Abstract
Purpose
The aim of this brief personal, high level review is to consider the state of the art for biological dosimetry for radiation routine and emergency response, and the potential future progress in this fascinating and active field. Four areas in which biomarkers may contribute to scientific advancement through improved dose and exposure characterization, as well as potential contributions to personalized risk estimation, are considered: emergency dosimetry, molecular epidemiology, personalized medical dosimetry, and space travel.
Conclusion
Ionizing radiation biodosimetry is an exciting field which will continue to benefit from active networking and collaboration with the wider fields of radiation research and radiation emergency response to ensure effective, joined up approaches to triage; radiation epidemiology to assess long term, low dose, radiation risk; radiation protection of workers, optimization and justification of radiation for diagnosis or treatment of patients in clinical uses, and protection of individuals traveling to space.
Graphical Abstract
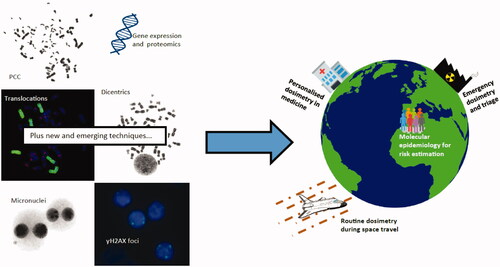
Well established and newer biodosimetric techniques show promise for a number of future dosimetry or wider research application. PCC: Premature chromosome condensation.
Introduction
Biological dosimetry relies on measurement of changes induced by ionizing radiation in blood or other materials from suspected exposed individuals (IAEA Citation2011). The most common techniques are those based on cytogenetic changes induced by radiation in blood cells, including formation of dicentric chromosome aberrations (Oestreicher et al. Citation2017) or micronuclei (Depuydt et al. Citation2017). Fluorescence in situ hybridization (FISH) for identification of translocations (Barquinero et al. Citation2017) and premature chromosome condensation (Terzoudi et al. Citation2017) are also widely used, particularly for long term post exposure and high-dose dose estimation, respectively. Since these long-established cytogenetic methods were deployed into routine use, a number of other biomarkers have been identified and are at various stages of development, for example the gamma-H2AX assay (Moquet et al. Citation2017) and measurement of transcriptional changes (e.g. Cruz-Garcia Citation2020). In addition, measurement of electron paramagnetic resonance in teeth (Umakoshi et al. Citation2017) or fingernails (Swarts et al. Citation2018) can also provide rapid, individual dose estimation.
On a day to day basis, the most frequent application of biodosimetry is in routine dosimetry, to confirm or refute suspected occupational (chiefly industrial) exposures registered on personal dosimeters, or to verify (or not) exposure in cases where individuals are not wearing personal dosimetry badges or similar. In the majority of these cases, biodosimetry does not indicate radiation exposure thereby providing evidence to resolve false alarms and reassurance to all concerned. However, when suspected overexposures are confirmed, biodosimetry plays a crucial role in assessing the dose and exposure circumstances, in order to inform the most appropriate course of medical intervention and/or the potential long term, chronic, risks (Sun et al. Citation2016). In addition, originally prompted by the Chernobyl accident and more recently by heightened awareness of terrorism (e.g. dirty bombs), a large amount of international effort has been focused on preparation and testing of mutual assistance networks in preparedness for a large scale, mass casualty, radiation accident (Kulka and Wojcik Citation2017; Kulka et al. Citation2018). Increasing the speed and capacity of the response is the key focus here (Wathen et al. Citation2020).
In recent years, advances in several techniques have highlighted the potential for biological dosimetry to be utilized in molecular epidemiological studies (e.g. Hall et al. Citation2017), or even for personalized dosimetry to aid treatment planning decisions/assessment of longer-term risks on an individual basis (Bucher et al. Citation2021; Cruz-Garcia Citation2021).
Space travel is another area of current interest for radiation protection, not least because there is a great deal of uncertainty associated with current risk estimates (Cucinotta et al. Citation2017).
The aim of this short topical review is to consider the state of the art for biological dosimetry for radiation routine and emergency response, and to look at some of the potential future avenues of development in this fascinating and active field.
Future directions 1: emergency dosimetry
A large scale, mass casualty, radiation accident or incident, is any situation in which large numbers of individuals are potentially exposed, overwhelming the local clinical resources. In such cases, triage of suspected exposed persons will be urgently required, in order to quickly identify those who have received large, potentially life-threatening doses in order to prioritize care for these individuals. Normally such triage is carried out on the basis of exposure location and clinical signs and symptoms of the prodromal phase of Acute Radiation Syndrome (ARS; del Rosario Pérez et al. Citation2010; Port et al. Citation2019). In most emergency response plans and associated exercises, biodosimetry rapid response currently contributes after this initial triage, to help separate the individuals who have been exposed to intermediate doses in the region 1 to ∼3 Gy, who won’t necessarily be exhibiting clinical signs and symptoms, but who do require more urgent care than those who have received doses <∼1 Gy (del Rosario Pérez et al. Citation2010; Rea et al. Citation2010; Jaworska et al. Citation2015). In an emergency it is very difficult to distinguish prodromal signs from other coincidental conditions including anxiety around potential exposures in the ‘worried well’. This is where the current and future biomarkers can resolve such confounding cases and, as a form of secondary triage, remove some of the pressure on the clinical services.
