Abstract
Anti-inflammatory treatment in chronic inflammatory lung diseases usually involves glucocorticosteroids. With patients suffering from serious side effects or becoming resistant, specific nutrients, that are suggested to positively influence disease progression, can be considered as new treatment options. The dietary inflammatory index is used to calculate effects of dietary components on inflammation and lung function to identify most potent dietary components, based on 162 articles. The positive effects of n-3 PUFAs and vitamin E on lung function can at least partially be explained by their anti-inflammatory effect. Many other dietary components showed only small or no effects on inflammation and/or lung function, although the number of weighted studies was often too small for a reliable assessment. Optimal beneficial dietary elements might reduce the required amounts of anti-inflammatory treatments, thereby decreasing both side effects and development of resistance as to improve quality of life of patients suffering from chronic inflammatory lung diseases.
Introduction
Lung diseases including asthma, chronic obstructive pulmonary disease (COPD) and interstitial lung diseases (ILD) such as sarcoidosis and idiopathic pulmonary fibrosis (IPF) are characterised by chronic inflammation (O’Byrne & Postma Citation1999; Rothkrantz-Kos et al. Citation2003; King Citation2005; Moldoveanu et al. Citation2009). Glucocorticosteroids are considered to be the mainstay in anti-inflammatory treatment for various inflammatory diseases, including these lung diseases (Barnes et al. Citation2003; Barnes & Adcock Citation2009). However, some patients show no response to these drugs and the use of glucocorticosteroids was shown to cause serious side effects (Barnes et al. Citation2003; King Citation2005; Barnes & Adcock Citation2009). Preventing such resistance and enhancing the effectiveness of glucocorticosteroids with nutrition present an interesting opportunity for treatment of these chronic inflammatory lung diseases.
Inflammation, the immediate response in tissue to injury, pathogen infiltration or irritants, is characterised by redness, swelling, heat, pain and loss of function (Coussens & Werb Citation2002; Calder Citation2006; Moldoveanu et al. Citation2009). When tissue is affected, chemical signals initiate activation and migration of leukocytes (neutrophils, monocytes and eosinophils) from the venous system to the damaged site and extracellular matrix. Following activation of adhesion molecules within the vascular endothelium, leukocyte integrins are triggered to be activated and upregulated. These attracted neutrophils are immobilised on the surface of the vascular endothelium and subsequently transmigrate to sites of injury (Coussens & Werb Citation2002). The progress of inflammation is shaped by chemotactic cytokines (chemokines) as TNF-α and TGF-β1, which stimulate monocyte migration to the injured tissue. These monocytes are involved in tissue repair, and defence mechanisms involve phagocytosis, the production of reactive oxygen species, and elimination of the pathogen as well as of cellular and tissue debris (Coussens & Werb Citation2002; Calder Citation2006; Pawelec et al. Citation2014). The inflammatory response may also affect healthy tissue and is therefore tightly controlled by the production of anti-inflammatory cytokines to terminate the process when the injury is repaired (Coussens & Werb Citation2002; Fullerton et al. Citation2013; Pawelec et al. Citation2014). When the process is not ended correctly due to either persistence of pro-inflammatory cytokines, failure or incomplete actions of anti-inflammatory cytokines or even persistence of these anti-inflammatory cytokines, inflammation can result in chronic inflammation (Coussens & Werb Citation2002; Moldoveanu et al. Citation2009; Fullerton et al. Citation2013; Pawelec et al. Citation2014).
With inflammation being a key factor in the aetiology of asthma, COPD, sarcoidosis and IPF, other aspects distinguish these diseases. COPD is characterised by a poorly reversible and usually progressive airflow limitation as a result of fixed narrowing of small airways, emphysema and luminal obstruction (Barnes et al. Citation2003; Rabe et al. Citation2007). In COPD, the parenchymal cells of the lungs are affected by inflammation, resulting in decreased airflow (Barnes et al. Citation2003; Moldoveanu et al. Citation2009). Asthma is typified by chronic inflammation of the airways only, with variable and widespread episodes of airway hyper-responsiveness leading to shortness of breath, wheezing, chest tightness and coughing due to chronic inflammation (Barnes et al. Citation2003; Bateman et al. Citation2008; Moldoveanu et al. Citation2009). ILD refers to a group of over 200 pulmonary diseases which are characterised by inflammation and fibrosis of the septal interstitium of the lungs, next to inflamed alveoli and distal airways (King Citation2005; Bourke Citation2006). Two of the more common diseases of this group are sarcoidosis and IPF (King Citation2005). Sarcoidosis is characterised by granuloma in multiple organs following inflammation, but most frequently affects the lungs and the lymphatic system (Baughman et al. Citation2003). IPF is a chronic disorder where fibrotic tissue accumulates in the lungs (Gross & Hunninghake Citation2001; King Citation2005; Patel et al. Citation2012). Following inflammation, injury in the lungs results in progressive collagen accumulation, leading to breathing difficulties and finally resulting in respiratory failure (Gross & Hunninghake Citation2001; Selman et al. Citation2001; King Citation2005; Patel et al. Citation2012).
Since inflammation is a key factor in chronic lung diseases as described above, anti-inflammatory therapies have been widely explored. Glucocorticoids are the mainstay in anti-inflammatory therapy. Glucocorticosteroids bind to the glucocorticosteroid receptor (GR) in the cytoplasm after diffusion across the cell membrane, which is translocated to the nucleus following activation by ligand binding (Barnes & Adcock Citation2009). The glucocorticosteroids–GR complex binds to the glucocorticoid response element (GRE) in the promotor region of glucocorticoid-responsive genes. When this complex binds to the GRE, gene transcription of previously activated inflammatory genes is directly inactivated (Barnes & Adcock Citation2009). The anti-inflammatory effect of GRs is also thought to be caused by various indirect effects, such as interfering with these inflammatory transcription factors, which normally activate gene expression, through binding to their promotor sites, or by inducing inhibitors of transcription factors (O’Byrne & Postma Citation1999). Studies into resistance to glucocorticosteroids in mainly asthma patients have led to the identification of six possible molecular mechanisms contributing to this resistance: (i) genetic susceptibility; (ii) GR modification due to phosphorylation, nitrosylation or ubiquitination altering their binding affinity; (iii) increased expression of the GR-isoform GR-β or disruption of the isoform GRα; (iv) increased activation of pro-inflammatory transcription factors (e.g. AP1, JNK), preventing interaction of GR with GRE and other transcription factors due to binding to the GR; (v) abnormal histone acetylation leading to no transactivation of genes; or (vi) increased P-glycoprotein transporting drugs out of cells (Barnes & Adcock Citation2009).
Since oxidative stress is one of the factors affecting histone acetylation, it is suggested to contribute to glucocorticosteroid resistance by suppressing the anti-inflammatory effect of the drug (Barnes & Adcock Citation2009; Ruijters et al. Citation2014a). Antioxidants can prevent this resistance, as was demonstrated in vitro with cocoa-derived epicatechin which was able to reduce cortisol resistance and protect the anti-inflammatory effects of dexamethasone (Ruijters et al. Citation2014a, Citation2014b). The role of nutrition in inflammatory diseases has been the subject of research previously: a diet rich in fruit, vegetables, cereals and fish (the so called prudent dietary pattern) was associated with a lower risk on COPD whereas a higher risk was associated with consumption of a Western diet (rich in refined grains, preserved meat, potatoes and sweets) (Varraso et al. Citation2007, Citation2010). Other studies showed a protective effect on oxidative processes and inflammation by different dietary components as fruit and vegetables, flavonoids, vitamin C, vitamin E, β-carotene, fatty acids and various minerals (Romieu Citation2005; Geraets et al. Citation2009; Hazewindus et al. Citation2014). The link between oxidative stress and several inflammatory lung diseases suggests a pivotal role for nutrition in their treatment (Romieu Citation2005).
