Abstract
A reemergence of research implementing radiation delivery at ultra-high dose rates (UHDRs) has triggered intense interest in the radiation sciences and has opened a new field of investigation in radiobiology. Much of the promise of UHDR irradiation involves the FLASH effect, an in vivo biological response observed to maintain anti-tumor efficacy without the normal tissue complications associated with standard dose rates. The FLASH effect has been validated primarily, using intermediate energy electron beams able to deliver high doses (>7 Gy) in a very short period of time (<200 ms), but has also been found with photon and proton beams. The clinical implications of this new area of research are highly significant, as FLASH radiotherapy (FLASH-RT) has the potential to enhance the therapeutic index, opening new possibilities for eradicating radio-resistant tumors without toxicity. As pioneers in this field, our group has developed a multidisciplinary research team focused on investigating the mechanisms and clinical translation of the FLASH effect. Here, we review the field of UHDR, from the physico-chemical to the biological mechanisms.
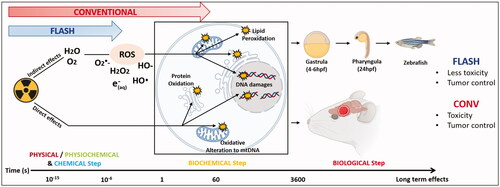
Graphical Abstract
Introduction
Today, radiation therapy is used in combination with other therapies to treat over 50% of cancer patients. Radiotherapy remains highly efficient at eradicate cancer cells and despite significant technological improvements related to tumor targeting by image-guidance, the dose required for tumor control is still limited by normal tissue toxicity. In addition, some tumors remain highly radio resistant, escaping modern treatment plans to recur and metastasize. Therefore, one major challenge in our field is to develop novel radiotherapy strategies to overcome these limitations.
In this context, our team conceptualized and developed FLASH radiotherapy (FLASH-RT), an innovative and transforming approach in the field of radiation oncology based on dose delivery at ultra-high dose rate (UHDR), typically over 1000 times higher than irradiation at conventional dose rates (CONV). The main interest of FLASH-RT lies on its differential impact on tumors vs. normal tissue, where at a given cytotoxic isodose for tumors, FLASH-RT spares normal tissue, an effect our group has coined as the FLASH effect. We have since confirmed the FLASH effect in several experimental animal models (mice, rat, zebrafish, pig, cats) and multiple organs (lung, skin, gut, brain) (reviews in Bourhis et al. Citation2019a, Citation2019b; Vozenin et al. Citation2019). The FLASH effect has now been reproduced by many other groups and will be reviewed in section ‘Biological steps after irradiation at conventional and ultra-high dose rate’.
Back in the 1970s, description of normal tissue protection triggered by UHDR irradiation was reported in the intestine and skin (Hornsey and Bewley Citation1971; Field and Bewley Citation1974; Hendry et al. Citation1982). Unfortunately at the time, most investigations in normal and tumor cells were performed in vitro and no differential effects were reported, leading to an abandonment of research using UHDR irradiation. It took more than four decades until we rediscovered FLASH-RT. In addition to its radiobiological advantages, several clinical benefits may be derived from such a technology. The very short exposure time provides the capability to deliver FLASH-RT using a few, relatively large fractions (hypofractionation), thereby reducing the number of treatment sessions, reducing the number of clinical visits, as well as reducing workload and cost. In addition, organ or tumor motion is minimized, thereby resolving a long-standing and confounding factor that still limits the precision of image-guided radiotherapy.
Today, development of FLASH-RT requires the contribution of a multidisciplinary team composed of physicists, chemists, biologists and clinicians. Definition of the physical parameters capable to produce the FLASH effect in biological tissue is needed and one major challenge of this research is related to accurate and online dosimetry measurements (recently reviewed by Schüller et al. Citation2020). More importantly, only a few devices are able to deliver an UHDR irradiation across a large volume of tissue (review in Mazal et al. Citation2020; Wilson et al. Citation2020; Med Phys special issue 2021) and therefore major technological improvements are still needed to upgrade current devices to safely and accurately deliver UHDR irradiation in the clinic. Although these physics related aspects are crucial for clinical translation of FLASH-RT, they have been discussed recently in a special issue of Med Phys (2021) and are beyond the scope of the present article dedicated to the mechanistic understanding of the FLASH effect.
Physico-chemical steps after irradiation at conventional and ultra-high dose rate
The main difference between irradiation at CONV dose rate and UHDR is related to the time of exposure and must impact initial radiation chemistry as summarized in . We thought that understanding differences in reactions that might occur (or not) at early chemical steps after exposure of carefully selected model systems to radiation would enable us to identify the primary events of the FLASH effect.
Figure 1. Physico-chemical effects after water exposure to UHDR and CONV. The graph summarizes the early interaction of ionizing radiation with matter that are chemical changes. They have been extensively investigated in the past and are generally described in liquid water, which happens to be a major component of living organisms. Water molecules absorb the majority of the ionizing energy in a process called water radiolysis. The first physical phase is initiated at the time ‘0’ at which the energy is deposited within the target and occurs during the first femtoseconds post-irradiation. Then after picosecond and millisecond, formation of free radicals including reactive oxygen species (ROS) occurs via reactions that form the chemical non-homogenous and homogeneous phases. Experimentally, G°-value can be determined to evaluate the initial yields of reactive species produced during the homogenous stage of chemistry (100 ns to 1 µs). ROS continue to diffuse and interact with soluble substances (like oxygen) at later time points and can then be evaluated as G-values, also named radiolytic yields. Experiments done in water have shown that UHDR produces less H2O2 than conventional irradiation.

During the physical stage, energetic particles interact with water within a femtosecond. The absorbed energy takes two pathways: electronic excitation (H2O*): the transfer of an electron from a fundamental state to the exited state. Ionization (H2O·+), where one electron (e–) is removed from the water molecule (Nikjoo et al. Citation1998). In water, a strong polar solvent, the geminate recombination of electrons with their positive parent cation is less favored; they become separated within 10−15 s. The resultant ionized and excited molecules are highly unstable and dissipate excess energy in the form of energy transfer to neighboring molecules. This physicochemical stage occurs between 10−15 and 10−12 s and surprisingly, is not well characterized experimentally in liquid phase. However, by analogy with the behavior of water molecules in gas phase under photolysis, three steps of de-excitation events were proposed: proton transfer to a neighboring molecule, dissociation of excited water molecule and electron thermalization and solvation. Proton transfer from the positive radical water ion H2O·+ to neighboring water molecule is an important event since it leads to the production of the hydroxyl (HO•) radical. The electronically excited molecules, H2O* can return to their fundamental state without dissociation by heat loss. Alternatively, higher excitation states can dissipate energy by emitting an electron, thus becoming an ion and dissociate into hydrogen and hydroxyl radicals. Another dissociation pathways can lead to formation of dihydrogen and oxygen in a singlet state O•(1D). Singlet oxygen is not stable, and reacts in a third step with water to form molecular hydrogen (H2) and two hydroxyl radicals (2HO•) or hydrogen peroxide (H2O2). The formation of oxygen in a triplet state (O3P) was also observed but in very low yield (Auclair Citation2001). The dissociation reactions take place in ∼10−13 s, which is on the same scale as the vibrational state of a water molecule (10−13 to 10−14 s).