In the US, a ‘rapid on-site screening test’ requiring only a drop of blood from a finger prick is under development, with the aim to rapidly separate those who have been exposed to little or no dose, i.e. the worried well, from those with higher doses who are in more urgent need of clinical care (Wathen et al. Citation2020). The assay is based on detection with enzyme-linked immunosorbent assay (ELISA) and classification of proteomic responses to radiation in blood plasma (namely salivary alpha amylase, AMY1; Flt3 ligand, FLT3L, and monocyte chemotactic protein 1, MCP1), which are detectable approximately 24 hours − 14 days post exposure. The device has been validated in patients receiving total body irradiation therapy for cancer, and is currently able to discriminate between doses of < and ≥2 Gy (Balog et al. Citation2020).
Gene expression is also a highly promising candidate for rapid, on-site assessment of radiation responsive changes. For example, Cruz-Garcia and colleagues recently presented details of a potential portable device which uses nanopore sequencing analysis (which facilitates long read sequencing; full details given in the Cruz-Garcia et al. Citation2020) to detect radiation induced genetic changes in 46 genes in human peripheral blood mononuclear cells. These included five radiation-responsive transcripts identified as such for the first time, further demonstrating the fast moving nature of this field. The results were validated by quantitative polymerase chain reaction (qPCR) analysis, and this technique was able to successfully distinguish between doses below and above 2 Gy (Cruz-Garcia et al. Citation2020).
Following initial on-site assessment, the aim would be to couple the results of on-site rapid biodosimetric assessments. Ideally this would utilize high throughput versions of existing or new technologies for provision of more accurate dose estimation and assessment of exposure circumstances (e.g. the % of the body exposed), for those individuals who have had high or intermediate doses likely to lead to ARS or increased longer term risks, respectively. A number of potential automated technologies are in development, which look to exploit recent advances in high throughput gene expression analysis or the Dicentric or Cytokinesis-block Micronucleus (CBMN) Assays (Wathen et al. Citation2020; Royba et al. Citation2019; Repin et al. Citation2020; Shirley et al. Citation2020).
However, in order to ensure biodosimetry is able to characterize the full range of potential exposures scenarios, multiparametric assays will be needed (Jaworska et al. Citation2015; Wojcik et al. Citation2017). Tichy and colleagues, for example, recently demonstrated the potential for combined analysis based on a number of cytogenetic and genetic biomarkers for characterization of exposures in vivo (Tichy et al. Citation2018). Given the active nature of this field, including the high rate at which new biomarkers are developing, further work is urgently needed to assess how best to use these biomarkers in parallel. In terms of the ‘fortuitous’ physical retrospective dosimeter, the future development of EPR on alanine in chip cards carried as standard by members of the public is an incredibly exciting prospect (NIST Citation2021).
Furthermore, biomarkers of organ injury have been suggested as a more accurate initial biodosimetry triage tool (Swartz et al. Citation2020). However, in order to do this effectively, information regarding the heterogeneity of the exposure, for example, is needed. Such information can be provided by more traditional biomarkers, further demonstrating the need for multiparametric assessment of biological changes in vivo (Swartz et al. Citation2020).
Future directions 2: molecular epidemiology for risk estimation
In recent years, a large amount of effort has focused on use of FISH as a well-established biomarker of dose that can be used many years post exposure, to support epidemiological studies (e.g. Sotnik et al. Citation2015). In 2012, Pernot et al. considered in detail the potential for biomarkers to contribute to lower radiation dose epidemiological studies – which is where many open questions in radiation protection remain (Pernot et al. Citation2012). The authors highlighted the need for careful study design depending on the type of exposure under investigation (particularly regarding dose assessment but also around the logistics of sample collection, hence prospective cohort studies are recommended), as well as the need to consider the logistics and confounding factors. For most molecular epidemiology studies, at very least, age and sex matched controls must be used, but it is also necessary to account for smoking and health status for some biomarkers including for DNA damage, and for others – for example extracellular 8-oxo-dG – the confounders are more or less unknown. An updated publication, in 2017, makes clear that integration of biomarkers of individual exposure, effects and susceptibility is required in order for low dose radiation epidemiology to effectively assess the risks < 100 mSv. Of the large number of biomarkers assessed, including those based on cytogenetic changes, DNA damage, germline variants, induced mutations, epigenetic and metabolic changes, the authors identified a range of biomarkers suitable for qualification, discovery or validation. However, only one biomarker – Transcriptomic changes in radiation specific mRNA – was judged to be sufficiently developed to be prioritized for further research (Hall et al. Citation2017).