This study therefore focussed on the effects of dietary components (including non-nutrients, nutrients, food items and diets) on inflammatory and immunological markers and respiratory function in chronic inflammatory lung diseases described above. Since this study aims to direct further research into dietary components which affect inflammation and/or function, all types of research – ranging from in vitro experiments to human trials – were included. Following the dietary inflammatory index as described by Shivappa et al., an inflammatory and respiratory effect score was calculated to identify potent dietary components in the treatment of chronic inflammatory lung diseases (Shivappa et al. Citation2014).
Methods
The dietary inflammatory index developed by Cavicchia et al. (Citation2009) and optimised by Shivappa et al. (Citation2014) measures the inflammatory effect of nutrients, relating it to the total dietary intake based on food consumption data (Cavicchia et al. Citation2009; Shivappa et al. Citation2014). The dietary inflammatory index was developed to score the overall effect of diet on inflammation (Cavicchia et al. Citation2009; Shivappa et al. Citation2014). With the index, the diet of an individual can be scored on a scale from maximally anti-inflammatory (with a score of −1) up to maximally pro-inflammatory (with a score of +1) by taking into account all potential dietary components affecting inflammation (Cavicchia et al. Citation2009; Shivappa et al. Citation2014). In the optimised dietary inflammatory index developed by Shivappa et al., the individual intake was compared with referent intakes provided by different food consumption data sets (Shivappa et al. Citation2014).
The dietary inflammatory index and its scoring system were the starting point for this literature review focussing on the effects of dietary components on inflammatory lung diseases. With the aim of this study being to identify components which can influence chronic inflammatory lung diseases and their underlying inflammatory processes, different experimental setups (such as in vitro studies, animal studies, observational studies and intervention trials) with potential varying quality of studies were required. The dietary inflammatory index provided a tool to score the effects found in these different studies.
Literature review strategy
Various search engines were used (Google Scholar®, Pubmed®, Science Direct®) to identify peer-reviewed studies published in English on the effects of single (non-)nutrients or whole diet on lung function or inflammatory markers in chronic inflammatory lung diseases. In the search strategy, the four diseases were combined with terms for nutrition (nutrients, diet, whole diet, nutrition) and “anti-inflammatory”. Cancer was explicitly excluded in the search strategy, as well as reviews, since only original research was used to calculate the effects of nutrition on these lung diseases.
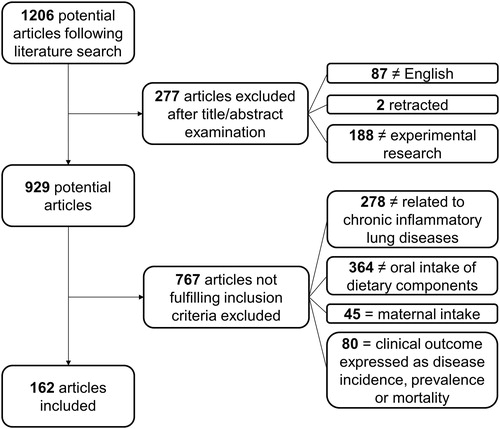
In total, 1206 articles were screened (). Studies were included when they studied one of the four diseases or models resembling these diseases and when studying either markers of lung function (forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, forced expiratory flow (FEF), peak expiratory flow (PEF)) or inflammatory and immunological markers (pro-inflammatory: interleukin (IL)-1β, IL-4 in asthma, IL-5, IL-6, IL-8, tumour necrosis factor (TNF)-α, leukotriene (LT) B4 and/or C-reactive protein (CRP); anti-inflammatory: IL-4 and IL-10) and models (type 1 helper T cells (Th1)/type 2 helper T cells (Th2) ratio; influx of immune/inflammatory cells in lung). As IL-4 mediates pro-inflammatory functions in asthma, it is used as pro-inflammatory immunological marker, but is regarded as anti-inflammatory marker in other diseases because of its suppressing effects on pro-inflammatory cytokine production (Steinke & Borish Citation2001; Woodward et al. Citation2010).
Studies were excluded when they studied: (i) other inflammation-associated diseases (such as CVD); (ii) other lung diseases (e.g. cystic fibrosis, rhinitis); (iii) effects of intake or exposure to substances other than well-defined dietary components (as drugs, environmental agents, herbal extracts); and (iv) nutrition status or plasma levels of dietary components instead of intake (as vitamin D status). Last, articles focussing on disease prevalence, incidence or occurrence were excluded leading to 162 included articles.
Scoring algorithm
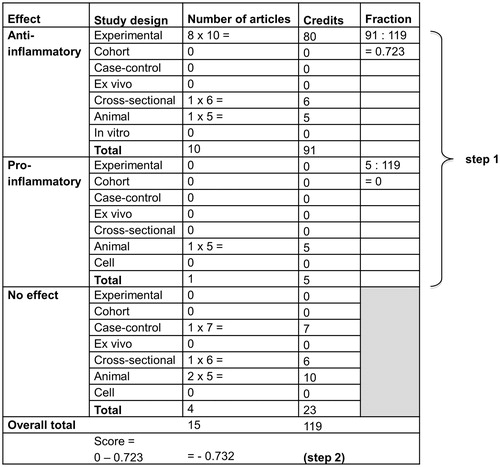
Included articles were scored based on (i) the outcome and (ii) the design of the study. Following the set-up of the dietary inflammatory index, the outcomes were labelled as “+1” when showing a pro-inflammatory effect or a decrease in lung function, “−1” with anti-inflammatory effects or improved lung function and “0” when no significant effect of the dietary component was reported (Shivappa et al. Citation2014). Scores were checked for consistency by all authors. When more effects (as independent intake of different dietary components) were described in one study or multiple methods were used (as in vitro and animal models), all effects were taken into consideration in the calculation of the final effect by weighing the study more than once. The second step was weighing the studies based on their design, which resulted in a specific amount of credits ().
Table 1. Study design weights (adapted from Shivappa et al. Citation2014).
Subsequently, the dietary component-specific effect score was calculated as follows: (i) the credits attributed to the weighted pro- and anti-inflammatory studies were divided by the weighted number of studies and (ii) the anti-inflammatory fraction was subtracted from the pro-inflammatory fraction (as exemplified in ). As previously done in calculating the dietary inflammatory index, the median of the total credits across all dietary components was used as a cut-off point to indicate the literature pool (Shivappa et al. Citation2014). When the number of weighted articles was below the cut-off point of 15 (inflammation) or 10 credits (lung function), the score was adjusted: the credits were divided by 15 or 10 and subsequently multiplied by the dietary component-specific effect score following from the first two steps (Shivappa et al. Citation2014).