The electrons emitted in the medium, depending on their kinetic energy, migrate either further and form secondary ionizations themselves or lose energy in multiple steps (Spotheim-Maurizot Citation2008). First, by vibrational and rotational relaxation then becoming thermalized in 10−12 s. Thermalized electrons orient the dipole moments of neighboring water molecules, forming a ‘cage’, and are referred to as aqueous electrons e–(aq).
For low LET radiations, such as accelerated electrons, end products of the physico-chemical stage (H•, e-(aq), HO•, H2, H2O2) are clustered together in small widely separated spurs. On average, one spur can contain two or three ion pairs (Baldacchino et al. Citation2019). Next, the non-homogeneous chemical stage starts between 10−12 and 10−6 s. Radical species diffuse and react with each other at different rates. Eventually, certain radical products encounter others from different spurs and form new radical species. While, some escape the spur to the bulk liquid and become homogeneously distributed with the rest of the radical species. Over 10−7 to 10−6 s, molecular yields are computed for photons, energetic electrons and ions, and are termed G-values, classically referred to as ‘primary yields’. G-values have been measured by pulse radiolysis or through scavenging methods (Hiroki et al. Citation2002; Wasselin-Trupin et al. Citation2002) and correspond to the yields of formation at the start of the homogeneous stage (i.e. not at time ‘0’). They are expressed in mol J−1 or in molecule/100 eV in the original literature. In summary, a G-value of a given species is given by the relationship between the dose D and the concentration C in units of mol dm−3 with a density correction ρ: C=ρ×D×G. Noteworthy too is that G-values are usually measured in deaerated samples to remove any oxygen contribution, known to have a significant impact on free radicals reactions due to its ultra-high reactivity for unpaired electrons.
In the case of CONV-RT, the result of the competition between recombination and diffusion strongly depends on how the energy deposition is done. It means that the initial distribution of ionizations in space, which is reflected by the LET, will dictate whether more or less radicals will escape the spurs, and alter the yields of molecular products on the scale of 10−7 s. The question is what will happen in the case of exposure to UHDR? Under this scenario, a recombination event can be expected to transpire within a time scale that is short enough to interfere with early chemical reactions, when radicals form and disappear. For example, with the eRT6/Oriatron (PMB, Peynier, France), energetic electrons with an LET = 0.2 keV μm−1 are deposited within a 1.8 µs pulse (Jaccard et al. Citation2018) (when the dose is applied in a single pulse) or in milliseconds (if multiple pulses are required). This time frame is relatively long for early chemical reactions and does not support the occurrence of radical–radical recombination, as pulse delivery extends to the end of the homogenous phase of chemistry between 100 ns–1 µs. At this stage, radical–radical interactions have taken place and free radicals begin to diffuse. Thus, recombination reactions are unlikely to explain a potential radiochemical basis of the FLASH effect.
In summary, the physicochemical cascade activated after UHDR and CONV dose rate needs to be carefully studied in order to determine whether initial radiolytic yield could be or not involved in the subsequent biological FLASH effect. It is important to explore furthermore other sources like protons where radiochemical processes can differ regarding the particle type, energy and LET. A radiation chemistry study describing the effect or not of dose rate in other sources is relevant especially that in literature such studies have not been done before. This would provide a guide/link to explain or not downstream biological effects observed after UHDR. Although, one should note that such studies require rigorous experimental conditions in order to have solid results and would be able then to compare with literature. Among the relevant parameters, the role of oxygen tension in mediating a certain fraction of the FLASH effect has come under intense scrutiny. The impact of oxygen in mediating the FLASH effect was first proposed based on the observation that hyperoxygenation was able to reverse normal tissue protection in the brain (Montay-Gruel Citation2019). Since then, numerous theoretical models were published, all based on calculations focused on FLASH-induced oxygen depletion (Pratx and Kapp Citation2019; Hu et al. Citation2020; Labarbe et al. Citation2020; Petersson et al. Citation2020; Zhou et al. Citation2020). Interestingly, recent experimental work done by direct oxygen measurements after CONV vs. UHDR irradiation refutes this depletion theory (Cao et al. Citation2021; Jansen et al. Citation2021) and shows that UHDR is not likely to deplete sufficient oxygen in tissues to elicit a transient state of hypoxic radioprotection. Nonetheless, FLASH may alter the yields of other free radicals and downstream processes to impact later biological responses (Montay-Gruel et al. Citation2019). Clearly, a major limitation in the field, is the lack of direct control of tissue oxygenation status, a complex biological process that is difficult to model ex vivo. The physicochemical cascade summarized in needs to be carefully studied in order to determine whether initial radiolytic yield could be or not involved in the subsequent biological FLASH effect.
DNA damage after irradiation at conventional and ultra-high dose rate
In living organisms, irradiation triggers the direct ionization of biomolecule (nucleic acids, lipids, proteins) and the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that can alter molecules essential for life. Oxidative reactions in the presence of oxygen predominate in the irradiated cell and are driven by the most reactive radical, the hydroxyl radical (von Sonntag Citation1991). Interactions of HO• with proteins can change signaling, gene expression, and protein half-lives. The action of hydroxyl radicals on lipids results in peroxidation chain reactions. Although these damages have the potential to disrupt cell metabolism and proliferation, irreparable damage to DNA in the form of DSB is seen as the primary determinant of cell survival.
The action of HO• on DNA is primarily mediated through the abstraction of a hydrogen atom from a 2-deoxyribose moiety exposed to solvent (more often at C4′ position), resulting in a frank single strand break (SSB) or the formation of an abasic site (Pogozelski and Tullius Citation1998). The hydroxyl radical can also oxidize a nucleoside, with no preference for purines or pyrimidines, forming a variety of products that are generally stable but also able, in some cases, to induce the loss of the base or an SSB. Hydrogen radicals and aqueous electrons also react with nucleobases in reductive reactions, mostly in anaerobic conditions and especially with pyrimidines, but their actions on the sugar-phosphate backbone are less important. Direct effects act most likely by ionization of the phosphate group (von Sonntag Citation1991). Double-strand breaks (DSBs) are then the consequence of either a radical transfer from a cleaved strand to the complementary strand or the action of multiple hydroxyl radicals in spatially defined regions (spurs, blobs) at track ends that result in locally multiply damaged sites or ‘clustered’ damage (Ward Citation1985, Citation1981; Siddiqi and Bothe Citation1987; Krisch et al. Citation1991). Non-DSB clusters, defined by the presence of multiple damages (SSB, abasic sites, or nucleoside lesions) concentrated within a turn of the DNA helix, can be lethal as well (Ward Citation1985; Shikazono et al. Citation2009).