Further, Simon and Bouville (Citation2016) recently reviewed the potential for biodosimetry in long term studies of population risk. The authors concluded that it may be possible for individual biodosimetry to completely replace the need for model based dose reconstruction, but highlighted the need to improve the cost, detection limit, interindividual variation, and to develop biodosimetric tools which can be used with high accuracy over long time periods post exposure.
Most recently, epigenetics has been an area of growing interest, not least on the basis of the observation that cellular aging is independent of DNA damage response and telomere length (Lowe et al. Citation2016). Horvath and Raj discussed the potential for biomarkers of aging based on DNA methylation, known as ‘epigenetic clocks,’ which may ultimately contribute to life span prediction, and which thus could be incredibly powerful in the context of multiparametric health outcome prediction following exposure to radiation too – as such markers could conceivably inform personal, systemic, longer term radiation risk (Horvath and Raj Citation2018; Sehl et al. Citation2020).
Another hugely promising area within larger scale studies is the potential to use machine learning or artificial intelligence to assess the large amounts of data which are necessarily produced during such research programmes. Very recently, Jang et al. (Citation2021) proposed a new deep learning system for automated dose estimation based on the dicentric assay, and Luxton and colleagues demonstrated the successful use of machine learning in identification of telomere length data, as a predictor of individual radiosensitivity and risk (Luxton et al. Citation2021). Marcos-Zambrano and colleagues recently looked at the potential to use machine learning to identify biomarkers and toward disease prediction and treatment in the field of microbiome studies (Marcos-Zambrano et al. Citation2021). The authors demonstrated the potential of machine learning to provide new insights and highlighted that many algorithms already developed could be applied to different fields. The biodosimetry community should pay a good deal of attention to the emerging research in this area.
Future directions 3: personalized dosimetry in the medical context
Biomarkers are not new to the clinic, however, it is only in recent years that the potential for radiation biomarkers has properly begun to be assessed. Port and colleagues recently reviewed the evidence and concluded that radiation dose alone is not of clinical use. However, with recent developments as outlined above, biodosimetry now provides the opportunity to identify a variety of different exposure characteristics at the individual level (Port et al. Citation2019), as well as the possibility to contribute to understanding of the mechanisms underpinning responses, and this makes it a potentially very powerful tool indeed.
In 2018, Moquet and colleagues validated use of the dicentric assay for in vivo irradiated patients, and demonstrated excellent agreement between treatment planning doses and partial body doses calculated using the dicentric assay as well as with a new blood dosimetry model (Moquet et al. Citation2018). A number of other authors have demonstrated that cytogenetic markers can provide important clinical information, for example, Bucher and colleagues recently showed that DNA damage assessed by phosphorylation of H2AX and chromosome aberrations assessed by mFISH can contribute to identification of radiation-sensitive ataxia-telangiectasia patients (Bucher et al. Citation2021).
Balajee and Hande (Citation2018), recently considered the future of cytogenetic applications in clinical research, highlighting that humans have over 20,000 genes – and that we are only beginning to understand how our individual genetic and cytogenetic responses contribute to our individual radiation responses. The data-based challenges are clear.
Artificial intelligence is already in development in the clinic, for example, Imamura and colleagues recently demonstrated how neural networking might be used to support early prediction of amyotrophic lateral sclerosis (Imamura et al. Citation2021), and machine learning has been shown to be of use in the diagnosis of sarcoma, on the basis of DNA methylation data (Koelsche et al. Citation2021). In the field of radiomics, for example in oncology, machine or deep learning is now routinely used for data analysis. The potential to further develop such approaches in personalized medicine has been highlighted by a number of authors (reviewed by Guiot et al. Citation2021). In most cases, the interindividual variation makes early identification of these diseases difficult, however, artificial intelligence facilitates targeted analysis of complex data sets to identify patterns of relevance to the disease or its treatment. Indeed, two of the key barriers in development of biomarkers of radiation effect, particularly in the promising omics areas where large amounts of data can be generated, are the interindividual variation and confounders (Cruz-Garcia Citation2018, Citation2020). Thus, there is a huge amount of potential for artificial intelligence to help identify individual, personalized responses and risks. It is not so difficult to imagine that it won’t be too long before such tools are in common usage, both for the occupationally exposed operators as well as for patients, to help inform optimization and justification of the use of radiation in personalized medicine.
