Abstract
Background
Epidemiological studies have indicated that exposure of the heart to doses of ionizing radiation as low as 0.5 Gy increases the risk of cardiac morbidity and mortality with a latency period of decades. The damaging effects of radiation to myocardial and endothelial structures and functions have been confirmed radiobiologically at high dose, but much less are known at low dose. Integration of radiation biology and epidemiology data is a recommended approach to improve the radiation risk assessment process. The adverse outcome pathway (AOP) framework offers a comprehensive tool to compile and translate mechanistic information into pathological endpoints which may be relevant for risk assessment at the different levels of a biological system. Omics technologies enable the generation of large volumes of biological data at various levels of complexity, from molecular pathways to functional organisms. Given the quality and quantity of available data across levels of biology, omics data can be attractive sources of information for use within the AOP framework. It is anticipated that radiation omics studies could improve our understanding of the molecular mechanisms behind the adverse effects of radiation on the cardiovascular system. In this review, we explored the available omics studies on radiation-induced cardiovascular disease (CVD) and their applicability to the proposed AOP for CVD.
Results
The results of 80 omics studies published on radiation-induced CVD over the past 20 years have been discussed in the context of the AOP of CVD proposed by Chauhan et al. Most of the available omics data on radiation-induced CVD are from proteomics, transcriptomics, and metabolomics, whereas few datasets were available from epigenomics and multi-omics. The omics data presented here show great promise in providing information for several key events (KEs) of the proposed AOP of CVD, particularly oxidative stress, alterations of energy metabolism, extracellular matrix (ECM), and vascular remodeling.
Conclusions
The omics data presented here shows promise to inform the various levels of the proposed AOP of CVD. However, the data highlight the urgent need of designing omics studies to address the knowledge gap concerning different radiation scenarios, time after exposure, and experimental models. This review presents the evidence to build a qualitative omics-informed AOP and provides views on the potential benefits and challenges in using omics data to assess risk-related outcomes.
Introduction
Experimental evidence for the cardiovascular impacts of ionizing irradiation has been described since the late 1890s in poikilotherm species and the early 1920s in homeotherm species (Desjardins Citation1937). The circulatory system has long been considered to be relatively radioresistant, such that higher doses (e.g. therapeutic radiation exposure with 40–80 Gy in 1.8–2 Gy daily fractions) are required to cause damage, in particular degenerative changes (ICRP Citation1984). However, since the late 1990s, there has been mounting epidemiological evidence for increased risk of diseases of the circulatory system (DCS) at dose and dose rates much lower than previously thought (Kreuzer et al. Citation2015; Little et al. Citation2021; Tapio et al. Citation2021). The International Commission on Radiological Protection (ICRP), in 2011, recommends the nominal threshold of 0.5 Gy to the heart for cardiovascular disease (CVD) and to the brain for cerebrovascular disease (CeVD) (Stewart et al. Citation2012), independent of rate of dose delivery, i.e. assuming no dose rate effect. In 2021, the United Nations Scientific Committee on Effects of Atomic Radiation (UNSCEAR) established an expert group to prepare a report on the scientific basis of radiogenic DCS, and ICRP established Task Group 119 to prepare a report on the implications of such radiogenic DCS in the radiation protection system.
In the context of radiation protection, especially for stochastic effects (i.e. cancer and heritable effects) of low linear energy transfer radiation, the dose <0.1 Gy is defined as low dose, 0.1–1 Gy as moderate dose, 1–5 Gy as high dose, and >5 Gy as very high dose. For dose rate, <0.005 Gy/h is defined as low dose rate, 0.005–0.1 Gy/h as moderate dose rate, and >0.1 Gy/h as high dose rate (Rühm et al. Citation2015; Little et al. Citation2021, Citation2022b). The epidemiological studies performed on exposed populations such as Japanese atomic bomb survivors serve as the basis of linear-non-threshold (LNT) model applied in radiation risk assessment of cancer for more than half a century. Such approach facilitates linear extrapolation of data from high and medium doses to low dose radiation. However, there are considerable uncertainties in assessing the risk-related outcomes at low doses or low dose rates (Preston Citation2017; Shore et al. Citation2018). Several national and international organizations of radiation protection have recommended integrating the data of radiation biology and epidemiology to improve the radiation risk assessment process (Hamada and Fujimichi Citation2014; NCRP Citation2020; Chauhan, Beaton, et al. Citation2022; NASEM Citation2022). The integration of experimental and epidemiological data has been introduced by several radiation biologically based dose-response (BBDR) models to estimate cancer risk (Preston Citation2015, Citation2017; Rühm et al. Citation2017; Preston et al. Citation2021). Among non-cancer endpoints of radiation exposure, disease of the circulatory system, including CVD, is the main domain for which radiation experimental data (e.g. on potential biomarkers) are becoming increasingly available and could be incorporated into epidemiological studies. However, compared to carcinogenesis, only a few mechanistic risk models have been developed for radiation-induced CVD. These early models were mainly concerned with the impact of cholesterol metabolism on atherosclerosis but have not been fitted to radiation epidemiological cohorts (Cobbold et al. Citation2002; Little et al. Citation2009; Mc Auley Citation2022). The recently developed BBDR model for late CVD risk after radiotherapy (RT) applied to breast cancer patients aims to address this issue using integration of experimental and clinical data (Simonetto, Kaiser, et al. Citation2022).
The AOP approach has been developed in the chemical toxicology field to assemble current knowledge on key events (KEs) that underlie disease progression (Villeneuve et al. Citation2014) and has been identified as being instrumental for the successful implementation of mechanistic data into research and regulatory framework (Ankley et al. Citation2010). An AOP is an analytical construct that begins with a molecular initiating event (MIE). It describes the sequential chain of KEs and causally linked key event relationships (KERs) at different levels of biological organization as it leads to an adverse outcome (AO) of interest to regulatory decision-making (Ankley et al. Citation2010).
In 2012, the Organization for Economic Co-operation and Development (OECD) launched a formal framework to provide the structure for building high-quality AOPs (OECD Citation2018). The framework allows the assembly of knowledge across scientific disciplines using evidence streams defined by the modified Bradford-Hill (B-H) criteria (Becker et al. Citation2015). This informs the weight of evidence of an AOP, derived from information on biological plausibility, empirical evidence, the essentiality of KEs and any documented uncertainties. As fluid documents, AOPs can continually evolve as new data or methods become available.
Because KEs are conceptually defined by interactions of different genes, proteins, or metabolites, omics data have the potential to contribute to the development and evaluation of AOP. The advances in high-throughput molecular analysis have completely reformed our understanding of biological processes. Mainstream omics are powerful analytical tools for the study of various biomolecules in a biological system at a given time and status (Vailati-Riboni et al. Citation2017). The DNA, epigenetic modifications, RNA, proteins, lipids, extracellular signaling molecules, and metabolites are the target molecules for genomics, epigenomics, transcriptomics, proteomics, lipidomics, secretomics, and metabolomics, respectively. The application of omics is rapidly emerging in different research areas and clinical investigations.
The first application of omics in radiation biology was published more than two decades ago, but omics profiling is yet young in radiation research (Azimzadeh, Gomolka, et al. Citation2021; Subedi et al. Citation2022). With the omics studies in radiation biology, the researchers can address several issues related to the biological effects of radiation exposure, radiation biomarkers, the individual difference in radiation response, and risk assessment (Pernot et al. Citation2012; Amundson Citation2021; Hladik et al. Citation2022; Subedi et al. Citation2022).
Omics data can contribute to a deeper understanding of molecular events associated with AOPs. The chemical and ecological fields have recently directed efforts toward integrating omics data within AOPs to refine and strengthen their predictive utility. The results from different omics studies have already been used in the development of AOP in chemical risk assessment in toxicology (Ankley et al. Citation2010; Villeneuve et al. Citation2014; Brockmeier et al. Citation2017). However, the applicability of omics data for regulatory purposes remains debatable, mainly due to the lack of standardized approaches for conducting and interpreting omics experiments on the one hand, and the gap between quantitative omics results and measurable phenotypic endpoints on the other hand (Sauer et al. Citation2017).
An optimal example for the application of omics data in the chemical toxicology field is benchmark dose (BMD) modeling to identify a point of departure at which a defined change from the control occurs that signifies a relevant phenotypic change (Filipsson et al. Citation2003). Studies have demonstrated transcriptional BMD values to be highly correlated with toxic doses of chemicals derived using traditional animal models. More recently, the approach has been applied to radiation datasets and has shown promise in identifying the point of departures of molecular pathway with reproducibility across datasets generated at different institutions (Chauhan et al. Citation2016; Chauhan, Rowan-Carroll, et al. Citation2019). Such an approach would be valuable to integrate within the KERs of an AOP and could be explored through case studies.
The AOP framework has increasingly gained attention from the radiation community as a means to advance the mechanistic understanding of the health effects of exposure to ionizing radiation at low dose and low dose rates (Chauhan, Said, et al. Citation2019; Chauhan, Hamada, et al. Citation2021; Chauhan, Wilkins, et al. Citation2021; Hamada et al. Citation2021; Preston et al. Citation2021; Chauhan, Hamada, et al. Citation2022; Hamada et al. Citation2022, in press). The overarching objective of such an approach is to identify the sequence of critical KEs leading to an AO and to identify robust bioindicators for the adverse pathological events. Subsequently, the biological dose-response data provided by bioindicators should be combined with epidemiological data in BBDR models to improve risk assessment.
In this context, a recent workshop on AOPs, organized by the Multidisciplinary European Low Dose Initiative (MELODI) and the European Radioecology Alliance associations (ALLIANCE), was held virtually on 12–16 April 2021. A working group was formed of experts in the field of CVD from both regulatory and research institutions, discussed the development of an AOP network for radiation-induced CVD (Chauhan, Hamada, et al. Citation2021). The group members deliberated on various exposure scenarios, potential toxicity mechanisms, the MIE, KEs, and their potential relationships. Based on these initial expert discussions, the group proposed an AOP for CVD (Chauhan, Hamada, et al. Citation2021). The AOP network is preliminary and describes biologically plausible mechanisms for cardiac toxicity at multiple levels of organization from molecular, cellular, and tissue to organ levels. It comprises three main branches: endothelial dysfunction, metabolic and extracellular matrix (ECM) remodeling leading to vascular, myocardial pathology, and fibrosis. The potential cellular interplay in irradiated cardiac tissue was also considered by proposing some bidirectional relationships between KEs (Chauhan, Hamada, et al. Citation2021). The next steps involve a documented assessment of the literature to support causal linkages of the KERs.
Here, we provide a summary of omics data that may inform the proposed radiation-induced CVD AOP. In particular, we describe the characteristics of omics profiles available in radiation research, including transcriptome, proteome, secretome, epigenome, metabolome and those from multi-omics analyses. We discuss the potential and deficiencies of omics data that can be used in the development of AOP for radiation-induced cardiovascular effects. The review also points to scientific and analytical domains of radiation research where sufficient omics data are not yet generated and interpreted.
Search criteria
All searches were performed in NCBI PubMed (https://pubmed.ncbi.nlm.nih.gov/advanced/) (accessed 25 April 2022). A combination of keywords, including the name of the omics platform, cardiovascular, and radiation, was searched in title/abstract of studies published from 2000 to 2022 as follows:
For genomics: (‘genome*’[Title/Abstract] OR (‘GWAS’[Title/Abstract] AND ‘cardiovascular’[Title/Abstract] AND (‘radiation’[Title/Abstract])
For Epigenetics: (‘epigenom*’[Title/Abstract] OR ‘dna methylation’[Title/Abstract]) AND ‘cardiovascular’[Title/Abstract] AND ‘radiation’[Title/Abstract])
For transcriptomics: (‘transcriptom*’[Title/Abstract] OR ‘microarray’[Title/Abstract] OR ‘rna seq’[Title/Abstract]) AND ‘cardiovascular’[Title/Abstract] AND ‘radiation’[Title/Abstract]
For miRNA array: (‘miRNA*’[Title/Abstract] AND ‘cardiovascular’[Title/Abstract] AND (‘radiation’[Title/Abstract])
For Proteomics: (‘proteom*’[Title/Abstract] AND ‘cardiovascular’[Title/Abstract] AND (‘radiation’[Title/Abstract])
For Secretome: (‘secretom*’[Title/Abstract] OR ‘exosome*’[Title/Abstract] OR ‘extracellular vesicle’ [Title/Abstrcact]) AND ‘cardiovascular’[Title/Abstract] AND ‘radiation’[Title/Abstract]
For metabolomics and lipidomics: (‘metabolom*’[Title/Abstract] OR ‘lipidom*’[Title/Abstract]) AND ‘cardiovascular’[Title/Abstract] AND ‘radiation’[Title/Abstract]
To cover a full range of data, no exclusion criteria were applied for experimental models, radiation exposure scenarios (e.g. dose, dose rate, and radiation quality), and time after exposure. This is consistent with guidelines for AOP development, whereby all studies irrespective of dose range were included, similar to an earlier commentary (Chauhan, Hamada, et al. Citation2021).
Results of the literature search
The PubMed search using the searching matrices applied in the title/abstract returned 108 articles. These articles were subjected to full-text review by authors to ascertain if they had original omics analyses. Among these, 41 articles (2 epigenomic, 11 transcriptomic, 2 miRNA, 23 proteomic, 1 secretomic, and 2 metabolomic analyses) qualified for inclusion. As the automatic PubMed search does not necessarily identify all relevant existing articles (Little et al. Citation2021, Citation2022a, Citation2022b; Subedi et al. Citation2022), additional articles and reviews (3 epigenomics, 7 transcriptomics, 7 miRNA, 7 proteomics, 13 metabolomics, and 8 secretomics) were manually selected by authors and included in the final list. Finally, 80 articles were discussed in the context of AOP CVD proposed by Chauhan, Hamada, et al. (Citation2021). Proteomics (27 studies), transcriptomics (16 studies), and metabolomics (15 studies) accounted for a large part (>70%) of all omics data included here. Only a few profiles were available for epigenomics (3 studies), miRNA (7 studies), and secretomics (6 studies) analyses of the cardiovascular system after irradiation. The articles for multi-omics (5 studies), and systems biology (1 study) were also manually included indicating significant research and knowledge gaps in these areas.
Genomics
Genomics is the analysis of an individual’s whole DNA, such as gene variants and single nucleotide polymorphisms (SNPs). The evolving large amount of data on human genetic information provides insights into susceptibilities and has the potential to be incorporated into risk assessments (Mortensen et al. Citation2018).
Various risk factors (e.g. high cholesterol and hypertension) and genetic factors (e.g. gene variants) are strongly recognized in CVD research. For example, the heritability of coronary artery disease (CAD) has been estimated at 40%, suggesting a strong genetic contribution to the disease (McPherson and Tybjaerg-Hansen Citation2016). Genome-wide association studies (GWAS) have identified ∼300 risk loci for CAD and myocardial infarction (Chen Z and Schunkert Citation2021). Moreover, large-scale, genome-wide, and targeted genetic association data have been combined to develop a genomic risk score for CAD (Inouye et al. Citation2018). To address the question of how the GWAS-identified risk variants function to increase the risk of CAD, Selvarajan et al. presented an integrative genomics approach representing a comprehensive effort in identifying putative causal regulatory regions and target genes that could predispose to clinical manifestation of CAD by affecting liver function (Selvarajan et al. Citation2021). The contribution of genetic predisposition to radiation sensitivity or radiation resistance in cancer is well accepted; however, the knowledge in the area of radiation-induced non-cancer effects is not very advanced (Foray et al. Citation2016; Rajaraman et al. Citation2018). In recent years, some associations between SNPs and long-term normal tissue radiation toxicities in the bowel, esophagus, lung, and skin were identified (Kerns et al. Citation2020). However, studies on genomics and radiation-induced cardiotoxicity are completely lacking. Neither our own search strategy nor other review work identified suitable studies (Tapio et al. Citation2021).
Epigenomics
Epigenetic modifications are dynamic modifications of the genome (e.g. DNA methylation and histone modification) that confer heritable changes in gene expression without altering the genome sequence (Dupont et al. Citation2009). GWAS analyses have identified several CAD and myocardial infarction-associated variants located in the non-coding regions of the genome, emphasizing the role of epigenetics in pathogenesis (Örd et al. Citation2021). A series of recent systematic reviews highlight epigenetic aspects in gene expression linked to the pathophysiology of CVD and related risk factors such as atherosclerosis, inflammation, hypertension, and diabetes (Muka, Nano, et al. Citation2016; Gonzalez-Jaramillo et al. Citation2019; Sallam et al. Citation2022).
Gene-specific studies suggest a higher frequency of methylation marks in CVD for genes involved in cholesterol transport and for mitochondrial cytochrome c oxidases but not for mitochondrial ATP synthases (Muka, Koromani, et al. Citation2016; Sallam et al. Citation2022). Higher frequency of methylations was also associated with myocardial infarction related to one-carbon metabolism while stroke was associated with lower frequency methylation at the TNF-alpha promoter (Muka, Koromani, et al. Citation2016). Heart diseases were associated with a greater frequency of methylation at the F7 promoter and genomic regions associated with genes for the ATP binding cassettes, estrogen receptor-alpha, inhibitors of metalloproteinases, and phospholipase A2 Group VII (Muka, Koromani, et al. Citation2016). Functional analysis of the differentially methylated genes (DMGs) revealed an association with various pathophysiological mechanisms of CVD, such as inflammatory responses, lipid metabolism, oxidative stress, and endothelial dysfunction, as well as with signaling pathways, such as JAK/STAT, PI3K, and interleukin (Sallam et al. Citation2022). The single-cell sequencing in cells derived from human atherosclerotic lesions demonstrated that genetic variants associated with CAD are mainly enriched in vascular endothelial and smooth muscle cell-specific open chromatin (Örd et al. Citation2021), further highlighting the importance of gene regulation.
Radiation-induced epigenetic alterations, especially changes in DNA methylation on both a global and a gene-specific scale are described in several normal (fibroblasts) and tumor (e.g. colorectal carcinoma) cells and tissues (e.g. thymus and spleen) (Pogribny et al. Citation2005; Koturbash et al. Citation2007; Aypar et al. Citation2011; Goetz et al. Citation2011; Luzhna et al. Citation2015; Koturbash et al. Citation2016; Miousse et al. Citation2017). Radiation-induced DNA methylation seems to be highly complex and depends on several variables such as radiation quality, dose, dose rate, time of sampling, and the biological model.
Investigation of epigenetics in radiation-induced CVD is relatively new; however, a potential contribution of epigenetic mechanisms in radiation-induced CVD has been recently proposed (Sallam et al. Citation2022). Among the various types of epigenetic processes, only DNA methylation has been investigated in a few studies on irradiated hearts (Impey et al. Citation2016; Koturbash et al. Citation2016; Seawright et al. Citation2017).