The appearance and type of DNA damage are related to the concentration and diffusion velocity of selective reactive species, i.e. the competition between reactions with DNA, with oxygen and recombination at track ends. DNA damage varies with the LET of the incident radiation. Increasing the LET leads to clusters with higher levels of damage multiplicity or complexity (Georgakilas et al. Citation2013). The modification of reaction equilibrium in the presence of specific scavengers also influences the rate and type of strand breaks. In that respect, the recombination of HO• with the aqueous electron is an important reaction. Interestingly, in the presence of oxygen, its combination with the products of water radiolysis causes its depletion. More specifically, oxygen acts as a potent scavenger of aqueous electrons to yield superoxide radicals. A model defining the distribution of DNA damage attributable to different reactive species with respect to the partial pressure of oxygen in water has been proposed (Barilla and Lokajíček Citation2000). Oxygen is also a potent radiosensitizer, able to fix radiolytic lesions that inhibits their efficient repair (so called oxygen fixation hypothesis).
DNA damage study with plasmids
Ongoing experiments aim to assess the direct and indirect effects of UHDR vs. conventional dose rate on DNA damage by systematically varying parameters such as beam structure or the biochemical environment. Between oligonucleotides and cellular DNA, a widespread model for the study of DNA damage in the absence of DNA repair involves isolated plasmid DNA. From bacterial preparations, the supercoiled plasmid conformation changes after the induction of an SSB or DSB to an open circular or linear form, respectively. Quantification of the conversion of supercoiled DNA to these topologically distinct conformations after irradiation can be accomplished by standard agarose gel electrophoresis (). However, at higher doses the fragmentation of the plasmid by damage multiplicity requires more sensitive methodologies, such as atomic force microscopy (Pachnerová Brabcová et al. Citation2019). Plasmid irradiations have been widely used to understand the effects of different parameters on DNA damage, such as the type of radiation, the ratio of direct to indirect effects, the effect of supercoiling density, or the physicochemical mechanisms delineated by the presence of radiosensitizers or radioprotectors. However, regarding the dose rate dependence of strand breaks yields, only one recent study aimed to assess the relative biological effect using very high energy electron and UHDR (Small et al. Citation2021). Under atmospheric conditions, no significant dependence of DSB on dose rate was observed. The addition of base excision repair enzymes increases the sensitivity of such approaches (Sutherland et al. Citation2001). Mathematical models also provide for the capability to compute strand break yields of selective plasmid conformations as a function of dose (Cowan et al. Citation1987; McMahon and Currell Citation2011). However, these models are insensitive to the time signatures used in UHDR, and results obtained should be interpreted with these caveats in mind (Moeckli et al. Citation2020).
Figure 2. Plasmid DNA as a tool to study DNA damage after CONV vs. UHDR irradiation. The induction of SSB and DSB after irradiation of a supercoiled plasmid releases mechanical constraints in the molecule and causes its spatial conformation to change. These isomers (circular (relaxed) or linear form after SSB or DSB, respectively) have different migration speeds in a gel matrix and are therefore easily separated and quantified by a simple agarose gel electrophoresis (AGE). To investigate the mechanisms underlying the FLASH effect, plasmids can be irradiated dry, or in atmospheric, physiologic or hypoxic aqueous solutions, in presence of various radiosensitizers or scavengers. In addition, several beam parameters such as instantaneous dose, dose per pulse, frequency, and total irradiation time can be investigated.

Biological steps after irradiation at conventional and ultra-high dose rate
The beneficial effect of FLASH-RT irradiation, named the ‘FLASH effect’ involves normal tissue sparing at doses known to provide tumor control. The benefits of FLASH-RT have now been described in many organs, in various animal models and from various groups over the world. Despite differences in nature and structure of the radiation beams, the FLASH effect has been validated in preclinical experiments with electron, proton and photon beams operating at dose rate above 40 Gy/s (Montay-Gruel et al. Citation2017, Citation2018; Diffenderfer et al. Citation2020; Cunningham et al. Citation2021). The field is advancing rapidly, with clinical trials in domestic animals (Vozenin et al. Citation2019; Konradsson et al. Citation2021). A feasibility study in a human patient (Bourhis et al. Citation2019b) and a FAST01-trial, concurrent with a wealth of mechanistic studies in multiple model systems.
The FLASH effect in experimental mouse models
Normal lung and tumors
Our group was the first to demonstrate the FLASH effect in the lung (Favaudon et al. Citation2014). Delayed pulmonary fibrosis after exposure to 17 Gy electron at UHDR irradiation was reduced whereas conventionally irradiated C57Bl6 mice developed massive fibrotic lesions. This sparing effect was associated with a reduction of apoptosis in blood vessels and bronchi. At the tumor level, 15 Gy UHDR irradiation was as efficient as CONV-RT in controlling the growth of orthotopic TC-1 tumor cells implanted in the lung. Dose escalation up to 28 Gy was made feasible and resulted in tumor control. Cell repopulation following radiation exposure was then studied in the normal lung (Fouillade et al. Citation2020). As a surrogate for DNA damage and senescence, DNA repair foci (53BP1) were used. Findings revealed that both processes were minimized after 17 Gy electron at UHDR in normal cells in situ. Interestingly, the number of 53BP1 foci was also reduced in vitro in human fibroblast cell lines after 5.2 Gy at UHDR vs. CONV-RT whereas no difference between the two modalities was reported in human lung adenocarcinoma cells, A549. Subsequent RNA sequencing analysis revealed that fibrogenic and proinflammatory gene expression (TGF-β1, Cebpb) was attenuated after UHDR vs. CONV-RT, an effect attributed to the preservation of stem/progenitor cells in the lung. These studies suggest a differential impact of UHDR irradiation at the genomic level which might impact the response in normal and tumor cells.
Normal brain and GBM
The biological FLASH effect has been investigated extensively in the brain, another late responding organ (), using 10 Gy whole brain irradiation. Systematic investigations using behavioral testing as a functional outcome enabled the determination of a dose rate threshold necessary to trigger the FLASH effect at 100 Gy/s when a small volume (1.7 cm diameter) was irradiated (Montay-Gruel et al. Citation2017). Subsequent analyses were performed at higher dose rates (107 Gy/s) which is the maximum achievable dose rate of the eRT6/Oriatron (PMB, Peynier, France) electron beam. Using these UHDRs, neuroinflammation was minimized, neurogenesis and neuronal morphology were preserved after exposure to UHDR while conventional dose rate (0.01 Gy/s) induced persistent structural degradation and apoptosis of hippocampal cells and memory loss (Montay-Gruel et al. Citation2019). Interestingly, carbogen-breathing during UHDR irradiation reversed the neuroprotection. Neuroprotection was further validated at lower dose rates ranging between 200 and 300 Gy/s and with a single fraction of 30 Gy (Simmons et al. Citation2019). Reactive astrogliosis, microglial and C3 complement activation were also found to be reduced following UHDR irradiation (Montay-Gruel et al. Citation2020), along with a preservation of vascular integrity and the blood–brain barrier (Allen et al. Citation2020) (), data that provided new physiopathological insights. More recently, with the goal to overcome radiation-induced toxicities of pediatric patient stricken with medulloblastoma and to improve their long-term quality of life, the effect of UHDR has been evaluated using juvenile mice. Memory loss, anxiety-like behaviors and the neurogenic niche were spared after whole brain irradiation at 8 Gy using electron at UHDR (Alaghband et al. Citation2020). In parallel, while UHDR spared normal brain tissue toxicity and reduced neuro-inflammation, its efficacy on glioblastoma (GBM) was found to be similar to irradiation at conventional dose rate, suggesting that the anti-tumor efficacy of radiotherapy was independent of dose rate (Montay-Gruel et al. Citation2021). As fractionated regimens are the standard of care for GBM treatment in current clinical practice, 4 × 3.5 Gy, 2 × 7 Gy, and 3 × 10 Gy regimens were investigated. UHDR reduced neurocognitive decline in GBM tumor bearing mice while tumor control was comparable to CONV-RT (Montay-Gruel et al. Citation2021).
