?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Cognitive difficulties can be subtle and only come to light when patients return home from inpatient care and start to participate in society. Subjective cognitive complaints often interfere with participation, hence capturing cognitive complaints systematically is important. We developed a patient- and relative-reported measure to assess cognitive complaints during daily life activities across the memory, attention and executive domain for patients with acquired brain injury (ABI). The inventory Cognitive Complaints - Participation (CoCo-P) was created based on a literature review, consultations with experts, semi-structured interviews with patients, and a quantitative study. The inventory was administered to patients with ABI (n = 46), their relatives (n = 33) and healthy controls (n = 102) to finalize the inventory. We examined the reported complaints per daily life activity and cognitive domain of patients and healthy controls, and we compared the patients’ and relatives’ reports. The majority of patients (87–96%) experienced cognitive complaints, mostly related to attention, at work/education, during leisure activities, and in contact with family/friends and community. Patients reported more cognitive complaints than relatives. The CoCo-P seems appropriate to capture cognitive complaints in daily life in patients with mild ABI. Additional research is needed in terms of reliability and validity.
Introduction
Acquired brain injury (ABI), mostly caused by stroke or traumatic brain injury (TBI) (Cicerone et al., Citation2000), frequently results in impairments in memory (Das Nair & Lincoln, Citation2007; Spreij, Visser-Meily, Van Heugten, & Nijboer, Citation2014), attention (Virk, Williams, Brunsdon, Suh, & Morrow, Citation2015), and executive function (Chung, Pollock, Campbell, Durward, & Hagen, Citation2013; Cicerone, Levin, Malec, Stuss, & Whyte, Citation2006). Cognitive impairments can be subtle and often only come to light when patients return home from the hospital or rehabilitation centre and start to participate in society (e.g., work, travel). Participation refers to the engagement of a person in daily life activities in a social context (Viscogliosi, Desrosiers, Belleville, Caron, & Ska, Citation2011). The presence of cognitive impairment is strongly associated with restrictions in participation (Ezekiel et al., Citation2018; Jette, Keysor, Coster, Ni, & Haley, Citation2005; Mole & Demeyere, Citation2018; Viscogliosi et al., Citation2011) and is the greatest burden to patients and their families (Ponsford, Olver, Ponsford, & Nelms, Citation2003).
Assessment of cognitive impairments is mostly done with neuropsychological tests. These tests, however, often fail to objectify subtle disorders and to determine which daily life difficulties the patient is likely to encounter (Bielak, Hatt, & Diehl, Citation2017; Chaytor & Schmitter-Edgecombe, Citation2003). In addition, cognitive impairments are not necessarily an indication of cognitive complaints, and vice versa (Clarke, Genat, & Anderson, Citation2012; Duits, Munnecom, Van Heugten, & Van Oostenbrugge, Citation2008; Landre, Poppe, Davis, Schmaus, & Hobbs, Citation2006; Rijsbergen, Van Mark, De Kort, & Sitskoorn, Citation2014). Cognitive complaints may also interfere with participation (Benedictus, Spikman, & Van Der Naalt, Citation2010; Robison et al., Citation2009; van der Naalt, van Zomeren, Sluiter, & Minderhoud, Citation1999); hence systematically capturing cognitive complaints is important (Rijsbergen et al., Citation2014).
However, suitable inventories that measure cognitive complaints during daily life activities are not available. Several instruments, like the Stroke Impact Scale (scale – memory and thinking) (Duncan, Wallace, Studenski, Lai, & Johnson, Citation2001), Cognitive Failure Questionnaire (Broadbent, Cooper, FitzGerald, & Parkes, Citation1982), Brain Injury Complaint Questionnaire (Vallat-Azouvi et al., Citation2018), and the Checklist for Emotional and Cognitive Consequences (CLCE-24) (van Heugten, Rasquin, Winkens, Beusmans, & Verhey, Citation2007) are available to identify cognitive complaints, yet the items are not directly related to daily life activities. On the contrary, several instruments particularly focus on daily life activities in a social context (i.e., participation), such as the Frenchay Activities Index (Holbrook & Skilbeck, Citation1983), Instrumental Activities of Daily Living (Lawton & Brody, Citation1969), Assessment of Life Habits (Fougeyrollas & Noreau, Citation2002), and the Utrecht Scale for Evaluation of Rehabilitation – Participation (USER-P) (Post et al., Citation2012), yet the focus is not on cognition as the reported restrictions may also be caused by motor, emotional and/or behavioural problems.
The primary aim of this study was to develop an inventory for patients with ABI to measure cognitive complaints across several cognitive domains as well as across several daily life activities. In a sequence of steps (Wiklund et al., Citation2016), an inventory suitable for patients with ABI was developed: (1) a literature search explored the availability of inventories measuring cognitive complaints on level of participation; (2) modifications were made to suit our target population after consulting an expert panel; (3) semi-structured interviews were held with patients (n = 7) to evaluate face validity (i.e., subjective evaluation whether the test seems to measure what it reports to measure); and (4) a quantitative study was conducted to finalize the inventory by administering the inventory in patients with ABI, their relatives and healthy controls. A secondary aim was to develop a version for relatives as impairment in self-awareness and the overestimation of cognitive abilities are common issues in ABI patients (Fischer, Trexler, & Gauggel, Citation2004; Kelley et al., Citation2014; Prigatano, Altman, & O’Brien, Citation1990; Sbordone, Seyranian, & Ruff, Citation1998). Based on the finalized inventory, we compared the reported complaints across daily life activities (e.g., work, travel), cognitive domains (i.e., memory, attention, executive function) and the level of fatigue between patients and healthy controls. Finally, we compared the patients’ and relatives’ reports regarding the cognitive complaints and the perceived level of fatigue.
Methods
Development of the cognitive complaints - participation (CoCo-P)
Literature search and gap analysis
A literature search was conducted and identified multiple inventories measuring cognitive complaints and/or participation (See Appendix A1 for an overview). Only the Cognitive Impairment in Daily Life (CID) (Johansson, Marcusson, & Wressle, Citation2016) was considered to meet the criteria to measure cognitive complaints, across cognitive domains, directly related to several daily life activities. This inventory was, however, developed for patients with a neurodegenerative disorder, such as mild cognitive impairment and dementia. As ABI and neurodegenerative disorders significantly differ in pathology, demographics (e.g., age) and cognitive sequelae, we set out to develop a new inventory based on the structure of the CID.