Figure 2. Example of the calculation of the nutrient-specific effect score for n-3 PUFAs in two steps: (i) calculation of the pro- and anti-inflammatory fraction and (ii) the nutrient specific raw inflammatory effect score (adapted from Shivappa et al. Citation2014).
Results
Since some of the 162 articles measured both inflammatory markers and respiratory function and some studies focussed on multiple dietary components, 101 articles were included in calculating the inflammatory effect scores of dietary components and 88 articles were included to calculate the respiratory function effect scores.
As shown in , various dietary components showed an anti-inflammatory effect when consumption occurred during chronic inflammatory lung diseases. EGCG (epigallocatechin gallate), n-3 PUFAs (polyunsaturated fatty acids with a double bond at the third carbon atom from the ω-end of the carbon chain), probiotics and vitamin E have been studied most thoroughly and have an inflammatory effect score of −1, −0.743, −0.538 and −1, respectively. This indicates that these dietary components gave rise to an anti-inflammatory effect in most of the included studies. Other components eliciting high anti-inflammatory effects were prebiotics (−0.667), quercetin (−0.864), resveratrol (−0.889), vitamin C (−0.667) and vitamin D (−1), although the number of weighted articles studying these dietary components in chronic inflammatory lung diseases was lower and sometimes fell below the cut-off point of literature robustness of 15 credits. No significant anti-inflammatory effects were found following intake of caffeine, high fat, the Mediterranean diet and an adjusted n-6:n-3 PUFA ratio. A pro-inflammatory effect was found in chronic inflammatory lung diseases with fish consumption (0.294) and n-6 PUFA (polyunsaturated fatty acids with a double bond at the sixth carbon atom from the ω-end of the carbon chain) intake (0.677).
Table 2. Nutrient specific inflammatory effect score.
Several dietary components were shown to elicit positive effects on respiratory function in chronic inflammatory lung diseases (). Most studied were the effects of intake of caffeine (−0.455), n-3 PUFAs (−0.374), vitamin C (−0.378) and vitamin E (−0.238). Consumption of bread (−0.800), fruit (−0.758), fruit and vegetables (−0.722), n-3 and n-6 PUFAs (−0.667), probiotics (−0.714), soy isoflavone (−1), synbiotics (−1), theophylline (−1) and white wine (−0.800) was suggested to result in the most significant improvements in respiratory function, although the weighted number of studies showing these effects is only ranging from 8 up to 36 credits. No effect was found following intake of calcium, carotenoids, conjugated linoleic acid, creatine, dairy, dietary salt, flavanols, flavones, iron, margarine and oils, meat, monosodium glutamate, n-9 PUFAs, sodium and potassium, olive oil, phosphorus, potassium, potatoes, red wine, selenium, sodium, tea, total energy intake, vegetables as well as vitamin C combined with vitamin E and selenium. A decline in function was observed in studies assessing the effects of n-6 PUFAs (0.162).
Table 3. Nutrient specific respiratory effect score.
Discussion
This study summarises the effects of intake of dietary components on chronic inflammatory lung diseases based on 162 studies in an attempt to identify components which should be studied into more detail, to review their exact effect on these diseases. The scores calculated with the dietary inflammatory index of these dietary components provide an indication of their effect on chronic inflammatory lung diseases. The scores were corrected for literature robustness by taking into account the median of credits attributed to the evidence by weighing the articles. This resulted in a cut-off point of 15 credits for inflammatory and immunological markers and a cut-off point of 10 credits for respiratory function. When the number of credits attributed to a dietary component was falling below the cut-off point, the effect found was divided by this cut-off point, as described in the “Methods” section, to correct for literature robustness. When a health benefit of a food product is reviewed however by agencies (as the European Food Safety Authority or the U.S. Food and Drug Administration) to advice on their legal status, multiple human intervention studies demonstrating the beneficial effect are required (Ellwood et al. Citation2010; EFSA NDA Panel Citation2011; U.S. Food and Drug Administration Citation2014). Translating this into a number of credits, a cut-off point of 20 could be recommended, below which the outcome should be corrected for literature robustness. Although this number of 20 credits is not necessarily based on two intervention studies but could also be reached by comparing seven in vitro studies (), a number of 20 credits could already establish a more reliable indication of an effect.
Effects of dietary components on markers and function
Considering the number of credits, most convincing evidence has been found for the positive effects following intake of EGCG, n-3 PUFAs, probiotics and vitamin E on inflammatory and immunological markers in chronic inflammatory lung diseases (). The calculated effects of these dietary components range from −0.538 up to −1. The effects on lung function have been studied mostly for caffeine, n-3 PUFAs, vitamin C and vitamin E (). With scores ranging from −0.238 to −0.455, their beneficial effects on respiratory function appeared to be less explicit than the effects of the most thoroughly studied dietary components on inflammatory and immune markers.
The calculated scores for the effects of n-3 PUFAs and vitamin E on chronic inflammatory lung diseases suggest that improvements in respiratory function are at least partially attributable to improvement of inflammatory and immunological markers. Intake of n-3 PUFAs may result in decreased inflammation and improved lung function by (i) competition with n-6 PUFAs for metabolism by specific enzymes (cyclooxygenase, lysyl oxidase or cytochrome P450 oxygenase), resulting in alternative, less pro-inflammatory and even anti-inflammatory eicosanoids (metabolites); (ii) binding to and activation of receptors bound to the plasma membrane or found in the cytosol as G-protein coupled receptors (mediating the anti-inflammatory effects of n-3 PUFAs) and PPAR (peroxisome proliferator-activated receptor) transcription factors (inhibiting NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation and thereby pro-inflammatory gene transcription); and (iii) inducing the anti-inflammatory pathways by resolvins and protectins derived from n-3 PUFAs (Yates & Calder Citation2014). The effect on the eicosanoid metabolisation was observed to be crucial in chronic inflammatory lung diseases: various LTs derived from arachidonic acid (n-6 PUFAs) are important mediators in asthma and are associated with inflammation in COPD (Okamoto et al. Citation2000; Yates & Calder Citation2014). N-3 PUFAs suppress the generation of LTB4, which promotes the production of inflammatory cytokines, and reduce production of IL-1, IL-6 and TNF-α by leukocytes (Okamoto et al. Citation2000; Calder Citation2006). This mechanism of action could also explain both the pro-inflammatory effects (0.667) and negative effects on respiratory function (0.162) following n-6 PUFA intake, due to the formation of more pro-inflammatory metabolites resulting from the ingestion of n-6 PUFAs.
