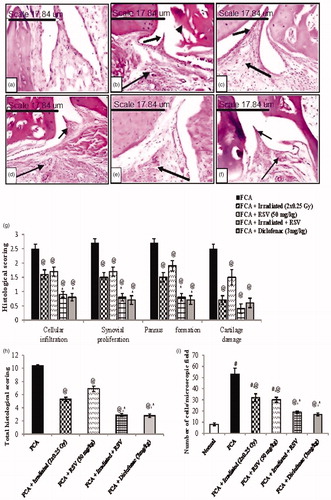
On the same line, total histopathological scoring of the FCA group showed a significant increase as compared to the normal group. Each of irradiated (2 × 0.25 Gy) and RSV groups resulted in a mild decrement in the total scoring as compared to the FCA group, while the combination and diclofenac (3 mg/kg) treated groups highly alleviated the FCA-induced synovial proliferation, pannus formation, inflammatory cell infiltration and cartilage damage ().
As for the inflammatory cells count, FCA immunization significantly increased the number of inflammatory cells reaching 53 ± 5.385 per scope. Either exposure to low dose radiation (2 × 0.25 Gy) or RSV administration reduced the cellular infiltration by 40% and 43%, respectively, as compared to the FCA group (p < .05). Application of the combination treatment increased the percentage of inhibition to 64% (p < .05), reaching the same extent to that observed in the diclofenac treated group and showed a significant difference from the irradiated group (p < .05) ().
Effect of low dose radiation and resveratrol on the NF-κB p65 expression of synovial tissues in FCA-induced arthritis
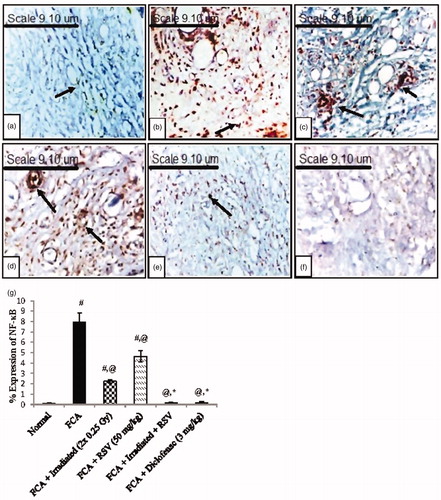
A negative expression of NF-κB p65 was observed in the synovial tissues of the normal group (), while a positive expression was shown in the FCA group () as compared to the normal group (p < .05). Each of irradiated (2 × 0.25 Gy) () and RSV () groups showed a significant suppression to the NF-κB p65 expression in the synovial tissue reaching 72% and 42%, respectively, as compared to the FCA group (p < .05). However, the combination of irradiation exposure and RSV administration led to a higher percentage of inhibition in NF-κB p65 expression reaching 98%, which indicated a nearly negative expression (p < .05 () and a similar degree of inhibition was observed in the diclofenac (3 mg/kg) treated group (). As shown in , the mean expression of NF-κB p65 in the combination treated group showed a significant difference from the irradiated group (p < .05).
Figure 6. Immuno-histochemical analysis (magnification x400) of the NF-κB p65 expression in synovial tissues of (a) Normal group, (b) FCA group, (c) FCA + irradiated (2 × 0.25 Gy, days 14&21) group, (d) FCA + RSV (50 mg/kg) group, (e) FCA + irradiated + RSV group and (f) FCA + diclofenac (3 mg/kg) group. (g) Quantitative analysis of NF-κB p65 expression in synovial tissues of normal, arthritic and treated groups. Results are expressed as mean percentage of expression area ± SEM (n = 8/group). #p < .05 compared to Normal group. @p < .05 compared to FCA group. *p < .05 compared to irradiated group.
Discussion
Exposure to low dose radiation modulates a variety of inflammatory processes. Previous studies have shown that the 0.5 Gy radiation dose level was effective in attenuating the progression of experimentally induced RA models (Nakatsukasa et al. Citation2008; Frey et al. Citation2009; Nakatsukasa et al. Citation2010). Accordingly, the dose level of 0.5 Gy was applied in the current study in different regimens either single (1 × 0.5 Gy at day 14) or split (2 × 0.25 Gy at days 14&21 and 21&28). Based on the findings of the inflammatory and oxidative stress parameters, the split regimen was shown to be more effective than the single exposure, especially on days 14&21 than on days 21&28, which could be likely due to the timing effect as the intervals between the endpoint and the last dose of irradiation was not equal (8 days vs 1 day). In the same concept, the study of Liebmann et al. (Citation2004) showed that the therapeutic effect of low dose radiation is time and fractionation dependent.