Koturbash et al. analyzed changes in cardiac DNA methylation patterns in C57BL/6J male mice exposed to space radiation: protons (0.1 Gy), and iron ions (0.5 Gy). The authors described hypomethylation of retrotransposon LINE-1 at 7 and 90 d after heavy iron irradiation. This event is associated with alterations in the one-carbon metabolism pathway particularly the metabolism of methionine that contributes to the synthesis of S-adenosylmethionine (SAM), the main donor of methyl groups for DNA methylation (Koturbash et al. Citation2016). Seawright et al. analyzed the methylation potential of cardiac tissue in female, C57BL/6J mice at 7 d, 1, 4, and 9 months after exposure to low-dose-rate γ-irradiation (4 cGy at 0.01 cGy/h). The analysis showed the reduction in the SAM: S-adenosylhomocysteine (SAH) ratios at 7 d and 1 month after exposure suggesting impaired DNA methylation (Seawright et al. Citation2017). Impey et al. showed persistent changes in the DNA methylation pattern of the left ventricle of 6-month-old C57BL/6J male mice, 22 weeks after total body irradiation (TBI) with 1 Gy of protons. The gene ontology analysis of these DNA regions indicated changes in the signaling pathways enriched for genes contributing to the heart development and differentiation (Impey et al. Citation2016). Yao et al. have recently analyzed the changes in cardiac DNA methylation and RNA expression profiles in a rat model of radiation-induced fibrosis (Yao et al. Citation2022). The study showed a negative regulation between methylation changes and RNA expression levels of 44 genes in the heart at 6 months after local exposure to 18 Gy suggesting a possible molecular mechanism involved in radiation-induced heart fibrosis.
It is important to note that the CVD pathways that are described in the context of methylation changes (Sallam et al. Citation2022) are well known in the pathogenesis of irradiated hearts (Baselet et al. Citation2019; Ramadan, Claessens, et al. Citation2021; Tapio et al. Citation2021). This encourages further investigation of DNA methylation as a possible contributor to radiation-induced CVD.
Transcriptomics
The transcriptome is defined as the complete collection of RNA transcripts that are produced in a cell- and circumstance-specific manner by the genome. Transcriptomics is the study of this collection using various high-throughput methods, such as microarray analysis and RNA sequencing (Wang Z et al. Citation2009). The comparison of transcriptomes allows the identification of genes that are differentially expressed in distinct cell populations and/or conditions.
In the field of radiation-induced CVD, 16 transcriptomic studies have thus far been published from 2005 to 2022. A summary of these studies is presented in .
Table 1. List of the transcriptomic studies on radiation-induced CVD.
Transcriptomic studies on irradiated endothelial cells demonstrate an acute activation of the typical DNA damage response associated with cell cycle repression, decreased cell proliferation, induction of oxidative stress signaling, and an increased inflammatory state (Baselet, Belmans, et al. Citation2017; Baselet et al. Citation2019). While it is still to be understood what causes this pro-inflammatory reaction in endothelial cells after radiation exposure, a plausible explanation would be the release of damage-associated molecular patterns (DAMPs) by stressed and dying endothelial cells (Sun et al. Citation2013). In the collected transcriptomic studies, only one article identified pathways linked to apoptosis after exposure to 20 Gy of X-rays (Wu Q et al. Citation2020). Increased pro-inflammatory response of endothelial cells is linked to radiation-induced chronic oxidative stress, which has been shown to affect the endothelial function by activating redox-sensitive transcription factors, such as NF-κB, AP-1, and Nrf2 (Marui et al. Citation1993; Gaboury et al. Citation1994; Rahman et al. Citation1999; Griendling et al. Citation2000; Awad et al. Citation2013). In good agreement with these findings, alterations in expression levels of the genes related to inflammatory pathways including MAPK, TGF beta, and NF-kB-related signaling were identified in the listed transcriptomic studies (Boerma et al. Citation2005; Halle et al. 2010b; Seemann et al. Citation2013; Coleman et al. Citation2015; Subramanian et al. Citation2017). In the end, this inflammatory cascade could lead to the activation of leukocyte adhesion and migration to cardiovascular tissue (Gupta and Gangenahalli Citation2019). Inflammation also plays a key role in the development and progression of atherosclerosis (Libby Citation2002). A comparison of the transcriptome of arterial biopsies from irradiated patients with control subjects revealed alterations in a group of genes involved in the NF-kB-related pathway, suggesting persistent inflammation years after irradiation (60.4 Gy (range 50–68 Gy) (Halle et al. Citation2010a).
Senescent endothelial cells are an emerging contributor to the pathogenesis of atherosclerosis (Wang Y et al. Citation2016) and are present in human atherosclerotic plaques (Minamino et al. Citation2002). As early as 14 d after exposure to a single dose of 2 Gy X-rays, premature endothelial senescence was identified (Baselet, Belmans, et al. Citation2017). Different pathways contribute to radiation-induced senescence, such as persistent activation of p53 signaling after 8.5 Gy (Boerma et al. Citation2005) or 2 Gy (Baselet, Azimzadeh, et al. Citation2017), which is usually associated with persistent DNA damage (Rufini et al. Citation2013). Furthermore, chronic inflammation, observed acutely after radiation exposure, can in theory also be a cause of cellular senescence (Freund et al. Citation2010; Kojima et al. Citation2013). In this context, chronic inflammation may cause cellular senescence by inducing the expression of p53 and related family members p21, p16, and p14 by persistent NF-κB activation and oxidative stress (Ren et al. Citation2009), which has also been identified by the listed transcriptomic studies. However, this cannot explain the induction of senescence at low X-ray doses, at which endothelial activation was not observed (Baselet, Belmans, et al. Citation2017). Identification of radiation-induced endothelial senescence at doses above 0.5 Gy agreed with other findings. Chronic low-dose-rate γ radiation (4.1 mGy/h) led to premature senescence in HUVECs by the induction of the p53/p21 pathway (Yentrapalli, Azimzadeh, Barjaktarovic, et al. Citation2013). Further analysis revealed PI3K/Akt/mTOR pathway inactivation, which may directly induce premature senescence by increasing the expression of p21 (Yentrapalli, Azimzadeh, Sriharshan, et al. Citation2013). At the gene expression level in these cells, the IGFBP5-related pathways were affected after irradiation (1.4 and 4.1 mGy/h), which is known to inhibit cell proliferation through a p53-dependent mechanism (Kim et al. Citation2007; Rombouts et al. Citation2014). Similarly, the downregulation of the genes related to PI3K/Akt/mTOR/eNOS and alteration of several senescence-associated genes was observed in the transcriptome profile of the endothelial cells isolated from mouse heart 16 weeks after local exposure of the heart to 16 Gy (Azimzadeh et al. Citation2015).
Exposure of the Est2-immortalized human coronary artery endothelial cells (HCAECs), to a dose of 10 Gy of X-rays has been shown to induce premature aging by epigenetic activation of CD44 expression (Lowe and Raj Citation2014). The target transcriptomics analysis on these cells, 14 d after irradiation (10 Gy), indicated significant changes in the set of genes related to the interferon-mediated pathway (Philipp et al. Citation2017).
The transcriptome profiles of other irradiated cardiac cells have not been frequently studied. The transcriptomic response of human-induced pluripotent stem cells (hiPSC)-derived cardiomyocytes to 5 Gy was also examined by RNA sequencing. The study identified alterations in genes involved in oxidative stress and cardiac calcium homeostasis (Becker et al. Citation2018). Analysis of the genes of neonatal rat cardiac myocytes and fibroblasts after 8.5 Gy of X-ray irradiation revealed alterations in the genes responsible for oxidative stress, the p53 signaling pathway, as well as cholesterol and fibrosis (Boerma et al. Citation2005). Fibrosis was also an identified endpoint in whole heart tissue transcriptome performed on 8–12 weeks old male C57BL/6 mice several weeks after local exposure to 16 Gy (Seemann et al. Citation2013; Subramanian et al. Citation2017). The studies identified alterations in several genes related to TGF beta signaling, leading to increased collagen deposition and the onset of fibrosis (Seemann et al. Citation2013; Subramanian et al. Citation2017).
The transcriptomic data showed changes in the expression levels of several genes involved in the response of the cardiovascular system to radiation including DNA damage, cell–cell adhesion, inflammation, fibrosis, and senescence. It is important to note that radiation-induced changes in the transcriptome related to dose and dose rate, as well as time after irradiation and radiation quality, need to be further investigated.
miRNA
Besides protein-encoding RNAs, a plethora of non-coding RNAs is transcribed. One group are small, non-coding microRNAs (miRNAs) involved in post-transcriptional gene regulation. The impact of miRNAs on the cellular response to radiation has been described (Moertl al. 2016). They have been suggested to be predictive biomarkers of response to RT as well as potential drug targets to modulate radiosensitivity (Moertl et al. Citation2016). miRNAs also contribute to a broad spectrum of physiological processes in the cardiovascular system and aberrant expressions are linked with pathologic developments (Nouraee and Mowla Citation2015; Zhou et al. Citation2018). The role of miRNA alterations in initiation, progression, diagnosis, and prognosis of a broad range of CVD including heart failure, acute myocardial infarction, and arrhythmia is described (Zhou et al. Citation2018). Several mRNA and proteins involved in cardiac cell biology, cell survival and apoptosis, autophagy, and cytoskeleton organization have been reported as targets for up- or downregulation of miRNAs in the heart (Zhou et al. Citation2018).
There were 7 studies published from 2011 to 2022 that analyzed the deregulation of miRNAs in cardiovascular cells in in vitro or in vivo models after irradiation. A summary of these studies is presented in . Several of these studies investigated the role of selected CVD-related miRNAs. Alterations in miRNAs including miRNA-1, -15 b, -21, -208, -133, -29, -199 b, -221, -222, and -155 have been detected in various irradiated cells/tissues of the cardiovascular system (Kura et al. Citation2017; Chen Y et al. Citation2021).
Table 2. List of the miRNA analyses of radiation-induced CVD.
Espluggas et al. analyzed the radiation-induced changes in miRNA-146a, -155, -221, and -222 in HUVECs at 2 and 24 h after 2 Gy exposure (Esplugas, Bellés, et al. Citation2019). These miRNAs are involved in CVD and contribute to endothelial dysfunction, lipid metabolism, inflammation, oxidative stress, apoptosis, and angiogenesis. In good agreement with these findings, the changes in the same miRNAs were found in the blood of RT-treated women with breast cancer (Esplugas, Arenas, et al. Citation2019).
The changes in miRNA-17-5p, -21, -7 b, -125a, -146 b-5p, and -10a were investigated in irradiated primary HCAEC at 4 and 24 h after irradiation with 0.2 Gy. Among these miRNAs, miRNA-21 was significantly downregulated at 4 h after irradiation and miRNA-146b was significantly upregulated at 24 h after irradiation (Barjaktarovic, Anastasov, et al. Citation2013). The authors found a negative correlation between miR-21 levels and the structural proteins as the predicted targets.
An increased level of miRNA-21 was identified in cardiac mouse tissue after local high dose (16 Gy) irradiation (Subramanian et al. Citation2017). Kura et al. reported downregulation of miRNA-1 and miRNA 15b and upregulation of miRNA-21 in the rat heart exposed to a single dose of 25 Gy (Kura et al. Citation2016). Azimzadeh et al. analyzed the alterations of miRNA in heart autopsies of plutonium enrichment plant workers by TaqMan-miRNA assays (Azimzadeh, Azizova, et al. Citation2017; Azimzadeh et al. Citation2020). The workers included in the study were occupationally exposed to low and moderate external radiation doses (<0.1 Gy, 0.1–0.5 Gy, and >0.5 Gy) throughout their working life and were later diagnosed and later died with ischemic heart disease similar to the unexposed control group. Increased levels of miRNA-21 and −146 as potential biomarkers of CVD were also reported in an analysis of a group of workers exposed to the highest chronic dose (>0.5 Gy).
Only a few studies applied array platforms to analyze the effect of irradiation on global miRNA profiles. Aryankalayil et al. used Agilent Mouse miRNA arrays to investigate changes in miRNA in mouse cardiac tissue at 48 h after TBI with 1, 2, 4, 8, and 12 Gy. The authors identified significant upregulation of miR-149-3p, -6538, -8101, -7118-5p, -211-3p, and -3960 in irradiated hearts at the highest dose (12 Gy) (Aryankalayil et al. Citation2021).
Wagner-Ecker et al. analyzed 315 miRNAs in human microvascular endothelial cells (HDMEC) after 2 Gy irradiation. The authors found upregulated miRNA let-7g, -16, -20a, -21, and -29c, while miR-18a, -125a, -127, -148 b, -189, and -503 were downregulated 6 h after exposure to 2 Gy. Individual analysis of miR-125a, -127, -189, and let-7g by overexpression/inhibition identified miRNA changes as regulators of radiosensitivity in HDMEC (Wagner-Ecker et al. Citation2010).
Kraemer et al. compared global miRNA expression of sham-irradiated and irradiated EA.hy926 cells and HUVECs using TaqMan® Low-Density Array A version 2 (Applied Biosystems, Foster City, USA), at 4 and 24 h after single irradiation with 2.5 Gy. The authors found a wide range of changes in various miRNAs, including downregulation of 4 miRNAs (let-7d, -519e, -323-3p, and -517 b) and upregulation of 1 miRNA (miR-518b) both 4 and 24 h after irradiation. The changes of other identified miRNA were transient at both time points (Kraemer et al. Citation2011). The predicted molecular targets of miRNAs affected by irradiation are mainly involved in the signaling pathways contributing to cardiac hypertrophy, fibrosis, and oxidative stress as well as endothelial structure and function (Sriharshan Citation2014; Kura et al. Citation2017).
The miRNA response of other cardiac cells, including cardiomyocytes and fibroblasts, to radiation exposure, remains to be investigated. The role of miRNA changes has been studied in skin fibroblasts, highlighting the potential regulatory role of miRNA in radiation response. In a microarray approach including 462 miRNAs, Maes et al. found the dose- and time-dependent changes in human fibroblasts in response to low (0.1 Gy) and high (2.0 Gy) X-ray doses (Maes et al. Citation2008). Changed miRNAs were predicted to act in typical radiation response pathways, such as apoptosis, cell cycle regulation, and DNA repair but also in cell adhesion or cytoskeleton biosynthesis (Maes et al. Citation2008).
In summary, changes in the miRNA profiles after irradiation is an established component in cellular radiation response in several cell types of the cardiovascular system. However, the responses are highly specific for doses, time points, and cell types. Moreover, the complexity of miRNA functions makes the biological interpretation of their alterations challenging because one miRNA may target many mRNAs and one mRNA may be targeted by multiple miRNAs.
Proteomics and post-translational modifications
Alteration in the proteome of cells and tissues is tightly linked to the physiological and pathological changes in a biological system. This makes proteomics analysis a comprehensive tool enabling the identification of the biological effect of radiation exposure. The application of proteomics approaches is well established in the radiation biology field (Azimzadeh et al. Citation2014; Azimzadeh and Tapio Citation2017).
Among the studies reported from 2011 to 2022 that applied proteomics platforms to investigate the cell and tissue response to radiation exposure, 27 studies analyzed the proteome response of cardiac tissue, vascular system or cardiac cells, and cell compartments using a broad range of quantitative proteomics platforms. A summary of these studies and their findings are presented in .
Table 3. List of the proteomics studies on radiation-induced CVD.
Mitochondrial proteins were the most sensitive protein cluster affected in irradiated hearts (Barjaktarovic et al. Citation2011; Azimzadeh et al. Citation2013; Azimzadeh, Azizova, et al. Citation2017; Azimzadeh, Subramanian et al. Citation2021). The analysis of cardiac mitochondrial proteome in C57BL/6 mice at 4 and 40 weeks after local heart exposure to moderate or high doses of X-rays (0.2 or 2 Gy) showed significant downregulation of several subunits of mitochondrial complexes I, III, and V after irradiation. The number and extent of downregulation increased over time (Barjaktarovic et al. Citation2011; Barjaktarovic, Shyla, et al. Citation2013). Mitochondrial proteins were also downregulated in the proteome profile of the murine hearts at 16 and 20 weeks after a high dose (16 Gy) of local heart irradiation (Azimzadeh et al. Citation2013; Xu et al. Citation2021). Cardiac mitochondrial proteins were also mainly affected short-term (4 and 24 h) after TBI (3 Gy) (Azimzadeh et al. Citation2012). The long-term effect of chronic exposure on cardiac mitochondria proteome and acetylome was exclusively studied in apolipoprotein E (ApoE)-deficient C57BL/6J female mice exposed to low-dose-rate (0.02 Gy/d) gamma radiation for 300 days using label-free proteomics (Barjaktarovic et al. Citation2019). This study showed that changes in mitochondrial acetylation after irradiation were associated with impaired cardiac metabolism (Barjaktarovic et al. Citation2019). The effect of chronic exposure was also analyzed in heart autopsies of Russian Mayak nuclear workers who were exposed only to external gamma radiation. A dose-dependent downregulation of mitochondrial proteins was observed in the heart of all exposed groups (<0.1 Gy, 0.1–0.5 Gy, and >0.5 Gy, chronic TBI) in comparison to the controls (Azimzadeh, Azizova, et al. Citation2017). The radiation-induced downregulation of mitochondrial proteins is accompanied by alteration in ultrastructure and reduced activity of oxidative phosphorylation subunits (Barjaktarovic et al. Citation2011; Barjaktarovic, Shyla, et al. Citation2013; Barjaktarovic et al. Citation2019).
Radiation-induced mitochondrial dysfunction increases the reactive oxygen species (ROS) production (Barjaktarovic et al. Citation2011; Barjaktarovic, Shyla, et al. Citation2013) and the level of oxidative stress-induced protein modifications (Azimzadeh et al. Citation2013; Azimzadeh et al. Citation2015). Although the oxidative stress is immediately measurable in the irradiated heart in the form of oxidized proteins (Azimzadeh et al. Citation2011; Barjaktarovic et al. Citation2011; Azimzadeh et al. Citation2013; Barjaktarovic, Shyla, et al. Citation2013; Azimzadeh, Subramanian et al. Citation2021), the expression of antioxidant defense proteins differs in the cardiac proteome in a dose- and time-dependent manner (Azimzadeh and Tapio Citation2017). Analysis of the mouse heart proteome, short (24 h) after 3 Gy TBI showed upregulation of acute-phase proteins involved in the oxidative stress response including peroxiredoxin, hemopexin, ferritin, transferrin, and murinoglobulin1 (Azimzadeh et al. Citation2011). Proteomics analysis of mouse hearts irradiated locally at a high dose (16 Gy) showed radiation-induced upregulation of proteins involved in oxidative stress response 16 weeks after irradiation (Azimzadeh et al. Citation2013) but downregulation after 40 weeks (Subramanian et al. Citation2017; Azimzadeh, Subramanian et al. Citation2021). The analysis of the proteome of heart autopsies from Mayak workers showed a dose-dependent reduction of several antioxidant defense proteins including catalase, superoxide dismutase, peroxiredoxins, and glutathione-S-transferase in the highest dose group (>0.5 Gy, chronic TBI) (Azimzadeh, Azizova, et al. Citation2017).