Future directions 4: routine dosimetry, particularly for space travel
The most exciting potential future direction for biodosimetry is in space travel, which is likely to become more common in the coming decades. Although adequate radiation protection will be key to protecting those traveling for exploration, business or pleasure, active measurement of doses and exposure conditions will also be key to individual radiation protection and assessment of the potential individual risks (Durante and Cucinotta Citation2011). Chromosome aberrations including FISH are well established for assessment of dose to astronaut doses (e.g. George et al. Citation2013). While space travel in low earth orbit was not found to increase intrachromasomal exchanges (Horstmann et al. Citation2005), complex rearrangements are associated with heavy ion exposure (Durante Citation2004). Further, while it is well known that astronauts are willing to accept the greater degree of general risk associated with space travel in view of the greater rewards, sufficient risk mediation is still a high priority (Patel et al. Citation2020). Further, on return to Earth, astronauts will wish to return to a normal life, with a normal level of risk, however there is evidence that space travel does increase risks of a number of different health effects in later life, not least cancer (Hamm et al. Citation1998; Edmondson et al. Citation2020) but also other effects, for example on the immune, respiratory, endocrine, visual, cardiovascular and central nervous systems. Further work is clearly needed to ensure the risks are fully understood before space travel can become more common (Jandial et al. Citation2018; Walsh et al. Citation2021). The potential for biomarkers to help develop personalized pharmacological interventions to support astronauts post mission is already being explored (Gertz et al. Citation2020).
The rapid, on-site technologies as outlined in the above sections, can be adapted for space travel to include details of exposure characteristics too. For example, Sridharan et al. (Citation2020), recently demonstrated that three proteins known to be phosphorylated in the presence of DNA double strand breaks, indicated highly variable kinetics of repair depending on the ion, energy, fluence and time point, following exposure to radiation from a variety of different ions of a range of LETs. This information not only demonstrates the potential of these assays to inform exposure conditions, but also the potential to identify interindividual responses, which will be key to knowing how to protect individual space travelers. Indeed, a ‘Star Trek’ style ‘tricorder’ is already in development (FFMD Citation2021). Then, incorporation of biomarkers into such a system, perhaps also making use of cell free DNA or exosomes as recently trialed by Bezdan et al. (Citation2020), does not seem so far away.
Conclusions
There are a large number of active areas of biodosimetry and biomarker research. Of the most recent technological developments, Sproull et al. (Citation2017) recently identified techniques relying on genomics (Cruz-Garcia et al. Citation2020), proteomics (Balog et al. Citation2020) or EPR (Umakoshi et al. Citation2017), as most promising due to the existence of field deployable prototypes utilizing these technologies. Metabolomics (Pannkuk et al. Citation2017) were also highlighted as being promising on the basis of the current ability to use plasma citrulline for evaluation of radiation-induced damage in the gastrointestinal system (Onal et al. Citation2011). As discussed above, however, multiparametric assessment of a variety of different biomarkers will be necessary to provide the sensitivity and specificity required to cover the full range of different potential exposure scenarios (Jaworska et al. Citation2015; Wojcik et al. Citation2017).
It is also essential that future research also focuses on development of biomarkers suitable for assessment of mixed field radiation exposures as well as internal contamination, as these remain areas in which current knowledge is limited (Giussani et al. Citation2020).
In terms of radiation emergency response, it is highly likely that in the near future, rapid, field deployable technologies capable of sampling blood or other tissues and providing triage dose estimates or categorization (e.g. < or > = 2 Gy) will be validated and incorporated into emergency response plans. A variety of rapid, automated techniques based on a mixture of cytogenetic, genetic, proteomic, metabolomic changes as discussed above, will then be employed off site for more detailed dose and exposure scenario characterization, with international networking for large scale responses as required. Such developments will also lead to more accurate techniques for personalized radiation dose and risk estimation.
Transcriptional changes have clearly been identified as the most promising to address the open questions in low dose radiation epidemiology, and this area continues to be the priority for research here – in parallel with development and/or application of advanced artificial intelligence based computational techniques, which will be essential in order to analyze the increasing amounts of data produced. All of the above will also potentially help underpin personalized radiation protection for individuals traveling to space for exploration, business or pleasure.
While this manuscript has been prepared specifically for the special issue celebrating the work of women scientists in the field of radiation biology, the work of women has not been specifically highlighted here, rather, the contributions of the many brilliant women who have been active in the field of biodosimetry should be evident from the publications referenced herein. However, readers may find interesting the fact that almost all of the regional and international biodosimetry leads do happen to currently be female (Kulka et al. Citation2018). Ultimately, of course, men and women will continue to work together to contribute to all of the areas outlined in this paper. Watch this space as the exciting developments unfold!