Figure 3. The FLASH effect in normal brain and GBM. Taking the brain as organ-model, this figure shows the cascade of biological events that occur after tissue exposure to conventional dose rate and UHDR. Exposure of the brain to conventional dose rate irradiation (0.1 Gy/s) (left side of the scheme) is associated with early loss of vascular integrity due to endothelial cell damages as well as neuroinflammatory processes involving at longer terms astrogliosis, microglial activation and local immune cell infiltration. This pathogenic process perpetuates in time and ultimately results in neurocognitive disorders associated with loss of neurons and decreased neurogenesis. Interestingly, the delivery of radiation at UHDR (>100 Gy/s) (right side of the scheme) does not activate any of these pathogenic pathways. It spares the vascular network, does not induce neuroinflammation and preserves the neurogenic niche. One possible mechanism to explain the sparing effect of UHDR on normal tissue is the decreased formation of free radicals including reactive oxygen species (ROS) that occurs via early chemical reactions following irradiation (see and ) but other mechanisms can occur and are under investigations. Interestingly, while the normal brain is not damaged by UHDR irradiation, GBM tumor cells are equally sensitive to UHDR and irradiation at conventional dose rate, suggesting that tumor sensitivity is independent on the dose rate. Many factors might be involved in tumor sensitivity to UHDR, including gene expression. A putative susceptibility profile in T-acute lymphoblastic leukemia tumors was related to the expression of GADD45 and FAT1. In contrast, AGAP9 and PDLIM1 expressions seem to be associated to a resistant profile to UHDR (Chabi et al. Citation2020). Furthermore, Spitz et al. proposed another hypothesis explaining the differential impact of UHDR vs. conventional dose rate irradiation on cancer and normal tissue responses (as described in the summary at the end of the biological section).

Normal skin and subcutaneous tumors
In a subcutaneous mouse model of Lewis lung carcinoma (LLC), the normal vasculature was also shown to be preserved after exposure to 15 Gy electron at UHDR (352 Gy/s) whereas critical vascular collapse was observed after exposure to CONV dose rate (0.06 Gy/s) (Kim et al. Citation2020). The preservation of the vasculature after UHDR exposure was associated with reduced phosphorylation of the myosin light chain (p-MLC) known to be involved in the contraction of endothelial cells and reduction of immune cell infiltration. Interestingly, combination of CONV-RT with an MLC kinase inhibitor (ML-7) replicated UHDR results identifying the MLC pathway as one potential molecular target of irradiation at UHDR. In another study, a dose escalation study was performed and showed that 30 and 40 Gy irradiation at UHDR (electron, 180 Gy/s) resulted in reduced skin ulceration in contrast to CONV-RT (0.07 Gy/s) (Soto et al. Citation2020). Recent studies performed with proton beams, reported similar results with 35 Gy delivered with a FLASH scanning proton pencil beam (FLASH-Proton-PBS) (Cunningham et al. Citation2021) and a FLASH transmission proton beam (Velalopoulou et al. Citation2021). In Cunningham et al. study, skin toxicity and leg contraction were decreased significantly after exposure to UHDR (115 Gy/s) (Cunningham et al. Citation2021). Furthermore, this study showed similar tumor control of subcutaneous MOC1 and MOC2 head and neck cancer cells after UHDR protons and CONV protons (CONV-PRT) in immunocompetent mice. In Velalopoulou et al. study, 30 Gy using UHDR proton radiotherapy (UHDR-PRT, 69–124 Gy/s) spared the skin leg and mesenchymal tissues of muscles and bones from severe toxicities. In contrast, CONV-PRT (0.39–0.65 Gy/s) increased TGF-β1 both in murine and canine skin. UHDR-PRT and CONV-PRT equally controlled subcutaneous and intramuscular sarcoma tumors (Velalopoulou et al. Citation2021).
Gastrointestinal track and abdominal tumors
In addition to the benefits reported in late responding organs, UHDR irradiation was also beneficial in acute responding organs such as the gastrointestinal track and the hematopoietic system (see section ‘The FLASH effect validated in patient and human samples’, and Chabi et al. Citation2020). Intestinal function, epithelial integrity and regenerating crypts were preserved while DNA damage and apoptosis in the columnar cells of the crypt were reduced after exposure to 14 Gy at UHDR (216 Gy/s, electron). Again, anti-tumor efficacy in a preclinical mouse model of ovarian cancer (ID8) was comparable to that obtained with CONV-RT (0.08 Gy/s) (Levy et al. Citation2020). The beneficial effects of UHDR were again validated with proton and photon beams using pancreatic tumor models. The radiation-induced gastrointestinal syndrome did not occur upon UHDR irradiation using 18 Gy proton radiotherapy (UHDR-PRT) (Diffenderfer et al. Citation2020) and with 15 Gy X-rays (Gao et al. Citation2020) suggesting that the FLASH effect is relatively independent of the ionizing radiation modality. Recently, using GI as model, the FLASH effect was confirmed with spread-out Bragg peak irradiation. Using a pulsed synchrocyclotron, Evan et al. showed that mice irradiated at 10–16 Gy UHDR (96 Gy/s) exhibited enhanced survival with LD50 reaching 14.1 Gy with UHDR vs. 13.5 Gy with conventional dose rate (Evans et al. Citation2021). Consistently, Kim et al. study compares the outcome of the proton transmission at UHDR (UHDR-PRT transmission) vs. the spread-out Bragg peak (UHDR-PRT SOBP) in mouse intestine and pancreatic tumor control. Toxicity was significantly decreased in both configurations, i.e. 15 Gy UHDR-PRT SOBP (108.2 ± 8.3 Gy/s) vs. UHDR-PRT transmission (107.1 ± 15.2 Gy/s). In contrast, conventional dose rate proton transmission (CONV-PRT transmission, 0.83 ± 0.19 Gy/s) and SOBP (CONV-PRT SOBP, 0.82 ± 0.14 Gy/s) both generated important damages with reduced regenerating and proliferating crypts. Importantly, 18 Gy at UHDR and conventional dose rate irradiation were equipotent to control subcutaneous MH641905 mouse pancreatic tumors in both transmission and spread-out Bragg peak dose regions. All mice treated with CONV-PRT and UHDR-PRT transmission survived the treatment. In SOBP, 70% of the mice treated at conventional dose rate died 20 days after irradiation whereas UHDR induced only 15% of lethality (Kim et al. Citation2021). Ultimately, Ruan et al. also reported a decreased gastrointestinal toxicity and better crypt survival at UHDR (electron, 7.5–12.5 Gy, 2–6 × 106 Gy/s) with a relatively low dose-modifying factor of 1.1, in comparison to conventional dose rate (CONV, 0.25 Gy/s). The FLASH effect was lost when delivery time between two pulses and pulse repetition number were increased, highlighting the relevance of parameterization studies to define the FLASH effect (Ruan et al. Citation2021).