For vitamin E intake, a calculated inflammatory effect score of −1 and a respiratory function score of −0.283 were found, indicating a beneficial effect on chronic inflammatory lung diseases. Vitamin E can be found in eight isoforms, with α-tocopherol and γ-tocopherol being the most abundant (Cook-Mills & Avila Citation2014). Human tissue concentrations of α-tocopherol are 10 times higher than γ-tocopherol concentrations due to the higher preference of HDL (high-density lipoprotein) and LDL (low-density lipoprotein) particles for α-tocopherol and a higher rate of degradation of γ-tocopherol (Cook-Mills & Avila Citation2014). The specific effects of the different isoforms are highly debated (van Acker et al. Citation1993; Wagner et al. Citation2007; Wagner et al. Citation2008; Geiser et al. Citation2013; Cook-Mills & Avila Citation2014). A total of 113 credits were taken into account for the function score, of which 108 credits were based on studies assessing total vitamin E intake (Britton et al. Citation1995; Grievink et al. Citation1998; Tabak et al. Citation1999; Butland et al. Citation2000; McKeever et al. Citation2002; Daga et al. Citation2003; Pearson et al. Citation2004; de Luis et al. Citation2005; Kan et al. Citation2008; Nadeem et al. Citation2008; Mabalirajan et al. Citation2009; de Batlle et al. Citation2010; Ng et al. Citation2014) and five credits focussed on the effect of α-tocopherol resulting in a significant improvement of respiratory function in mice (Okamoto et al. Citation2006). Inflammatory and immune responses were mainly studied with γ-tocopherol administration (39 of the 49 credits) (Wagner et al. Citation2007, Citation2008; Wiser et al. Citation2008; Geiser et al. Citation2013; Hernandez et al. Citation2013), where five credits originated from studies focussed on total vitamin E intake (Mabalirajan et al. Citation2009) and five from studies on α-tocopherol intake (Okamoto et al. Citation2006). Vitamin E can affect the metabolism of arachidonic acid by decreasing cyclooxygenase enzymes and lowering LT synthesis, next to decreasing the serum levels of immunoglobulin E which is typically elevated in asthmatics (Fogarty et al. Citation2000; Okamoto et al. Citation2006). Both α- and γ-tocopherol scavenge reactive oxygen species, although the clinical relevance of this antioxidant effect is questioned and the positive effects of vitamin E supplementation are attributed to other mechanisms of action such as (i) upregulating PPARγ, (ii) inhibiting cyclooxygenase and lysyl oxidase and (iii) inhibiting nitration reactions by γ-tocopherol (Wagner et al. Citation2008). Both isoforms show similar inhibiting capacities of protein kinase B, which induces production of pro-inflammatory cytokines and activates NF-κB (Cook-Mills & Avila Citation2014). But whereas α-tocopherol showed anti-inflammatory effects and reduced airway hyper-reactivity in mice, γ-tocopherol was observed in mice to induce pro-inflammatory effects and enhanced airway hyper reactivity during eosinophilic airway inflammation (Cook-Mills & Avila Citation2014). On the other hand, γ-tocopherol was observed to react with reactive nitrogen species (found in eosinophils and neutrophils) and blocked acute neutrophil inflammation (Patel et al. Citation2007; Wagner et al. Citation2007; Cook-Mills & Avila Citation2014). Therefore γ-tocopherol is suggested to exert a broader anti-inflammatory profile than α-tocopherol (Wagner et al. Citation2007). Consequently, both isoforms are considered to contribute to the anti-inflammatory effects of vitamin E on chronic inflammatory lung diseases.
Although the effects of probiotics on respiratory function have been assessed in relatively few studies (35 credits), the calculated anti-inflammatory effects (−0.538) are reflected in a positive effect on respiratory function (−0.714). Probiotics is the collective term for 400–500 species of live bacteria which survive digestion and are consequently colonised in the gastrointestinal tract, providing a health benefit to the host (Heller & Duchmann Citation2003; Osborn & Sinn Citation2007). Since all probiotics do not influence the immune system similarly, the elicited health benefits can differ per bacterial strain and metabolites (Frei et al. Citation2015). The differences in effectiveness of strains also influenced the calculated score in this study: where some tested bacteria strains showed anti-inflammatory properties (e.g. Lactobacillus rhamnosus GG and Lactobacillus reuteri) (Feleszko et al. Citation2007; Forsythe et al. Citation2007; Karimi et al. Citation2009; Harb et al. Citation2013), other strains did not affect inflammation significantly (e.g. Bifidobacterium longum and Lactobacillus casei) (Lim et al. Citation2009; Lyons et al. Citation2010). The beneficial effects found following probiotic administration appear to be triggered via different mechanisms, which are still poorly understood (Frei et al. Citation2015). Various aspects as dendritic cells, epithelial cells, T regulatory cells, effector lymphocytes, natural killer T cells and B cells seem to be influenced by consumption of probiotics, resulting in reduced inflammation (Strickland & Holt Citation2011; Jan et al. Citation2012; Frei et al. Citation2015).
A link between the calculated improved function (−0.378) and the anti-inflammatory and immunological effect (−0.667) of vitamin C is complicated to assess due to the limited number of credits. Vitamin C intake is suggested to reduce inflammation due to its antioxidant capacity, by lowering the oxidative damage in the lungs and by blocking activation of the NF-κB pathway by inhibiting TNF-α formation (Tecklenburg et al. Citation2007; Milan et al. Citation2013). Other suggested mechanisms are the antiviral properties of vitamin C or its potency to alter the arachidonic acid pathway (Cohen Citation1997; Milan et al. Citation2013).
The relationship between improvement of inflammatory and immunological markers and resulting respiratory function appeared less evident for many other studied dietary components, such as caffeine and EGCG. With a score of −0.455 based on 110 credits, caffeine intake was shown to result in an improvement in lung function. Still, only one in vitro study (assigned three credits) assessed the inflammatory and immunological effects caused by caffeine consumption, and found no significant improvements (Geraets et al. Citation2006). The positive effect of caffeine on lung function is attributed to its bronchodilating effect (Gong et al. Citation1986; Kivity et al. Citation1990; Duffy & Phillips Citation1991; Welsh et al. Citation2010). Caffeine, as one of the methylxanthines, can affect various cellular processes and thereby instigate bronchodilation: it is an inhibitor of cyclic nucleotide phosphodiesterases, of intracellular translocation of calcium, it can increase the intracellular accumulation of cyclic nucleotides, block the adenosine receptor as competitive antagonist and it is able to directly decrease the binding of actin to phosphorylated myosin heads of muscles (Welsh et al. Citation2010; Tazzeo et al. Citation2012; Chawla & Suleman Citation2013). These cellular actions lead to bronchodilation, the widening of the airways, and thereby to improved lung function (Welsh et al. Citation2010).
The inflammatory effect score of EGCG of −1.000 indicated an anti-inflammatory potential, although the positive effect on respiratory function (−0.500) has only been established in one animal study so far (Bani et al. Citation2006). The polyphenol EGCG is not only an antioxidant but can also enhance endothelial-type and neuronal-type nitric oxide synthase enzymes (Bani et al. Citation2006). A decline in these enzymes is a feature of inflammation, leading to decreased nitric oxide, which is required to prevent activation of NF-κB and thereby pro-inflammatory gene transcription (Chen et al. Citation2002; Wheeler et al. Citation2004; Bani et al. Citation2006). In different animal and in vitro studies, a negative effect of EGCG on chemokines as well as matrix metalloproteinases (extracellular matrix proteins) has been observed, suppressing collagen production in fibroblasts (Kim et al. Citation2006; Qin et al. Citation2011; Chan et al. Citation2012; Lee et al. Citation2013).
Still, many of the studied dietary components showed no effect on either inflammatory markers and/or respiratory function. With using the median as cut-off point, the number of weighted articles fell below this established cut-off point. However, the calculated effects of many dietary components were only just above the cut-off points not reaching the “ideal amount” of 20 credits. This shows the need for more well-designed studies to assess the effects of dietary intake on patients suffering from chronic inflammatory lung diseases, with determining not only inflammatory and immunological markers but also the effect on respiratory function.