Physiological cardiac function depends on consistent energy production through oxidative phosphorylation in mitochondria: therefore, alterations in cardiac energy metabolism have been reported in various cardiac diseases (Wang Y et al. Citation2013; Guo et al. Citation2017). In good agreement with this, alteration in the energy metabolism has been described in the irradiated heart (Azimzadeh et al. Citation2012; Azimzadeh et al. Citation2013; Barjaktarovic, Shyla, et al. Citation2013; Azimzadeh, Azizova, et al. Citation2017; Subramanian et al. Citation2017; Xu et al. Citation2021). Analysis of the formalin-fixed paraffin-embedded (FFPE) heart proteome shortly (24 h) after (3 Gy) TBI of mice showed that several enzymes involved in fatty acid oxidation, pyruvate metabolism, and citric acid cycle were significantly downregulated in irradiated hearts (Azimzadeh et al. Citation2012). Similarly, lipid metabolism enzymes were downregulated in the proteome of locally irradiated (2 Gy) mouse hearts at 40 weeks after radiation (Barjaktarovic, Shyla, et al. Citation2013). The majority of the dysregulated proteins in the heart after local high-dose irradiation (16 Gy) were also associated with the regulatory network of peroxisome proliferator-activated receptor alpha (PPAR alpha) (Azimzadeh et al. Citation2013), a key regulator of cardiac fatty acid oxidation, lipoprotein assembly and lipid transport (Watanabe et al. Citation2000; Finck Citation2007). The bioinformatic analysis of the proteome of the irradiated heart predicted the deactivation of PPAR alpha (Azimzadeh et al. Citation2013; Subramanian et al. Citation2017; Azimzadeh, Subramanian et al. Citation2021). In good agreement with this finding, the analysis of the cardiac proteome of the PPAR alpha null mice, at 20 weeks after exposure to the local high dose (16 Gy) showed the least changes, demonstrating the central role of PPAR alpha in cardiac response to ionizing radiation (Subramanian et al. Citation2018; Azimzadeh, Subramanian et al. Citation2021). Disruption of the PPAR alpha pathway has previously been described in a variety of heart diseases (Dewald et al. Citation2005; Finck Citation2007). It has been recently shown that the effect of high-dose irradiation on the heart can be ameliorated by the activation of PPAR alpha (Azimzadeh, Subramanian et al. Citation2021).
The impact of vascular injury and endothelial dysfunction in the pathogenesis of radiation-induced CVD has been described in animal and human data (Boerma and Hauer-Jensen Citation2010; Baker et al. Citation2011; Seemann et al. Citation2012; Azimzadeh et al. Citation2013). The effects of radiation exposure on the proteome of different endothelial cell models have been studied. Proteomics analysis of both in vivo and in vitro models indicated significant changes in the proteins involved in the structure and function of cardiac endothelial cells. The Impairment of PI3K/Akt/NO signaling and alteration in NO availability are characteristics of endothelial dysfunction (Wiench et al. Citation2013). The RhoA pathway was affected in the proteome of irradiated transformed human umbilical vein endothelial cell line (HUVEC; EA.hy926) after high (2.5 Gy) (Sriharshan et al. Citation2012) and moderate-dose radiation (0.2 Gy) (Pluder et al. Citation2011). Both RhoGDI and PI3K/Akt/NO signaling pathways were affected following irradiation in the proteome of endothelial cells exposed to the dose of 0.5 Gy as well as the proteome of endothelial cells isolated directly from locally irradiated (16 Gy) mouse hearts (Azimzadeh et al. Citation2015; Azimzadeh, Subramanian, et al. Citation2017). Proteomics analysis of the chronically irradiated HUVECs (1.4 and 2.4 mGy/h) indicated the involvement of PI3K/Akt/mTOR pathway inhibition in the radiation-induced endothelial senescence (Yentrapalli, Azimzadeh, Sriharshan, et al. Citation2013).
Cardiac tissue integrity, as well as cardiac cell interaction and communication is supported by the ECM (Chang et al. Citation2016). Changes in cardiac ECM are known to contribute to heart pathologies (Westermann et al. Citation2008; Diez Citation2010) including cardiac fibrosis (Westermann et al. Citation2008) and cardiac remodeling (Chang et al. Citation2016; Lindsey et al. Citation2016). Deposition of collagen, excessive fibroblast proliferation, and upregulation of TGF beta are all characteristics of radiation-induced heart fibrosis (Gao et al. Citation2012; Seemann et al. Citation2012; Sun et al. Citation2016). The expression of ECM proteins, such as biglycan, decorin, fibrinogen, and collagen was shown to be increased in the cardiac proteome, at 16, 20, and 40 weeks after local heart exposure to 16 Gy (Azimzadeh et al. Citation2013; Subramanian et al. Citation2017; Azimzadeh, Subramanian et al. Citation2021; Xu et al. Citation2021). Proteomics analysis of the FFPE hearts of Mayak workers (>500 mGy, chronical TBI) showed significant changes in the expression of ECM proteins in the chronically irradiated hearts. The collagen isoforms were all upregulated in irradiated samples (Azimzadeh et al. Citation2020).
Taken together, the proteome response of irradiated heart is well characterized and supports several KEs in CVD AOP including mitochondrial dysfunction, metabolic alterations, and structural remodeling.
Secretome
The secretome is the entirety of all biomolecules released from a cell to the extracellular space, where it has indispensable functions in cell-to-cell and cell-to-ECM communication. The exploration of the secretome includes molecular and functional effects of released factors and offers unique insights into the molecular interactions between cells. Autocrine, paracrine, and endocrine secretome-mediated intercellular communication occurs by the release of individual soluble components, such as chemokines, cytokines, and growth factors, but also by extracellular vesicles (EVs) that transport a variety of active macromolecules, including nucleic acids, metabolites, and lipids (Mukherjee and Mani Citation2013).
For the dynamic equilibrium of cells that compose the cardiovascular system, fine-tuned communication via the secretome is essential (Tirziu et al. Citation2010; Liu Q et al. Citation2021). Under stress conditions, an orchestrated crosstalk between cardiovascular cells is of particular importance to sustain efficient responses (Martins-Marques et al. Citation2021). Especially for regenerative processes after heart injury, effects from secreted factors are intensively discussed. In this context EVs secreted from mesenchymal stem cells indicated high potential (Gallina et al. Citation2015). Intercellular communication is one of the proposed mechanisms of radiation-induced atherosclerosis (Ramadan et al. Citation2019, Citation2020; Ramadan, Baatout, et al. Citation2021).
The secretome is a vital component for communication during the radiation response of cells to radiation-induced damage as well as for mediating non-targeted effects (Yahyapour et al. Citation2018; Dawood et al. Citation2021). A specific type of secretome is released by radiation-induced prematurely senescent cells and consists of a collection of cytokines, chemokines, proteases, and growth factors. This senescence-associated secretory phenotype (SASP) can contribute to different cellular responses (Coppe et al. Citation2008). Amongst others, the adoption of this phenotype is described in endothelial cells, fibroblasts, and cardiomyocytes (Li M et al. Citation2018).
Several studies described radiation-induced changes in secreted chemokines and interleukins from cardiovascular cells. For example, the interleukins IL-6 and IL-8 and the chemokines CXCL5/8 are released from endothelial cells after various radiation doses and timepoints (Van Der Meeren et al. Citation1999; Baselet et al. Citation2019). Likewise, for fibroblasts, the secretion of individual SASP-factors, like IL-1, IL-6, and IL-8 were described after irradiation (Coppe et al. Citation2008; Isermann et al. Citation2020). Only two studies were found that employed an omics approach for the unbiased identification of radiation-induced changes in the secretome. Philip et al. quantified around 1000 secreted endothelial proteins, from which 338 were dysregulated 14 d after exposure to 10 Gy. Pathway analysis of these proteins revealed inflammatory response and identified interferon Type I as the main pathways of the radiation-deregulated proteins (Philipp et al. Citation2017). Proteomic data were also reported for radiation effects on the secretome of fibroblasts where more than 500 upregulated proteins in the secretome of irradiated fibroblasts with 10 Gy were documented in the ‘SASP atlas’ (Basisty et al. Citation2020). They also made a distinction between radiation-dysregulated soluble factors and factors released within EVs. The main pathways covered by the secreted factors included tissue and cell structure effects, such as ECM organization, actin cytoskeleton, integrin interactions, and peptidase regulation. In contrast to endothelial cells and fibroblasts, there was no information on the effect of irradiation on the secretome of cardiomyocytes.
Factors secreted from irradiated cardiovascular cells can affect recipient cells. We found one study which performed a medium transfer from irradiated endothelial cells to non-irradiated cells followed by proteome analysis. Induction of interferon Type I-related proteins and activation of the STAT3 pathway were identified as the main affected pathways in endothelial cells 14 d after exposure to 10 Gy X-rays (Philipp et al. Citation2017). Most other studies were restricted to the description of functional alterations as consequences of secretome exposure and provided no omics data.
Radiation effects on the cardiovascular structure may also be triggered by secreted factors from cell types that are not the main constituents of the cardiovascular system, such as adipocytes, mesenchymal stem cells, tumor cells, and blood cells. For example, endothelial cells and cardiomyocytes were recipients of EVs from mesenchymal stem cells and tumor cells. RNA sequencing of these EVs identified a large number of radiation-deregulated miRNAs which were related to CVD (Jabbari et al. Citation2019; Luo et al. Citation2021). On the other hand, several studies identified peripheral blood mononuclear cells (PBMC)-secreted EVs as mediators of radiation effects in cardiovascular cells. By RNA-sequencing and label-free proteomics, Moertl et al. showed dose-dependent changes in the miRNA and proteome cargo of EVs derived from irradiated PBMCs. Integrated pathway analysis of the radiation-triggered EV proteins and miRNAs consistently predicted an association of deregulated molecules with apoptosis, cell death, and survival (Moertl et al. Citation2020). Further omics analysis demonstrated large increases in the miRNA and protein content of EVs secreted from PBMCs after high dose exposure (60 Gy) (Wagner et al. Citation2018). Consistently, both studies showed that radiation-triggered EV changes induced pro-survival and angiogenic effects on endothelial cells. In the meantime, the secretome of irradiated PBMCs was shown to improve cardiac performance in a porcine infarction model. Transcriptional analyses of PBMC secretome effects identified the regulation of genes that were essential for cardiomyocyte function and the downregulation of pro-inflammatory genes (Mildner et al. Citation2022).
In summary, there is very limited omics data on the secretomes released from cardiovascular cells after irradiation. However, this knowledge is an indispensable tool to understand the crosstalk between different components of the heart in response to radiation exposure.
Metabolomics and lipidomics
Metabolomics and its subcategory lipidomics aim to identify and in some cases quantify small molecules called metabolites in cells, tissues, and biofluids. These small molecules can serve as the building blocks to maintain structural, genetic, signaling, and metabolic integrity. Both metabolomics and lipidomics have gained traction in radiation biology and are now considered optimal integral-omics technology in a systems biology approach. Targeted and untargeted approaches in both metabolomics and lipidomics have distinct pros and cons, including positive metabolite identification and coverage of different classes, or sensitivity and absolute quantification levels. Although the applied detection methods in the mainstream of metabolomics have also evolved and become more sensitive, there are still challenges where some methods (e.g. nuclear magnetic resonance [NMR]) that provide important structural information need to be yet improved as they identify only the most abundant molecules.
Among studies that applied metabolomics or lipidomics in radiation research from 2011 to 2022, 9 publications (including 2 reviews) focused on profiling small molecules related to CVD. A summary of these studies and their findings is presented in .
Table 4. List of the metabolomics studies on radiation-induced CVD.
Gramytyka et al. exposed human cardiomyocytes to 2 Gy (CLINAC 600 6 MV photons, 1 Gy/min) and evaluated metabolic levels at 48 h afterwards with high-resolution magic-angle spinning (HR-MAS) NMR (Gramatyka et al. Citation2018). Significant differences were identified between exposed and unexposed groups, specifically related to oxidative stress, energy metabolism, membrane integrity, and amino acids (Gramatyka et al. Citation2018). The investigators extended their work to study mice following TBI (0.2 or 2 Gy, Varian CLINAC 23006 MV photons, 300 MU/min) and analyzed the heart metabolites at 48 h or 20 weeks after exposure. Metabolomic analysis with 1H NMR spectroscopy showed that most changes occurred at the earlier time point with the higher dose, with pantothenate, glutamate, alanine, malonate, acetylcarnitine, glycine, and adenosine as the most abundant markers. Glutamine and acetylcarnitine were significantly reduced in the 2 Gy irradiated group at 20 weeks after exposure, while the exposure to 0.2 Gy showed no statistically significant effects on heart metabolomics. Treatment of mice with resveratrol before exposure to 2 Gy was able to reduce effects in glycerophosphocholine and choline compounds (Gramatyka et al. Citation2020). Rats exposed to gamma rays (6 Gy TBI with 60Co) showed that lipid peroxidation metabolites of heart tissue were significantly increased at 24 h after exposure, although the overall levels were measured with a standard malondialdehyde (MDA) assay (de Freitas et al. Citation2013). The analysis of the heart metabolites in mice exposed to TBI at 9.6 Gy (0.6 Gy/min, 60Co) showed changes in various fatty acids and inflammatory mediator hydroxyprostaglandin E1 in the heart (Cheema A. K. et al. Citation2020). The heart showed significant early responses to radiation, which were dampened quickly by day 9 after exposure. The authors showed that the administration of the radioprotective agent, amifostine partially corrects the effect of TBI on the heart metabolome (Cheema A. K. et al. Citation2020).
A few studies have also examined the metabolome of heart tissue of nonhuman primates (NHPs). Cheema et al. identified increased levels of long-chain acylcarnitines, particularly sterearoylcarnitine, and linoleylcarnitine, in the heart tissue of surviving and non-surviving NHPs following 7.2 Gy of TBI (60Co, 0.6 Gy/min) (Cheema A. K. et al. 2019). Alteration of long-chain acylcarnitines was associated with increased hypoxia and may impact both fatty acid β-oxidation and proper mitochondrial function (Cheema A. K. et al. Citation2019). In addition, a decrease of fatty acid amines, the mitochondrial-regulated metabolites with anti-inflammatory properties, strongly suggests that mitochondria are implicated in radiation responses in heart tissue (Cheema A. K. et al. Citation2019). Two other studies in NHPs, after partial body irradiation of 12 Gy (6 MV LINAC photons, 0.8 Gy/min) identified 8 enriched metabolic pathways in the left side and 3 in the right side of the heart at 4 days after exposure, with some changes persisting at 3 weeks after exposure (Kumar et al. Citation2021; Zalesak-Kravec et al. Citation2021). Kumar et al. showed that fatty acid β-oxidation (short, long, and very long-chain fatty acids), phospholipid biosynthesis, phenylacetate metabolism, and pyrimidine metabolism were significantly enriched (p < .05) in the left side of the heart, while glucose-alanine cycle, arginine, and proline metabolism, and selenoamino acid metabolism were significantly enriched in the right side (Kumar et al. Citation2021). Metabolomics, in combination with proteomic data from the same model at 3 weeks after exposure, showed dysregulation of inflammatory, energy metabolism, and myocardial remodeling pathways, as well as retinoid homeostasis (Zalesak-Kravec et al. Citation2021).
These studies have focused on high doses and dose rates. Radiation exposure and CVD remain a concern as well for astronauts and deep space exploration. The mitochondrial-related pathways were shown to be affected by both spaceflight (da Silveira et al. Citation2020) and space radiation (Vernice et al. Citation2020; Barnette et al. Citation2021; Laiakis et al. Citation2021).
One study has investigated whether exposure to 16O (0.1, 0.25, or 1 Gy, 600 MeV/n) would alter specific metabolic pathways in heart tissue. The investigators (Miousse et al. Citation2019) identified changes in the transsulfuration pathway with high levels of cystathione, while the homocysteine remethylation pathway was unaffected.
Taken together, the metabolomic studies reported here highlight the involvement of mitochondrial and metabolic alterations in cardiac injury due to radiation exposure.
Biofluid
Metabolomics analysis of minimally invasive and easily accessible biofluids provides a reliable platform to monitor radiation-induced biological effects including CVD. As with tissues and cells, the vast majority of the literature on biofluids (e.g. serum, plasma, urine, saliva, and feces) focuses on high-dose exposures for biodosimetry, with increased emphasis on connecting biomarkers of delayed effects of acute radiation exposure (Pannkuk et al. Citation2017; Satyamitra et al. Citation2020; Vicente et al. Citation2020). The metabolomic and lipidomic studies on radiation biomarkers in biofluids at the low dose and low dose rates are limited. Nine studies that analyzed biofluids following irradiation (from 2014 to 2022) are summarized in .
Table 5. List of the biofluid analyses on radiation-induced CVD.
McGarrah et al. (Citation2018) reported a list of such targets in plasma including branched-chain amino acids, branched-chain α-ketoacids, ketone oxidation, long-chain acylcarnitines, tricarboxylic acid (TCA) cycle intermediates, certain amino acids, pyrimidines, pentose phosphate pathway intermediates, short-chain dicarboxylacylcarnitine, and trimethylamine N-oxide (TMAO). In particular, TMAO (a gut microbiota-derived metabolite) is emerging as a strong biomarker for CVD (Schiattarella et al. Citation2017), in addition to changes in short-chain fatty acids and secondary bile acids (Brown and Hazen Citation2018). Lipidomic changes have also been shown in patients with CAD and coronary microvascular dysfunction (Lindner et al. Citation2021) while decreased total bile acids are consistently shown in patients and animal models with CAD (Li, Shu et al. Citation2020; Chong Nguyen et al. Citation2021). The majority of these metabolites, lipid species, and classes could easily be measured with targeted metabolomic and lipidomic approaches.
Atherogenic lipid profiles with low high-density lipoprotein (HDL) cholesterol and hypertriglyceridemia were also found in Japanese atomic bomb survivors (Akahoshi et al. Citation2003). These observations have been suggested as a mechanism to link radiation exposure and coronary heart disease.
A select number of studies with internal contamination (injectable 137Cs or uranium in drinking water) have assessed biomarkers in serum, plasma, or urine. Goudarzi et al. identified perturbations in amino acid metabolism, fatty acids, TCA cycle, glycolysis, and phosphatidylcholines (PCs) in mice injected with 137CsCl (2–30 d, 1.95–9.91 Gy cumulative dose) (Goudarzi et al. Citation2015). A similar approach by Li et al. described oleamide and sphingosine-1-phosphate as metabolites that were perturbed irrespective of dose rate (varying dose rates from 0.16 to 1.36 Gy/d) (Li, Lin et al. Citation2020). Sphingosine-1-phosphate is of particular importance because of its significant role in vascular and immune systems and the metabolism of HDL (Proia and Hla Citation2015; Jiang XC Citation2017; Cartier and Hla Citation2019). Chronic low dose exposure to uranium on the other hand showed significant changes in the nicotinate-nicotinamide pathway and unsaturated fatty acid biosynthesis, reflected in the urine of male and female rats (Grison et al. Citation2019), which could be due to the dual toxicity of uranium (radioactive and a heavy metal). The estimated absorbed dose rate to the kidneys at 9 months of age was estimated to be 5.4 × 10−7 Gy/d, with a cumulative dose of 0.15 mGy under the assumption of a constant dose rate over 9 months.