Negative studies
While studies on the FLASH effect have now been extended and validated across multiple animal models, other studies investigating UHDR irradiation have not reported beneficial effects. Exposure of 24 hour post-fertilization (hpf) zebrafish embryos with a proton beam at 100 Gy/s and 0.08 Gy/s showed no difference in survival and morphology (Beyreuther et al. Citation2019). With synchrotron X-ray at 37–41 Gy/s and 0.06 Gy/s, no difference was reported after total body, thoracic and abdominal irradiation (Smyth et al. Citation2018). Similarly, cardiac and splenic irradiation of mice with electron beams, 35 Gy/s and 0.1 Gy/s caused lymphopenia (Venkatesulu et al. Citation2020). While the basis of these negative results have to be carefully explored, certain caveats may well be related to the low dose rates and experimental set-up used in these studies.
The FLASH effect validated in patient and human samples
With the principal aim to transfer FLASH-RT into early clinical trials, our group investigated the FLASH effect on the skin of a mini-pig. A dose escalation study in cat-cancer patients with squamous cell carcinoma of the nasal planum identified 34 Gy as a tolerated and efficacious dose (Vozenin et al. Citation2019) and a phase III validation trial is currently ongoing. A dose escalation trials in dog patients with various superficial solid tumors has been published with only three/six month follow up (Konradsson et al. Citation2021). In addition, a feasibility study on dog-patients with osteosarcoma evaluated acute production of TGF-β and found minimal production following 12 Gy UHDR protons irradiation (Velalopoulou et al. Citation2021). Similarly, one feasibility study in one human patient is available (Bourhis et al. Citation2019a, Citation2019b) and one with UHDR protons is ongoing (FATS01); however, only few results are available with human cells and samples. Recently, we evaluated the effect of FLASH-RT on three patient-derived xenograft (PDX) of human T-acute lymphoblastic leukemia (T-ALL). We found that two out of three were sensitive to UHDR irradiation. Their genomic imprint revealed a putative susceptibility profile in T-ALL tumors and suggests that T-ALL sensitivity to UHDR could be related to the expression of certain genes including GADD45, involved in the control of the cell cycle G2/M checkpoint and FAT1 that regulates wnt pathway and cell division. In contrast, AGAP9 and PDLIM1 expressions seem to be associated to a resistant profile to UHDR (Chabi et al. Citation2020). Further studies are ongoing to elucidate this question.
Table 1 and Table 2 (Supplementary material) summarize positive and negative studies investigating the FLASH effect.
Interestingly, UHDR and conventional dose rate irradiation are equipotent to control tumors, suggesting that tumor but not normal tissue sensitivity is dose rate independent. The mechanistic basis of this differential effect triggered by UHDR on tumors vs. normal tissue is under scrutiny. One interesting hypothesis has been proposed by Spitz et al. and is based on differential distribution of organic hydroperoxides after UHDR vs. conventional dose rate irradiation. Hydroperoxides, derived from Fenton and peroxidation chain reactions are produced immediately at equal levels in normal and tumor tissue following UHDR. In normal tissues, antioxidants pathways remove more effectively than the organic hydroperoxides as compared to the tumor (higher levels of redox-active iron). Thus, explaining the beneficial therapeutic index of the FLASH effect in normal tissue compared to tumor. Whereas, at conventional dose rates, levels of hydroperoxides seem too low to uncover the differences in the tumor vs. normal tissue in oxidative metabolism (Spitz et al. Citation2019).
Example of experiments integrating biology and chemistry to investigate dose rate effect: from murine cognition and zebrafish embryo development to plasmid damage and H2O2 Yield
In this paper, we reviewed the current knowledge and approaches available to investigate the impact of dose rate from chemical systems to complex biological models. In this paragraph, we will give an example of integrated experiments performed to investigate the FLASH effect. We started from the first systematic investigation of the FLASH effect, that we performed several years ago using normal brain toxicity as the main outcome for the FLASH sparing effect (Montay-Gruel et al. Citation2017). The dose rate de-escalation study started with 10 Gy delivered in one pulse of 1.8 micros (5.6 × 106 Gy/s) until 0.1 Gy/s, the latter of which corresponds to conventional dose rates used in the clinic. Full preservation of neurocognitive function was obtained above 100 Gy/s, whereas it dropped at 30 Gy/s and was abolished at 0.1 Gy/s (). Then to derive different dose rate threshold data for a separate endpoint, dose rate escalation was performed at a fixed 10 Gy total dose in zebrafish embryos with body length measurement as an outcome (). Interestingly, zebrafish embryos corroborated data derived from mice () with a remarkable consistency (correlation coefficient >0.94). Next, experiments were pursued with pBR322 plasmids irradiated in aqueous solutions equilibrated at physiological oxygen conditions (4%). Notably, no difference in SSB yields (open circular DNA) was measured after UHDR compared to CONV-RT, whereas DSB yields (linear DNA) after 10 Gy were close to the detection limit of AGE (). Importantly, assuming that 4% oxygen mimics normal tissue oxygenation, these results suggest that UHDR irradiation does not modify DNA damage under physiological conditions. Finally, H2O2 production in terms of G(H2O2) (μM/10 Gy) was measured in cell-free systems and at physiological oxygen levels. Interestingly, the radiolytic production of H2O2 was reduced at the highest dose rates 5.6 × 106 Gy/s and 100 Gy/s, whereas an increase in G(H2O2) was observed with lower dose rates () as previously described (Montay-Gruel et al. Citation2019). The beam parameters required to perform these studies are summarized in .