Induction of arthritis led to the development of edema, which was attributed to the infiltration of inflammatory cells, synovial proliferation and cartilage damage (Liu, Xu, et al. Citation2017). The failure of either irradiation or RSV administration to produce any significant change in the paw volume was supported by the histopathological findings that indicated mild suppression of the leukocytes infiltration.
The pathogenesis of RA is orchestrated by pro-inflammatory cytokines which control the severity of the disease (McInnes and Schett Citation2007). The elevated serum levels of pro-inflammatory cytokines; TNF-α and IL-1β in the FCA group were in harmony with the study of Voon et al. (2017). The down-regulation of the pro-inflammatory cytokines by low dose radiation was in agreement with the results of Nakatsukasa et al. (Citation2008), where gamma-irradiation of collagen-induced arthritic rats reduced the paw swelling by suppressing the production of TNF-α and IL-6 production. Furthermore, the therapeutic effect of RSV in arthritic-induced rats was previously correlated to the suppression of pro-inflammatory cytokines (Chen et al. Citation2013; Yang et al. Citation2018).
Moreover, NF-κB was considered a key factor in RA pathogenesis as its activation correlates with the transcription of genes encoding pro-inflammatory cytokines, adhesion molecules, and chemokines (Simmonds and Foxwell Citation2008). In resting state, NF-κB p65 subunit is located in the cytosol complexed with the inhibitory protein IκB. Once activated, IκB undergoes phosphorylation by the IκB kinase (IKK) and subsequent degradation to produce free p65 which is then translocated into the nucleus and binds to its target genes inducing transcription of pro-inflammatory cytokines (Siebenlist et al. Citation1994; Lappas et al. Citation2002). In the current study, the expression of NF-κB p65 (the active form) induced by FCA immunization was paralleled with the resulted up-regulation in the pro-inflammatory cytokines level and considered as an indicative to the nuclear localization of the p65 (Liu, Xu, et al. Citation2017). The impact of low dose radiation on the NF-κB activation was supported by previous in vitro studies where activated macrophage exposed to low dose radiation exhibited a reduced secretion of IL-1β correlated with reduced activation and translocation of NF-κB subunit p65 (Lodermann et al. Citation2012; Wunderlich et al. Citation2015). Regarding the effect of RSV, Lei et al. (Citation2012) suggested that RSV reversed the inflammatory effect of IL-1β on articular chondrocytes by suppressing NF-κβ expression and transactivation. Interference with the NF-κB activation by RSV was previously attributed to the inhibition of IKK signaling (Holmes-McNary and Baldwin Citation2000).
Oxidative stress is another key factor that is implicated in the inflammatory process of RA. During inflammation, reactive oxygen species (ROS) are produced by activated macrophages during the oxidative burst and led to lipid peroxides formation (Filippin et al. Citation2008), which is reflected by a rise in TBARS level (Esterbauer et al. Citation1991). The increase in the TBARS serum level observed in the FCA group was consistent with the results of El-Ghazaly et al. (Citation2011). The influence of low dose radiation on the ROS production was previously illustrated in the in vitro study of Schaue et al. (Citation2002) in which activated macrophages exposed to low dose radiation of 0.3–0.6 Gy exhibited a reduced oxidative burst capacity and consequently a reduced level of ROS. Moreover, the anti-oxidant effect of RSV was previously attributed to the inhibition of neutrophil infiltration as a source of ROS and/or enhancing of anti-oxidant enzymes (Riveiro-Naveira et al. Citation2016).