Expert panel and revision
We arranged two meetings with an expert panel that consisted of healthcare professionals (rehabilitation physicians and occupational therapists). Based on their expertise, we aimed to select daily life activities (e.g., work, finances, driving) in which our target population (i.e., outpatients with ABI, living at home) frequently reports complaints. Also, the response options were adjusted and based on the USER-P (Post et al., Citation2012) and reflected different grades of independence and effort (0 [independent without effort], 1 [independent with effort], 2 [with help], 3 [not possible]). It included a fourth response option (4 [not applicable]), as some activities (e.g., driving a car, cooking) are not applicable for some patients. Emoticons were used in the response options, in addition of the written words, to denote the different points on the scales.
Next, we arranged two meetings with cognitive neuroscientists. Attention, memory and reasoning abilities (i.e., problem solving ability that requires both memory and executive functioning; Spielberger, Citation2004) are the basic functions required to complete tasks and solve everyday problems (Bielak et al., Citation2017). We established on three cognitive models presenting memory (Squire, Citation1992, Citation2004), attention (Petersen & Posner, Citation1990, Citation2012; Posner & Rothbart, Citation2007) and executive function (Ylvisaker, Szekeres, & Feeney, Citation1998) to use as theoretical framework for the selection of the items. We selected items focusing on memory (i.e., retrospective memory, prospective memory), attention (i.e., arousal, orienting, monitoring, sustained) or executive function (i.e., planning, self-evaluating, initiative, flexibility) across each daily life activity. Language and visual-perceptual functions were not included in the inventory. Language disorders (e.g., aphasia) and lower-level visual disorders (e.g., scotoma, diplopia) are often prominent in daily life and relatively more easily recognized by clinicians and patients. Lower-level visual disorders are also frequently regarded as pre-cognition. In contrast, higher-order perceptual disorders (e.g., prosopagnosia, simultanagnosia) are more challenging to capture. Luckily, suitable inventories for both lower- and higher-level visual-perceptual disorders as well as language disorders are already available, such as the Cerebral Visual Disorders (CVD) (Kerkhoff, Schaub, & Zihl, Citation1990), the Screening Test for Cognitive Communication (STCC) (Paemeleire, Citation2014), and the Communicative Participation Item Bank (CPIB) (Baylor, Burns, Eadie, Britton, & Yorkston, Citation2011). Based on the expert meeting, a first draft was conducted.
Patient panel and revision
The draft version was administered in seven patients, and semi-structured interviews were conducted to evaluate face validity. See for the demographical and clinical characteristics of these patients. Five patients were visited at home and two patients performed the evaluation by e-mail. We asked patients whether any important daily life activities were missing. We included five questions that could be answered on a Visual Analogue Scale (VAS) ranging from 0–10: (1) How clear was the instruction?; (2) How clear were the items?; (3) How clear were the response options?; (4) How familiar were the daily life activities?; and (5) How do you evaluate the length of the inventory? Additional remarks were administered.
Table 1. Demographical and clinical characteristics of the patients that were interviewed.
For each question, the mean VAS score was above 9, for the exception of one question (How clear were the items?) that had a mean score of 7.1. Based on their suggestions, we adjusted the formulation of several items. Also, the time frame was not clear, so we clarified that the items reflected the patients’ current state (i.e., post ABI onset). The response options were appropriate and well understood by the patients.
Face validity was considered adequate as all patients considered the daily life activities relevant and the items representative for their difficulties. Three patients did feel emotional and behavioural changes were missing in the inventory. We considered their suggestion, however, we felt that including those topics was not in line with our main scope of the inventory (i.e., cognitive complaints post-ABI). Fatigue was also reported as a common complaint especially after consecutive activities, which is in line with previous research (Visser-Keizer, Hogenkamp, Westerhof-Evers, Egberink, & Spikman, Citation2015). Therefore, we included an item measuring fatigue after each daily life activity by using a VAS (range 0–10 cm). Patients are asked to indicate in what extend a daily life activity is tiring along a visual analogue line that extends between two extremes (i.e., “not tiring at all” to “extremely tiring”).
Preliminary inventory used in quantitative study
A preliminary version of the inventory was developed based on the expert meetings and semi-structured interviews with patients. The patient-reported and relative-reported measures contained 42 items focusing on memory, attention or executive function over 11 daily life activities (i.e., work/education, leisure activities, travel, driving, finances, use of medication, family life, contact with family/friends, contact with community, cooking, grocery shopping). After each activity the level of fatigue was measured using a VAS. See for an overview of the preliminary version that was used in the quantitative study.
Table 2. Preliminary version used in the quantitative study: overview of the items for each daily life activity across the cognitive (sub)domains.
Quantitative study
Participants
Patients with ABI, their relatives and healthy controls were asked to participate. We recruited patients with ABI who received outpatient rehabilitation in either the University Medical Centre Utrecht or De Hoogstraat Rehabiliation Centre, the Netherlands. Patients had to meet the following inclusion criteria: (1) aged between 18–80 years old; and (2) fluent in Dutch. Patients were asked if a close relative was willing to participate. Furthermore, the healthy controls had to meet the following inclusion criteria: (1) aged between 18–80 years old; (2) fluent in Dutch, and (3) no history of neurological and/or psychiatric disorders. Healthy controls were recruited among acquaintances of the researchers and by using advertisements in online newsletters and websites. All participants gave written informed consent. The experiment was performed in accordance with the Declaration of Helsinki. The research protocol was approved by the Medical Ethics Committee of the University Medical Centre (METC protocol number 17-407/C).
Procedure
Patients (and relatives) were invited by a rehabilitation physician or a neuropsychologist to participate. After confirmation, the CoCo-P along with the informed consent form was sent by post. Patients were instructed to bring the completed forms to a scheduled appointment or return them by post. Healthy controls returned the completed forms by post.
Finalizing the inventory based on the data of the quantitative study
To finalize the inventory, we revised the response distributions of each item within healthy controls and patients (See Appendices A2 and A3). The response options (four-point scale) were dichotomized into “no complaints” (i.e., [0] independent, without effort) and “complaints” (i.e., [1] independent, with effort, [2] with help or [3] not possible). The presence of floor or ceiling effects were important determinants. Items were deleted from the final version and further analyses when: (1) >20% of healthy controls reported “complaints” on the item in question (which means the item can be considered “quite challenging,” even for healthy controls); and (2) <10% patients reported “complaints” on the item in question (which means the item can be considered “not challenging enough”). More than 20% of the healthy controls reported complaints on item 14 (i.e., remembering the time of arrival and departure), 17 (i.e., remembering unfamiliar routes), and 32 (i.e., remembering names of people I just met). These items were excluded as they were not suitable in differentiating between patients with ABI and healthy controls. Regarding item 14 and 17, this finding might be explained by the fact that nowadays technology (e.g., application on phone, navigational system) is used during these activities. So performing these activities on its own merits might be considered challenging. The exclusion of item 32 caused the daily life activity “contact with community” to contain only one item (i.e., item 33). For this reason, item 33 was added to “contact with family/friends,” and the daily life activity was renamed into “contact with family/friends and community.” Only two patients (<10%) reported complaints on item 23 (i.e., taking my medication). Due to the lack of variance, this item was excluded from the final inventory and further analyses.