Figure 4. Dose rate de-escalation was performed in mice, zebrafish embryos, plasmid and water using 10 Gy. (a) Recognition ratio (RR) evaluation two months post irradiation for control mice group and groups that received 10 Gy WBI delivery with a dose rate delivered in a single 1.8 µs electron pulse and ranging from (0.1, 10, or 100, 33, 10, 0.1 Gy/s). No memory alteration was observed in the groups irradiated with 100 Gy/s or higher, RR was comparable to the control group. Whereas a significant drop in the RR was observed for the group irradiated at 33 Gy/s. The drop became even slightly larger as the dose rate was further lowered (adapted from Montay-Gruel Citation2017). (b) Wild-type zebrafish embryos were irradiated four hours post fertilization and body length measurement five days post fertilization was used to assess radiation-induced injury. Similarly, with the murine recognition ratio results, both highest dose rates (5.6 × 106 Gy/s and 100 Gy/s) showed less alteration in body length as compared to non-irradiated embryos. Whereas, less protection was observed at lower dose rates and no protection at CONV-RT dose rate. Mean ± SD. p Values derived from Mann–Whitney’s test: *p<.05; **p<.01; ***p<.001 (N = 12–16 embryos/group). (c) Dose rate de-escalation involved irradiation at 10 Gy of pBR322 plasmids in aqueous solutions equilibrated at physiological oxygen conditions (4%). Notably, no difference in DNA damage was measured after UHDR compared to CONV-RT. Mean ± SD. p Values were derived from one-way ANOVA and Tukey’s multiple comparisons test. (d) H2O2 was quantified with the fluorogenic assay AmplexRed after 10 Gy irradiation of water samples equilibrated at 4% O2 following dose rate de-escalation similarly like described in the previous models. The radiolytic yield of H2O2 was significantly lower for the highest dose rates. Whereas, an increase in G(H2O2) was observed when the dose rate is lowered. This differential production reveals a decrease in ROS production following UHDR. Mean ± SD. p Values were derived from Mann–Whitney’s U test: **p<.01.

The reduced production of H2O2 was consistent with the lower toxicities observed in normal tissues. In this context, the role for oxygen, reduced ROS production, altered redox biology and modifications of the biological cascade downstream remain relevant and constitute a key focus of our ongoing research efforts. However, other important physiological factors such as temperature, proliferation status, and metabolism (among others) are likely to play a certain role in mediating the FLASH effect, and will require further experimental validation.
Table1-2_3_SupplementaryMaterial_20.10.2021.xlsx
Download MS Excel (23.3 KB)Acknowledgements
The authors thank Charles Limoli for discussions in the preparation of this MS as well as help with English edition. We would like to thank Jonathan Ollivier for the help doing zebrafish experiments as well as Benoit Petit for the animal handling. We would like to thank the team of Institute of Radiation Physics (IRA, CHUV) especially François Bochud, Claude Bailat, Pascal Froidevaux, Jerome Damet, Raphael Moeckli, Veljko Grilj, Patrik Jorge Gonçalves and Jean-François Germond for the development of the physics part of the FLASH-RT program at CHUV as well as Jean Bourhis for his support. We would like to thank the Epalinges Animal Facility for the animal care-taking as well as Francesca Amati at the zebrafish facility. Figures were created with BioRender.com.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Houda Kacem
Houda Kacem, PhD Student Research interest I am investigating the oxygen contribution and production of reactive oxygen species after FLASH irradiation in cell free systems and biological systems such as normal/tumor cells and zebrafish model. Background I'm a chemist by training. I have graduated from the international Erasmus Master's program SERP+, at the University of Paris-Saclay (France) and the University of Porto (Portugal).
Aymeric Almeida
Aymeric Almeida, PhD Student Research interest I'm investigating the anti tumor immune response and downstream immune cascade, activated or not, in lung tumor, glioblastoma and normal tissue after irradiation at ultra high dose rate (FLASH). Background I studied at l'Université Claude Bernard Lyon where I obtained my bachelor in physiology and subsequently did a Masters in Immunology & Cancer at the University of Lausanne.
Nicolas Cherbuin
Nicolas Cherbuin, Technician RTT Research interest I have an interest in research for the benefit of radiation protection of patients, workers and the public, and therefore involved in projects ranging from radiobiology to occupational dosimetry monitoring. Background with a Bachelor's in Medical Radiology Technics study (HES-SO, HESAV) gained in 2008, I worked in the Service of nuclear medicine of the Lausanne University Hospital, particularly working on radiation therapies. In 2014, I moved to the hospital's Institute of radiation physics, as a scientific collaborator and radiation protection expert. I completed the joint HES-SO/UNIL Master's in Health Sciences in 2021, in the course of which I had the chance to begin an ongoing collaboration with the Vozenin Lab.
Marie-Catherine Vozenin
Marie-Catherine Vozenin, PhD Associate professor in the service of Radio-oncology, CHUV/UNIL Head of the radio-Oncology Laboratory, CHUV Adjunct Professor, University of California Irvine Research interest: The research project that I develop with my team aim at finding innovative tools able to protect normal tissue and enhance tumor control. In this context, we have developed a novel of modality of radiation therapy called FLASH radiotherapy that minimizes normal tissue toxicity and eradicates tumors in various organs including the brain, lung and skin, and in various species including mice, zebrafish, pigs and cats. Much of our recent work has focused on investigating the entirely different biological response induced after FLASH exposure. Importantly, we have worked to secure the translation of FLASH-RT into clinical trials for human patient with cancer.
References
- Alaghband Y, Cheeks SN, Allen BD, Montay-Gruel P, Doan N-L, Petit B, Jorge PG, Giedzinski E, Acharya MM, Vozenin M-C, et al. 2020. Neuroprotection of radiosensitive juvenile mice by ultra-high dose rate FLASH irradiation. Cancers. 12(6):1671.
- Allen BD, Acharya MM, Montay-Gruel P, Jorge PG, Bailat C, Petit B, Vozenin M-C, Limoli C. 2020. Maintenance of tight junction integrity in the absence of vascular dilation in the brain of mice exposed to ultra-high-dose-rate FLASH irradiation. Radiat Res. 194(6):625–635.
- Auclair G. 2001. Determination of primary yields in the alpha radiolysis of alkaline water [Determination des rendements radiolytiques primaires alpha en milieu alcalin]. Paris, France: University Pierre et Marie Curie. Available from INIS: http://inis.iaea.org/search/search.aspx?orig_q=RN:46133716
- Baldacchino G, Brun E, Denden I, Bouhadoun S, Roux R, Khodja H, Sicard-Roselli C. 2019. Importance of radiolytic reactions during high-LET irradiation modalities: LET effect, role of O2 and radiosensitization by nanoparticles. Cancer Nano. 10(1):3.
- Barilla J, Lokajíček M. 2000. The role of oxygen in DNA damage by ionizing particles. J Theor Biol. 207(3):405–414.
- Beyreuther E, Brand M, Hans S, Hideghéty K, Karsch L, Leßmann E, Schürer M, Szabó ER, Pawelke J. 2019. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother Oncol. 139:46–50.
- Bourhis J, Montay-Gruel P, Gonçalves Jorge P, Bailat C, Petit B, Ollivier J, Jeanneret-Sozzi W, Ozsahin M, Bochud F, Moeckli R, et al. 2019a. Clinical translation of FLASH radiotherapy: why and how? Radiother Oncol. 139:11–17.
- Bourhis J, Sozzi WJ, Jorge PG, Gaide O, Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond J-F, et al. 2019b. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol. 139:18–22.