One of the functions of inflammatory macrophages is the expression of inducible nitric oxide synthase (iNOS), which is involved in the synthesis of nitric oxide (NO) (Abramson et al. Citation2001). NO plays a pathological role in RA as its continued overproduction contributes to persistent inflammation and tissue destruction (Evans and Stefanovic-Racic Citation1996). In the current study, the elevation in the NOx serum level in the FCA group was in agreement with the results of Liu, Hou, et al. (Citation2017). The down-regulation action of the low dose radiation on the NOx was in line with previous in vitro study carried out on activated macrophages, showed that exposure to low dose radiation suppresses the iNOS-protein expression and cumulative NO production (Hildebrandt et al. Citation2003). In regard with RSV, the in vitro study of Lei et al. (Citation2012) reported the inhibition of iNOS expression and NO production in articular chondrocytes treated with RSV.
The current histopatholgical examinations showed infiltration of inflammatory cells and focal necrosis of cartilage by adjuvant immunization. Mechanistically, inflammatory cytokines stimulate the expression of cellular adhesion molecules and production of chemokines by tissue resident macrophages, which lead to more cellular infiltration into the joint cavity (Osborn et al. Citation1989; Taylor et al. Citation2000) and consequent production of free radicals which plays a critical role in the process of cartilage damage (Filippin et al. Citation2008). In addition, cytokines also provoke the release of degrading enzymes such as matrix metalloproteinase from chondrocyte and synovial fibroblast resulting in cartilage damage (Choy and Panayi Citation2001). The inhibition of leukocytes infiltration by low dose radiation was supported by the in-vitro study of Rodel et al. (Citation2008) where a reduced chemokine secretion paralleled with a hampered leukocytes adhesion to the endothelial cells occurred with a pronounced effect at 0.7 Gy exposure. On the same line, the inhibitory effect of RSV on the leukocytes infiltration was in agreement with the study of Riveiro-Naveira et al. (Citation2016) in which RSV supplementation significantly decreased the number of T cells and macrophages infiltrated into the synovium through down regulation of chemokines. The protective effect of RSV on arthritic cartilage was first reported in the study of Elmali et al. (Citation2007); in which intra-articular administration of RSV decreased cartilage destruction, inhibited proteoglycan depletion and lowered synovial proliferation
Although the clinical application of low dose radiation in RA treatment is more concerned with local radiation (Micke and Seegenschmiedt Citation2002), application of whole-body irradiation might be considered of potential interest in the future for severe cases of arthritis. This was demonstrated by Kojima et al. (Citation2018), who clinically observed the therapeutic effect of whole-body irradiation on a RA patient. Furthermore, given the risks of whole-body irradiation, even at low doses, and the limitations of modest such as the one used here, additional studies should be performed to determine if similar improvements can be seen when combining RA treatments with more localized fields.
Conclusion
Interestingly, low dose radiation exposure in combination with RSV administration as an adjunctive therapy resulted in a more potent anti-arthritic activity. That pronounced effect could be explained on the basis of the common multi-targeting anti-inflammatory and anti-oxidant pathways underlying the mechanism of action of each. Accordingly, the results of the current study suggest that applying an adjunctive therapy such as RSV throughout the exposure course of low dose radiation may be a safer and more promising strategy to enhance the low dose radiation therapeutic efficacy in reducing RA severity.
Acknowledgement
The authors wish to acknowledge Dr. Kawkab Abdel-Aziz Ahmed and Dr. Ahmed H. Osman, Professors of Pathology, Faculty of Veterinary Medicine, Cairo University, for their professional help in carrying out the histological examinations.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Mona A. El-Ghazaly
Mona A. El-Ghazaly, PhD, is a Professor of Pharmacology, Department of Drug Radiation Research, National Centre for Radiation Research and Technology (NCRRT), Atomic Energy Authority, Cairo, Egypt.
Noha A. Fadel
Noha A. Fadel, PhD, is an Assistant lecturer of Pharmacology, Department of Drug Radiation Research, National Centre for Radiation Research and Technology (NCRRT), Atomic Energy Authority, Cairo, Egypt.
Doaa H. Abdel-Naby
Doaa H. Abdel-Naby, PhD, is a Lecturer of Pharmacology, Department of Drug Radiation Research, National Centre for Radiation Research and Technology (NCRRT), Atomic Energy Authority, Cairo, Egypt.