The daily life activity “use of medication” (i.e., items 21 [planning prescription refill]; item 22 [remembering taking my medications]; item 24 [intake of medication at fixed times]) seemed not applicable in our patient population. However, we did not exclude this activity from our inventory because 20–33% of the patient that used medication did report complaints on these items.
As a result of a review of available literature, expert meetings with health professionals and cognitive neuroscientists, semi-structured interviews with patients, and a quantitative study, the final version of the inventory was developed. The Cognitive Complaints - Participation (CoCo-P) is a patient-reported and/or relative-reported measure that contains 38 items focusing on memory, attention or executive function over 10 daily life activities (i.e., work/education, leisure activities, travel, driving, finances, use of medication, family life, contact with family/friends and community, cooking, grocery shopping). An English translation of the inventory is presented in Appendix A4 (see supplementary material). Note that the results in this study are obtained with the original Dutch version.
Statistical analyses on data of the quantitative study with the final inventory
Demographic and clinical characteristics
We collected data on sex, age and level of education. Level of education was assessed using a Dutch classification system (Verhage, Citation1965), that consists of 7 levels, with 1 being the lowest (less than primary school) and 7 being the highest (academic degree). These levels were converted into three categories for analysis: low (Verhage 1–4), average (Verhage 5), and high (Verhage 6–7). Non-parametric tests (Kruskal–Wallis non-parametric ANOVA and Chi-square test for categorical variables) were used to compare demographic characteristics between the patients and healthy controls. Additionally, we extracted the following characteristics from the medical files: ABI type (i.e., stroke, TBI, brain tumour resection), time since ABI onset, lesion side, and the current state regarding work employment. If a neuropsychological assessment was scheduled within three months around the administration of the inventory, we collected the patient’s neuropsychological performance on four tests (i.e., Mini-Mental State Examination – 2nd Version [MMSE-2], Rey Auditory Verbal Learning Test [RAVLT], Digit Span, Trail Making Test B [TMT]) to give an indication of the cognitive sequelae on group level.
Reported complaints per daily life activity
We presented the results in percentages of patients or healthy controls reporting complaints per daily life activity (10 activities). The four-point scale was dichotomized into “no complaints” and “complaints.” If any complaints were reported ([1] independent with effort, [2] with help, [3] not possible) on at least one of the items within the activity, the participant was classified into the “complaints” category. The percentages of patients and healthy controls who reported that the activity was “not applicable” were reported. In addition, we created a hierarchy among the complaints and differentiated between the level of restrictions, dependence, and incapability. Patients were considered restricted, when any restrictions were reported ([1] independent with effort) on at least one item within the activity. Patients were considered dependent, when help was needed ([2] with help) on at least one item within the activity. Patients were considered uncapable, when they reported to be uncapable to perform the task ([3] not possible) on at least one of the items within the activity.
Furthermore, the level of fatigue (VAS score) was compared between the patients and the healthy controls per daily life activity using a Mann–Whitney U tests (adjusted p for 10 tests = .005).
Reported complaints per cognitive domain
We presented the results in percentages of patients or healthy controls reporting complaints per cognitive domain (3 domains). Similar to the procedure mentioned above, we created a hierarchy among the complaints and differentiated between the level of restrictions, dependence, and incapability.
Furthermore, we computed a total complaint score (sum score) based on all items as global indication of cognitive complaints. In addition, complaints scores per cognitive domain were computed (i.e., memory complaint score, attention complaint score, executive complaint score). Only items that were applicable for the individual were included (i.e., items rated [0] independent without effort, [1] independent with effort, [2] with help, [3] not possible). To obtain the same range between the scores, the complaints scores were converted to a 0–100 scale with the formula:
Higher scores indicated a higher degree of reported complaints. The median and the interquartile range were computed for patients and healthy controls. A Wilcoxon signed-rank test (two related samples) was used to compare the complaint scores within the patient group (adjusted p for 3 tests = .017).
Comparison between patients’ and relatives’ reports
A Wilcoxon signed-rank test (two related samples) was used to compare the complaints scores (3 domains) between patients and their relatives (adjusted p for 3 tests = .017). In addition, a Wilcoxon signed-rank test was used to compare the level of fatigue (mean VAS) per daily life activity (10 activities) as reported by patients and their relatives (adjusted p for 10 tests = .005).
Results
Demographic and clinical characteristics
We invited 76 ABI patients to participate and 28 patients declined for several different reasons (e.g., no time, personal reasons). In total, we recruited 48 ABI patients and 107 healthy controls. We had to exclude 2 patients and 4 healthy controls from the current study as no written informed consent was obtained (only verbal consent was given). One healthy control was excluded because she had a neurological disorder (i.e., mild Transient Ischemic Attack [TIA]) in the past. Finally, we included 46 patients and 102 healthy controls for the analyses. From the 46 patients, 33 relatives were included. See for demographic and clinical characteristics. Brain lesion was mostly due to a TBI (57%). All patients were in the chronic phase of rehabilitation (>3 months post ABI onset), and 52% of the patients was either back to work or in process of reintegration. Between the patients and healthy controls, there was no significant difference regarding sex (χ2(1) = .48, p = .488), nor age (U = 2161.00, z = −.77, p = .443), nor education (χ2(2) = 4.81, p = .090). Patients reported a higher level of cognitive complaints (as measured with the total complaints score) compared to healthy controls (U = 216.00, z = −8.95, p < .001).
Table 3. Demographic and clinical characteristics of the participants in the quantitative study.
Reported complaints per daily life activity
The highest percentage of patients reported complaints during “contact with friend/family and community” (96%), “leisure activities” (89%), and “work/education” (87%) (see ). The highest percentage of healthy controls reported complaints during “work/education” (32%), “contact with family/friends and community” (32%), and “cooking” (24%). The percentage of patients reporting restrictions (22–46%), dependence (0–24%), and incapability (2–50%) varied greatly between daily activities (see and ). The percentage of healthy controls reporting restrictions (8–31%), dependence (0–6%), and incapability (0–3%) varied less. Regarding fatigue, patients reported more fatigue during each daily life activity compared to healthy controls (see ).