- Cao X, Zhang R, Esipova TV, Allu SR, Ashraf R, Rahman M, Gunn JR, Bruza P, Gladstone DJ, Williams BB, et al. 2021. Quantification of oxygen depletion during FLASH irradiation in vitro and in vivo. Int J Radiat Oncol Biol Phys. 111(1):240–248.
- Chabi S, Van To TH, Leavitt R, Poglio S, Jorge PG, Jaccard M, Petersson K, Petit B, Roméo P-H, Pflumio F, et al. 2020. Ultra-high-dose-rate FLASH and conventional-dose-rate irradiation differentially affect human acute lymphoblastic leukemia and normal hematopoiesis. Int J Radiat Oncol. 109(3):819–829.
- Cowan R, Collis CM, Grigg GW. 1987. Breakage of double-stranded DNA due to single-stranded nicking. J Theor Biol. 127(2):229–245.
- Cunningham S, McCauley S, Vairamani K, Speth J, Girdhani S, Abel E, Sharma RA, Perentesis JP, Wells SI, Mascia A, et al. 2021. FLASH proton pencil beam scanning irradiation minimizes radiation-induced leg contracture and skin toxicity in mice. Cancers. 13(5):1012.
- Diffenderfer ES, Verginadis II, Kim MM, Shoniyozov K, Velalopoulou A, Goia D, Putt M, Hagan S, Avery S, Teo K, et al. 2020. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int J Radiat Oncol Biol Phys. 106(2):440–448.
- Evans T, Cooley J, Wagner M, Yu T, Zwart T. 2021. Demonstration of the FLASH effect within the spread-out Bragg peak after abdominal irradiation of mice. Int J Part Ther.
- Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, Poupon M-F, Brito I, Hupé P, Bourhis J, et al. 2014. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 6(245):245ra93.
- Field SB, Bewley DK. 1974. Effects of dose-rate on the radiation response of rat skin. Int J Radiat Biol Relat Stud Phys Chem Med. 26(3):259–267.
- Fouillade C, Curras-Alonso S, Giuranno L, Quelennec E, Heinrich S, Bonnet-Boissinot S, Beddok A, Leboucher S, Karakurt HU, Bohec M, et al. 2020. FLASH irradiation spares lung progenitor cells and limits the incidence of radio-induced senescence. Clin Cancer Res. 26(6):1497–1506.
- Gao F, Yang Y, Zhu H, Wang J, Xiao D, Zhou Z, Dai T, Zhang Y, Feng G, Li J, et al. 2020. First demonstration of the FLASH effect with ultrahigh dose-rate high energy X-rays. bioRxiv.
- Georgakilas AG, O'Neill P, Stewart RD. 2013. Induction and repair of clustered DNA lesions: what do we know so far? Radiat Res. 180(1):100–109.
- Hendry JH, Moore JV, Hodgson BW, Keene JP. 1982. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res. 92(1):172–181.
- Hiroki A, Pimblott SM, LaVerne JA. 2002. Hydrogen peroxide production in the radiolysis of water with high radical scavenger concentrations. J Phys Chem A. 106(40):9352–9358.
- Hornsey S, Bewley DK. 1971. Hypoxia in mouse intestine induced by electron irradiation at high dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med. 19(5):479–483.
- Hu A, Qiu R, Wu Z, Li C, Zhang H, Li J. 2020. Oxygen depletion hypothesis remains controversial: a mathematical model of oxygen depletion during FLASH radiation. ArXiv200110788 Phys. Q-Bio.
- Jaccard M, Durán MT, Petersson K, Germond J-F, Liger P, Vozenin M-C, Bourhis J, Bochud F, Bailat C. 2018. High dose-per-pulse electron beam dosimetry: commissioning of the Oriatron eRT6 prototype linear accelerator for preclinical use. Med Phys. 45(2):863–874.
- Jansen J, Knoll J, Beyreuther E, Pawelke J, Skuza R, Hanley R, Brons S, Pagliari F, Seco J. 2021. Does FLASH deplete oxygen? Experimental evaluation for photons, protons and carbon ions. ArXiv210212762 Phys.
- Kim MM, Verginadis II, Goia D, Haertter A, Shoniyozov K, Zou W, Maity A, Busch TM, Metz JM, Cengel KA, et al. 2021. Comparison of FLASH proton entrance and the spread-out Bragg peak dose regions in the sparing of mouse intestinal crypts and in a pancreatic tumor model. Cancers. 13(16):4244.
- Kim Y-E, Gwak S-H, Hong B-J, Oh J-M, Choi H-S, Kim MS, Oh D, Lartey F, Rafat M, Schüler E, et al. 2020. Effects of ultra-high doserate FLASH irradiation on the tumor microenvironment in Lewis lung carcinoma: role of myosin light chain. Int J Radiat Oncol Biol Phys. 109(5):1440–1453.
- Konradsson E, Arendt ML, Bastholm Jensen K, Børresen B, Hansen AE, Bäck S, Kristensen AT, Munck af Rosenschöld P, Ceberg C, Petersson K. 2021. Establishment and initial experience of clinical FLASH radiotherapy in canine cancer patients. Front Oncol. 11:658004.
- Krisch RE, Flick MB, Trumbore CN. 1991. Radiation chemical mechanisms of single- and double-strand break formation in irradiated SV40 DNA. Radiat Res. 126(2):251–259.
- Labarbe R, Hotoiu L, Barbier J, Favaudon V. 2020. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol. 153:303–310.
- Levy K, Natarajan S, Wang J, Chow S, Eggold JT, Loo PE, Manjappa R, Melemenidis S, Lartey FM, Schüler E, et al. 2020. Abdominal FLASH irradiation reduces radiation-induced gastrointestinal toxicity for the treatment of ovarian cancer in mice. Sci Rep. 10(1):21600.
- Mazal A, Vera Sanchez JA, Sanchez-Parcerisa D, Udias JM, España S, Sanchez-Tembleque V, Fraile LM, Bragado P, Gutierrez-Uzquiza A, Gordillo N, et al. 2020. Biological and mechanical synergies to deal with proton therapy pitfalls: minibeams, FLASH, arcs, and gantryless rooms. Front Oncol. 10:613669.
- McMahon SJ, Currell FJ. 2011. A robust curve-fitting procedure for the analysis of plasmid DNA strand break data from gel electrophoresis. Radiat Res. 175(6):797–805.
- Moeckli R, Germond J-F, Bailat C, Bochud F, Vozenin M-C, Bourhis J. 2020. In regard to van Marlen et al. Int J Radiat Oncol Biol Phys. 107(5):1012–1013.
- Montay-Gruel P, Acharya MM, Gonçalves Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K, Gondré M, Ollivier J, et al. 2021. Hypofractionated FLASH-RT as an effective treatment against glioblastoma that reduces neurocognitive side effects in mice. Clin Cancer Res. 27(3):775–784.