Hassan A. Abd El-Rehim
Hassan A. Abd El-Rehim, PhD, is a Professor of Radiation Chemistry, Department of Polymers, National Centre for Radiation Research and Technology (NCRRT), Atomic Energy Authority, Cairo, Egypt.
Hala F. Zaki
Hala F. Zaki, PhD, is a Professor of Pharmacology, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Cairo University, Egypt.
Sanaa A. Kenawy
Sanaa A. Kenawy, PhD, is a Professor of Pharmacology, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Cairo University, Egypt.
References
- Abramson SB, Amin AR, Clancy RM, Attur M. 2001. The role of nitric oxide in tissue destruction. Best Pract Res Clin Rheumatol. 15(5):831–845.
- Agarwal SK. 2011. Core management principles in rheumatoid arthritis to help guide managed care professionals. J Manag Care Pharm. 17(9 Supp B):S03–S08.
- Arenas M, Sabater S, Hernandez V, Rovirosa A, Lara PC, Biete A, Panes J. 2012. Anti-inflammatory effects of low-dose radiotherapy. Indications, dose, and radiobiological mechanisms involved. Strahlenther Onkol. 188(11):975–981.
- Bancroft JD, Stevens A, Turner DR. 1996. Theory and practice of histological techniques. New York: Churchill Livingstone.
- Calabrese EJ, Calabrese V. 2013. Low dose radiation therapy (LD-RT) is effective in the treatment of arthritis: animal model findings. Int J Radiat Biol. 89(4):287–294.
- Chen X, Lu J, An M, Ma Z, Zong H, Yang J. 2014. Anti-inflammatory effect of resveratrol on adjuvant arthritis rats with abnormal immunological function via the reduction of cyclooxygenase-2 and prostaglandin E2. Mol Med Rep. 9(6):2592–2598.
- Chen XY, Wang ZC, Li J, Liu XL, Sun YH. 2013. Regulation of synoviocyte activity by resveratrol in rats with adjuvant arthritis. Exp Ther Med. 6(1):172–176.
- Choudhary M, Kumar V, Gupta P, Singh S. 2014. Investigation of antiarthritic potential of Plumeria alba L. leaves in acute and chronic models of arthritis. Biomed Res Int. 2014:1–12.
- Choy EH, Panayi GS. 2001. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 344(12):907–916.
- El-Ghazaly MA, El-Naby DH, Khayyal MT. 2011. The influence of irradiation on the potential chondroprotective effect of aqueous extract of propolis in rats. Int J Radiat Biol. 87(3):254–262.
- El-Ghazaly MA, Kenawy S, Khayyal MT, Roushdy H, Saleh S. 1985. Effect of exposure to radiation on the inflammatory process and its influence by diclofenac. Br J Pharmacol. 85(1):45–50.
- Elmali N, Baysal O, Harma A, Esenkaya I, Mizrak B. 2007. Effects of resveratrol in inflammatory arthritis. Inflammation. 30(1-2):1–6.
- Esterbauer H, Schaur RJ, Zollner H. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 11(1):81–128.
- Evans CH, Stefanovic-Racic M. 1996. Nitric oxide in arthritis. Methods. 10(1):38–42.
- Filippin LI, Vercelino R, Marroni NP, Xavier RM. 2008. Redox signalling and the inflammatory response in rheumatoid arthritis. Clin Exp Immunol. 152(3):415–422.
- Frey B, Frey B, Gaipl US, Frey B, Gaipl US, Sarter K, Zaiss MM, Stillkrieg W, Rödel F, Schett G, et al. 2009. Whole body low dose irradiation improves the course of beginning polyarthritis in human TNF-transgenic mice. Autoimmunity. 42(4):346–348.
- Frey B, Hehlgans S, Rodel F, Gaipl US. 2015. Modulation of inflammation by low and high doses of ionizing radiation: implications for benign and malign diseases. Cancer Lett. 368(2):230–237.
- Gibofsky A. 2014. Epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis: a synopsis. Am J Manag Care. 20(7 Suppl):S128–S135.
- Hildebrandt G, Loppnow G, Jahns J, Hindemith M, Anderegg U, Saalbach A, Kamprad F. 2003. Inhibition of the iNOS pathway in inflammatory macrophages by low-dose X-irradiation in vitro. Is there a time dependence? Strahlenther Onkol. 179(3):158–166.