Figure 1. Percentage of patients reporting complaints per daily life activity. A hierarchy was created among the complaints and differentiated between the level of restrictions, dependence, and incapability.
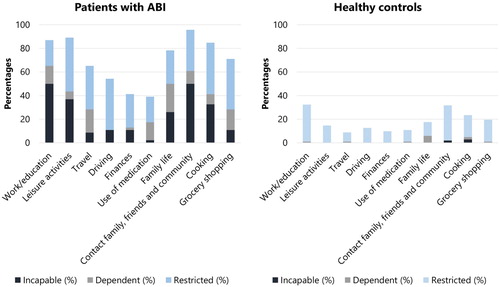
Table 4. Percentage of patients and healthy controls reporting complaints per daily life activity.
Table 5. Comparison of the level of fatigue (mean VAS scores) per daily life activity between patients and healthy controls.
Reported complaints per cognitive domain
A high percentage of patients reported complaints regarding memory (94%), attention (98%) and executive function (96%), when compared to reported complaints regarding memory (38%), attention (47%), executive function (36%) of healthy controls (see ). The highest percentage of patient reported incapability (37–65%), when compared to restrictions (24–37%) and dependence (9–20%). The highest percentage of healthy controls reported restrictions (36–44%), when compared to dependence (2–6%) and incapability (0–3%).
Table 6. Percentage of patients and healthy controls reporting complaints per cognitive domain.
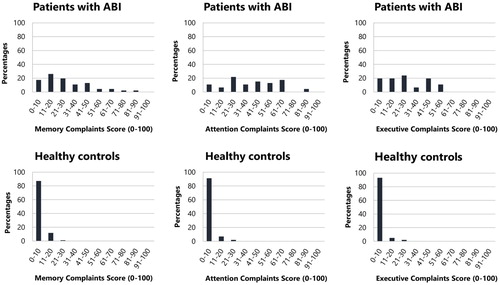
Regarding the complaints score of the patients, the median was 26 for memory, 42 for attention, and 23 for executive function. The median for the healthy controls was 0 for each cognitive domain. See for the distribution of the complaints score per cognitive domain for both groups. The complaints score was higher for attention compared to memory (z = −3.96, p < .001) and executive functions (z = −5.82, p < .001) within patients. Demographical characteristics (i.e., sex, age and level of education) did not influence the complaints scores (memory, attention, executive function) within the current sample of patient with ABI (see Appendix A5 in supplementary material).
Comparison between patients’ and relatives’ reports
The complaints scores of patients were significantly higher for memory and attention, compared to the complaints scores of relatives (see ). Patients and relatives had a similar complaints score for executive functions. Patients and relatives did not differ on the perceived level of fatigue during the 10 daily life activities (see ).
Table 7. Comparison of the complaints scores (higher scores indicate a higher level of complaints) between patients’ and relatives’ reports.
Table 8. Comparison of the level of fatigue (mean VAS score) as reported by patients and their relatives, split per daily life activity.
Discussion
Based on available literature, expert meetings with health professionals and cognitive neuroscientists, semi-structured interviews with patients, and a quantitative study, the inventory Cognitive Complaints - Participation (CoCo-P) was developed as a patient- and relative-reported measure to assess cognitive complaints during daily life activities. The majority of patients (87–96%) who participated in the quantitative study experienced cognitive complaints at work/education, during leisure activities, and/or in contact with family/friends and community. This is probably due to the dynamic and demanding nature of such daily life activities, where one is required to perform multiple operations simultaneously while dealing with environmental distractions (e.g., background noise) and time pressure. Performing adequately in those demanding situations requires more from attentional processes (McCulloch, Citation2007). Previous literature also reports that the presence of cognitive complaints negatively affects the possibility to return to work (Benedictus et al., Citation2010; van der Naalt et al., Citation1999) and the possibility to resume leisure and social activities post ABI (Robison et al., Citation2009). We found much lower percentages of healthy controls reporting cognitive complaints. However, we found a similar pattern regarding the most affected daily life activities. The highest percentages of heathy controls (31–32%) reported complaints during work/education and in contact with family/friends and community. It is therefore likely that those daily life activities do require more from cognitive processes, compared to other daily life activities. Also, patients reported more fatigue during all daily life activities compared to healthy controls. The fatigue VAS score was considered as an independent measure to give insight in the level of fatigue during daily life activities regardless of the presence or absence of cognitive complaints. Furthermore, we found that complaints related to attention were more frequently reported compared to complaints related to memory or executive functions by patients with ABI. These findings are consistent with a previous review that found a percentage of 29–92% of stroke patients reporting complaints (measured by questionnaires or interviews) about concentration, mental speed and memory (Rijsbergen et al., Citation2014).
Patients reported more cognitive complaints regarding memory and attention (as measured with the complaints scores) than their relatives. This might reflect too little knowledge about the possible consequences of ABI among relatives (Hochstenbach, Prigatano, & Mulder, Citation2005). Subtle problems and the impact on daily life may not be recognized or understood by relatives, leading to an overestimation of patients’ ability (Fordyce & Roueche, Citation1986; Hochstenbach et al., Citation2005). For instance, relatives have overestimated patients with ABI in their communication abilities (McClenahan, Johnston, & Densham, Citation1990, Citation1992; Seel et al., Citation1997), or overall functioning (Cavallo, Kay, & Ezrachi, Citation1992; Cusick, Gerhart, & Mellick, Citation2000). Previous research shows that agreement tends to be lower for invisible symptoms (e.g., memory problems), but higher for observable symptoms (e.g., writing) (Hochstenbach et al., Citation2005; Vallat-Azouvi et al., Citation2018). Fatigue was probably more observable for relatives, hence patients and relatives reported a comparable level of fatigue. A note of caution is due here since we cannot state which underlying process causes the discrepancy between the patients’ and relatives’ reports. Future research could shed light on this matter.
Strengths and limitations
Involvement of experts, patients and relatives
The strength of this study is the process of development, where we followed a sequence of steps including the consultation of experts and patients. The inventory is based on well-known cognitive models (theory-based) (Petersen & Posner, Citation1990, Citation2012; Posner & Rothbart, Citation2007; Squire, Citation1992, Citation2004; Ylvisaker et al., Citation1998), but also based on the clinical input of healthcare professional and patients (experienced-based). Especially, the patients’ engagement in research can potentially lead to an improved development of patient-reported outcomes (Domecq et al., Citation2014; Wiklund et al., Citation2016). However, we did not involve the relatives in the development of the relative-reported inventory. Relatives were not interviewed regarding missing daily life activities and specific items. This could be considered as a limitation. However, we do not expect that the involvement of relatives would have resulted in great modifications, because the activities of the CoCo-P can be considered the most characteristic for participation. For instance, previous research suggest that homemaking for others (e.g., cooking), interpersonal relations (e.g., contact with friends and family), major life areas (e.g., work) and community-based roles outside of home or work (e.g., leisure) represent participation (Post et al., Citation2012; Whiteneck & Dijkers, Citation2009). These activities are included in the CoCo-P. In addition, the involved patients with mild impairments were considered capable to evaluate the completeness of the daily life activities.