Limitations
The number of studies exploring the effects of the different dietary components and food items on chronic inflammatory lung diseases was limited and conflicting results were found. Ideally, the number of weighed articles would be above 20 credits. In our study, the cut-off points were established by calculating the median of credits assigned to the group of dietary components, in total 15 credits and 10 credits. For many dietary components with credits ranging between the cut-off point and the ideal number of 20 credits, the effects might be more reliable when they would be corrected for literature robustness as well. To interpret the results of this study that showed possible health enhancing effects for specific components, some further limitations have to be taken into account.
The calculated inflammatory and respiratory effect scores give an indication of the effect of consuming specific dietary components when suffering from chronic inflammatory lung diseases. The calculation is based on the dietary inflammatory index, to measure the inflammatory effect of dietary components (Cavicchia et al. Citation2009; Shivappa et al. Citation2014). Shivappa et al. (Citation2014) related the calculated effect to the total dietary intake based on food consumption data. This was not done in the present study since our main interest was to identify potent nutrients or food items affecting chronic inflammatory lung diseases. Not the amounts consumed by the general population, but intake resulting in a positive effect for patients was the main point of interest. Including a variety of experimental setups and abstaining from a quality control of these included studies could introduce an additional bias to this research. However, with the main aim being to identify dietary compounds potent to affect inflammation and/or function, the scores described in this study should serve as a starting point for future research rather than provide a calculation of an effect that can be reached.
By studying the effect of intake of single dietary components, the components most capable of positively influencing the progress of chronic inflammatory lung diseases were identified. However, methods to measure nutrient intake such as food questionnaires can result in measurement errors and bias by under- or overestimation of consumption (Freedman et al. Citation2011). Conflicting results between studies on similar ingredients can also be explained by heterogeneity, resulting from: (i) variations in the intake of the active ingredient (due to different compositions of the matrix of the active ingredient, the dosage regimen or duration of intake); (ii) set-up and quality of the study; and (iii) variations between patients (in genetics, dietary status, physical activity and dietary intake).
Following food consumption, not only single dietary components potentially affect health but all bioactive components are able to influence disease progress. Therefore the whole diet should be taken into consideration: absorption of bioactive ingredients can be influenced, but more importantly the intake of various bioactive ingredients could result in synergistic effects as suggested with adherence to the Mediterranean diet, a diet high in fruit, vegetable and fibre intake (Jacobs & Steffen Citation2003; Liu Citation2003; Jacobs & Tapsell Citation2007; Romieu et al. Citation2009).
Finally, publication and selection bias could have influenced the inclusion of 162 articles from over 1000 articles uncovered by the literature review strategy. With many studies showing significant anti-inflammatory or positive function effects, the question can be raised whether studies showing no or negative effects on inflammation or respiratory function are published to a similar extent as studies showing these positive effects, the so-called publication bias. Furthermore, the search strategy and inclusion criteria for this study could have led to selection bias of dietary components or models, although the possibility of this bias was reduced by conducting various searches by different authors and thorough discussion of inclusion and exclusion criteria.
Conclusions
The calculated inflammatory and respiratory function effect scores showed predominantly beneficial influences of various dietary components and food items on inflammatory and immunological responses as well as on lung function in patients suffering from chronic inflammatory lung diseases. Although inflammatory and immunological markers are not the only factors influencing disease progress, the positive effects of n-3 PUFAs and vitamin E on these markers were accompanied by improved lung function scores. Therefore, the consumption of these components could improve quality of life of patients and reduce the need for pharmaceutical anti-inflammatory therapies, thereby reducing both side effects and development of resistance.
Disclosure statement
The authors declare that there are no conflicts of interest.
References
- Agarwal S, Hordvik S, Morar S. 2006. Nutritional claims for functional foods and supplements. Toxicology. 221:44–49.
- Agrawal T, Gupta GK, Agrawal DK. 2013. Vitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine model. Clin Exp Allergy. 43:672–683.
- Arm JP, Horton CE, Mencia-Huerta JM, House F, Eiser NM, Clark TJ, Spur BW, Lee TH. 1988. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax. 43:84–92.
- Arshi S, Fallahpour M, Nabavi M, Bemanian MH, Javad-Mousavi SA, Nojomi M, Esmaeilzadeh H, Molatefi R, Rekabi M, Jalali F, Akbarpour N. 2014. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthma. Ann Allergy Asthma Immunol. 113:404–409.
- Baldrick FR, Elborn JS, Woodside JV, Treacy K, Bradley JM, Patterson CC, Schock BC, Ennis M, Young IS, McKinley MC. 2012. Effect of fruit and vegetable intake on oxidative stress and inflammation in COPD: a randomised controlled trial. Eur Respir J. 39:1377–1384.
- Bani D, Giannini L, Ciampa A, Masini E, Suzuki Y, Menegazzi M, Nistri S, Suzuki H. 2006. Epigallocatechin-3-gallate reduces allergen-induced asthma-like reaction in sensitized guinea pigs. J Pharmacol Exp Ther. 317:1002–1011.
- Bao Z-S, Hong L, Guan Y, Dong X-W, Zheng H-S, Tan G-L, Xie Q-M. 2011. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int Immunopharmacol. 11:899–906.
- Barnes PJ, Adcock IM. 2009. Glucocorticoid resistance in inflammatory diseases. Lancet. 373:1905–1917.
- Barnes PJ, Shapiro SD, Pauwels RA. 2003. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 22:672–688.
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, et al. 2008. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 31:143–178.
- Baughman RP, Lower EE, Du Bois RM. 2003. Sarcoidosis. Lancet. 361:1111–1118.
- Becker AB, Simons KJ, Gillespie CA, Simons FER. 1984. The bronchodilator effects and pharmacokinetics of caffeine in asthma. N Engl J Med. 310:743–746.
- Bime C, Wei CY, Holbrook J, Smith LJ, Wise RA. 2012. Association of dietary soy genistein intake with lung function and asthma control: a post-hoc analysis of patients enrolled in a prospective multicentre clinical trial. Prim Care Respir J. 21:398–404.
- Birrell MA. 2005. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-B-independent mechanism. FASEB J. 19:840–841.
- Böcking C, Harb H, Ege MJ, Zehethofer N, Fischer K, Krauß J, Holst O, Nüsing RM, Lindner B, von Mutius E, et al. 2014. Bioavailability and allergoprotective capacity of milk-associated conjugated linoleic acid in a murine model of allergic airway inflammation. Int Arch Allergy Immunol. 163:234–242.
- Boots AW, Drent M, de Boer VCJ, Bast A, Haenen GRMM. 2011. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 30:506–512.
- Boots AW, Drent M, Swennen ELR, Moonen HJJ, Bast A, Haenen GRMM. 2009. Antioxidant status associated with inflammation in sarcoidosis: a potential role for antioxidants. Respir Med. 103:364–372.
- Bourke SJ. 2006. Interstitial lung disease: progress and problems. Postgrad Med J. 82:494–499.
- Britton JR, Pavord ID, Richards KA, Knox AJ, Wisniewski AF, Lewis SA, Tattersfield AE, Weiss ST. 1995. Dietary antioxidant vitamin intake and lung function in the general population. Am J Respir Crit Care Med. 151:1383–1387.