- Holmes-McNary M, Baldwin AS Jr. 2000. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 60(13):3477–3483.
- Kinne RW, Brauer R, Stuhlmuller B, Palombo-Kinne E, Burmester GR. 2000. Macrophages in rheumatoid arthritis. Arthritis Res. 2(3):189–202.
- Klareskog L, Catrina AI, Paget S. 2009. Rheumatoid arthritis. Lancet. 373(9664):659–672.
- Kojima S, Thukimoto M, Cuttler JM, Inoguchi K, Ootaki T, Shimura N, Koga H, Murata A. 2018. Recovery from rheumatoid arthritis following 15 months of therapy with low doses of ionizing radiation: a case report. Dose Response. 16(3):155932581878471–1559325818784719.
- Kubota S, Kurihara T, Mochimaru H, Satofuka S, Noda K, Ozawa Y, Oike Y, Ishida S, Tsubota K. 2009. Prevention of ocular inflammation in endotoxin-induced uveitis with resveratrol by inhibiting oxidative damage and nuclear factor-kappaB activation. Invest Ophthalmol Vis Sci. 50(7):3512–3519.
- Lan Z, Wei M, Chen L, Xie G, Liu X, Zhang X. 2016. Role of Sinomenine on Complete Freund’s Adjuvant-Induced Arthritis in Rats. IUBMB Life. 68(6):429–435.
- Lappas M, Permezel M, Georgiou HM, Rice GE. 2002. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 67(2):668–673.
- Lei M, Wang JG, Xiao DM, Fan M, Wang DP, Xiong JY, Chen Y, Ding Y, Liu SL. 2012. Resveratrol inhibits interleukin 1beta-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-kappaB activity. Eur J Pharmacol. 674(2–3):73–79.
- Liebmann A, Hindemith M, Jahns J, Madaj-Sterba P, Weisheit S, Kamprad F, Hildebrandt G. 2004. Low-dose X-irradiation of adjuvant-induced arthritis in rats. Efficacy of different fractionation schedules. Strahlenther Onkol. 180(3):165–172.
- Liu JY, Hou YL, Cao R, Qiu HX, Cheng GH, Tu R, Wang L, Zhang JL, Liu D. 2017. Protodioscin ameliorates oxidative stress, inflammation and histology outcome in Complete Freund’s adjuvant induced arthritis rats. Apoptosis. 22(11):1454–1460.
- Liu XY, Xu L, Wang Y, Li JX, Zhang Y, Zhang C, Wang SS, Zhang XM. 2017. Protective effects of total flavonoids of Astragalus against adjuvant-induced arthritis in rats by regulating OPG/RANKL/NF-kappaB pathway. Int Immunopharmacol. 44:105–114.
- Lodermann B, Wunderlich R, Frey S, Schorn C, Stangl S, Rodel F, Keilholz L, Fietkau R, Gaipl US, Frey B. 2012. Low dose ionising radiation leads to a NF-kappaB dependent decreased secretion of active IL-1beta by activated macrophages with a discontinuous dose-dependency. Int J Radiat Biol. 88(10):727–734.
- Lopez M, Martin M. 2011. Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother. 16(4):138–146.
- McInnes IB, Schett G. 2007. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 7(6):429–442.
- Micke O, Seegenschmiedt MH. 2002. Consensus guidelines for radiation therapy of benign diseases: a multicenter approach in Germany. Int J Radiat Oncol Biol Phys. 52(2):496–513.
- Miranda KM, Espey MG, Wink DA. 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5(1):62–71.
- Nakatsukasa H, Tsukimoto M, Ohshima Y, Tago F, Masada A, Kojima S. 2008. Suppressing effect of low-dose gamma-ray irradiation on collagen-induced arthritis. J Radiat Res. 49(4):381–389.
- Nakatsukasa H, Tsukimoto M, Tokunaga A, Kojima S. 2010. Repeated gamma irradiation attenuates collagen-induced arthritis via up-regulation of regulatory T cells but not by damaging lymphocytes directly. Radiat Res. 174(3):313–324.
- Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R. 1989. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 59(6):1203–1211.