Patient sample
The sample size of the patient group was relatively small. In addition, the group was relatively high-educated and mildly cognitively impaired. Even though the CoCo-P appears to be suitable for all patients with ABI, it remains to be seen how feasible it is for low-educated patients or for patients with moderate to severe cognitive impairments. One might argue that a subjective evaluation of daily life difficulties might be more challenging for patients with a lower education (Boynton, Wood, & Greenhalgh, Citation2004) or for patients with severe injury-related cognitive impairments (Barrett, Citation2009; Reeves et al., Citation2018). Items such as “Do you have attentional problems?” are often considered abstract and challenging by patients. In the CoCo-P, however, the items describe specific cognitive tasks during daily life activities, which is expected to be less challenging. In addition, the frequencies of the cognitive complaints (as measured with the CoCo-P) remain unknown in an ABI population with more severe impairments. Future research should include a larger, more heterogenous sample of patients with respect to type of ABI and severity. This will especially allow the exploration of possible differences in frequencies of complaints between diagnosis-related groups (e.g., stroke, TBI) varying in ABI severity (i.e., mild, moderate, severe).
Given the aim of the current study (developing an inventory to capture cognitive complaints during daily life activities for patient with ABI) the inclusion of patients with mild cognitive impairment could be considered as a strength, as the discrepancy between relatively good test results (on neuropsychological tests) and reported complaints is strikingly common within this group. A novel inventory for systematically assessing cognitive complaints in this group is crucial. This group is also a growing population in rehabilitation medicine, because of the improved neurological treatment (e.g., mechanical thrombectomy, intravenous thrombolytic treatment) and the increased use of early multidisciplinary rehabilitation interventions (Barreto, Citation2011; Campbell, Donnan, Mitchell, & Davis, Citation2016; Cifu & Stewart, Citation1999; Maulden, Gassaway, Horn, Smout, & DeJong, Citation2005).
Another limitation is the fact that we did not exclude patients with comorbid disorders (e.g., psychiatric or neurological), which might have influenced the frequencies of cognitive complaints. For example, affective disturbances (e.g., depression, irritability, anxiety) can influence subjective reports (Clarke et al., Citation2012). However, comorbidity is common after ABI (Garrelfs, Donker-Cools, Wind, & Frings-Dresen, Citation2015), so inviting all patients in the outpatient rehabilitation programme probably increased the representativeness of our sample.
Cognitive domains and subdomains
We selected items focusing on memory (i.e., retrospective memory, prospective memory), attention (i.e., arousal, orienting, monitoring, sustained) or executive function (i.e., planning, self-evaluating, initiative, flexibility) based on well-known cognitive models (Petersen & Posner, Citation1990, Citation2012; Posner & Rothbart, Citation2007; Squire, Citation1992, Citation2004; Ylvisaker et al., Citation1998). The cognitive domains, however, might lack relevant subdomains. For instance, items are missing related to processing speed (as part of attention) and inhibition (as part of executive function), which are commonly impaired in ABI patients (Chung et al., Citation2013; Cicerone et al., Citation2000; Veltman, Brouwer, van Zomeren, & van Wolffelaar, Citation1996). Furthermore, one could argue that the items belong to more than one cognitive (sub)domain, because the cognitive tasks described in the items involve multiple cognitive processes.
Future research
Future research will address the reliability and the validity of the CoCo-P. The reliability could be evaluated in terms of the internal consistency by using the McDonald’s omega (McDonald’s ω), which is considered the best estimate when the scale in question is multidimensional (Dunn, Baguley, & Brunsden, Citation2014; Watkins, Citation2017; Zinbarg, Revelle, Yovel, & Li, Citation2005). The calculation of the McDonald’s ω requires the application of a factor analytic model, which requires a large sample size (Watkins, Citation2017; Zinbarg, Yovel, Revelle, & McDonald, Citation2006). A factor analytic model will identify the structure of the inventory by revealing whether the items reflect the three underlying and independent cognitive domains (i.e., memory, attention, executive function). Next, the validity should be addressed in terms of construct validity (i.e., examination whether the inventory measures the theoretical constructs of interest) by estimating its association with other patient-reported measures (e.g., USER-P, CLCE-24). Furthermore, the complaints scores per cognitive domain could be compared with the scores on a neuropsychological assessment, which would reveal the relation between the reported complaints and underlying cognitive impairments. Finally, we found that healthy controls are unlikely to show a complaint score higher than 5 per cognitive domain (highest Q3 = 5 for the memory complaint score). The complaints scores are easy to use clinically and seems appropriate in differentiating between cognitively healthy controls and patients reporting cognitive complaints. However, more established analyses are needed to determine a valid cut-off score. Future research should focus on investigating the sensitivity and specificity (positive and negative predicted value) in relation to an external instrument measuring cognitive complaints (e.g., CLCE-24).
Clinical implications
Especially during outpatients rehabilitation, the primary goals are to maximize functional independence and participation (Post et al., Citation2012; Wade & de Jong, Citation2000). Neuropsychological assessment examines the cognitive impairments that could hamper participation. Cognitive complaints may also negatively affect participation. For this reason, previous research emphasized the need for patient-reported measures to capture and quantify the difficulties patients encounter in daily life (Carrigan & Barkus, Citation2016; Meadows, Citation2011; Wiklund et al., Citation2016). The CoCo-P can complement a neuropsychological assessment by capturing the subjective cognitive complaints in a standardized manner, and, just as important, by assessing the impact of cognitive complaints on participation. The CoCo-P can be used in a multidisciplinary team (e.g., neuropsychologists, occupational therapists) to determine the focus of the intervention (activity-focused/domain-focused). Finally, the CoCo-P can be used as a metric to assess cognitive complaints longitudinally and to evaluate the effect of the intervention.