While internal exposures demonstrated significant changes in metabolomics and lipidomics, external exposures to low dose rate photons showed similar results. TCA cycle and fatty acids were the most dysregulated pathways in the urine of high dose rate exposed mice (3.09 mGy/min) (Goudarzi et al. Citation2014), with a novel metabolite, hexosamine-valine-isoleucine-OH (Hex-V-I), increased 150-fold in low dose rate exposed (variable 0.1–1 Gy/d) mice vs. an 80-fold increase in mice exposed at higher dose rate (∼0.8 Gy/min) (Pannkuk et al. Citation2021). The role of this novel metabolite remains to be elucidated, including whether persistent changes may have a role in the delayed effects of acute radiation exposure. A common theme emerging from the published studies is alterations in energy metabolism showing the most significant changes in TCA and mitochondria (Jang et al. Citation2016; Pannkuk et al. Citation2017; Kawamura et al. Citation2018; Livingston et al. Citation2020). Studies on human exposures are limited to biofluids, focusing primarily on samples from cancer patients with doses outside the scope of this review.
Nonetheless, mitochondrial dysregulation, and pro-inflammatory metabolites were prominent results in biofluids (serum and urine) from TBI human cohorts (Laiakis et al. Citation2014; Laiakis et al. Citation2017; Vera et al. Citation2020).
Interestingly, alterations in fatty acids, various lipids, glycerol, glycolate, and choline-containing phospholipids were described in serum analysis of breast cancer and head and neck cancer patients who received RT (Shaikh et al. Citation2017; Jelonek et al. Citation2020; Boguszewicz et al. Citation2021). Changes in circulating levels of lipids were reported as a biosignature of radiation exposure in TBI patients before stem cell transplantation (Laiakis et al. Citation2017).
In contrast to urine and blood, fecal material and saliva were two largely unexplored biomaterials in low-dose radiation research, although evidence is accumulating showing that radiation doses as low as 0.5 Gy have significant effects. BALB/c mice exposed to photons (0.5 Gy) showed microbiome changes linked to the glucagon signaling pathway, central carbon metabolism in cancer, and TCA cycle (Laiakis et al. Citation2014; Liu X et al. Citation2019), while salivary metabolomics in C57BL/6 mice exposed to photons (0.5, 3, or 8 Gy) showed that the overall metabolic profile remained distinct from sham-exposed animals one week after exposure (Laiakis et al. Citation2016).
Integrative multi-omics
A deep understanding of the biological effects of radiation exposure is greatly facilitated by a systems biology multi-omics approach. As described in the previous sections, each omics technique provides a picture of one specific aspect of how the biological system changes in response to radiation exposure. To achieve a comprehensive understanding of the systemic impact on cells or tissue, it is necessary to integrate findings from different omics approaches to resolve the complexity of the organization of biological processes. The current status of the systems biology multi-omics approach in CVD research has been recently well-reviewed, including the limitations and advantages (Joshi et al. Citation2021). Currently, specific multi-omics studies to look at radiation impact on the cardiovascular system are lacking. Only a few studies have so far been performed using at least two omics platforms (Subramanian et al. Citation2017; Papiez et al. Citation2018; Cheema et al. Citation2022). As stated above, literature exists for multi-omics studies related to CVD in general. On the other hand, multi-omics studies on radiation are limited but show the power of these studies.
Papiez et al. analyzed the proteomic and RNA sequencing data of the postmortem cardiac left ventricle samples from Mayak workers exposed to a dose >0.5 Gy (Papiez et al. Citation2018). The analysis revealed that a systemic response as a function of dose caused alterations in glycolysis, oxidative phosphorylation, the TCA cycle, and PPAR alpha signaling pathways to be dominantly impacted in the cardiac tissue from the Mayak nuclear workers. The study confirmed the previous findings on Mayak heart autopsies (Azimzadeh, Azizova, et al. Citation2017) where the metabolic and mitochondrial proteins were highly impacted in the cardiac tissue from the nuclear workers exposed to radiation.
The cardiac proteome and transcriptome were analyzed in male C57BL/6 mice at 40 weeks after an acute local high-dose heart exposure (16 Gy) (Subramanian et al. Citation2017). Although the integrative analysis of gene expression array and proteomics data showed a modest direct correlation between gene and protein expression, it demonstrated that cardiac TGF beta signaling and PPAR alpha signaling were affected by irradiation at both the mRNA and protein levels (Subramanian et al. Citation2017).
Proteomics and transcriptomics were also used to compare the endothelial cell responses at 1 and 7 d after exposure to a single 2 Gy dose of X-rays or iron ions (Baselet, Azimzadeh, et al. Citation2017). The analysis showed alterations in genes and proteins involved in cell-cell adhesion, endocytosis signaling, and inflammation. The inflammatory response and adherence properties of endothelial cells were decreased after iron ion exposure, whereas they were increased by X-rays (Baselet, Azimzadeh, et al. Citation2017).
A recent study utilized a multi-omics approach with metabolomics, proteomics, and lipidomics to assess acute radiation injury in the serum of male and female NHP models following exposure to 6–8.5 Gy with 60Co (Cheema et al. Citation2022). The study showed alterations in the circulating levels of lipids, metabolites, and proteins. The analysis demonstrated a blood-based multi-omics biomarker panel that contained four analytes that could stratify individuals as a function of dose. The panel of analytes was comprised of PC (16:0/22:6), acetyl carnitine, suberyl glycine, and serpin family A protein. Among these metabolites, acetyl carnitine (Dundar et al. Citation2016), suberyl glycine (Lu et al. Citation2017), and serpin family A protein (Li et al. Citation2016) have been previously reported as decent cardiac toxicity markers. Interestingly, all 4 analytes are heavily related to the metabolic, mitochondrial, and glycolytic functions. By using this multi-omics approach, the authors were able to provide a focused and targeted list of biomarkers that can potentially be utilized for therapy with further research.
The ultimate example of a comprehensive multi-omic analysis of radiation impact on the cardiovascular system is the NASA Twin Study which entailed studying samples obtained from two identical twins, with one twin being exposed to space radiation at low Earth orbit onboard the International Space Station (ISS) compared to the identical twin on Earth (Garrett-Bakelman et al. Citation2019). This is an important example of demonstrating how a comprehensive multi-omics approach can provide a complete map of the systemic biological impact. In this study, Garrett-Bakelman et al. performed transcriptomics, epigenetics, proteomics, metabolomics, and microbiomics on multiple time points from blood (i.e. multiple cell types isolated from the blood), urine, and fecal samples. Although the focus of that study was not specifically on cardiovascular health risks associated with spaceflight, utilizing this multi-omics approach revealed that cardiovascular changes occur during spaceflight. Specifically, markers associated with inflammatory cytokines and chemokines and oxidative stress were increased for the twin in flight, while no changes occurred for the control ground twin. In combination with the proteomic data, the authors found alterations in the proteins involved in the vascular structure and function. From this comprehensive multi-omics study, it was determined that vascular changes or adaptations occurring during spaceflight might have an impact on cardiovascular health due to the combination of space radiation and microgravity.
Discussion
Radiation omics support CVD AOP development
The application of omics in the context of hazard and risk assessment has already been discussed in toxicology (Bridges et al. Citation2017; Sauer et al. Citation2017; Aguayo-Orozco et al. Citation2019). Omics data offer great potential for the discovery of reliable biomarkers and biomarker arrays, as well as for integration into weight-of-evidence approaches to interpreting the mode of action of toxic substances (Bridges et al. Citation2017; Sauer et al. Citation2017; Aguayo-Orozco et al. Citation2019). Interest in the application of the AOP approach to radiation protection has increased greatly, drawing on experience gained in the field of chemical toxicology (NCRP Citation2020; Chauhan, Hamada, et al. Citation2021; Chauhan, Leblanc, et al. Citation2021; Chauhan, Sherman, et al. Citation2021; Chauhan, Stricklin, et al. Citation2021; Chauhan, Wilkins, et al. Citation2021; Chauhan, Beaton, et al. Citation2022). This review aimed to describe the potential of radiation omics to be incorporated into the AOP framework.
The studies reviewed here are representative of omics data that have been generated over the last two decades in the context of radiation-induced CVD. The literature review was conducted by experts in related fields and the relevance of the findings related to adverse effects introduced in CVD AOP (Chauhan, Hamada, et al. Citation2021) was highlighted. Data discussed here collectively showed the potential to support different KEs in the hypothetical CVD AOP previously proposed by the authors (Chauhan, Hamada, et al. Citation2021). However, the studies discussed here need to be comprehensively evaluated to fulfill B-H criteria. The development and progression of radiation-induced CVD is a multidimensional phenomenon, so studying the interaction between genes, proteins, metabolites, etc., at all levels of biological organization is crucial.
Interestingly, some of the omics findings are consistent following different radiation scenarios at different time points, suggesting common AOs of radiation exposure to the heart ( and ). There were several omics data showing the changes in oxidative stress, mitochondrial dysfunction, metabolic, and ECM remodeling, and endothelial signaling pathway. The change in cardiac energy metabolism is known as a hallmark of different heart pathology including those induced by irradiation. Identification of the different genes, proteins, and metabolites associated with heart metabolism offers an attractive panel of potential candidates for biomarker profiling, diagnosis, and risk assessment of radiation-induced CVD.
Figure 1. Categories of radiation omics studies to support proposed CVD AOP. Available types of omics data are represented across key events in the CVD AOP network as colored boxes (modified from Chauhan, Hamada, et al. (Citation2021)).
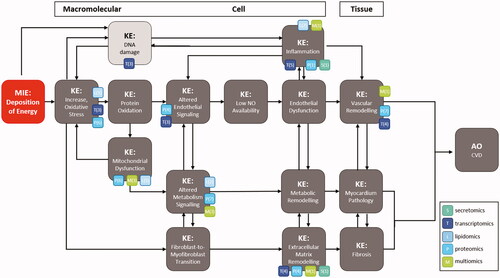
Transcriptomics data discussed here point to the importance of DNA damage in the progression of vascular pathology and open the debate on the inclusion of these cellular events as KEs in the proposed CVD AOP. Changes in the inflammatory response have also been reported by several radiation omics studies, but further studies are needed to establish a relationship between inflammatory status and biological effects of radiation exposure.
The proposed CVD AOP contains several bidirectional relationships between major KEs emphasizing molecular and cellular interplay in the cardiovascular system. The omics data on secretomes and exosomes can provide evidence for such cross-talks between pathological processes contributing to heart injury after irradiation. Such data may fill the knowledge gaps in the potential interplay between endothelial dysfunction, and cardiac metabolic and structural changes.
Systems biology: a path forward for comprehensive AOP
The integration of findings generated by different omics platforms is key for a systems biology approach to gain a full understanding of how radiation exposure impacts the cardiovascular system. Integrating systems biology into the AOP framework can potentially enrich AOP features by expanding the linear abstraction behind KEs to a more complex network of those biological events. To achieve a comprehensive picture of the radiation effect, non-omics findings, such as pathologic images, clinical measures, and epidemiological information as well as other biochemical and biological assays and arrays are necessary to fill the gaps in assessing risk-related outcomes. illustrates a proposed multidisciplinary and multidimensional systems biology approach, ranging from high-throughput data to modeling. The approach is designed to integrate omics and non-omics results to understand the biological response of a biosystem (e.g. cardiovascular system) to radiation exposure (). Applying the systems biology approach within the established AOP framework should facilitate the development of a reproducible bioassay to measure endpoints relevant to risk assessment ().
Figure 2. The application of omics data in the development of AOP for radiation-induced CVD. The different levels of omics data obtained from the cell, animal, and human samples can enrich the AOP framework alongside available knowledge from non-omics data, epidemiological findings, and mechanistic, and mathematical modeling. Together, this information can be integrated across biological levels of organization and provide confident bioindicators for the development of bioscreening tools that can support radiation risk assessment. MIE: molecular initiating events; KE: key events; AO: adverse outcome. The figure is created with BioRender.com.
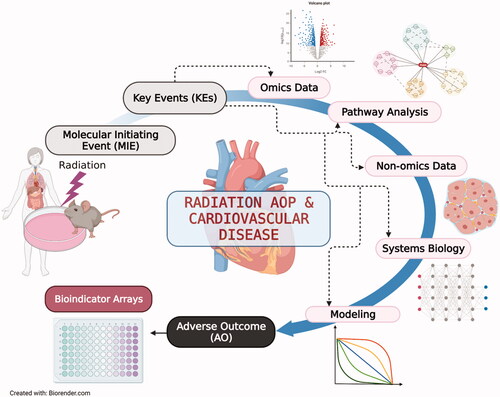
The evolving radiomics are a good example to meet such an interactive approach (Sotoudeh et al. Citation2021). The platform extracts a wide range of features of the medical images and translates them to algorithms that can be combined with those from other analytical platforms including omics (Zanfardino et al. Citation2019). The compiled information can further support the various components of the AOP framework where omics results alone cannot do so.
Understanding CVD as a complex disease requires the investigation at different biological levels of the cardiovascular system. The application of systems biology has recently received increasing attention. The platform provides a pragmatic but sophisticated approach to understanding the changes in the heart tissue during the development and progression of CVD. The various cellular components of the cardiovascular system have been studied by systems biology (Joshi et al. Citation2021). The integration of omics data and non-omics clinical findings through advanced computational methods and mathematical algorithms provides a highly interactive biological network that can advance our understanding of CVD.
Although there are not many multi-omics studies currently published on the impact of radiation on the cardiovascular system, there are many individual omics studies with strong potential to be integrated into a systems biology approach. A systems biology approach needs an optimal collection of radiobiological samples and data following the FAIR Data Principles (Findable, Accessible, Interoperable, and Reusable) to facilitate sharing and integration of data in the radiation research field (Subedi et al. Citation2022). To perform optimal re-analysis of data, accessibility to the user-friendly and well-designed repository is crucial. The STOREDB database (https://www.storedb.org) hosted by the German Federal Office for Radiation Protection (BfS) is one of a few available databanks designed for such purposes which include managing, sustaining, and sharing data generated by different platforms of radiation research (Schofield et al. Citation2019).
Although maintaining linear and simplified biological pathways is the main philosophy behind the AOP approach, systems biology can provide the opportunity to give more mechanistic information, fill gaps in biological data, and translate them at different levels of the organism from molecule to cell up to the individual organism. The integrated approaches may facilitate profiling of the radiation response in a dose- and time-dependent manner to identify the critical exposure criteria leading to the adverse effect on individuals and populations. To fully translate this information at a population level, further research is needed on molecular epidemiology to test and validate the proposed connections between the AOPs and actual health outcomes. Such ‘big data’ approaches would typically use information from omics platforms, biobanks, and health registries. Such a comprehensive platform can identify attractive genes, proteins, or metabolites as measurable bioindicators for screening and risk assessment.
In this context, a recent study used a systems (radiation) biology approach, by integrating experimental data on different cellular endpoints, including cellular functions, such as proliferation, senescence, and angiogenic properties, as well as transcriptomics and proteomics data, in order to more comprehensively understand the mechanisms underlying the endothelial response after chronic low-dose gamma irradiation (0, 1.4, 2.1, and 4.1 mGy/h) (Babini et al. Citation2022).
The mathematical model applied in the study to integrate different datasets reproduced the experimental results and confirmed a dose-dependence for radiation-induced premature senescence, however, the molecular mechanisms differed with dose rates. The re-analysis of cell proliferation rate and the senescence status (percentage of cells responding positively to the senescence-associated β-galactosidase) using a mathematical model (a logistic curve) confirmed a decreasing trend of cell proliferation and increase of senescence as a function of the dose rate.
The study also found that different dose rates could trigger different molecular mechanisms in cells during the onset of senescence (e.g. the dose rate of 4.1 mGy/h affected cell-to-cell communication, cellular adhesion, and inflammation, while the dose rate of 2.4 mGy/h affected the expression of cell cycle-related inhibitors such as the cyclin-dependent kinase inhibitor p21 and the PI3K/Akt/mTOR pathway) (Babini et al. Citation2022).
Toward regulatory implementation: challenges for radiation omics to inform the AOP framework
The omics data presented here emphasized that the development of the AOP network will need measurements at several dose and time points and in different cell types because the omics profiles of phenotypic alterations may differ by the stage in the pathogenesis of the disease (NCRP Citation2020). Moreover, before incorporation into the AOP framework omics data need to be evaluated for a series of assessments adapted to B-H criteria including persistence and significance, reproducibility and consistency, biological plausibility, and human health relevance (Bridges et al. Citation2017). To meet regulatory expectations, omics analyses and related results must be standardized universally and validated effectively. Such uniform conditions enable omics science to produce consistent, reliable, reproducible, and repeatable data to be applied to decision-makers and regulatory applications. In addition, there is a need to improve understanding of the relationships between molecular changes observed in omics data and disease outcomes, as molecular (particularly transcriptional) responses are often early events that lead to overt phenotypic (apical) effects associated with disease progression.
As stated above, the poor availability of low-dose and low-dose-rate data complicates investigations in this area. It is more likely that the available high-dose data can be initially incorporated into the AOP. However, it remains debatable whether, in the absence of data for low dose irradiation, this knowledge can be applied at lower dose and dose rate/fractionation (Little Citation2016; Little et al. Citation2020).
It is important to note that ICRP recommended the nominal threshold of 0.5 Gy for DCS, independent of the rate of dose delivery (Stewart et al. Citation2012). This recommendation aimed basically to serve as a precaution to medical practitioners for risks posed by dose to the heart and brain of the patients. Thus far, no dose limit has been recommended for DCS, so there is no need of discussion for regulatory implementation at this stage. Nevertheless, ICRP Task Group 119 established in 2021 considers implications of the current knowledge on radiogenic DCS for the ICRP System of Radiological Protection. Such discussions are ongoing as part of the steps in preparing the next basic recommendations (Clement et al. Citation2021). The AOP approach is supported by the ICRP (Laurier et al. Citation2021) and the UNSCEAR Expert Group on Diseases of the Circulatory System (CircuDis), which encourages further efforts to develop an AOP network for DCS in which omics data can play a central role.
The omics studies discussed here emphasized the urgent need to apply an optimal animal model to study radiation-induced CVD. Such an in vivo system would be important to improve the knowledge base for environmental radiation protection, but should also provide transferable and translational data to apply to human health and human risk assessment. Although the omics data from rodent models are very informative, it is not always easy to correlate the affected pathways with pathological endpoints that are measurable in humans. The genetically modified and diseased animals are also not the best model because they do not necessarily reflect the changes that would be induced in normal animals following irradiation (Chauhan, Hamada, et al. Citation2021). The unexpected observations, including adaptive or suppressive effects in these mutants at low dose and/or low dose rates, must also be interpreted with caution (Hamada et al. Citation2014).
An optimal experimental model should be able to account for individual susceptibility and sensitivity in response to radiation exposure, a critical aspect of human health risk assessment. Individual sensitivity to radiation varies and is correlated with genetic factors and lifestyle (De Stefano et al. Citation2021). Although the contribution of genetic predisposition to radiation sensitivity or radiation resistance in cancer is well accepted, the knowledge in the area of the radiation-induced non-cancer effect is not yet very well developed (Foray et al. Citation2016; Rajaraman et al. Citation2018; Barnard and Hamada Citation2022). The background status of the biosystem (from cell to tissue) at the time of irradiation may also impact the biological effect of radiation exposure. Radiation exposure is arrhythmogenic in healthy individuals (Azizova et al. Citation2022), but anti-arrhythmogenic in individuals with cardiac arrhythmias receiving cardiac RT (Zhang et al. Citation2021).