- Pan K, Xia X, Guo WH, Kong LY. 2015. Suppressive effects of total alkaloids of Lycopodiastrum casuarinoides on adjuvant-induced arthritis in rats. J Ethnopharmacol. 159:17–22.
- Pearson CM. 1964. Experimental Models in Rheumatoid Disease. Arthritis Rheum. 7(1):80–86.
- Riveiro-Naveira RR, Valcarcel-Ares MN, Almonte-Becerril M, Vaamonde-Garcia C, Loureiro J, Hermida-Carballo L, Lopez-Pelaez E, Blanco FJ, Lopez-Armada MJ. 2016. Resveratrol lowers synovial hyperplasia, inflammatory markers and oxidative damage in an acute antigen-induced arthritis model. Rheumatology (Oxford). 55(10):1889–1900.
- Rodel F, Frey B, Gaipl U, Keilholz L, Fournier C, Manda K, Schollnberger H, Hildebrandt G, Rodel C. 2012. Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem. 19(12):1741–1750.
- Rodel F, Hofmann D, Auer J, Keilholz L, Rollinghoff M, Sauer R, Beuscher HU. 2008. The anti-inflammatory effect of low-dose radiation therapy involves a diminished CCL20 chemokine expression and granulocyte/endothelial cell adhesion. Strahlenther Onkol. 184(1):41–47.
- Schaue D, Marples B, Trott KR. 2002. The effects of low-dose X-irradiation on the oxidative burst in stimulated macrophages. Int J Radiat Biol. 78(7):567–576.
- Siebenlist U, Franzoso G, Brown K. 1994. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 10(1):405–455.
- Simmonds RE, Foxwell BM. 2008. Signalling, inflammation and arthritis: NF-kappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford). 47(5):584–590.
- Taylor PC, Peters AM, Paleolog E, Chapman PT, Elliott MJ, McCloskey R, Feldmann M, Maini RN. 2000. Reduction of chemokine levels and leukocyte traffic to joints by tumor necrosis factor alpha blockade in patients with rheumatoid arthritis. Arthritis Rheum. 43(1):38–47.
- Uchiyama M, Mihara M. 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 86(1):271–278.
- Valcarcel-Ares MN, Riveiro-Naveira RR, Vaamonde-Garcia C, Loureiro J, Hermida-Carballo L, Blanco FJ, Lopez-Armada MJ. 2014. Mitochondrial dysfunction promotes and aggravates the inflammatory response in normal human synoviocytes. Rheumatology (Oxford). 53(7):1332–1343.
- Vastano BC, Chen Y, Zhu N, Ho CT, Zhou Z, Rosen RT. 2000. Isolation and identification of stilbenes in two varieties of Polygonum cuspidatum. J Agric Food Chem. 48(2):253–256.
- Voon FL, Sulaiman MR, Akhtar MN, Idris MF, Akira A, Perimal EK, Israf DA, Ming-Tatt L. 2017. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur J Pharmacol. 794:127–134.
- Wei ZF, Jiao XL, Wang T, Lu Q, Xia YF, Wang ZT, Guo QL, Chou GX, Dai Y. 2013. Norisoboldine alleviates joint destruction in rats with adjuvant-induced arthritis by reducing RANKL, IL-6, PGE(2), and MMP-13 expression. Acta Pharmacol Sin. 34(3):403–413.
- Weng L, Williams RO, Vieira PL, Screaton G, Feldmann M, Dazzi F. 2010. The therapeutic activity of low-dose irradiation on experimental arthritis depends on the induction of endogenous regulatory T cell activity. Ann Rheum Dis. 69(8):1519–1526.
- Wenjin C, Jianwei W. 2017. Protective effect of gentianine, a compound from Du Huo Ji Sheng Tang, against Freund’s complete adjuvant-induced arthritis in rats. Inflammation. 40(4):1401–1408.
- Wunderlich R, Ernst A, Rodel F, Fietkau R, Ott O, Lauber K, Frey B, Gaipl US. 2015. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 179(1):50–61.
- Yang G, Chang CC, Yang Y, Yuan L, Xu L, Ho CT, Li S. 2018. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J Agric Food Chem. 66(49):12953–12960.