- Montay-Gruel P, Acharya MM, Petersson K, Alikhani L, Yakkala C, Allen BD, Ollivier J, Petit B, Jorge PG, Syage AR, et al. 2019. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A. 116(22):10943–10951.
- Montay-Gruel P, Bouchet A, Jaccard M, Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis J, et al. 2018. X-rays can trigger the FLASH effect: ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother Oncol. 129(3):582–588.
- Montay-Gruel P, Markarian M, Allen BD, Baddour JD, Giedzinski E, Jorge PG, Petit B, Bailet C, Vozenin M-C, Limoli C, et al. 2020. Ultra-high-dose-rate FLASH irradiation limits reactive gliosis in the brain. Radiat Res. 194(6):636–645.
- Montay-Gruel P, Petersson K, Jaccard M, Boivin G, Germond J-F, Petit B, Doenlen R, Favaudon V, Bochud F, Bailat C, et al. 2017. Irradiation in a flash: unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother Oncol. 124(3):365–369.
- Nikjoo H, Uehara S, Wilson WE, Hoshi M, Goodhead DT. 1998. Track structure in radiation biology: theory and applications. Int J Radiat Biol. 73:355–364.
- Pachnerová Brabcová K, Sihver L, Ukraintsev E, Štěpán V, Davídková M. 2019. How detection of plasmid DNA fragmentation affects radiation strand break yields. Radiat Prot Dosimetry. 183(1–2):89–92.
- Petersson K, Adrian G, Butterworth K, McMahon SJ. 2020. A quantitative analysis of the role of oxygen tension in FLASH radiation therapy. Int J Radiat Oncol Biol Phys. 107(3):539–547.
- Pogozelski WK, Tullius TD. 1998. Oxidative strand scission of nucleic acids: routes initiated by hydrogen abstraction from the sugar moiety. Chem Rev. 98(3):1089–1108.
- Pratx G, Kapp DS. 2019. A computational model of radiolytic oxygen depletion during FLASH irradiation and its effect on the oxygen enhancement ratio. Phys Med Biol. 64(18):185005.
- Ruan J-L, Lee C, Wouters S, Tullis ID, Verslegers M, Mysara M, Then CK, Smart SC, Hill MA, Muschel RJ, et al. 2021. Irradiation at ultra-high (FLASH) dose rates reduces acute normal tissue toxicity in the mouse gastrointestinal system. Int J Radiat Oncol Biol Phys.
- Schüller A, Heinrich S, Fouillade C, Subiel A, De Marzi L, Romano F, Peier P, Trachsel M, Fleta C, Kranzer R, et al. 2020. The European Joint Research Project UHDpulse – metrology for advanced radiotherapy using particle beams with ultra-high pulse dose rates. Phys Med. 80:134–150.
- Shikazono N, Noguchi M, Fujii K, Urushibara A, Yokoya A. 2009. The yield, processing, and biological consequences of clustered DNA damage induced by ionizing radiation. J Radiat Res. 50(1):27–36.
- Siddiqi MA, Bothe E. 1987. Single- and double-strand break formation in DNA irradiated in aqueous solution: dependence on dose and OH radical scavenger concentration. Radiat Res. 112(3):449–463.
- Simmons DA, Lartey FM, Schüler E, Rafat M, King G, Kim A, Ko R, Semaan S, Gonzalez S, Jenkins M, et al. 2019. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother Oncol. 139:4–10.
- Small KL, Henthorn NT, Angal-Kalinin D, Chadwick AL, Santina E, Aitkenhead A, Kirkby KJ, Smith RJ, Surman M, Jones J, et al. 2021. Evaluating very high energy electron RBE from nanodosimetric pBR322 plasmid DNA damage. Sci Rep. 11(1):3341.
- Smyth LML, Donoghue JF, Ventura JA, Livingstone J, Bailey T, Day LRJ, Crosbie JC, Rogers PAW. 2018. Comparative toxicity of synchrotron and conventional radiation therapy based on total and partial body irradiation in a murine model. Sci. Rep. 8:12044.
- Soto LA, Casey KM, Wang J, Blaney A, Manjappa R, Breitkreutz D, Skinner L, Dutt S, Ko RB, Bush K, et al. 2020. FLASH irradiation results in reduced severe skin toxicity compared to conventional-dose-rate irradiation. Radiat Res. 194(6):618–624.
- Spitz DR, Buettner GR, Petronek MS, St-Aubin JJ, Flynn RT, Waldron TJ, Limoli CL. 2019. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol. 139:23–27.
- Spotheim-Maurizot M, editor. 2008. Radiation chemistry: from basics to applications in material and life sciences, L’actualité chimique livres. Les Ulis, France: EDP Sciences.
- Sutherland JC, Monteleone DC, Trunk JG, Bennett PV, Sutherland BM. 2001. Quantifying DNA damage by gel electrophoresis, electronic imaging and number-average length analysis. Electrophoresis. 22(5):843–854.
- Velalopoulou A, Karagounis IV, Cramer GM, Kim MM, Skoufos G, Goia D, Hagan S, Verginadis II, Shoniyozov K, Chiango J, et al. 2021. FLASH proton radiotherapy spares normal epithelial and mesenchymal tissues while preserving sarcoma response. Cancer Res. 81(18):4808–4821.
- Venkatesulu BP, Sharma A, Pollard-Larkin JM, Sadagopan R, Symons J, Neri S, Singh PK, Tailor R, Lin SH, Krishnan S. 2020. Author correction: ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci Rep. 10:11018.
- von Sonntag C. 1991. The chemistry of free-radical-mediated DNA damage. Basic Life Sci. 58:287–317, discussion 317–321.
- Vozenin M-C, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond J-F, Petit B, Burki M, Ferrand G, Patin D, et al. 2019. The advantage of FLASH radiotherapy confirmed in mini-pig and cat-cancer patients. Clin Cancer Res. 25(1):35–42.
- Vozenin M-C, Hendry JH, Limoli CL. 2019. Biological benefits of ultra-high dose rate FLASH radiotherapy: sleeping beauty awoken. Clin Oncol (R Coll Radiol). 31(7):407–415.
- Ward JF. 1981. Some biochemical consequences of the spatial distribution of ionizing radiation-produced free radicals. Radiat Res. 86(2):185–195.
- Ward JF. 1985. Biochemistry of DNA lesions. Radiat Res Suppl. 8:S103–S111.
- Wasselin-Trupin V, Baldacchino G, Bouffard S, Hickel B. 2002. Hydrogen peroxide yields in water radiolysis by high-energy ion beams at constant LET. Radiat Phys Chem. 65(1):53–61.
- Wilson JD, Hammond EM, Higgins GS, Petersson K. 2020. Ultra-high dose rate (FLASH) radiotherapy: silver bullet or fool’s gold? Front Oncol. 9:9.
- Zhou S, Zheng D, Fan Q, Yan Y, Wang S, Lei Y, Besemer A, Zhou C, Enke C. 2020. Minimum dose rate estimation for pulsed FLASH radiotherapy: a dimensional analysis. Med Phys. 47(7):3243–3249.