The availability of human samples for omics studies is a well-known concern in CVD research, where the collection of fresh-frozen biomaterial is inconvenient for ethical reasons. In this context, the role of radiation biobanks is an important consideration for future studies. Radiobiological and clinical archives could provide a valuable source of human biomaterial for understanding the adverse effects of radiation exposure on the cardiovascular system. Omics profiling of archived biomaterial in radiation research has progressed over the past decade but still needs to be further improved (Azimzadeh, Gomolka et al. Citation2021). Although only a few omics datasets were generated on human biomaterial (Laiakis et al. Citation2014; Azimzadeh, Azizova, et al. Citation2017; Papiez et al. Citation2018; Garrett-Bakelman et al. Citation2019; Azimzadeh et al. Citation2020), these studies highlighted the impact of retrospective analysis of archival samples to investigate the adverse effect of clinical or occupational radiation exposure.
In the absence of sufficient frozen samples to study the effects of radiation exposure on cardiac tissue, formalin-fixed, paraffin-embedded (FFPE) samples may indeed represent the optimal material for such retrospective analyses given their large number, simple storage, and plentiful diagnostic records. This stresses the urgent need to improve the radiation biology biobank policy and its infrastructure (Azimzadeh, Gomolka et al. Citation2021).
The application of high-throughput omics data generated on in vitro systems is an important vision for toxicologists to find an alternative to animal testing (Sauer et al. Citation2017). However, applying an ideal cellular model to represent the cardiovascular system is not an easy task. The available commercial cellular systems (endothelial cells, fibroblasts, cardiomyocytes, and smooth muscle cells) are not the best alternative for complex and dynamic features of cardiac tissue. Consequently, the bioindicators and biomarkers identified in the in vitro systems cannot be used directly as a surrogate in humans. Using isolated cells from irradiated hearts, 3D models of interactive cells and cell co-culture in omics analysis may help to address this issue.
It is important to note that applications of omics platforms are still in their infancy in radiation research. Epigenetics, post-translational modifications (PTMs), and the secretome of cell communication are unexplored potential that needs to be further investigated to identify early and late health effects of radiation exposure.
Integration of biological and epidemiological data, a vision for risk assessment
Translating the qualitative understanding of detrimental effects into quantitative estimates of health risk remains a pertinent challenge of the AOP framework. The epidemiology-based risk assessment approach used to extrapolate from AOs assessed at higher doses to low doses is associated with significant uncertainties in estimating risk at low doses and low dose rates of ionizing radiation (Preston Citation2017; Preston et al. Citation2021).
Approaches to integrating radiation biology data and epidemiology findings for risk assessment, especially at low doses are discussed in Report No. 186 of the US National Council on Radiation Protection and Measurements (NCRP) for cancer and CVD (NCRP Citation2020). The biological data generated by advanced omics technologies will not only provide a better understanding of the cellular and molecular processes affected by low doses and low dose rates, but may also facilitate the development of biomarkers for radiation-related health effects. Integration of such mechanistic data with epidemiological evidence is expected to improve risk assessment for cancer and CVD (Hamada and Fujimichi Citation2014; NCRP Citation2020; Chauhan, Beaton, et al. Citation2022; NASEM Citation2022).
Among epidemiological studies with biological data or available biosamples for further examinations (reviewed in NCRP Citation2020), Japanese atomic bomb survivors and Mayak production association workers are the main cohorts to offer potential biomarker information usable as parameters in a predictive model for the effect of radiation on cardiovascular system (NCRP Citation2020; Preston et al. Citation2021).
The Report has clearly emphasized the potential of AOPs to inform conceptual designs of biologically based risk models (BBRMs). For radiation risk of cancer, the Report already illustrated promising BBRM prototypes which were developed for organs, such as the colon, thyroid, and lung. Kaiser et al. propose systematic approaches and appropriate study designs to harness knowledge stored in AOPs for quantitative risk assessment using (cancer-related) BBRMs as hinges (Kaiser et al. Citation2021). However, for radiation-induced CVD, the development of such BBRMs is still in its infancy. Previous attempts focused on the impact of cholesterol metabolism on atherosclerosis and CVD risk but did not perform model fits against epidemiological datasets (Cobbold et al. Citation2002; Little et al. Citation2009; Mc Auley Citation2022). On the one hand, the sheer complexity of the involved pathogenic processes prevents the development of models with a reasonably low number of parameters. Furthermore, biological and epidemiological evidence for a response at doses below 0.5 Gy is uncertain (Little et al. Citation2021).
High dose, fractionated RT results in an increase in the rate of major coronary events by 7.4% per Gy in breast cancer patients (Darby et al. Citation2013). The majority of these events arise from atherosclerosis as the main underlying disease (Rehammar et al. Citation2017). Thus, the risk projections from BBRMs for therapeutic doses in clinical applications may be useful. Consequently, in the EU-funded MEDIRAD project, the development of a BBRM for acute coronary events (ACEs) in breast cancer patients after RT has been proposed (http://www.medirad-project.eu/work-packages; last accessed on 25 April 2022). Motivated by the AOP framework, it was planned to inform the conceptual model design with data at molecular and vascular levels in a ‘bottom-up’ approach. The molecular data consisted of epigenetic (DNA methylation) and transcriptomic (miRNAs) analyses obtained from circulating blood cells which have been measured in animal experiments and the patient samples. For the characterization of a detrimental effect at the vascular level, imaging data from echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI) have been recorded. Before joining them into a common database the data are screened separately for each area of origin with standard statistical methods such as multivariate regression of single outcomes. More suited to unravel the complexity of causal relationships between different levels is bioinformatical network analysis (Schäfer and Strimmer Citation2005; van Wieringen and Chen Citation2021). This analytical tool should identify radiation-induced clusters of biomarkers linking molecular and vascular levels. Such clusters might pertain to atherogenic processes like chronic inflammation. They generate hypotheses on radiation targets in the atherogenic chain which can be implemented and tested in BBRMs.
Starting from radiation epidemiology in a ‘top-down’ approach, the development of a BBRM was organized into three levels. In Level I, the baseline model without risk factors described the progression and age-risk pattern of atherosclerosis in a Markov chain Monte Carlo simulation with coronary artery intimal surface area as the object of time evolution (Simonetto et al. Citation2021). During aging, this area is subsequently covered with atherosclerotic lesions of increasing severity. Complicated lesions appear at the end of the chain and the rate of myocardial infarction increases proportionally to the area of these lesions. Model parameters are derived from simultaneous fits to incidence rates in the German KORA cohort (Kuch et al. Citation2008) and the age-dependent prevalence and spatial extent of atherosclerotic lesions in the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) (Citation1993) study (PDAY Citation1993).
In Level II, the model included classical risk factors, such as smoking, hypertension, and dyslipidemia from the KORA cohort (Simonetto, Heier, et al. Citation2022). The main target of detriment in the chain of the atherogenic process was identified by goodness-of-fit. Whereas the impact of dyslipidemia was realized along the whole atherogenic chain, smoking made its strongest impact at the chain end with complicated lesions. Finally, in Level III, the KORA cohort was replaced by a Dutch cohort of breast cancer patients to obtain a risk model for ACEs after RT. Using the same method of effect identification as in Level II, therapeutic radiation released the strongest impact on complicated lesions. Due to low case numbers, the exact shape of the post-RT age-risk pattern could not be determined. However, the risk increased significantly already in the first five years after RT (Simonetto, Kaiser, et al. Citation2022). These findings are in line with the observation that RT doses to atherosclerotic plaques in the left anterior descending coronary artery are enhancing cardiac toxicity (van den Bogaard et al. Citation2021). Moreover, Simonetto et al. proposed that the dose-response for rupture of large atherosclerotic lesions might be non-linear. These studies show that susceptibility to radiation-induced ACEs depends markedly on the atherosclerotic state of a patient (Simonetto et al. Citation2020). Thus, for risk reduction heart sparing techniques or proton therapy has been proposed for patients burdened with plaques within the volume of high RT doses. The outlined approach of ‘Development of biologically-based models to evaluate radiation-induced disease risk’ has been added to the MEDIRAD recommendations (http://medirad-project.eu/recommendations/).
Conclusions
There is a body of available data on the various omics applied in radiation research that can broadly inform the AOP framework. By comprehensive screening, omics platforms provide the opportunity to identify biological pathways indicative for risk-related outcomes. Such valuable information can be used to understand the mechanistic links between dose, dose-rate, and radiation quality and disease development and progression. Further research is needed to clarify the issues for the application of omics for regulatory purposes. The quality, accuracy, and reproducibility of omics data need to be improved. The omics data needs to be universally standardized, interpreted, and validated before they can be incorporated into the AOP framework to address radiation protection purposes. A deeper understanding of the molecular mechanism of the biological effects of radiation offers potential measurable bioindicators of radiation exposure, late and long-term effects, susceptibility, and sensitivity. In the long term, this information proposes the development of a reliable and universal bioassay for the human health effects of radiation exposure. It is important to note that the acceptance of the AOP framework in the radiation protection community will be influenced by its ability to improve quantitative risk assessment. Designed mechanistic models, potentially based on different levels of biological organization, may help in this respect.
Supplementary_Table_1.xlsx
Download MS Excel (11.8 KB)Acknowledgments
The authors are thankful to Ms Sarita Sanchez Cuadro (Health Canada, Canada) for her assistance with the graphics. The authors would like to thank the Multidisciplinary European Low dose Initiative (MELODI) for funding support for the MELODI Workshop 2021 and for open access publication of this paper.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Omid Azimzadeh
Omid Azimzadeh, Ph.D, PD, is a senior researcher in the section of Radiation Biology at the German Federal Office for Radiation Protection (BfS) and an Adjunct Professor (Docent) of Radiation Biology at Technical University Munich. He is a member of the working group CircuDis (Circulatory Diseases from Radiation Exposure) at the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) and the High Level Group on Low Dose Research (HLG-LDR) Rad/Chem AOP Joint Topical Group.
Simone Moertl
Simone Moertl, Ph.D., PD, is head of the section of Radiation Biology at the Federal Office for Radiation Protection (BfS) and an Adjunct Professor (Docent) of Radiation Biology at Technical University Munich. Until September 2019 she led the group of Clinical Radiobiology at the Institute of Radiation Biology, Helmholtz Zentrum München. Her research focuses on the cellular radiation response of tumors and normal tissues with the aim to understand the mechanisms of individual radiation sensitivity. She is particularly interested in the vesicle-mediated transfer (e.g. by exosomes) of RNAs between cells. She is a member of the High Level Group on Low Dose Research (HLG-LDR) Rad/Chem AOP Joint Topical Group.
Raghda Ramadan
Raghda Ramadan, Ph.D., is a scientist in the Radiobiology Unit at the Belgian Nuclear Research Center (SCK CEN). She coordinates the research line focusing on cardiovascular risks after ionizing radiation exposure. She is a member of a working group CircuDis (Circulatory Diseases from Radiation Exposure) at the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR).
Bjorn Baselet
Bjorn Baselet, Ph.D., is a senior researcher in the Radiobiology group at the Belgian Nuclear Research Center (SCK CEN). His research interests lie in the field of space biology, looking into the adverse effects of space stressors, such as cosmic radiation exposure on normal tissues with a special emphasis on the immunological and cardiovascular system. Besides his research activities, he is a dedicated mentor of undergraduate and graduate students. Furthermore, he is a member of a working group on ionizing radiation at the Belgian High Health Council and received the 2020 MELODI award for his work on radiation-induced cardiovascular disease and immune dysfunction.
Evagelia C. Laiakis
Evagelia C. Laiakis, Ph.D., is an Associate Professor in the Department of Oncology at Georgetown University. She received her Ph.D. degree from the University of Maryland at Baltimore. She is an elected Council Member of the National Council on Radiation Protection and Measurements and has been serving at PAC-1 since 2016. She is also the recipient of the 2019 Jack Fowler Award from Radiation Research Society, and a dedicated mentor to students and postdocs. Her research focuses on metabolomic and lipidomic responses to ionizing radiation, with particular emphasis on the development of early biodosimetry applications in biofluids.
Soji Sebastian
Soji Sebastian, Ph.D., in Cell Biology, is currently a research scientist in the Radiobiology and Health Branch at Canadian Nuclear Laboratories (CNL) and adjunct professor in the department of cellular and molecular medicine, University of Ottawa. He has been in the field of stem cell biology and radiobiology using multi-omics approach to address epigenetic basis of tissue regeneration. At CNL, he is investigating the epigenetic effects of ionizing radiation in skeletal muscle, cardiac, and lung cellular models. Experimental approaches span from molecular in vitro studies to integrative in vivo stem cell biology and mass spectrometry.
Danielle Beaton
Danielle Beaton, Ph.D., is a Research Scientist at Canadian Nuclear Laboratories. Her current research focuses on the effects of low dose radiation on biological systems. She is on the NEA HLG-LDR committee and assists the co-chairs of the HLG-LDR Rad/Chem AOP Joint Topical Group.
Jaana M. Hartikainen
Jaana M. Hartikainen, Ph.D., is a Senior Researcher in the School of Medicine at University of Eastern Finland (UEF). She is an Adjunct Professor (Docent) in Molecular Genetics and has 25 years of research experience in cancer genetics and molecular biology. She leads the research project focusing on non-coding RNAs in breast cancer and is also involved in teaching and thesis supervision in the School of Medicine at UEF.
Jan Christian Kaiser
Jan Christian Kaiser, Ph.D., heads the research group ‘Integrative Modeling’ in the Institute of Radiation Medicine at the Helmholtz-Zentrum München. His main interests relate to the integration of radiation biology and epidemiology for health risk estimation with biologically based models addressing the pathogenesis of cancer and cardiovascular diseases.
Afshin Beheshti
Afshin Beheshti, Ph.D., is a Principal Investigator and Bioinformatician at KBR at NASA Ames Research Center, a Visiting Researcher at Broad Institute of MIT and Harvard, and President of a nonprofit formed in March 2020 working on COVID-19 called COVID-19 International Research Team (COV-IRT, www.cov-irt.org). His research interests include applying systems biology approaches to a broad range of topics which include: mitochondria, noncoding RNAs/miRNAs, radiation biology, space biology, cancer, COVID-19, and cardiovascular disease.
Sisko Salomaa
Sisko Salomaa, Ph.D., is a Professor of radiobiology at the University of Eastern Finland and Coordinator of the National Radiation Safety Research Program in STUK. As part of setting up the European low dose program (MELODI), she coordinated the DoReMi Network of Excellence 2010–2015. She is a representative of Finland to UNSCEAR and was a member of ICRP C1.
Vinita Chauhan
Vinita Chauhan, Ph.D., is a Senior Research Scientist at the Consumer and Clinical Radiation Protection Bureau of Health Canada. She is a Canadian delegate of the High Level Group on Low Dose Research (HLG-LDR) and Extended Advisory Group on Molecular Screening and Toxicogenomics (EAGMST) of the OECD. She co-chairs the HLG-LDR Rad/Chem AOP Joint Topical Group and is the co-founder of the Canadian Organization of Health Effects from Radiation Exposure (COHERE) initiative.
Nobuyuki Hamada
Nobuyuki Hamada, RT, Ph.D., is a Senior Research Scientist at CRIEPI and a Visiting Professor at Hiroshima University Research Institute for Radiation Biology and Medicine. He serves on ICRP Task Groups 102, 111 and 119, NCRP PAC 1, OECD/NEA/CRPPH/HLG-LDR/Rad/Chem AOP Joint Topical Group, IRPA Task Group on Tissue Reactions, and Consultation Committee on AOP development for space flight health outcomes (Canadian project).
References
- Aguayo-Orozco A, Taboureau O, Brunak S. 2019. The use of systems biology in chemical risk assessment. Curr Opin Toxicol. 15:48–54.
- Akahoshi M, Amasaki Y, Soda M, Hida A, Imaizumi M, Nakashima E, Maeda R, Seto S, Yano K. 2003. Effects of radiation on fatty liver and metabolic coronary risk factors among atomic bomb survivors in Nagasaki. Hypertens Res. 26(12):965–970.
- Amundson SA. 2021. Transcriptomics for radiation biodosimetry: progress and challenges. Int J Radiat Biol. 1–9. doi:10.1080/09553002.2021.1928784
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 29(3):730–741.
- Aryankalayil MJ, Martello S, Bylicky MA, Chopra S, May JM, Shankardass A, MacMillan L, Sun L, Sanjak J, Vanpouille-Box C, et al. 2021. Analysis of lncRNA-miRNA-mRNA expression pattern in heart tissue after total body radiation in a mouse model. J Transl Med. 19(1):336.
- Awad EM, Khan SY, Sokolikova B, Brunner PM, Olcaydu D, Wojta J, Breuss JM, Uhrin P. 2013. Cold induces reactive oxygen species production and activation of the NF-kappa B response in endothelial cells and inflammation in vivo. J Thromb Haemost. 11(9):1716–1726.
- Aypar U, Morgan WF, Baulch JE. 2011. Radiation-induced epigenetic alterations after low and high LET irradiations. Mutat Res. 707(1–2):24–33.
- Azimzadeh O, von Toerne C, Subramanian V, Sievert W, Multhoff G, Atkinson MJ, Tapio S. 2021. Data-independent acquisition proteomics reveals long-term biomarkers in the. Serum of C57BL/6J mice following local high-dose heart irradiation. Front Public Health. 9:678856.
- Azimzadeh O, Atkinson MJ, Tapio S. 2014. Proteomics in radiation research: present status and future perspectives. Radiat Environ Biophys. 53(1):31–38.
- Azimzadeh O, Azizova T, Merl-Pham J, Blutke A, Moseeva M, Zubkova O, Anastasov N, Feuchtinger A, Hauck SM, Atkinson MJ, et al. 2020. Chronic occupational exposure to ionizing radiation induces alterations in the structure and metabolism of the heart: a proteomic analysis of human formalin-fixed paraffin-embedded (FFPE) cardiac tissue. Int J Mol Sci. 21(18):6832.
- Azimzadeh O, Azizova T, Merl-Pham J, Subramanian V, Bakshi MV, Moseeva M, Zubkova O, Hauck SM, Anastasov N, Atkinson MJ, et al. 2017. A dose-dependent perturbation in cardiac energy metabolism is linked to radiation-induced ischemic heart disease in Mayak nuclear workers. Oncotarget. 8(6):9067–9078.
- Azimzadeh O, Gomolka M, Birschwilks M, Saigusa S, Grosche B, Moertl S. 2021. Advanced omics and radiobiological tissue archives: the future in the past. Appl Sci. 11(23):11108.
- Azimzadeh O, Scherthan H, Sarioglu H, Barjaktarovic Z, Conrad M, Vogt A, Calzada-Wack J, Neff F, Aubele M, Buske C, et al. 2011. Rapid proteomic remodeling of cardiac tissue caused by total body ionizing radiation. Proteomics. 11(16):3299–3311.
- Azimzadeh O, Scherthan H, Yentrapalli R, Barjaktarovic Z, Ueffing M, Conrad M, Neff F, Calzada-Wack J, Aubele M, Buske C, et al. 2012. Label-free protein profiling of formalin-fixed paraffin-embedded (FFPE) heart tissue reveals immediate mitochondrial impairment after ionising radiation. J Proteomics. 75(8):2384–2395.
- Azimzadeh O, Sievert W, Sarioglu H, Merl-Pham J, Yentrapalli R, Bakshi MV, Janik D, Ueffing M, Atkinson MJ, Multhoff G, et al. 2015. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res. 14(2):1203–1219.