- Broekhuizen R, Wouters EFM, Creutzberg EC, Weling-Scheepers CAPM, Schols AMWJ. 2005. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 60:376–382.
- Broughton K, Johnson C, Pace B, Liebman M, Kleppinger K. 1997. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr. 65:1011–1017.
- Bukowskyj M, Nakatsu K. 1987. The bronchodilator effect of caffeine in adult asthmatics. Am Rev Respir Dis. 135:173–175.
- Butland BK, Fehily AM, Elwood PC. 2000. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax. 55:102–108.
- Calder PC. 2006. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 83:S1505–S1519.
- Carey IM, Strachan DP, Cook DG. 1998. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med. 158:728–733.
- Cavicchia PP, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Hébert JR. 2009. A new dietary inflammatory index predicts interval changes in serum high-sensitivity C-reactive protein. J Nutr. 139:2365–2372.
- Chan KH, Chan SCH, Yeung SC, Man RYK, Ip MSM, Mak JCW. 2012. Inhibitory effect of Chinese green tea on cigarette smoke-induced up-regulation of airway neutrophil elastase and matrix metalloproteinase-12 via antioxidant activity. Free Radic Res. 46:1123–1129.
- Charng Y-C, Lin C-C, Hsu C-H. 2006. Inhibition of allergen-induced airway inflammation and hyperreactivity by recombinant lactic-acid bacteria. Vaccine. 24:5931–5936.
- Chawla J, Suleman A. 2013. Neurologic effects of caffeine. Available from: http://emedicine.medscape.com/article/1182710-overview#aw2aab6b4
- Chen PC, Wheeler DS, Malhotra V, Odoms K, Denenberg AG, Wong HR. 2002. A green tea-derived polyphenol, epigallocatechin-3-gallate, inhibits IkappaB kinase activation and IL-8 gene expression in respiratory epithelium. Inflammation. 26:233–241.
- Cohen HA. 1997. Blocking effect of vitamin C in exercise-induced asthma. Arch Pediatr Adolesc Med. 151:367–370.
- Colacone A, Bertolo L, Wolkove N, Cohen C, Kreisman H. 1990. Effect of caffeine on histamine bronchoprovocation in asthma. Thorax. 45:630–632.
- Cook DG, Carey IM, Whincup PH, Papacosta O, Chirico S, Bruckdorfer KR, Walker M. 1997. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax. 52:628–633.
- Cook-Mills JM, Avila PC. 2014. Vitamin E and D regulation of allergic asthma immunopathogenesis. Int Immunopharmacol. 23:364–372.
- Coussens LM, Werb Z. 2002. Inflammation and cancer. Nature. 420:860–867.
- Crivelli M, Wahlländer A, Jost G, Preisig R, Bachofen H. 1986. Effect of dietary caffeine on airway reactivity in asthma. Respiration. 50:258–264.
- Culpitt SV. 2003. Inhibition by red wine extract, resveratrol, of cytokine release by alveolar macrophages in COPD. Thorax. 58:942–946.
- Daga MK, Chhabra R, Sharma B, Mishra TK. 2003. Effects of exogenous vitamin E supplementation on the levels of oxidants and antioxidants in chronic obstructive pulmonary disease. J Biosci. 28:7–11.
- de Batlle J, Barreiro E, Romieu I, Mendez M, Gómez FP, Balcells E, Ferrer J, Orozco-Levi M, Gea J, Antó JM, Garcia-Aymerich J, et al. 2010. Dietary modulation of oxidative stress in chronic obstructive pulmonary disease patients. Free Radical Res. 44:1296–1303.
- de Batlle J, Sauleda J, Balcells E, Gómez FP, Méndez M, Rodriguez E, Barreiro E, Ferrer JJ, Romieu I, Gea J, et al. 2012. Association between Ω3 and Ω6 fatty acid intakes and serum inflammatory markers in COPD. J Nutr Biochem. 23:817–821.
- de Luis DA, Armentia A, Aller R, Asensio A, Sedano E, Izaola O, Cuellar L. 2005. Dietary intake in patients with asthma: a case control study. Nutrition. 21:320–324.
- de Vries A, Hazlewood L, Fitch PM, Seckl JR, Foster P, Howie SEM. 2009. High-fat feeding redirects cytokine responses and decreases allergic airway eosinophilia. Clin Exp Allergy. 39:731–739.
- Demissie K, Ernst P, Gray Donald K, Joseph L. 1996. Usual dietary salt intake and asthma in children: a case-control study. Thorax. 51:59–63.
- Dimeloe S, Richards DF, Urry ZL, Gupta A, Stratigou V, Farooque S, Saglani S, Bush A, Hawrylowicz CM. 2012. 1α,25-dihydroxyvitamin D3 promotes CD200 expression by human peripheral and airway-resident T cells. Thorax. 67:574–581.
- Donnelly LE, Newton R, Kennedy GE, Fenwick PS, Leung RHF, Ito K, Russel REK, Barnes PJ. 2004. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol. 287:774–783.
- Duffy P, Phillips YY. 1991. Caffeine consumption decreases the response to bronchoprovocation challenge with dry gas hyperventilation. Chest. 99:1374–1377.
- EFSA NDA Panel. 2011. General guidance for stakeholders on the evaluation of Article 13.1, 13.5 and 14 health claims. EFSA J. 9:2135. 1–24.
- Ellwood KC, Trumbo PR, Kavanaugh CJ. 2010. How the US Food and Drug Administration evaluates the scientific evidence for health claims. Nutr Rev. 68:114–121.
- Falk B, Gorev R, Zigel L, Ben-Amotz A, Neuman I. 2005. Effect of lycopene supplementation on lung function after exercise in young athletes who complain of exercise-induced bronchoconstriction symptoms. Ann Allerg Asthma Immunol. 94:480–485.
- Feleszko W, Jaworska J, Rha R-D, Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn U, Hamelmann E. 2007. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 37:498–505.
- Fogarty A, Lewis S, Weiss S, Britton J. 2000. Dietary vitamin E, IgE concentrations, and atopy. Lancet. 356:1573–1574.
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, Britton J. 2003. Oral magnesium and vitamin C supplements in asthma: a parallel group randomized placebo-controlled trial. Clin Exp Allergy. 33:1355–1359.
- Forsythe P, Inman MD, Bienenstock J. 2007. Oral treatment with live Lactobacillus reuteri inhibits the allergic airway response in mice. Am J Respir Crit Care Med. 175:561–569.
- Freedman LS, Schatzkin A, Midthune D, Kipnis V. 2011. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 103:1086–1092.
- Frei R, Akdis M, O’mahony L. 2015. Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr Opin Gastroenterol. 31:153–158.
- Fujita M, Ye Q, Ouchi H, Nakashima N, Hamada N, Hagimoto N, Kuwano K, Mason RJ, Nakanishi Y. 2004. Retinoic acid fails to reverse emphysema in adult mouse models. Thorax. 59:224–230.
- Fuld JP, Kilduff LP, Neder JA, Pitsiladis Y, Lean MEJ, Ward SA, Cotton MM. 2005. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax. 60:531–537.