Conclusion
In conclusion, the CoCo-P is a patient- and relative-reported measure to assess cognitive complaints during daily life activities in patients with ABI. The majority of patients (87–96%) experienced cognitive complaints at work/education, during leisure activities, and in contact with family/friends and community. The CoCo-P can be used to capture the subjective cognitive complaints in a standardized manner, and, just as important, to assess the impact of cognitive complaints on participation. The complaints scores per cognitive domain are easy to use clinically and seems appropriate to differentiate between cognitively healthy controls and patients reporting cognitive complaints in daily life. Future research will address the reliability and validity of the CoCo-P.
Supplemental Material
Download Zip (1,016.9 KB)Supplemental Material
Download Zip (871.6 KB)Acknowledgments
We would like to thank all patients, relatives, and healthy controls for participating in this study. We would like to thank the developers of the CID for sharing their knowledge. Finally, we would like to thank Vera Schepers, Willeke Kruithof, Jacqueline Sibbel, Rinske Maathuis, Tjamke Strikwerda, Carolien van Veen, Chris Dijkerman, Stefan van der Stigchel, Martine van Zandvoort, Irene Huenges Wajer and Albert Postma for their contribution as expert panel.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Barreto, A. D. (2011). Intravenous thrombolytics for ischemic stroke. Neurotherapeutics, 8(3), 388–399. doi: 10.1007/s13311-011-0049-x
- Barrett, A. M. (2009). Rose-colored answers: Neuropsychological deficits and patient-reported outcomes after stroke. Behavioural Neurology, 22(1–2), 17–23. doi: 10.3233/BEN-2009-0250
- Baylor, C., Burns, M., Eadie, T., Britton, D., & Yorkston, K. (2011). A qualitative study of interference with communicative participation across communication disorders in adults. American Journal of Speech-Language Pathology, 20(November), 269–288. doi: 10.1044/1058-0360(2011/10-0084)
- Benedictus, M. R., Spikman, J. M., & Van Der Naalt, J. (2010). Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Archives of Physical Medicine and Rehabilitation, 91(9), 1436–1441. doi: 10.1016/j.apmr.2010.06.019
- Bielak, A. A. M., Hatt, C. R., & Diehl, M. (2017). Cognitive performance in adults’ daily lives: Is there a lab-life gap? Research in Human Development, 14(3), 219–233. doi: 10.1080/15427609.2017.1340050
- Boynton, P. M., Wood, G. W., & Greenhalgh, T. (2004). Hands-on guide to questionnaire research: Reaching beyond the white middle classes. Bmj, 328(7453), 1433–1436. doi: 10.1136/bmj.328.7453.1433
- Broadbent, D. E., Cooper, P. F., FitzGerald, P., & Parkes, K. R. (1982). The cognitive failures questionnaire (CFQ) and its correlates. British Journal of Clinical Psychology, 21(1), 1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x
- Campbell, B. C. V., Donnan, G. A., Mitchell, P. J., & Davis, S. M. (2016). Endovascular thrombectomy for stroke : Current best practice and future goals. doi: 10.1136/svn-2015-000004
- Carrigan, N., & Barkus, E. (2016). A systematic review of cognitive failures in daily life: Healthy populations. Neuroscience and Biobehavioral Reviews, 63, 29–42. doi: 10.1016/j.neubiorev.2016.01.010
- Cavallo, M. M., Kay, T., & Ezrachi, O. O. (1992). Problems and changes after traumatic brain injury: Differing perceptions within and between families. Brain Injury, 6(4), 327–335.
- Chaytor, N., & Schmitter-Edgecombe, M. (2003). The ecological validity of neuropsychological tests: A review of the literature on everyday cognitive skills. Neuropsychology Review, 13(4), 181–197. doi: 10.1023/B:NERV.0000009483.91468.fb
- Chung, C., Pollock, A., Campbell, T., Durward, B., & Hagen, S. (2013). Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult nonprogressive acquired brain damage. Stroke, 44(7), doi: 10.1161/STROKEAHA.113.002049
- Cicerone, K., Dahlberg, C., Kalmar, K., Langenbahn, D. M., Malec, J. F., Bergquist, T. F., … Morse, P. A. (2000). Evidence-based cognitive rehabilitation: Recommendations for clinical practice. Archives of Physical Medicine and Rehabilitation, 81(12), 1596–1615. doi: 10.1053/apmr.2000.19240
- Cicerone, K., Levin, H., Malec, J., Stuss, D., & Whyte, J. (2006). Cognitive rehabilitation interventions for executive function: Moving from bench to bedside in patients with traumatic brain injury. Journal of Cognitive Neuroscience, 18(7), 1212–1222. doi: 10.1162/jocn.2006.18.7.1212
- Cifu, D. X., & Stewart, D. G. (1999). Factors affecting functional outcome after stroke: A critical review of rehabilitation interventions. Archives of Physical Medicine and Rehabilitation, 80(5 suppl.), doi: 10.1016/S0003-9993(99)90101-6
- Clarke, L. A., Genat, R. C., & Anderson, J. F. I. (2012). Long-term cognitive complaint and post-concussive symptoms following mild traumatic brain injury: The role of cognitive and affective factors. Brain Injury, 26(3), 298–307. doi: 10.3109/02699052.2012.654588
- Cusick, C. P., Gerhart, K. A., & Mellick, D. C. (2000). Participant-proxy reliability in traumatic brain injury outcome research. Journal of Head Trauma Rehabilitation, 15(1), 739–749. doi: 10.1097/00001199-200002000-00012
- Das Nair, R., & Lincoln, N. (2007). Cognitive rehabilitation for memory deficits following stroke. Cochrane Database of Systematic Reviews, 3. doi: 10.1002/14651858.CD002293.pub2
- Domecq, J. P., Prutsky, G., Elraiyah, T., Wang, Z., Nabhan, M., Shippee, N., … Murad, M. H. (2014). Patient engagement in research: A systematic review. BMC Health Services Research, 14, 1–9. doi: 10.1186/1472-6963-14-89
- Duits, A., Munnecom, T., Van Heugten, C., & Van Oostenbrugge, R. J. (2008). Cognitive complaints in the early phase after stroke are not indicative of cognitive impairment. Journal of Neurology, Neurosurgery and Psychiatry, 79(2), 143–146. doi: 10.1136/jnnp.2007.114595
- Duncan, P. W., Wallace, D., Studenski, S., Lai, S. M., & Johnson, D. (2001). Conceptualization of a new stroke-specific outcome measure: The stroke impact scale. Topics in Stroke Rehabilitation, 8(2), 19–33. doi: 10.1310/BRHX-PKTA-0TUJ-UYWT
- Dunn, T. J., Baguley, T., & Brunsden, V. (2014). From alpha to omega: A practical solution to the pervasive problem of internal consistency estimation. British Journal of Psychology, 105(3), 399–412. doi: 10.1111/bjop.12046
- Ezekiel, L., Collett, J., Mayo, N. E., Pang, L., Field, L., & Dawes, H. (2018). Factors associated with participation in life situations for adults with stroke: A systematic review. Archives of Physical Medicine and Rehabilitation, doi: 10.1016/j.apmr.2018.06.017
- Fischer, S., Trexler, L. E., & Gauggel, S. (2004). Awareness of activity limitations and prediction of performance impairments in patients with brain injuries and orthopedic disorders. Journal of the International Neuropsychological Society, 10(2), 190–199. doi: 10.1017/S1355617704102051
- Fordyce, D. J., & Roueche, J. R. (1986). Changes in perspectives of disability among patients, staff, and relatives during rehabilitation of brain injury. Rehabilitation Psychology, 31(4), 217–229.