- Azimzadeh O, Sievert W, Sarioglu H, Yentrapalli R, Barjaktarovic Z, Sriharshan A, Ueffing M, Janik D, Aichler M, Atkinson MJ, et al. 2013. PPAR alpha: a novel radiation target in locally exposed Mus musculus heart revealed by quantitative proteomics. J Proteome Res. 12(6):2700–2714. eng.
- Azimzadeh O, Subramanian V, Sievert W, Merl-Pham J, Oleksenko K, Rosemann M, Multhoff G, Atkinson MJ, Tapio S. 2021. Activation of PPARα by fenofibrate attenuates the effect of local heart high dose irradiation on the mouse cardiac proteome. Biomedicines. 9(12):1845.
- Azimzadeh O, Subramanian V, Ständer S, Merl-Pham J, Lowe D, Barjaktarovic Z, Moertl S, Raj K, Atkinson MJ, Tapio S. 2017. Proteome analysis of irradiated endothelial cells reveals persistent alteration in protein degradation and the RhoGDI and NO signalling pathways. Int J Radiat Biol. 93(9):920–928.
- Azimzadeh O, Tapio S. 2017. Proteomics landscape of radiation-induced cardiovascular disease: somewhere over the paradigm. Expert Rev Proteomics. 14(11):987–996.
- Azizova TV, Bannikova MV, Briks KV, Grigoryeva ES, Hamada N. 2022. Incidence risks for subtypes of heart diseases in a Russian cohort of Mayak Production Association nuclear workers. Radiat Environ Biophys. 61: in press.
- Babini G, Baiocco G, Barbieri S, Morini J, Sangsuwan T, Haghdoost S, Yentrapalli R, Azimzadeh O, Rombouts C, Aerts A, et al. 2022. A systems radiation biology approach to unravel the role of chronic low-dose-rate gamma-irradiation in inducing premature senescence in endothelial cells. PLoS One. 17(3):e0265281.
- Baker JE, Moulder JE, Hopewell JW. 2011. Radiation as a risk factor for cardiovascular disease. Antioxid Redox Signal. 15(7):1945–1956.
- Bakshi MV, Barjaktarovic Z, Azimzadeh O, Kempf SJ, Merl J, Hauck SM, Eriksson P, Buratovic S, Atkinson MJ, Tapio S. 2013. Long-term effects of acute low-dose ionizing radiation on the neonatal mouse heart: a proteomic study. Radiat Environ Biophys. 52(4):451–461.
- Barjaktarovic Z, Anastasov N, Azimzadeh O, Sriharshan A, Sarioglu H, Ueffing M, Tammio H, Hakanen A, Leszczynski D, Atkinson MJ, et al. 2013. Integrative proteomic and microRNA analysis of primary human coronary artery endothelial cells exposed to low-dose gamma radiation. Radiat Environ Biophys. 52(1):87–98.
- Barjaktarovic Z, Kempf SJ, Sriharshan A, Merl-Pham J, Atkinson MJ, Tapio S. 2015. Ionizing radiation induces immediate protein acetylation changes in human cardiac microvascular endothelial cells. J Radiat Res. 56(4):623–632.
- Barjaktarovic Z, Merl-Pham J, Azimzadeh O, Kempf SJ, Raj K, Atkinson MJ, Tapio S. 2017. Low-dose radiation differentially regulates protein acetylation and histone deacetylase expression in human coronary artery endothelial cells. Int J Radiat Biol. 93(2):156–164.
- Barjaktarovic Z, Merl-Pham J, Braga-Tanaka I, Tanaka S, Hauck SM, Saran A, Mancuso M, Atkinson MJ, Tapio S, Azimzadeh O. 2019. Hyperacetylation of cardiac mitochondrial proteins is associated with metabolic impairment and sirtuin downregulation after chronic total body irradiation of ApoE (-/-) mice. Int J Mol Sci. 20(20):5239.
- Barjaktarovic Z, Schmaltz D, Shyla A, Azimzadeh O, Schulz S, Haagen J, Dörr W, Sarioglu H, Schäfer A, Atkinson MJ, et al. 2011. Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS One. 6(12):e27811.
- Barjaktarovic Z, Shyla A, Azimzadeh O, Schulz S, Haagen J, Dörr W, Sarioglu H, Atkinson MJ, Zischka H, Tapio S. 2013. Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiother Oncol. 106(3):404–410.
- Barnard SGR, Hamada N. 2022. Individual response of the ocular lens to ionizing radiation. Int J Radiat Biol. 1–17. doi:10.1080/09553002.2022.2074166
- Barnette BL, Yu Y, Ullrich RL, Emmett MR. 2021. Mitochondrial effects in the liver of C57BL/6 mice by low dose, high energy, high charge irradiation. Int J Mol Sci. 22(21):11806.
- Baselet B, Azimzadeh O, Erbeldinger N, Bakshi MV, Dettmering T, Janssen A, Ktitareva S, Lowe DJ, Michaux A, Quintens R, et al. 2017. Differential impact of single-dose fe ion and x-ray irradiation on endothelial cell transcriptomic and proteomic responses. Front Pharmacol. 8(570):570.
- Baselet B, Belmans N, Coninx E, Lowe D, Janssen A, Michaux A, Tabury K, Raj K, Quintens R, Benotmane MA, et al. 2017. Functional gene analysis reveals cell cycle changes and inflammation in endothelial cells irradiated with a single x-ray dose. Front Pharmacol. 8:213.
- Baselet B, Sonveaux P, Baatout S, Aerts A. 2019. Pathological effects of ionizing radiation: endothelial activation and dysfunction. Cell Mol Life Sci. 76(4):699–728.
- Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, et al. 2020. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 18(1):e3000599.
- Becker RA, Ankley GT, Edwards SW, Kennedy SW, Linkov I, Meek B, Sachana M, Segner H, Van Der Burg B, Villeneuve DL, et al. 2015. Increasing scientific confidence in adverse outcome pathways: application of tailored bradford-hill considerations for evaluating weight of evidence. Regul Toxicol Pharmacol. 72(3):514–537.
- Becker BV, Majewski M, Abend M, Palnek A, Nestler K, Port M, Ullmann R. 2018. Gene expression changes in human iPSC-derived cardiomyocytes after X-ray irradiation. Int J Radiat Biol. 94(12):1095–1103.
- Boerma M, Hauer-Jensen M. 2010. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract. 2011:1–8.
- Boerma M, van der Wees CG, Vrieling H, Svensson JP, Wondergem J, van der Laarse A, Mullenders LH, van Zeeland AA. 2005. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics. 6:6.
- Boguszewicz Ł, Bieleń A, Ciszek M, Wendykier J, Szczepanik K, Skorupa A, Mrochem-Kwarciak J, Składowski K, Sokół M. 2021. NMR-based metabolomics in investigation of the radiation induced changes in blood serum of head and neck cancer patients and its correlation with the tissue volumes exposed to the particulate doses. Int J Mol Sci. 22(12):6310.
- Bridges J, Sauer UG, Buesen R, Deferme L, Tollefsen KE, Tralau T, van Ravenzwaay B, Poole A, Pemberton M. 2017. Framework for the quantitative weight-of-evidence analysis of 'omics data for regulatory purposes. Regul Toxicol Pharmacol. 91( 1):S46–s60.
- Brockmeier EK, Hodges G, Hutchinson TH, Butler E, Hecker M, Tollefsen KE, Garcia-Reyero N, Kille P, Becker D, Chipman K, et al. 2017. The role of omics in the application of adverse outcome pathways for chemical risk assessment. Toxicol Sci. 158(2):252–262.
- Brown JM, Hazen SL. 2018. Microbial modulation of cardiovascular disease. Nat Rev Microbiol. 16(3):171–181.
- Cartier A, Hla T. 2019. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science. 366(6463):eaar5551.
- Chang CW, Dalgliesh AJ, Lopez JE, Griffiths LG. 2016. Cardiac extracellular matrix proteomics: challenges, techniques, and clinical implications. Proteomics Clin Appl. 10(1):39–50.
- Chauhan V, Beaton D, Hamada N, Wilkins R, Burtt J, Leblanc J, Cool D, Garnier-Laplace J, Laurier D, Le Y, et al. 2022. Adverse outcome pathway: a path toward better data consolidation and global co-ordination of radiation research. Int J Radiat Biol. 1–10. doi:10.1080/09553002.2021.2020363
- Chauhan V, Hamada N, Garnier-Laplace J, Laurier D, Beaton D, Tollefsen KE, Locke PA. 2022. Establishing a communication and engagement strategy to facilitate the adoption of the adverse outcome pathways in radiation research and regulation. Int J Radiat Biol. 1–8. doi:10.1080/09553002.2022.2086716
- Chauhan V, Hamada N, Monceau V, Ebrahimian T, Adam N, Wilkins RC, Sebastian S, Patel ZS, Huff JL, Simonetto C, et al. 2021. Expert consultation is vital for adverse outcome pathway development: a case example of cardiovascular effects of ionizing radiation. Int J Radiat Biol. 97(11):1516–1525.
- Chauhan V, Kuo B, McNamee JP, Wilkins RC, Yauk CL. 2016. Transcriptional benchmark dose modeling: exploring how advances in chemical risk assessment may be applied to the radiation field. Environ Mol Mutagen. 57(8):589–604.
- Chauhan V, Leblanc J, Sadi B, Burtt J, Sauvé K, Lane R, Randhawa K, Wilkins R, Quayle D. 2021. COHERE - strengthening cooperation within the Canadian government on radiation research. Int J Radiat Biol. 97(9):1153–1165.
- Chauhan V, Rowan-Carroll A, Gagné R, Kuo B, Williams A, Yauk CL. 2019. The use of in vitro transcriptional data to identify thresholds of effects in a human lens epithelial cell-line exposed to ionizing radiation. Int J Radiat Biol. 95(2):156–169.
- Chauhan V, Said Z, Daka J, Sadi B, Bijlani D, Marchetti F, Beaton D, Gaw A, Li C, Burtt J, et al. 2019. Is there a role for the adverse outcome pathway framework to support radiation protection? Int J Radiat Biol. 95(2):225–232.
- Chauhan V, Sherman S, Said Z, Yauk CL, Stainforth R. 2021. A case example of a radiation-relevant adverse outcome pathway to lung cancer. Int J Radiat Biol. 97(1):68–84.
- Chauhan V, Stricklin D, Cool D. 2021. The integration of the adverse outcome pathway framework to radiation risk assessment. Int J Radiat Biol. 97(1):60–67.
- Chauhan V, Wilkins RC, Beaton D, Sachana M, Delrue N, Yauk C, O'Brien J, Marchetti F, Halappanavar S, Boyd M, et al. 2021. Bringing together scientific disciplines for collaborative undertakings: a vision for advancing the adverse outcome pathway framework. Int J Radiat Biol. 97(4):431–441.
- Cheema AK, Li Y, Girgis M, Jayatilake M, Fatanmi OO, Wise SY, Seed TM, Singh VK. 2020. Alterations in tissue metabolite profiles with amifostine-prophylaxed mice exposed to gamma radiation. Metabolites. 10(5):211.
- Cheema AK, Li Y, Moulton J, Girgis M, Wise SY Carpenter A, Fatanmi OO, Singh VK. 2022. Identification of novel biomarkers for acute radiation injury using multi-omics approach and nonhuman primate model. Int J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2022.05.046
- Cheema AK, Mehta KY, Rajagopal MU, Wise SY, Fatanmi OO, Singh VK. 2019. Metabolomic studies of tissue injury in nonhuman primates exposed to gamma-radiation. Int J Mol Sci. 20(13):3360.
- Chen Y, Cui J, Gong Y, Wei S, Wei Y, Yi L. 2021. MicroRNA: a novel implication for damage and protection against ionizing radiation. Environ Sci Pollut Res Int. 28(13):15584–15596.
- Chen Z, Schunkert H. 2021. Genetics of coronary artery disease in the post-GWAS era. J Intern Med. 290(5):980–992.
- Chong Nguyen C, Duboc D, Rainteau D, Sokol H, Humbert L, Seksik P, Bellino A, Abdoul H, Bouazza N, Treluyer JM, et al. 2021. Circulating bile acids concentration is predictive of coronary artery disease in human. Sci Rep. 11(1):22661.
- Clement C, Rühm W, Harrison J, Applegate K, Cool D, Larsson CM, Cousins C, Lochard J, Bouffler S, Cho K, et al. 2021. Keeping the ICRP recommendations fit for purpose. J Radiol Prot. 41(4):1390.
- Cobbold CA, Sherratt JA, Maxwell SR. 2002. Lipoprotein oxidation and its significance for atherosclerosis: a mathematical approach. Bull Math Biol. 64(1):65–95.
- Coleman MA, Sasi SP, Onufrak J, Natarajan M, Manickam K, Schwab J, Muralidharan S, Peterson LE, Alekseyev YO, Yan X, et al. 2015. Low-dose radiation affects cardiac physiology: gene networks and molecular signaling in cardiomyocytes. Am J Physiol Heart Circ Physiol. 309(11):H1947–1963.
- Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6(12):2853–2868.
- da Silveira WA, Fazelinia H, Rosenthal SB, Laiakis EC, Kim MS, Meydan C, Kidane Y, Rathi KS, Smith SM, Stear B, et al. 2020. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell. 183(5):1185–1201. e1120.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al. 2013. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 368(11):987–998. eng.
- Dawood A, Mothersill C, Seymour C. 2021. Low dose ionizing radiation and the immune response: what is the role of non-targeted effects? Int J Radiat Biol. 97(10):1368–1382.
- de Freitas RB, Boligon AA, Rovani BT, Piana M, de Brum TF, da Silva Jesus R, Rother FC, Alves NM, Teixeira da Rocha JB, Athayde ML, et al. 2013. Effect of black grape juice against heart damage from acute gamma TBI in rats. Molecules. 18(10):12154–12167.
- Stefano D, Leonardi I, Casciati S, Pasquali A, Giardullo E, Antonelli P, Novelli F, Babini F, Tanori G, Tanno M, et al. 2021. Contribution of genetic background to the radiation risk for cancer and non-cancer diseases in Ptch1+/- mice. Radiat Res. 197:43–56.
- Desjardins AU. 1937. The action of roentgen rays or radium on inflammatory processes. Radiology. 29(4):436–445.
- Dewald O, Sharma S, Adrogue J, Salazar R, Duerr GD, Crapo JD, Entman ML, Taegtmeyer H. 2005. Downregulation of peroxisome proliferator-activated receptor-alpha gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation. 112(3):407–415.
- Diez J. 2010. Altered degradation of extracellular matrix in myocardial remodelling: the growing role of cathepsins and cystatins. Cardiovasc Res. 87(4):591–592.
- Dundar HA, Kiray M, Kir M, Kolatan E, Bagriyanik A, Altun Z, Aktas S, Ellidokuz H, Yilmaz O, Mutafoglu K, et al. 2016. Protective effect of acetyl-L-carnitine against doxorubicin-induced cardiotoxicity in Wistar Albino rats. Arch Med Res. 47(7):506–514.
- Dupont C, Armant DR, Brenner CA. 2009. Epigenetics: definition, mechanisms and clinical perspective. Semin Reprod Med. 27(5):351–357.
- Esplugas R, Arenas M, Serra N, Bellés M, Bonet M, Gascón M, Vallvé JC, Linares V. 2019. Effect of radiotherapy on the expression of cardiovascular disease-related miRNA-146a, -155, -221 and -222 in blood of women with breast cancer. PLoS One. 14(5):e0217443.
- Esplugas R, Bellés M, Serra N, Arenas M, Hernández V, Vallvé JC, Linares V. 2019. Effect of radiation on the expression of CVD-related miRNAs, inflammation and endothelial dysfunction of HUVECs. Anticancer Res. 39(2):771–780.
- Filipsson AF, Sand S, Nilsson J, Victorin K. 2003. The benchmark dose method–review of available models, and recommendations for application in health risk assessment. Crit Rev Toxicol. 33(5):505–542.
- Finck BN. 2007. The PPAR regulatory system in cardiac physiology and disease. Cardiovasc Res. 73(2):269–277.
- Foray N, Bourguignon M, Hamada N. 2016. Individual response to ionizing radiation. Mutat Res Rev Mutat Res. 770(Pt B):369–386.
- Freeman JL, Weber GJ, Peterson SM, Nie LH. 2014. Embryonic ionizing radiation exposure results in expression alterations of genes associated with cardiovascular and neurological development, function, and disease and modified cardiovascular function in zebrafish. Front Genet. 5:268.
- Freund A, Orjalo AV, Desprez PY, Campisi J. 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 16(5):238–246.
- Furusawa Y, Zhao QL, Hattori Y, Tabuchi Y, Iwasaki T, Nomura T, Kondo T. 2016. Comprehensive and computational analysis of genes in human umbilical vein endothelial cells responsive to X-irradiation. Genom Data. 8:126–130.
- Gaboury JP, Anderson DC, Kubes P. 1994. Molecular mechanisms involved in superoxide-induced leukocyte-endothelial cell interactions in vivo. Am J Physiol. 266(2 Pt 2):H637–642.
- Gallina C, Turinetto V, Giachino C. 2015. A new paradigm in cardiac regeneration: the mesenchymal stem cell secretome. Stem Cells Int. 2015:765846.
- Gao S, Wu R, Zeng Y. 2012. Up-regulation of peroxisome proliferator-activated receptor gamma in radiation-induced heart injury in rats. Radiat Environ Biophys. 51(1):53–59.
- Garikipati VNS, Arakelyan A, Blakely EA, Chang PY, Truongcao MM, Cimini M, Malaredy V, Bajpai A, Addya S, Bisserier M, et al. 2021. Long-term effects of very low dose particle radiation on gene expression in the heart: degenerative disease risks. Cells. 10(2):387.
- Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, Macias BR, McKenna MJ, Meydan C, Mishra T, Nasrini J, et al. 2019. The NASA twins study: a multidimensional analysis of a year-long human spaceflight. Science. 364(0 ):eaau8650.
- Goetz W, Morgan MN, Baulch JE. 2011. The effect of radiation quality on genomic DNA methylation profiles in irradiated human cell lines. Radiat Res. 175(5):575–587.
- Gonzalez-Jaramillo V, Portilla-Fernandez E, Glisic M, Voortman T, Ghanbari M, Bramer W, Chowdhury R, Nijsten T, Dehghan A, Franco OH, et al. 2019. Epigenetics and inflammatory markers: a systematic review of the current evidence. Int J Inflam. 2019:6273680.
- Goudarzi M, Mak TD, Chen C, Smilenov LB, Brenner DJ, Fornace AJ. 2014. The effect of low dose rate on metabolomic response to radiation in mice. Radiat Environ Biophys. 53(4):645–657.
- Goudarzi M, Weber WM, Mak TD, Chung J, Doyle-Eisele M, Melo DR, Brenner DJ, Guilmette RA, Fornace AJ. Jr. 2015. Metabolomic and lipidomic analysis of serum from mice exposed to an internal emitter, cesium-137, using a shotgun LC-MS(E) approach. J Proteome Res. 14(1):374–384.