Acknowledgements
The authors of this work have been partly supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Chemical & Radiation Threats & Hazards, a partnership between PHE and Imperial College London. The views expressed are those of the authors and not necessarily those of the NIHR, PHE or the Department of Health and Social Care.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
Notes on contributors
Elizabeth A. Ainsbury
Elizabeth A. Ainsbury, PhD, MP is Senior Scientific Group Leader of the Cytogenetics and Pathology Group within the Radiation Effects Department of Public Health England’s Centre for Radiation, Chemical and Environmental Hazards, Oxfordshire, UK, which houses the UK’s Cytogenetic Dosimetry Service, and a Visiting Researcher at the Environmental Research Group within the School of Public Health, Faculty of Medicine at Imperial College of Science, Technology and Medicine, London, UK.
Jayne Moquet
Jayne Moquet is a Principal Radiation Protection Scientist of the Cytogenetics and Pathology Group within the Radiation Effects Department of Public Health England’s Centre for Radiation, Chemical and Environmental Hazards, Oxfordshire, UK.
Mingzhu Sun
Mingzhu Sun is a Higher Radiation Protection Scientist of the Cytogenetics and Pathology Group within the Radiation Effects Department of Public Health England’s Centre for Radiation, Chemical and Environmental Hazards, Oxfordshire, UK.
Stephen Barnard
Stephen Barnard a Senior Radiation Protection Scientist of the Cytogenetics and Pathology Group within the Radiation Effects Department of Public Health England’s Centre for Radiation, Chemical and Environmental Hazards, Oxfordshire, UK.
Michele Ellender
Michele Ellender is a Principal Radiation Protection Scientist of the Cytogenetics and Pathology Group within the Radiation Effects Department of Public Health England’s Centre for Radiation, Chemical and Environmental Hazards, Oxfordshire, UK.
David Lloyd
David Lloyd is a Visiting Worker of the Cytogenetics and Pathology Group within the Radiation Effects Department of Public Health England’s Centre for Radiation, Chemical and Environmental Hazards, Oxfordshire, UK.
References
- Balajee AS, Hande MP. 2018. History and evolution of cytogenetic techniques: current and future applications in basic and clinical research. Mutat Res Genet Toxicol Environ Mutagen. 836(Pt A):3–12.
- Balog RP, Bacher R, Chang P, Greenstein M, Jammalamadaka S, Javitz H, Knox SJ, Lee S, Lin H, Shaler T, Shura L, et al. 2020. Development of a biodosimeter for radiation triage using novel blood protein biomarker panels in humans and non-human primates. Int J Radiat Biol. 96(1):22–34.
- Barquinero JF, Beinke C, Borràs M, Buraczewska I, Darroudi F, Gregoire E, Hristova R, Kulka U, Lindholm C, Moreno M, et al. 2017. RENEB biodosimetry intercomparison analyzing translocations by FISH. Int J Radiat Biol. 93(1):30–35.
- Bezdan D, Grigorev K, Meydan C, Pelissier Vatter FA, Cioffi M, Rao V, MacKay M, Nakahira K, Burnham P, Afshinnekoo E, et al. 2020. Cell-free DNA (cfDNA) and exosome profiling from a year-long human spaceflight reveals circulating biomarkers. iScience. 23(12):10184425.
- Bucher M, Endesfelder D, Roessler U, Borkhardt A, Dückers G, Kirlum HJ, Lankisch P, Oommen PT, Niehues T, Rübe CE, et al. 2021. Analysis of chromosomal aberrations and γH2A.X foci to identify radiation-sensitive ataxia-telangiectasia patients. Mutat Res Genet Toxicol Environ Mutagen. 861-862:503301.
- Cruz-Garcia L, Badie C, Anbalagan S, Moquet J, Gothard L, O'Brien G, Somaiah N, Ainsbury EA. 2021. An ionising radiation-induced specific transcriptional signature of inflammation-associated genes in whole blood from radiotherapy patients: a pilot study. Radiat Oncol. 16(1):83.
- Cruz-Garcia L, O'Brien G, Donovan E, Gothard L, Boyle S, Laval A, Testard I, Ponge L, Woźniak G, Miszczyk L, et al. 2018. Influence of confounding factors on radiation dose estimation using in vivo validated transcriptional biomarkers. Health Phys. 115(1):90–101.
- Cruz-Garcia L, O'Brien G, Sipos B, Mayes S, Love MI, Turner DJ, Badie C. 2020. Generation of a transcriptional radiation exposure signature in human blood using long-read nanopore sequencing. Radiat Res. 193(2):143–154.
- Cucinotta FA, To K, Cacao E. 2017. Predictions of space radiation fatality risk for exploration missions. Life Sci Space Res (Amst). 13:1–11.