- Fullerton JN, O’Brien AJ, Gilroy DW. 2013. Pathways mediating resolution of inflammation: when enough is too much. J Pathol. 231:8–20.
- Geiser M, Lay JC, Bennett WD, Zhou H, Wang X, Peden DB, Alexis NE. 2013. Effects of ex vivo γ-tocopherol on airway macrophage function in healthy and mild allergic asthmatics. J Innate Immun. 5:613–624.
- Geraets L, Haegens A, Brauers K, Haydock JA, Vernooy JHJ, Wouters EFM, Bast A, Hageman GJ. 2009. Inhibition of LPS-induced pulmonary inflammation by specific flavonoids. Biochem Biophys Res Commun. 382:598–603.
- Geraets L, Moonen HJJ, Wouters EFM, Bast A, Hageman GJ. 2006. Caffeine metabolites are inhibitors of the nuclear enzyme poly(ADP-ribose)polymerase-1 at physiological concentrations. Biochem Pharmacol. 72:902–910.
- Ghayur MN, Gilani AH, Janssen LJ. 2008. Ginger attenuates acetylcholine-induced contraction and Ca2+ signalling in murine airway smooth muscle cells. Can J Physiol Pharm. 86:264–271.
- Gong H, Simmons MS, Tashkin DP, Hui KK, Lee EY. 1986. Bronchodilator effects of caffeine in coffee. A dose-response study of asthmatic subjects. Chest. 89:335–342.
- Gontijo-Amaral C, Ribeiro MAGO, Gontijo LSC, Condino-Neto A, Ribeiro JD. 2006. Oral magnesium supplementation in asthmatic children: a double-blind randomized placebo-controlled trial. Eur J Clin Nutr. 61:54–60.
- Grievink L, Smit HA, Ocké MC, van’T Veer P, Kromhout D. 1998. Dietary intake of antioxidant (pro)-vitamins, respiratory symptoms and pulmonary function: the MORGEN study. Thorax. 53:166–171.
- Gross TJ, Hunninghake GW. 2001. Idiopathic pulmonary fibrosis. N Engl J Med. 345:517–525.
- Harb H, van Tol EAF, Heine H, Braaksma M, Gross G, Overkamp K, Hennen M, Alrifai M, Conrad ML, Renz H, Garn H. 2013. Neonatal supplementation of processed supernatant from Lactobacillus rhamnosus GG improves allergic airway inflammation in mice later in life. Clin Exp Allergy. 43:353–364.
- Hazewindus M, Haenen GRMM, Weseler AR, Bast A. 2014. Protection against chemotaxis in the anti-inflammatory effect of bioactives from tomato ketchup. PLoS One. 9:e114387.
- Hazlewood LC, Wood LG, Hansbro PM, Foster PS. 2011. Dietary lycopene supplementation suppresses Th2 responses and lung eosinophilia in a mouse model of allergic asthma. J Nutr Biochem. 22:95–100.
- Heller F, Duchmann R. 2003. Intestinal flora and mucosal immune responses. Int J Med Microbiol. 293:77–86.
- Henderson JC, O’Connell F, Fuller RW. 1993. Decrease of histamine induced bronchoconstriction by caffeine in mild asthma. Thorax. 48:824–826.
- Hernandez M, Zhou H, Zhou B, Robinette C, Crissman K, Hatch G, Alexis NE, Peden D. 2009. Combination treatment with high-dose vitamin C and alpha-tocopherol does not enhance respiratory-tract lining fluid vitamin C levels in asthmatics. Inhal Toxicol. 21:173–181.
- Hernandez ML, Wagner JG, Kala A, Mills K, Wells HB, Alexis NE, Lay JC, Jiang Q, Zhang H, Zhou H, Peden DB. 2013. Vitamin E, γ-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic Biol Med. 60:56–62.
- Hill J, Micklewright A, Lewis S, Britton J. 1997. Investigation of the effect of short-term change in dietary magnesium intake in asthma. Eur Respir J. 10:2225–2229.
- Hirayama F, Lee AH, Oura A, Mori M, Hiramatsu N, Taniguchi H. 2010. Dietary intake of six minerals in relation to the risk of chronic obstructive pulmonary disease. Asia Pac J Clin Nutr. 19:572–577.
- Hisada T, Adcock IM, Nasuhara Y, Salmon M, Huang TJ, Barnes PJ, Chung KF. 1999. Inhibition of ozone-induced lung neutrophilia and nuclear factor-kappaB binding activity by vitamin A in rat. Eur J Pharmacol. 377:63–68.
- Hodge L, Salome CM, Hughes JM, Liu-Brennan D, Rimmer J, Allman M, Pang D, Armour C, Woolcock AJ. 1998. Effect of dietary intake of omega-3 and omega-6 fatty acids on severity of asthma in children. Eur Respir J. 11:361–365.
- Hougee S, Vriesema AJM, Wijering SC, Knippels LMJ, Folkerts G, Nijkamp FP, Knol J, Garssen J. 2010. Oral treatment with probiotics reduces allergic symptoms in ovalbumin-sensitized mice: a bacterial strain comparative study. Int Arch Allergy Immunol. 151:107–117.
- Hsieh C-C, Kuo C-H, Kuo H-F, Chen Y-S, Wang S-L, Chao D, Lee M-S, Hung C-H. 2014. Sesamin suppresses macrophage-derived chemokine expression in human monocytes via epigenetic regulation. Food Funct. 5:2494–2500.
- Hsu D-Z, Liu C-T, Chu P-Y, Li Y-H, Periasamy S, Liu M-Y. 2013. Sesame oil attenuates ovalbumin-induced pulmonary edema and bronchial neutrophilic inflammation in mice. J Biomed Biotechnol. 2013:905670.
- Hu G, Zhang X, Chen J, Peto R, Campbell TC, Cassano PA. 1998. Dietary vitamin C intake and lung function in rural China. Am J Epidemiol. 148:594–599.
- Hurst SM, McGhie TK, Cooney JM, Jensen DJ, Gould EM, Lyall KA, Hurst RD. 2010. Blackcurrant proanthocyanidins augment IFN-gamma-induced suppression of IL-4 stimulated CCL26 secretion in alveolar epithelial cells. Mol Nutr Food Res. 54:S159–S170.
- Iwamura C, Shinoda K, Yoshimura M, Watanabe Y, Obata A, Nakayama T. 2010. Naringenin chalcone suppresses allergic asthma by inhibiting the type-2 function of CD4 T cells. Allergol Int. 59:67–73.
- Jacobs DR, Tapsell LC. 2007. Food, not nutrients, is the fundamental unit in nutrition. Nutr Rev. 65:439–450.
- Jacobs DRJ, Steffen LM. 2003. Nutrients, foods, and dietary patterns as exposures in research: a framework for food synergy. Am J Clin Nutr. 78:508S–513S.
- Jan R-L, Yeh K-C, Hsieh M-H, Lin Y-L, Kao H-F, Li P-H, Chang Y-S, Wang J-Y. 2012. Lactobacillus gasseri suppresses Th17 pro-inflammatory response and attenuates allergen-induced airway inflammation in a mouse model of allergic asthma. Br J Nutr. 108:130–139.