- Gramatyka M, Skorupa A, Sokół M. 2018. Nuclear magnetic resonance spectroscopy reveals metabolic changes in living cardiomyocytes after low doses of ionizing radiation. Acta Biochim Pol. 65(2):309–318.
- Gramatyka M, Widłak P, Gabryś D, Kulik R, Sokół M. 2020. Resveratrol administration prevents radiation-related changes in metabolic profiles of hearts 20 weeks after irradiation of mice with a single 2 Gy dose. Acta Biochim Pol. 67(4):629–632.
- Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. 2000. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 20(10):2175–2183.
- Grison S, Kereselidze D, Cohen D, Gloaguen C, Elie C, Lestaevel P, Legendre A, Manens L, Habchi B, Benadjaoud MA, et al. 2019. Applying a multiscale systems biology approach to study the effect of chronic low-dose exposure to uranium in rat kidneys. Int J Radiat Biol. 95(6):737–752.
- Guo Y, Cui L, Jiang S, Zhang A, Jiang S. 2017. Proteomics of acute heart failure in a rat post-myocardial infarction model. Mol Med Rep. 16(2):1946–1956.
- Gupta S, Gangenahalli G. 2019. Analysis of molecular switch between leukocyte and substrate adhesion in bone marrow endothelial cells. Life Sci. 238:116981.
- Halle M, Gabrielsen A, Paulsson-Berne G, Gahm C, Agardh HE, Farnebo F, Tornvall P. 2010a. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol. 55(12):1227–1236.
- Hamada N, Fujimichi Y. 2014. Classification of radiation effects for dose limitation purposes: history, current situation and future prospects. J Radiat Res. 55(4):629–640.
- Hamada N, Fujimichi Y, Iwasaki T, Fujii N, Furuhashi M, Kubo E, Minamino T, Nomura T, Sato H. 2014. Emerging issues in radiogenic cataracts and cardiovascular disease. J Radiat Res. 55(5):831–846.
- Hamada N, Kawano K, Nomura T, Furukawa K, Yusoff F, Maruhashi T, Maeda M, Nakashima A, Higashi Y. 2022. Temporal changes in sparing and enhancing dose protraction effects of ionizing irradiation for aortic damage in wild-type mice. Cancers. 14(14):3319.
- Hamada N, Kawano KI, Nomura T, Furukawa K, Yusoff FM, Maruhashi T, Maeda M, Nakashima A, Higashi Y. 2021. Vascular damage in the aorta of wild-type mice exposed to ionizing radiation: sparing and enhancing effects of dose protraction. Cancers (Basel. 13(21):5344.
- Hladik D, Bucher M, Endesfelder D, Oestreicher U. 2022. The potential of omics in biological dosimetry. Radiation. 2(1):78–90.
- ICRP. 1984. Nonstochastic effects of ionizing radiation. Annals of the ICRP. Vol. 18. London: ICRP.
- Impey S, Pelz C, Tafessu A, Marzulla T, Turker MS, Raber J. 2016. Proton irradiation induces persistent and tissue-specific DNA methylation changes in the left ventricle and hippocampus. BMC Genomics. 17:273.
- Inouye M, Abraham G, Nelson CP, Wood AM, Sweeting MJ, Dudbridge F, Lai FY, Kaptoge S, Brozynska M, Wang T, et al. 2018. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J Am Coll Cardiol. 72(16):1883–1893.
- Isermann A, Mann C, Rube CE. 2020. Histone variant H2A.J marks persistent DNA damage and triggers the secretory phenotype in radiation-induced senescence. Int J Mol Sci. 21(23):9130.
- Jabbari N, Nawaz M, Rezaie J. 2019. Bystander effects of ionizing radiation: conditioned media from X-ray irradiated MCF-7 cells increases the angiogenic ability of endothelial cells. Cell Commun Signal. 17(1):165.
- Jang WG, Park JY, Lee J, Bang E, Kim SR, Lee EK, Yun HJ, Kang CM, Hwang GS. 2016. Investigation of relative metabolic changes in the organs and plasma of rats exposed to X-ray radiation using HR-MAS (1)H NMR and solution (1)H NMR. NMR Biomed. 29(4):507–518.
- Jelonek K, Krzywon A, Jablonska P, Slominska EM, Smolenski RT, Polanska J, Rutkowski T, Mrochem-Kwarciak J, Skladowski K, Widlak P. 2020. Systemic effects of radiotherapy and concurrent chemo-radiotherapy in head and neck cancer patients-comparison of serum metabolome profiles. Metabolites. 10(2):60.
- Jiang XCL. 2017. Sphingosine-1-phosphate and HDL metabolism. In Komoda T, editor. The HDL handbook; biological functions and clinical implications. Amsterdam, Netherlands: Elsevier.
- Joshi A, Rienks M, Theofilatos K, Mayr M. 2021. Systems biology in cardiovascular disease: a multiomics approach. Nat Rev Cardiol. 18(5):313–330.
- Kaiser JC, Blettner M, Stathopoulos GT. 2021. Biologically based models of cancer risk in radiation research. Int J Radiat Biol. 97(1):2–11.
- Kawamura K, Qi F, Kobayashi J. 2018. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J Radiat Res. 59(2):ii91–ii97.
- Kerns SL, Fachal L, Dorling L, Barnett GC, Baran A, Peterson DR, Hollenberg M, Hao K, Narzo AD, Ahsen ME, et al. 2020. Radiogenomics consortium genome-wide association study meta-analysis of late toxicity after prostate cancer radiotherapy. J Natl Cancer Inst. 112(2):179–190.
- Kim KS, Seu YB, Baek SH, Kim MJ, Kim KJ, Kim JH, Kim JR. 2007. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell. 18(11):4543–4552.
- Kojima H, Inoue T, Kunimoto H, Nakajima K. 2013. IL-6-STAT3 signaling and premature senescence. JAKSTAT. 2(4):e25763.
- Koturbash I, Boyko A, Rodriguez-Juarez R, McDonald RJ, Tryndyak VP, Kovalchuk I, Pogribny IP, Kovalchuk O. 2007. Role of epigenetic effectors in maintenance of the long-term persistent bystander effect in spleen in vivo. Carcinogenesis. 28(8):1831–1838.
- Koturbash I, Miousse IR, Sridharan V, Nzabarushimana E, Skinner CM, Melnyk SB, Pavliv O, Hauer-Jensen M, Nelson GA, Boerma M. 2016. Radiation-induced changes in DNA methylation of repetitive elements in the mouse heart. Mutat Res. 787:43–53.
- Kraemer A, Anastasov N, Angermeier M, Winkler K, Atkinson MJ, Moertl S. 2011. MicroRNA-mediated processes are essential for the cellular radiation response. Radiat Res. 176(5):575–586.
- Kreuzer M, Auvinen A, Cardis E, Hall J, Jourdain JR, Laurier D, Little MP, Peters A, Raj K, Russell NS, et al. 2015. Low-dose ionising radiation and cardiovascular diseases–Strategies for molecular epidemiological studies in Europe. Mutat Res Rev Mutat Res. 764:90–100.
- Kuch B, Heier M, von Scheidt W, Kling B, Hoermann A, Meisinger C. 2008. 20-year trends in clinical characteristics, therapy and short-term prognosis in acute myocardial infarction according to presenting electrocardiogram: the MONICA/KORA AMI Registry (1985–2004). J Intern Med. 264(3):254–264.
- Kumar P, Wang P, Farese AM, MacVittie TJ, Kane MA. 2021. Metabolomics of multiorgan radiation injury in non-human primate model reveals system-wide metabolic perturbations. Health Phys. 121(4):395–405.
- Kura B, Babal P, Slezak J. 2017. Implication of microRNAs in the development and potential treatment of radiation-induced heart disease. Can J Physiol Pharmacol. 95(10):1236–1244.
- Kura B, Yin C, Frimmel K, Krizak J, Okruhlicova L, Kukreja RC, Slezak J. 2016. Changes of microRNA-1, -15b and -21 levels in irradiated rat hearts after treatment with potentially radioprotective drugs. Physiol Res. 65(1):S129–S137.
- Laiakis EC, Mak TD, Anizan S, Amundson SA, Barker CA, Wolden SL, Brenner DJ, Fornace AJ. Jr. 2014. Development of a metabolomic radiation signature in urine from patients undergoing total body irradiation. Radiat Res. 181(4):350–361.
- Laiakis EC, Pannkuk EL, Chauthe SK, Wang YW, Lian M, Mak TD, Barker CA, Astarita G, Fornace AJ. Jr. 2017. A serum small molecule biosignature of radiation exposure from total body irradiated patients. J Proteome Res. 16(10):3805–3815.
- Laiakis EC, Shuryak I, Deziel A, Wang YW, Barnette BL, Yu Y, Ullrich RL, Fornace AJ, Jr., Emmett MR. 2021. Effects of low dose space radiation exposures on the splenic metabolome. Int J Mol Sci. 22(6):3070.
- Laiakis EC, Strawn SJ, Brenner DJ, Fornace AJ. Jr. 2016. Assessment of saliva as a potential biofluid for biodosimetry: a pilot metabolomics study in mice. Radiat Res. 186(1):92–97.
- Laurier D, Rühm W, Paquet F, Applegate K, Cool D, Clement C; International Commission on Radiological Protection (ICRP). 2021. Areas of research to support the system of radiological protection. Radiat Environ Biophys. 60(4):519–530.
- Li HH, Lin YT, Laiakis EC, Goudarzi M, Weber W, Fornace AJ. Jr. 2020. Serum metabolomic alterations associated with cesium-137 internal emitter delivered in various dose rates. Metabolites. 10(7):270.
- Li W, Shu S, Cheng L, Hao X, Wang L, Wu Y, Yuan Z, Zhou J. 2020. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. 292:193–200.
- Li M, You L, Xue J, Lu Y. 2018. Ionizing radiation-induced cellular senescence in normal, non-transformed cells and the involved DNA damage response: a mini review. Front Pharmacol. 9:522.
- Li X, Zhao D, Guo Z, Li T, Qili M, Xu B, Qian M, Liang H, E X, Chege Gitau S, et al. 2016. Overexpression of serpinE2/protease nexin-1 contribute to pathological cardiac fibrosis via increasing collagen deposition. Sci Rep. 6:37635.
- Libby P. 2002. Inflammation in atherosclerosis. Nature. 420(6917):868–874.
- Lindner JR, Davidson BP, Song Z, Maier CS, Minnier J, Stevens JF, Ferencik M, Taqui S, Belcik JT, Moccetti F, et al. 2021. Plasma lipidomic patterns in patients with symptomatic coronary microvascular dysfunction. Metabolites. 11(10):648.
- Lindsey ML, Hall ME, Harmancey R, Ma Y. 2016. Adapting extracellular matrix proteomics for clinical studies on cardiac remodeling post-myocardial infarction. Clin Proteomics. 13:19.
- Little MP. 2016. Radiation and circulatory disease. Mutat Res Rev Mutat Res. 770:299–318.
- Little MP, Azizova TV, Hamada N. 2021. Low- and moderate-dose non-cancer effects of ionizing radiation in directly exposed individuals, especially circulatory and ocular diseases: a review of the epidemiology. Int J Radiat Biol. 97(6):782–803.
- Little MP, Gola A, Tzoulaki I. 2009. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure. PLoS Comput Biol. 5(10):e1000539.
- Little MP, Pawel D, Misumi M, Hamada N, Cullings HM, Wakeford R, Ozasa K. 2020. Lifetime mortality risk from cancer and circulatory disease predicted from the Japanese atomic bomb Survivor life span study data taking account of dose measurement error. Radiat Res. 194(3):259–276.
- Little MP, Wakeford R, Bouffler SD, Abalo K, Hauptmann M, Hamada N, Kendall GM. 2022a. Cancer risks among studies of medical diagnostic radiation exposure in early life without quantitative estimates of dose. Sci Total Environ. 832:154723.
- Little MP, Wakeford R, Bouffler SD, Abalo K, Hauptmann M, Hamada N, Kendall GM. 2022b. Review of the risk of cancer following low and moderate doses of sparsely ionising radiation received in early life in groups with individually estimated doses. Environ Int. 159:106983.
- Liu Q, Piao H, Wang Y, Zheng D, Wang W. 2021. Circulating exosomes in cardiovascular disease: novel carriers of biological information. Biomed Pharmacother. 135:111148.
- Liu X, Zhou Y, Wang S, Guan H, Hu S, Huang R, Zhou P. 2019. Impact of low-dose ionising radiation on the composition of the gut microbiota of mice. Toxicol Sci. 171(1):258–268.
- Livingston K, Schlaak RA, Puckett LL, Bergom C. 2020. The role of mitochondrial dysfunction in radiation-induced heart disease: from bench to bedside. Front Cardiovasc Med. 7:20.
- Lowe D, Raj K. 2014. Premature aging induced by radiation exhibits pro-atherosclerotic effects mediated by epigenetic activation of CD44 expression. Aging Cell. 13(5):900–910.
- Lu Y, Zhu X, Li J, Fang R, Wang Z, Zhang J, Li K, Li X, Bai H, Yang Q, et al. 2017. Glycine prevents pressure overload induced cardiac hypertrophy mediated by glycine receptor. Biochem Pharmacol. 123:40–51.
- Luo L, Yan C, Fuchi N, Kodama Y, Zhang X, Shinji G, Miura K, Sasaki H, Li TS. 2021. Mesenchymal stem cell-derived extracellular vesicles as probable triggers of radiation-induced heart disease. Stem Cell Res Ther. 12(1):422.
- Luzhna L, Ilnytskyy Y, Kovalchuk O. 2015. Mobilization of LINE-1 in irradiated mammary gland tissue may potentially contribute to low dose radiation-induced genomic instability. Genes Cancer. 6(1–2):71–81.
- Maes OC, An J, Sarojini H, Wu H, Wang E. 2008. Changes in MicroRNA expression patterns in human fibroblasts after low-LET radiation. J Cell Biochem. 105(3):824–834.
- Martins-Marques T, Hausenloy DJ, Sluijter JPG, Leybaert L, Girao H. 2021. Intercellular communication in the heart: therapeutic opportunities for cardiac ischemia. Trends Mol Med. 27(3):248–262.
- Marui N, Offermann MK, Swerlick R, Kunsch C, Rosen CA, Ahmad M, Alexander RW, Medford RM. 1993. Vascular cell adhesion molecule-1 (VCAM-1) gene transcription and expression are regulated through an antioxidant-sensitive mechanism in human vascular endothelial cells. J Clin Invest. 92(4):1866–1874.
- Mc Auley MT. 2022. Modeling cholesterol metabolism and atherosclerosis. WIREs Mech Dis. 14(3):e1546.
- McGarrah RW, Crown SB, Zhang GF, Shah SH, Newgard CB. 2018. Cardiovascular metabolomics. Circ Res. 122(9):1238–1258.
- McPherson R, Tybjaerg-Hansen A. 2016. Genetics of coronary artery disease. Circ Res. 118(4):564–578.
- Mildner CS, Copic D, Zimmermann M, Lichtenauer M, Direder M, Klas K, Bormann D, Gugerell A, Moser B, Hoetzenecker K, et al. 2022. Secretome of stressed peripheral blood mononuclear cells alters transcriptome signature in heart, liver, and spleen after an experimental acute myocardial infarction: an in silico analysis. Biology (Basel). 11(1):116.
- Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. 2002. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 105(13):1541–1544.
- Miousse IR, Chang J, Shao L, Pathak R, Nzabarushimana É, Kutanzi KR, Landes RD, Tackett AJ, Hauer-Jensen M, Zhou D, et al. 2017. Inter-strain differences in LINE-1 DNA methylation in the mouse hematopoietic system in response to exposure to ionizing radiation. Int J Mol Sci. 18(7):1430.
- Miousse IR, Skinner CM, Sridharan V, Seawright JW, Singh P, Landes RD, Cheema AK, Hauer-Jensen M, Boerma M, Koturbash I. 2019. Changes in one-carbon metabolism and DNA methylation in the hearts of mice exposed to space environment-relevant doses of oxygen ions ((16)O). Life Sci Space Res (Amst). 22:8–15.
- Moertl S, Buschmann D, Azimzadeh O, Schneider M, Kell R, Winkler K, Tapio S, Hornhardt S, Merl-Pham J, Pfaffl MW, et al. 2020. Radiation exposure of peripheral mononuclear blood cells alters the composition and function of secreted extracellular vesicles. Int J Mol Sci. 21(0 ):2336.
- Moertl S, Mutschelknaus L, Heider T, Atkinson MJ. 2016. MicroRNAs as novel elements in personalized radiotherapy. Transl Cancer Res. 5(S6):S1262–S1269.
- Mortensen HM, Chamberlin J, Joubert B, Angrish M, Sipes N, Lee JS, Euling SY. 2018. Leveraging human genetic and adverse outcome pathway (AOP) data to inform susceptibility in human health risk assessment. Mamm Genome. 29(1–2):190–204.
- Muka T, Koromani F, Portilla E, O’Connor A, Bramer WM, Troup J, Chowdhury R, Dehghan A, Franco OH. 2016. The role of epigenetic modifications in cardiovascular disease: a systematic review. Int J Cardiol. 212:174–183.
- Muka T, Nano J, Voortman T, Braun KVE, Ligthart S, Stranges S, Bramer WM, Troup J, Chowdhury R, Dehghan A, et al. 2016. The role of global and regional DNA methylation and histone modifications in glycemic traits and type 2 diabetes: a systematic review. Nutr Metab Cardiovasc Dis. 26(7):553–566.
- Mukherjee P, Mani S. 2013. Methodologies to decipher the cell secretome. Biochim Biophys Acta. 1834(11):2226–2232.
- NASEM. 2022. Leveraging advances in modern science to revitalize low-dose radiation research in the United States. Washington (DC): The National Academies Press.
- NCRP. 2020. Report No. 186 – approaches for integrating information from radiation biology and epidemiology to enhance low-dose health risk assessment (2020). Bethesda (MD): NCRP.
- Nouraee N, Mowla SJ. 2015. miRNA therapeutics in cardiovascular diseases: promises and problems. Front Genet. 6:232.
- OECD. 2018. Handbook supplement to the guidance document for developing and assessing adverse outcome pathways. Paris, France: OECD.
- Örd T, Õunap K, Stolze LK, Aherrahrou R, Nurminen V, Toropainen A, Selvarajan I, Lönnberg T, Aavik E, Ylä-Herttuala S, et al. 2021. Single-cell epigenomics and functional fine-mapping of atherosclerosis GWAS loci. Circ Res. 129(2):240–258.
- Pannkuk EL, Fornace AJ, Jr., Laiakis EC. 2017. Metabolomic applications in radiation biodosimetry: exploring radiation effects through small molecules. Int J Radiat Biol. 93(10):1151–1176.
- Pannkuk EL, Laiakis EC, Girgis M, Garty GY, Morton SR, Pujol-Canadell M, Ghandhi SA, Amundson SA, Brenner DJ, Fornace AJ. Jr. 2021. Biofluid metabolomics of mice exposed to external low-dose rate radiation in a novel irradiation system, the variable dose-rate external (137)Cs irradiator. J Proteome Res. 20(11):5145–5155.
- Papiez A, Azimzadeh O, Azizova T, Moseeva M, Anastasov N, Smida J, Tapio S, Polanska J. 2018. Integrative multiomics study for validation of mechanisms in radiation-induced ischemic heart disease in Mayak workers. PLoS One. 13(12):e0209626.