- del Rosario Pérez M, Carr Z, Rojas-Palma C, van der Meer K, Smith K, Rahola T, Muikku M, Liland A, Jaworska A, Jerstad A. 2010. A new handbook on triage, monitoring and treatment of people following malevolent use of radiation. Health Phys. 98(6):898–902.
- Depuydt J, Baeyens A, Barnard S, Beinke C, Benedek A, Beukes P, Buraczewska I, Darroudi F, De Sanctis S, Dominguez I, et al. 2017. RENEB intercomparison exercises analyzing micronuclei (Cytokinesis-block Micronucleus Assay). Int J Radiat Biol. 93(1):36–47.
- Durante M. 2004. Heavy ion radiobiology for hadrontherapy and space radiation protection. Radiother Oncol. 73(Suppl 20):S158–S160.
- Durante M, Cucinotta F. 2011. Physical basis of radiation protection in space travel. Rev Mod Phys. 83(4):1245–1281.
- Edmondson EF, Gatti DM, Ray FA, Garcia EL, Fallgren CM, Kamstock DA, Weil MM. 2020. Genomic mapping in outbred mice reveals overlap in genetic susceptibility for HZE ion- and γ-ray-induced tumors. Sci Adv. 6(16):eaax5940.
- [FFMD] Final Frontier Medical Devices 2021. [accessed 2021 Apr 04]. https://space.xprize.org/prizes/tricorder/teams/final_frontier_medical_devices.
- George K, Rhone J, Beitman A, Cucinotta FA. 2013. Cytogenetic damage in the blood lymphocytes of astronauts: effects of repeat long-duration space missions. Mutat Res. 756(1-2):165–169.
- Gertz ML, Chin CR, Tomoiaga D, MacKay M, Chang C, Butler D, Afshinnekoo E, Bezdan D, Schmidt MA, Mozsary C, et al. 2020. Multi-omic, single-cell, and biochemical profiles of astronauts guide pharmacological strategies for returning to gravity. Cell Rep. 33(10):108429.
- Giussani A, Lopez MA, Romm H, Testa A, Ainsbury EA, Degteva M, Della Monaca S, Etherington G, Fattibene P, Güclu I, et al. 2020. Eurados review of retrospective dosimetry techniques for internal exposures to ionising radiation and their applications. Radiat Environ Biophys. 59(3):357–387.
- Guiot J, Vaidyanathan A, Deprez L, Zerka F, Danthine D, Frix AN, Lambin P, Bottari F, Tsoutzidis N, Miraglio B, et al. 2021. A review in radiomics: making personalized medicine a reality via routine imaging. Med Res Rev. doi:https://doi.org/10.1002/med.21846.
- Hall J, Jeggo PA, West C, Gomolka M, Quintens R, Badie C, Laurent O, Aerts A, Anastasov N, Azimzadeh O, et al. 2017. Ionizing radiation biomarkers in epidemiological studies - An update. Mutat Res. 771:59–84.
- Hamm PB, Billica RD, Johnson GS, Wear ML, Pool SL. 1998. Risk of cancer mortality among the Longitudinal Study of Astronaut Health (LSAH) participants. Aviat Space Environ Med. 69(2):142–144.
- Horstmann M, Durante M, Johannes C, Pieper R, Obe G. 2005. Space radiation does not induce a significant increase of intrachromosomal exchanges in astronauts' lymphocytes. Radiat Environ Biophys. 44(3):219–224.
- Horvath S, Raj K. 2018. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 19(6):371–384.
- [IAEA] International Atomic Energy Agency 2011. Cytogenetic dosimetry: applications in preparedness for and response to radiation Emergencies. Vienna (AT): EPR-Biodosimetry Series; [accessed 2021 mar 22]. https://www.iaea.org/publications/8735/cytogenetic-dosimetry-applications-in-preparedness-for-and-response-to-radiation-emergencies.
- Imamura K, Yada Y, Izumi Y, Morita M, Kawata A, Arisato T, Nagahashi A, Enami T, Tsukita K, Kawakami H, et al. 2021. Prediction model of amyotrophic lateral sclerosis by deep learning with patient induced pluripotent stem cells. Ann Neurol. 89(6):1226–1233.
- Jandial R, Hoshide R, Waters JD, Limoli CL. 2018. Space-brain: the negative effects of space exposure on the central nervous system. Surg Neurol Int. 9:9.
- Jang S, Shin SG, Lee MJ, Han S, Choi CH, Kim S, Cho WS, Kim SH, Kang YR, Jo W, et al. 2021. Feasibility study on automatic interpretation of radiation dose using deep learning technique for dicentric chromosome assay. Radiat Res. 195(2):163–172.