- Fougeyrollas, P., & Noreau, L. (2002). La Mesure des Habitude de Vie: Version abrégée (MHAVIE 3.1) [Assessment of life habits: Shortened version (LIFE-H 3.1)]. Lac St-Charles: RiPPH.
- Garrelfs, S. F., Donker-Cools, B. H. P. M., Wind, H., & Frings-Dresen, M. H. W. (2015). Return-to-work in patients with acquired brain injury and psychiatric disorders as a comorbidity: A systematic review. Brain Injury, 29(5), 550–557. doi: 10.3109/02699052.2014.995227
- Hochstenbach, J., Prigatano, G., & Mulder, T. (2005). Patients’ and relatives’ reports of disturbances 9 months after stroke: Subjective changes in physical functioning, cognition, emotion, and behavior. Archives of Physical Medicine and Rehabilitation, 86(8), 1587–1593. doi: 10.1016/j.apmr.2004.11.050
- Holbrook, M., & Skilbeck, C. (1983). An activities index for use with stroke patients. Age and Ageing, 12(2), 166–170.
- Jette, A. M., Keysor, J., Coster, W., Ni, P., & Haley, S. (2005). Beyond function: Predicting participation in a rehabilitation cohort. Archives of Physical Medicine and Rehabilitation, 86(11), 2087–2094. doi: 10.1016/j.apmr.2005.08.001
- Johansson, M. M., Marcusson, J., & Wressle, E. (2016). Development of an instrument for measuring activities of daily living in persons with suspected cognitive impairment. Scandinavian Journal of Occupational Therapy, 23(3), 230–239. doi: 10.3109/11038128.2016.1139621
- Kelley, E., Sullivan, C., Loughlin, J. K., Hutson, L., Dahdah, M. N., Long, M. K., … Poole, J. H. (2014). Self-awareness and neurobehavioral outcomes, 5 years or more after moderate to severe brain injury. Journal of Head Trauma Rehabilitation, 29(2), 147–152. doi: 10.1097/HTR.0b013e31826db6b9
- Kerkhoff, G., Schaub, J., & Zihl, J. (1990). Assessment of cerebral visual disorders by patient-questionnaire. Nervenarzt, 61(12), 711–718.
- Landre, N., Poppe, C. J., Davis, N., Schmaus, B., & Hobbs, S. E. (2006). Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Archives of Clinical Neuropsychology, 21(4), 255–273. doi: 10.1016/j.acn.2005.12.007
- Lawton, M., & Brody, E. (1969). Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist, 9(3), 179–186.
- Maulden, S. A., Gassaway, J., Horn, S. D., Smout, R. J., & DeJong, G. (2005). Timing of initiation of rehabilitation after stroke. Archives of Physical Medicine and Rehabilitation, 86(12 suppl.), 34–40. doi: 10.1016/j.apmr.2005.08.119
- McClenahan, R., Johnston, M., & Densham, Y. (1990). Misperceptions of comprehension difficulties of stroke patients by doctors, nurses and relatives. Journal of Neurology Neurosurgery and Psychiatry, 53(8), 700–701. doi: 10.1136/jnnp.53.8.700
- McClenahan, R., Johnston, M., & Densham, Y. (1992). Factors influencing accuracy of estimation of comprehension problems in patients following cerebrovascular accident, by doctors, nurses and relatives. International Journal of Language & Communication Disorders, 27(3), 209–219. doi: 10.3109/13682829209029421
- McCulloch, K. (2007). Attention and dual-task conditions: Physical therapy implications for individuals with acquired brain injury. Journal of Neurologic Physical Therapy, 31(3), 104–118. doi: 10.1097/NPT.0b013e31814a6493
- Meadows, K. A. (2011). Patient-reported outcome measures: An overview. British Journal of Community Nursing, 16(3), 146–151. doi: 10.12968/bjcn.2011.16.3.146
- Mole, J. A., & Demeyere, N. (2018). The relationship between early post-stroke cognition and longer term activities and participation: A systematic review. Neuropsychological Rehabilitation, 1–25. doi: 10.1080/09602011.2018.1464934
- Paemeleire, F. (2014). De screeningstest voor cognitie en communicatie (STCC): Een nieuw instrument voor volwassenen met NAH. Logopedie, 27(2), 51–65.
- Petersen, S. E., & Posner, M. I. (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42. doi: 10.1146/annurev-neuro-062111-150525
- Petersen, S. E., & Posner, M. I. (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35(1), 73–89. doi: 10.1146/annurev-neuro-062111-150525
- Ponsford, J., Olver, J., Ponsford, M., & Nelms, R. (2003). Long-term adjustment of families following traumatic brain injury where comprehensive rehabilitation has been provided. Brain Injury, 17(6), 453–468. doi: 10.1080/0269905031000070143
- Posner, M. I., & Rothbart, M. K. (2007). Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology, 58(1), 1–23. doi: 10.1146/annurev.psych.58.110405.085516
- Post, M. W. M., Van Der Zee, C. H., Hennink, J., Schafrat, C. G., Visser-Meily, J. M. A., & Van Berlekom, S. B. (2012). Validity of the Utrecht scale for evaluation of rehabilitation- participation. Disability and Rehabilitation, 34(6), 478–485. doi: 10.3109/09638288.2011.608148
- Prigatano, G. P., Altman, I. M., & O’Brien, K. P. (1990). Behavioral limitations that traumatic-brain-injured patients tend to underestimate. Clinical Neuropsychologist, 4(2), 163–176. doi: 10.1080/13854049008401509
- Reeves, M., Lisabeth, L., Williams, L., Katzan, I., Kapral, M., Deutsch, A., & Prvu-Bettger, J. (2018). Patient-reported outcome measures (PROMs) for acute stroke: Rationale, methods and future directions. Stroke, 49(6), 1549–1556. doi: 10.1161/strokeaha.117.018912
- Rijsbergen, M. W. A., Van Mark, R. E., De Kort, P. L. M., & Sitskoorn, M. M. (2014). Subjective cognitive complaints after stroke : A systematic review. Journal of Stroke and Cerebrovascular Diseases, 23(3), 408–420. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.003
- Robison, J., Wiles, R., Ellis-Hill, C., McPherson, K., Hyndman, D., & Ashburn, A. (2009). Resuming previously valued activities post-stroke: Who or what helps. Disability and Rehabilitation, 31(19), 1555–1566. doi: 10.1080/09638280802639327
- Sbordone, R. J., Seyranian, G. D., & Ruff, R. M. (1998). Are the subjective complaints of traumatically brain injured patients reliable? Brain Injury, 12(6), 505–515. doi: 10.1080/026990598122467
- Seel, R. T., Kreutzer, J. S., Sander, A. M., Rt, A. S., Js, K., & Concor-, S. A. M. (1997). Concordance of patients’ and family members’ ratings of neurobehavioral. Medicine, 78(November), 1254–1259.