- PDAY. 1993. Natural history of aortic and coronary atherosclerotic lesions in youth. Findings from the PDAY Study. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb. 13(9):1291–1298.
- Pernot E, Hall J, Baatout S, Benotmane MA, Blanchardon E, Bouffler S, El Saghire H, Gomolka M, Guertler A, Harms-Ringdahl M, et al. 2012. Ionizing radiation biomarkers for potential use in epidemiological studies. Mutat Res Rev Mutat Res. 751(2):258–286.
- Philipp J, Azimzadeh O, Subramanian V, Merl-Pham J, Lowe D, Hladik D, Erbeldinger N, Ktitareva S, Fournier C, Atkinson MJ, et al. 2017. Radiation-induced endothelial inflammation is transferred via the secretome to recipient cells in a STAT-mediated process. J Proteome Res. 16(10):3903–3916.
- Philipp J, Le Gleut R, Toerne CV, Subedi P, Azimzadeh O, Atkinson MJ, Tapio S. 2020. Radiation response of human cardiac endothelial cells reveals a central role of the cGAS-STING pathway in the development of inflammation. Proteomes. 8(4):30.
- Pluder F, Barjaktarovic Z, Azimzadeh O, Mörtl S, Krämer A, Steininger S, Sarioglu H, Leszczynski D, Nylund R, Hakanen A, et al. 2011. Low-dose irradiation causes rapid alterations to the proteome of the human endothelial cell line EA.hy926. Radiat Environ Biophys. 50(1):155–166.
- Pogribny I, Koturbash I, Tryndyak V, Hudson D, Stevenson SM, Sedelnikova O, Bonner W, Kovalchuk O. 2005. Fractionated low-dose radiation exposure leads to accumulation of DNA damage and profound alterations in DNA and histone methylation in the murine thymus. Mol Cancer Res. 3(10):553–561.
- Preston RJ. 2015. Integrating basic radiobiological science and epidemiological studies: why and how. Health Phys. 108(2):125–130.
- Preston RJ. 2017. Can radiation research impact the estimation of risk? Int J Radiat Biol. 93(10):1009–1014.
- Preston RJ, Rühm W, Azzam EI, Boice JD, Bouffler S, Held KD, Little MP, Shore RE, Shuryak I, Weil MM. 2021. Adverse outcome pathways, key events, and radiation risk assessment. Int J Radiat Biol. 97(6):804–814.
- Proia RL, Hla T. 2015. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J Clin Invest. 125(4):1379–1387.
- Rahman A, Bando M, Kefer J, Anwar KN, Malik AB. 1999. Protein kinase C-activated oxidant generation in endothelial cells signals intercellular adhesion molecule-1 gene transcription. Mol Pharmacol. 55(3):575–583.
- Rajaraman P, Hauptmann M, Bouffler S, Wojcik A. 2018. Human individual radiation sensitivity and prospects for prediction. Ann ICRP. 47(3–4):126–141.
- Ramadan R, Baatout S, Aerts A, Leybaert L. 2021. The role of connexin proteins and their channels in radiation-induced atherosclerosis. Cell Mol Life Sci. 78(7):3087–3103.
- Ramadan R, Claessens M, Cocquyt E, Mysara M, Decrock E, Baatout S, Aerts A, Leybaert L. 2021. X‑irradiation induces acute and early term inflammatory responses in atherosclerosis‑prone ApoE‑/‑ mice and in endothelial cells. Mol Med Rep. 23(6):399.
- Ramadan R, Vromans E, Anang DC, Decrock E, Mysara M, Monsieurs P, Baatout S, Leybaert L, Aerts A. 2019. Single and fractionated ionizing radiation induce alterations in endothelial connexin expression and channel function. Sci Rep. 9(1):4643.
- Ramadan R, Vromans E, Anang DC, Goetschalckx I, Hoorelbeke D, Decrock E, Baatout S, Leybaert L, Aerts A. 2020. Connexin43 hemichannel targeting with TAT-Gap19 alleviates radiation-induced endothelial cell damage. Front Pharmacol. 11(212):212.
- Rehammar JC, Jensen MB, McGale P, Lorenzen EL, Taylor C, Darby SC, Videbaek L, Wang Z, Ewertz M. 2017. Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005. Radiother Oncol. 123(2):299–305.
- Ren JL, Pan JS, Lu YP, Sun P, Han J. 2009. Inflammatory signaling and cellular senescence. Cell Signal. 21(3):378–383.
- Rombouts C, Aerts A, Quintens R, Baselet B, El-Saghire H, Harms-Ringdahl M, Haghdoost S, Janssen A, Michaux A, Yentrapalli R, et al. 2014. Transcriptomic profiling suggests a role for IGFBP5 in premature senescence of endothelial cells after chronic low dose rate irradiation. Int J Radiat Biol. 90(7):560–574.
- Rufini A, Tucci P, Celardo I, Melino G. 2013. Senescence and aging: the critical roles of p53. Oncogene. 32(43):5129–5143.
- Rühm W, Eidemüller M, Kaiser JC. 2017. Biologically-based mechanistic models of radiation-related carcinogenesis applied to epidemiological data. Int J Radiat Biol. 93(10):1093–1117.
- Rühm W, Woloschak GE, Shore RE, Azizova TV, Grosche B, Niwa O, Akiba S, Ono T, Suzuki K, Iwasaki T, et al. 2015. Dose and dose-rate effects of ionizing radiation: a discussion in the light of radiological protection. Radiat Environ Biophys. 54(4):379–401.
- Sallam M, Benotmane MA, Baatout S, Guns PJ, Aerts A. 2022. Radiation-induced cardiovascular disease: an overlooked role for DNA methylation? Epigenetics. 17(1):59–80.
- Satyamitra MM, Cassatt DR, Hollingsworth BA, Price PW, Rios CI, Taliaferro LP, Winters TA, DiCarlo AL. 2020. Metabolomics in radiation biodosimetry: current approaches and advances. Metabolites. 10(8):328.
- Sauer UG, Deferme L, Gribaldo L, Hackermüller J, Tralau T, van Ravenzwaay B, Yauk C, Poole A, Tong W, Gant TW. 2017. The challenge of the application of 'omics technologies in chemicals risk assessment: background and outlook. Regul Toxicol Pharmacol. 91(1):S14–s26.
- Schäfer J, Strimmer K. 2005. An empirical Bayes approach to inferring large-scale gene association networks. Bioinformatics. 21(6):754–764.
- Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. 2017. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: a systematic review and dose-response meta-analysis. Eur Heart J. 38(39):2948–2956.
- Schofield PN, Kulka U, Tapio S, Grosche B. 2019. Big data in radiation biology and epidemiology; an overview of the historical and contemporary landscape of data and biomaterial archives. Int J Radiat Biol. 95(7):861–878.
- Seawright JW, Samman Y, Sridharan V, Mao XW, Cao M, Singh P, Melnyk S, Koturbash I, Nelson GA, Hauer-Jensen M, et al. 2017. Effects of low-dose rate γ-irradiation combined with simulated microgravity on markers of oxidative stress, DNA methylation potential, and remodeling in the mouse heart. PLoS One. 12(7):e0180594.
- Seemann I, Gabriels K, Visser NL, Hoving S, te Poele JA, Pol JF, Gijbels MJ, Janssen BJ, van Leeuwen FW, Daemen MJ, et al. 2012. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol. 103(2):143–150.
- Seemann I, Te Poele JA, Luikinga SJ, Hoving S, Stewart FA. 2013. Endoglin haplo-insufficiency modifies the inflammatory response in irradiated mouse hearts without affecting structural and mircovascular changes. PLoS One. 8(7):e68922.
- Selvarajan I, Toropainen A, Garske KM, López Rodríguez M, Ko A, Miao Z, Kaminska D, Õunap K, Örd T, Ravindran A, et al. 2021. Integrative analysis of liver-specific non-coding regulatory SNPs associated with the risk of coronary artery disease. Am J Hum Genet. 108(3):411–430.
- Shaikh S, Channa NA, Talpur FN, Younis M, Tabassum N. 2017. Radiotherapy improves serum fatty acids and lipid profile in breast cancer. Lipids Health Dis. 16(1):92.
- Shore RE, Beck HL, Boice JD, Caffrey EA, Davis S, Grogan HA, Mettler FA, Preston RJ, Till JE, Wakeford R, et al. 2018. Implications of recent epidemiologic studies for the linear nonthreshold model and radiation protection. J Radiol Prot. 38(3):1217–1233.
- Simonetto C, Heier M, Peters A, Kaiser JC, Rospleszcz S. 2022. From atherosclerosis to myocardial infarction - a process-oriented model investigating the role of risk factors. Am J Epidemiol. doi:10.1093/aje/kwac038
- Simonetto C, Heier M, Rospleszcz S, Meisinger C, Then C, Seißler J, Peters A, Kaiser JC. 2020. Risk for cardiovascular events responds nonlinearly to carotid intima-media thickness in the KORA F4 study. Atherosclerosis. 296:32–39.
- Simonetto C, Kaiser JC, van den Bogaard VAB, Langendijk JA, Crijns APG. 2022. Breast cancer radiotherapy and the risk of acute coronary events–insights from a process oriented model. Int J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2022.06.082
- Simonetto C, Rospleszcz S, Heier M, Meisinger C, Peters A, Kaiser JC. 2021. Simulating the dynamics of atherosclerosis to the incidence of myocardial infarction, applied to the KORA population. Stat Med. 40(14):3299–3312.
- Smit T, Schickel E, Azimzadeh O, von Toerne C, Rauh O, Ritter S, Durante M, Schroeder IS. 2021. A human 3D cardiomyocyte risk model to study the cardiotoxic influence of x-rays and other noxae in adults. Cells. 10(10):2608.
- Sotoudeh H, Sarrami AH, Roberson GH, Shafaat O, Sadaatpour Z, Rezaei A, Choudhary G, Singhal A, Sotoudeh E, Tanwar M. 2021. Emerging applications of radiomics in neurological disorders: a review. Cureus. 13(12):e20080.
- Sriharshan A. 2014. Radiation-induced crosstalk between microRNAs and proteins of the endothelium: in silico analysis. J Proteomics Bioinform. 07(10):327–331.
- Sriharshan A, Boldt K, Sarioglu H, Barjaktarovic Z, Azimzadeh O, Hieber L, Zitzelsberger H, Ueffing M, Atkinson MJ, Tapio S. 2012. Proteomic analysis by SILAC and 2D-DIGE reveals radiation-induced endothelial response: four key pathways. J Proteomics. 75(8):2319–2330.
- Stewart FA, Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ, Aleman BM, Edgar AB, Mabuchi K, Muirhead CR, et al. 2012. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP. 41(1–2):1–322.
- Subedi P, Moertl S, Azimzadeh O. 2022. Omics in radiation biology: surprised but not disappointed. Radiation. 2(1):124–129.
- Subramanian V, Borchard S, Azimzadeh O, Sievert W, Merl-Pham J, Mancuso M, Pasquali E, Multhoff G, Popper B, Zischka H, et al. 2018. PPARalpha is necessary for radiation-induced activation of noncanonical TGFbeta signaling in the heart. J Proteome Res. 17(4):1677–1689.
- Subramanian V, Seemann I, Merl-Pham J, Hauck SM, Stewart FA, Atkinson MJ, Tapio S, Azimzadeh O. 2017. Role of TGF beta and PPAR alpha signaling pathways in radiation response of locally exposed heart: integrated global transcriptomics and proteomics analysis. J Proteome Res. 16(1):307–318.
- Sun W, Jiao Y, Cui B, Gao X, Xia Y, Zhao Y. 2013. Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Lab Invest. 93(6):626–638.
- Sun W, Ni X, Sun S, Cai L, Yu J, Wang J, Nie B, Sun Z, Ni X, Cao X. 2016. Adipose-derived stem cells alleviate radiation-induced muscular fibrosis by suppressing the expression of TGF-beta1. Stem Cells Int. 2016:5638204.
- Tapio S, Little MP, Kaiser JC, Impens N, Hamada N, Georgakilas AG, Simar D, Salomaa S. 2021. Ionizing radiation-induced circulatory and metabolic diseases. Environ Int. 146:106235.
- Tirziu D, Giordano FJ, Simons M. 2010. Cell communications in the heart. Circulation. 122(9):928–937.
- Vailati-Riboni M, Palombo V, Loor JJ. 2017. What are omics sciences? In: Ametaj BN, editor. Periparturient diseases of dairy cows: a systems biology approach. Cham, Switzerland: Springer International Publishing; p. 1–7.
- van den Bogaard VAB, Spoor DS, van der Schaaf A, van Dijk LV, Schuit E, Sijtsema NM, Langendijk JA, Maduro JH, Crijns APG. 2021. The importance of radiation dose to the atherosclerotic plaque in the left anterior descending coronary artery for radiation-induced cardiac toxicity of breast cancer patients? Int J Radiat Oncol Biol Phys. 110(5):1350–1359.
- Van Der Meeren A, Squiban C, Gourmelon P, Lafont H, Gaugler MH. 1999. Differential regulation by IL-4 and IL-10 of radiation-induced IL-6 and IL-8 production and ICAM-1 expression by human endothelial cells. Cytokine. 11(11):831–838.
- van Wieringen WN, Chen Y. 2021. Penalized estimation of the Gaussian graphical model from data with replicates. Stat Med. 40(19):4279–4293.
- Vera NB, Coy SL, Laiakis EC, Fornace AJ, Jr., Clasquin M, Barker CA, Pfefferkorn JA, Vouros P. 2020. Quantitation of urinary acylcarnitines by DMS-MS/MS uncovers the effects of total body irradiation in cancer patients. J Am Soc Mass Spectrom. 31(3):498–507.
- Vernice NA, Meydan C, Afshinnekoo E, Mason CE. 2020. Long-term spaceflight and the cardiovascular system. Precis Clin Med. 3(4):284–291.
- Vicente E, Vujaskovic Z, Jackson IL. 2020. A systematic review of metabolomic and lipidomic candidates for biomarkers in radiation injury. Metabolites. 10(6):259.
- Villeneuve DL, Crump D, Garcia-Reyero N, Hecker M, Hutchinson TH, LaLone CA, Landesmann B, Lettieri T, Munn S, Nepelska M, et al. 2014. Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci. 142(2):312–320.
- Wagner T, Traxler D, Simader E, Beer L, Narzt MS, Gruber F, Madlener S, Laggner M, Erb M, Vorstandlechner V, et al. 2018. Different pro-angiogenic potential of gamma-irradiated PBMC-derived secretome and its subfractions. Sci Rep. 8(1):18016.
- Wagner-Ecker M, Schwager C, Wirkner U, Abdollahi A, Huber PE. 2010. MicroRNA expression after ionizing radiation in human endothelial cells. Radiat Oncol. 5:25.
- Wang Y, Boerma M, Zhou D. 2016. Ionizing radiation-induced endothelial cell senescence and cardiovascular diseases. Radiat Res. 186(2):153–161.
- Wang Z, Gerstein M, Snyder M. 2009. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 10(1):57–63.
- Wang Y, Li C, Chuo W, Liu Z, Ouyang Y, Li D, Han J, Wu Y, Guo S, Wang W. 2013. Integrated proteomic and metabolomic analysis reveals the NADH-mediated TCA cycle and energy metabolism disorders based on a new model of chronic progressive heart failure. Mol Biosyst. 9(12):3135–3145. eng.
- Watanabe K, Fujii H, Takahashi T, Kodama M, Aizawa Y, Ohta Y, Ono T, Hasegawa G, Naito M, Nakajima T, et al. 2000. Constitutive regulation of cardiac fatty acid metabolism through peroxisome proliferator-activated receptor alpha associated with age-dependent cardiac toxicity. J Biol Chem. 275(29):22293–22299.
- Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, Lüllmann-Rauch R, Lettau O, Jacoby C, Schrader J, et al. 2008. Biglycan is required for adaptive remodeling after myocardial infarction. Circulation. 117(10):1269–1276.
- Wiench B, Chen YR, Paulsen M, Hamm R, Schroder S, Yang NS, Efferth T. 2013. Integration of different “-omics” technologies identifies inhibition of the IGF1R-Akt-mTOR signaling cascade involved in the cytotoxic effect of Shikonin against leukemia cells. Evid Based Complement Alternat Med. 2013:818709.
- Wu K, Chen Z, Peng Q, Chen G, Yan W, Chen X. 2019. Ku86 alleviates human umbilical vein endothelial cellular apoptosis and senescence induced by a low dose of ionizing radiation. J Int Med Res. 47(2):893–904.
- Wu Q, Fang Y, Zhang X, Song F, Wang Y, Chen H, Du J, Zheng CB, Shen B. 2020. Effect of X-rays on transcript expression of rat brain microvascular endothelial cells: role of calcium signaling in X-ray-induced endothelium damage. Biosci Rep. 40(4):BSR20193760.
- Xu P, Yi Y, Luo Y, Liu Z, Xu Y, Cai J, Zeng Z, Liu A. 2021. Radiation‑induced dysfunction of energy metabolism in the heart results in the fibrosis of cardiac tissues. Mol Med Rep. 24(6):842.
- Yahyapour R, Salajegheh A, Safari A, Amini P, Rezaeyan A, Amraee A, Najafi M. 2018. Radiation-induced non-targeted effect and carcinogenesis; implications in clinical radiotherapy. J Biomed Phys Eng. 8(4):435–446.
- Yao Y, Chen LF, Li J, Chen J, Tian XL, Wang H, Mei ZJ, Xie CH, Zhong YH. 2022. Altered DNA methylation and gene expression profiles in radiation-induced heart fibrosis of sprague-dawley rats. Radiat Res. 198(2):154–161.
- Yentrapalli R, Azimzadeh O, Barjaktarovic Z, Sarioglu H, Wojcik A, Harms-Ringdahl M, Atkinson MJ, Haghdoost S, Tapio S. 2013. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma-radiation. Proteomics. 13(7):1096–1107.
- Yentrapalli R, Azimzadeh O, Sriharshan A, Malinowsky K, Merl J, Wojcik A, Harms-Ringdahl M, Atkinson MJ, Becker KF, Haghdoost S, et al. 2013. The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS One. 8(8):e70024.
- Zalesak-Kravec S, Huang W, Wang P, Yu J, Liu T, Defnet AE, Moise AR, Farese AM, MacVittie TJ, Kane MA. 2021. Multi-omic analysis of non-human primate heart after partial-body radiation with minimal bone marrow sparing. Health Phys. 121(4):352–371.
- Zanfardino M, Franzese M, Pane K, Cavaliere C, Monti S, Esposito G, Salvatore M, Aiello M. 2019. Bringing radiomics into a multi-omics framework for a comprehensive genotype-phenotype characterization of oncological diseases. J Transl Med. 17(1):337.
- Zhang DM, Navara R, Yin T, Szymanski J, Goldsztejn U, Kenkel C, Lang A, Mpoy C, Lipovsky CE, Qiao Y, et al. 2021. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat Commun. 12(1):5558.
- Zhou SS, Jin JP, Wang JQ, Zhang ZG, Freedman JH, Zheng Y, Cai L. 2018. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 39(7):1073–1084.