- Jaworska A, Ainsbury EA, Fattibene P, Lindholm C, Oestreicher U, Rothkamm K, Romm H, Thierens H, Trompier F, Voisin P, et al. 2015. Operational guidance for radiation emergency response organisations in Europe for using biodosimetric tools developed in EU MULTIBIODOSE project. Radiat Prot Dosimetry. 164(1-2):165–169.
- Koelsche C, Schrimpf D, Stichel D, Sill M, Sahm F, Reuss DE, Blattner M, Worst B, Heilig CE, Beck K, et al. 2021. Sarcoma classification by DNA methylation profiling. Nat Commun. 12(1):498.
- Kulka U, Wojcik A. 2017. Special issue: Networking in biological and EPR/OSL dosimetry: the European RENEB platform for emergency preparedness and research. Int J Radiat Biol. 93(1):1.
- Kulka U, Wojcik A, Di Giorgio M, Wilkins R, Suto Y, Jang S, Quing-Jie L, Jiaxiang L, Ainsbury E, Woda C, et al. 2018. Biodosimetry and biodosimetry networks for managing radiation emergency. Radiat Prot Dosimetry. 182(1):128–138.
- Lowe D, Horvath S, Raj K. 2016. Epigenetic clock analyses of cellular senescence and ageing. Oncotarget. 7(8):8524–8531.
- Luxton JJ, McKenna MJ, Lewis AM, Taylor LE, Jhavar SG, Swanson GP, Bailey SM. 2021. Telomere length dynamics and chromosomal instability for predicting individual radiosensitivity and risk via machine learning. J Pers Med. 11(3):188.
- Marcos-Zambrano LJ, Karaduzovic-Hadziabdic K, Loncar Turukalo T, Przymus P, Trajkovik V, Aasmets O, Berland M, Gruca A, Hasic J, Hron K, et al. 2021. Applications of machine learning in human microbiome studies: a review on feature selection, biomarker identification, disease prediction and treatment. Front Microbiol. 12:634511.
- May JM, Bylicky M, Chopra S, Coleman CN, Aryankalayil MJ. 2021. Long and short non-coding RNA and radiation response: a review. Transl Res. 233:162–179.
- Moquet J, Barnard S, Staynova A, Lindholm C, Monteiro Gil O, Martins V, Rößler U, Vral A, Vandevoorde C, Wojewódzka M, et al. 2017. The second gamma-H2AX assay inter-comparison exercise carried out in the framework of the European biodosimetry network (RENEB). Int J Radiat Biol. 93(1):58–64.
- Moquet J, Higueras M, Donovan E, Boyle S, Barnard S, Bricknell C, Sun M, Gothard L, O'Brien G, Cruz-Garcia L, et al. 2018. Dicentric dose estimates for patients undergoing radiotherapy in the RTgene study to assess blood dosimetric models and the new Bayesian method for gradient exposure. Radiat Res. 190(6):596–604.
- [NIST] National Institute of Standards and Technologies, U.S. Department of Commerce 2021. NIST on a chip: Emergency Dosimetry; [accessed 2021 Apr 04]. https://www.nist.gov/noac/technology/radiation/emergency-dosimetry.
- Oestreicher U, Samaga D, Ainsbury E, Antunes AC, Baeyens A, Barrios L, Beinke C, Beukes P, Blakely WF, Cucu A, et al. 2017. RENEB intercomparisons applying the conventional Dicentric Chromosome Assay (DCA). Int J Radiat Biol. 93(1):20–29.
- Onal C, Kotek A, Unal B, Arslan G, Yavuz A, Topkan E, Yavuz M. 2011. Plasma citrulline levels predict intestinal toxicity in patients treated with pelvic radiotherapy. Acta Oncol. 50(8):1167–1174.
- Pannkuk EL, Fornace AJ, Jr, Laiakis EC. 2017. Metabolomic applications in radiation biodosimetry: exploring radiation effects through small molecules. Int J Radiat Biol. 93(10):1151–1176.
- Patel ZS, Brunstetter TJ, Tarver WJ, Whitmire AM, Zwart SR, Smith SM, Huff JL. 2020. Red risks for a journey to the red planet: the highest priority human health risks for a mission to Mars. NPJ Microgravity. 6(1):33.
- Pernot E, Hall J, Baatout S, Benotmane MA, Blanchardon E, Bouffler S, El Saghire H, Gomolka M, Guertler A, Harms-Ringdahl M, et al. 2012. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat Res. 751(2):258–286.
- Port M, Majewski M, Abend M. 2019. Radiation dose is of limited clinical usefulness in persons with acute radiation syndrome. Radiat Prot Dosimetry. 186(1):126–129.
- Rea ME, Gougelet RM, Nicolalde RJ, Geiling JA, Swartz HM. 2010. Proposed triage categories for large-scale radiation incidents using high-accuracy biodosimetry methods. Health Phys. 98(2):136–144.