- Spielberger, C. D. (2004). Encyclopedia of applied psychology. Academic Press. Retrieved from https://books.google.nl/books?id=q8tUSCDORzwC&printsec=frontcover&dq=spielberger+2004+applied+psychology&hl=nl&sa=X&ved=0ahUKEwjS3aCy8_7hAhVDYlAKHdeTAE8Q6AEIKTAA#v=snippet&q=reosoning&f=false
- Spreij, L. A., Visser-Meily, J. M. A., Van Heugten, C. M., & Nijboer, T. C. W. (2014). Novel insights into the rehabilitation of memory post acquired brain injury: A systematic review. Frontiers in Human Neuroscience, 8, 1–19. doi: 10.3389/fnhum.2014.00993
- Squire, L. R. (1992). Nondeclarative memory: Multiple brain systems supporting learning. Journal of Cognitive Neuroscience, 4(3), 232–243. doi: 10.1162/jocn.1992.4.3.232
- Squire, L. R. (2004). Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory, 82(3), 171–177. doi: 10.1016/j.nlm.2004.06.005
- Vallat-Azouvi, C., Paillat, C., Bercovici, S., Morin, B., Paquereau, J., Charanton, J., … Azouvi, P. (2018). Subjective complaints after acquired brain injury: Presentation of the brain injury complaint questionnaire (BICoQ). Journal of Neuroscience Research, 96(4), 601–611. doi: 10.1002/jnr.24180
- van der Naalt, J., van Zomeren, A. H., Sluiter, W. J., & Minderhoud, J. M. (1999). One year outcome in mild to moderate head injury: The predictive value of acute injury characteristics related to complaints and return to work. Journal of Neurology, Neurosurgery & Psychiatry, 66(2), 207–213. doi: 10.1136/jnnp.66.2.207
- van Heugten, C., Rasquin, S., Winkens, I., Beusmans, G., & Verhey, F. (2007). Checklist for cognitive and emotional consequences following stroke (CLCE-24): Development, usability and quality of the self-report version. Clinical Neurology and Neurosurgery, 109(3), 257–262. doi: 10.1016/j.clineuro.2006.10.002
- Veltman, J. C., Brouwer, W. H., van Zomeren, A. H., & van Wolffelaar, P. C. (1996). Central executive aspects of attention in subacute severe and very severe closed head injury patients: Planning, inhibition, flexibility, and divided attention. Neuropsychology, 10(3), 357–367. doi: 10.1037//0894-4105.10.3.357
- Verhage, F. (1965). Intelligence and age in a Dutch sample. Human Development, 8, 238–245. doi: 10.1159/000270308
- Virk, S., Williams, T., Brunsdon, R., Suh, F., & Morrow, A. (2015). Cognitive remediation of attention deficits following acquired brain injury : A systematic review and meta-analysis. 36, 367–377. doi: 10.3233/NRE-151225
- Viscogliosi, C., Desrosiers, J., Belleville, S., Caron, C. D., & Ska, B. (2011). Differences in participation according to specific cognitive deficits following a stroke. Applied Neuropsychology, 18(2), 117–126. doi: 10.1080/09084282.2010.547779
- Visser-Keizer, A. C., Hogenkamp, A., Westerhof-Evers, H. J., Egberink, I. J. L., & Spikman, J. M. (2015). Dutch multifactor fatigue scale: A new scale to measure the different aspects of fatigue after acquired brain injury. Archives of Physical Medicine and Rehabilitation, 96(6), 1056–1063. doi: 10.1016/j.apmr.2014.12.010
- Wade, D. T., & de Jong, B. A. (2000). Recent advances in rehabilitation. British Medical Journal, 320(20), 1385–1388. Retrieved from http://nnr.sagepub.com/content/16/2/211.short
- Watkins, M. W. (2017). The reliability of multidimensional neuropsychological measures: From alpha to omega. Clinical Neuropsychologist, 31(6–7), 1113–1126. doi: 10.1080/13854046.2017.1317364
- Whiteneck, G., & Dijkers, M. P. (2009). Difficult to measure constructs: Conceptual and methodological issues concerning participation and environmental factors. Archives of Physical Medicine and Rehabilitation, 90(11 suppl. 1), S22–S35. doi: 10.1016/j.apmr.2009.06.009
- Wiklund, I., Anatchkova, M., Oko-Osi, H., von Maltzahn, R., Chau, D., Malik, F. I., … Teerlink, J. R. (2016). Incorporating development of a patient-reported outcome instrument in a clinical drug development program: Examples from a heart failure program. Health and Quality of Life Outcomes, 14(1), 1–9. doi: 10.1186/s12955-016-0529-0
- Ylvisaker, M., Szekeres, S. F., & Feeney, T. (1998). Cognitive rehabilitation: Executive functions. In M. Ylvisaker (Ed.), Traumatic brain injury rehabilitation: Children and adolescents (pp. 221–269). Boston: Butterworth-Heineman.
- Zinbarg, R. E., Revelle, W., Yovel, I., & Li, W. (2005). Cronbach’s, α Revelle’s β and McDonald’s ω H: Their relations with each other and two alternative conceptualizations of reliability. Psychometrika, 70(1), 123–133. doi: 10.1007/s11336-003-0974-7
- Zinbarg, R. E., Yovel, I., Revelle, W., & McDonald, R. P. (2006). Estimating generalizability to a latent variable common to all of a scale’s indicators: A comparison of estimators for ωh. Applied Psychological Measurement, 30(2), 121–144. doi: 10.1177/0146621605278814