- Repin M, Pampou S, Brenner DJ, Garty G. 2020. The use of a centrifuge-free RABiT-II system for high-throughput micronucleus analysis. J Radiat Res. 61(1):68–72.
- Royba E, Repin M, Balajee AS, Shuryak I, Pampou S, Karan C, Brenner DJ, Garty G. 2020. The RABiT-II DCA in the rhesus macaque model. Radiat Res. doi: https://doi.org/10.1667/RR15547.1.
- Royba E, Repin M, Pampou S, Karan C, Brenner DJ, Garty G. 2019. RABiT-II-DCA: a fully-automated dicentric chromosome assay in multiwell plates. Radiat Res. 192(3):311–323.
- Sehl ME, Carroll JE, Horvath S, Bower JE. 2020. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer. 6:2323.
- Shirley BC, Knoll JHM, Moquet J, Ainsbury E, Pham ND, Norton F, Wilkins RC, Rogan PK. 2020. Estimating partial-body ionizing radiation exposure by automated cytogenetic biodosimetry. Int J Radiat Biol. 96(11):1492–1503.
- Simon SL, Bouville A. 2016. Long-term biodosimetry redux. Radiat Prot Dosimetry. 172(1-3):244–247.
- Sotnik NV, Azizova TV, Darroudi F, Ainsbury EA, Moquet JE, Fomina J, Lloyd DC, Hone PA, Edwards AA. 2015. Verification by the FISH translocation assay of historic doses to Mayak workers from external gamma radiation. Radiat Environ Biophys. 54(4):445–451.
- Sproull MT, Camphausen KA, Koblentz GD. 2017. Biodosimetry: a future tool for medical management of radiological emergencies. Health Secur. 15(6):599–610.
- Sridharan DM, Chien LC, Cucinotta FA, Pluth JM. 2020. Comparison of signaling profiles in the low dose range following low and high LET radiation. Life Sci Space Res (Amst). 25:28–41.
- Sun M, Moquet JE, Barnard S, Lloyd DC, Rothkamm K, Ainsbury EA. 2016. Doses in Radiation Accidents Investigated by Chromosomal Aberration Analysis XXV. London (UK): PHE CRCE 025; [accessed 2021 Mar 22]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/515260/PHE-CRCE-025.pdf.
- Swarts SG, Sidabras JW, Grinberg O, Tipikin DS, Kmiec MM, Petryakov SV, Schreiber W, Wood VA, Williams BB, Flood AB, et al. 2018. Developments in biodosimetry methods for triage with a focus on x-band electron paramagnetic resonance in vivo fingernail dosimetry. Health Phys. 115(1):140–150.
- Swartz HM, Flood AB, Singh VK, Swarts SG. 2020. Scientific and logistical considerations when screening for radiation risks by using biodosimetry based on biological effects of radiation rather than dose: the need for prior measurements of homogeneity and distribution of dose. Health Phys. 119(1):72–82.
- Terzoudi GI, Pantelias G, Darroudi F, Barszczewska K, Buraczewska I, Depuydt J, Georgieva D, Hadjidekova V, Hatzi VI, Karachristou I, et al. 2017. Dose assessment intercomparisons within the RENEB network using G0-lymphocyte prematurely condensed chromosomes (PCC assay). Int J Radiat Biol. 93(1):48–57.
- Tichy A, Kabacik S, O’Brien G, Pejchal J, Sinkorova Z, Kmochova A, Sirak I, Malkova A, Beltran CG, Gonzalez JR, et al. 2018. The first in vivo multiparametric comparison of different radiation exposure biomarkers in human blood. PLoS One. 2313(2):e0193412.
- Umakoshi M, Yamaguchi I, Hirata H, Kunugita N, Williams BB, Swartz HM, Miyake M. 2017. In vivo electron paramagnetic resonance tooth dosimetry: dependence of radiation-induced signal amplitude on the enamel thickness and surface area of ex vivo human teeth. Health Phys. 113(4):262–270.
- Walsh L, Hafner L, Straube U, Ulanowski A, Fogtman A, Durante M, Weerts G, Schneider U. 2021. A bespoke health risk assessment methodology for the radiation protection of astronauts. Radiat Environ Biophys. 60(2):213–231.
- Wathen LK, Eder PS, Horwith G, Wallace RL. 2020. Using biodosimetry to enhance the public health response to a nuclear incident. Int J Radiat Biol. 21:1–4.
- Wojcik A, Oestreicher U, Barrios L, Vral A, Terzoudi G, Ainsbury E, Rothkamm K, Trompier F, Kulka U. 2017. The RENEB operational basis: complement of established biodosimetric assays. Int J Radiat Biol. 93(1):15–19.