- Jang H-Y, Lim K, Lee S-M, Park B-H. 2014. Effects of n-3 PUFA on the CD4+ type 2 helper T-cell-mediated immune responses in Fat-1 mice. Mol Nutr Food Res. 58:365–375.
- Jaudszus A, Krokowski M, Möckel P, Darcan Y, Avagyan A, Matricardi P, Jahreis G, Hamelmann E. 2008. Cis-9,trans-11-conjugated linoleic acid inhibits allergic sensitization and airway inflammation via a PPARgamma-related mechanism in mice. J Nutr. 138:1336–1342.
- Jeong D-W, Yoo M-H, Kim TS, Kim J-H, Kim IY. 2002. Protection of mice from allergen-induced asthma by selenite: prevention of eosinophil infiltration by inhibition of NF-kappa B activation. J Biol Chem. 277:17871–17876.
- Kalhan R, Smith LJ, Nlend MC, Nair A, Hixon JL, Sporn PHS. 2008. A mechanism of benefit of soy genistein in asthma: inhibition of eosinophil p38-dependent leukotriene synthesis. Clin Exp Allergy. 38:103–112.
- Kan H, Stevens J, Heiss G, Rose KM, London SJ. 2008. Dietary fiber, lung function, and chronic obstructive pulmonary disease in the atherosclerosis risk in communities study. Am J Epidemiol. 167:570–578.
- Kanwar RK, Macgibbon AK, Black PN, Kanwar JR, Rowan A, Vale M, Krissansen GW. 2008. Bovine milk fat enriched in conjugated linoleic and vaccenic acids attenuates allergic airway disease in mice. Clin Exp Allergy. 38:208–218.
- Karimi K, Inman MD, Bienenstock J, Forsythe P. 2009. Lactobacillus reuteri-induced regulatory T cells protect against an allergic airway response in mice. Am J Respir Crit Care Med. 179:186–193.
- Kazaks AG, Uriu-Adams JY, Albertson TE, Shenoy SF, Stern JS. 2010. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. J Asthma. 47:83–92.
- Keranis E, Makris D, Rodopoulou P, Martinou H, Papamakarios G, Daniil Z, Zintzaras E, Gourgoulianis KI. 2010. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 36:774–780.
- Kim H-H, Bae Y, Kim S-H. 2013. Galangin attenuates mast cell-mediated allergic inflammation. Food Chem Toxicol. 57:209–216.
- Kim H-J, Kim Y-J, Lee S-H, Kang M-J, Yu H-S, Jung Y-H, Lee E, Seo J-H, Kwon J-W, Kim B-Jet al. 2013. Effects of Lactobacillus rhamnosus on asthma with an adoptive transfer of dendritic cells in mice. J Appl Microbiol. 115:872–879.
- Kim S-H, Park H-J, Lee C-M, Choi I-W, Moon D-O, Roh H-J, Lee H-K, Park Y-M. 2006. Epigallocatechin-3-gallate protects toluene diisocyanate-induced airway inflammation in a murine model of asthma. FEBS Lett. 580:1883–1890.
- King TE. Jr. 2005. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med. 172:268–279.
- Kirsch CM, Payan DG, Wong MY, Dohlman JG, Blake VA, Petri MA, Offenberger J, Goetzl EJ, Gold WM, et al. 1988. Effect of eicosapentaenoic acid in asthma. Clin Allergy. 18:177–187.
- Kirschvink N, Fiévez L, Bougnet V, Art T, Degand G, Smith N, Marlin D, Roberts C, Harris P, Lekeux P. 2002. Effect of nutritional antioxidant supplementation on systemic and pulmonary antioxidant status, airway inflammation and lung function in heaves-affected horses. Equine Vet J. 34:705–712.
- Kivity S, Ben Aharon Y, Man A, Topilsky M. 1990. The effect of caffeine on exercise-induced bronchoconstriction. Chest. 97:1083–1085.
- Knobloch J, Sibbing B, Jungck D, Lin Y, Urban K, Stoelben E, Strauch J, Koch A. 2010. Resveratrol impairs the release of steroid-resistant inflammatory cytokines from human airway smooth muscle cells in chronic obstructive pulmonary disease. J Pharmacol Exp Ther. 335:788–798.
- Kordansky DW, Rosenthal RR, Norman PS. 1979. The effect of vitamin C on antigen-induced bronchospasm. J Allergy Clin Immunol. 63:61–64.
- Kukkonen AK, Kuitunen M, Savilahti E, Pelkonen A, Malmberg P, Mäkelä M. 2011. Airway inflammation in probiotic-treated children at 5 years. Pediatr Allergy Immunol. 22:249–251.
- Kunitsugu I, Okuda M, Murakami N, Hashimoto M, Yamanishi R, Bando N, Sasaki S, Terao J, Sugiyama S, Hobara T. 2012. Self-reported seafood intake and atopy in Japanese school-aged children. Pediatr Int. 54:233–237.
- Kuo Y-T, Jan R-L, Kuo C-H, Kuo P-L, Wang W-L, Huang M-Y, Chen H-N, Hung C-H. 2012. Effects of vitamin D3 on the expression of growth-related oncogene-α in THP-1 cells and human primary monocytes. J Food Sci. 77:H47–H52.
- Lang CJ, Hansen M, Roscioli E, Jones J, Murgia C, Leigh Ackland M, Zalewski P, Anderson G, Ruffin R. 2010. Dietary zinc mediates inflammation and protects against wasting and metabolic derangement caused by sustained cigarette smoke exposure in mice. BioMetals. 24:23–39.
- Lee I-T, Lin C-C, Lee C-Y, Hsieh P-W, Yang C-M. 2013. Protective effects of (−)-epigallocatechin-3-gallate against TNF-α-induced lung inflammation via ROS-dependent ICAM-1 inhibition. J Nutr Biochem. 24:124–136.
- Lee M, Kim S, Kwon O-K, Oh S-R, Lee H-K, Ahn K. 2009. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 9:418–424.
- Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, Decallonne B, Bouillon R, Decramer M, Janssens W. 2012. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 156:105–114.
- Lim LH, Li HY, Huang CH, Lee BW, Lee YK, Chua KY. 2009. The effects of heat-killed wild-type Lactobacillus casei Shirota on allergic immune responses in an allergy mouse model. Int Arch Allergy Immunol. 148:297–304.
- Liu RH. 2003. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 78:517S–520S.
- Lu H, Xin Y, Tang Y, Shao G. 2012. Zinc suppressed the airway inflammation in asthmatic rats: effects of zinc on generation of eotaxin, MCP-1, IL-8, IL-4, and IFN-γ. Biol Trace Elem Res. 150:314–321.
- Lyons A, O’Mahony D, O’Brien F, MacSharry J, Sheil B, Ceddia M, Russell WM, Forsythe P, Bienenstock J, Kiely B, et al. 2010. Bacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy models. Clin Exp Allergy. 40:811–819.
- Mabalirajan U, Aich J, Leishangthem GD, Sharma SK, Dinda AK, Ghosh B. 2009. Effects of vitamin E on mitochondrial dysfunction and asthma features in an experimental allergic murine model. J Appl Physiol. 107:1285–1292.
- MacSharry J, O’Mahony C, Shalaby KH, Sheil B, Karmouty-Quintana H, Shanahan F, Martin JG. 2012. Immunomodulatory effects of feeding with Bifidobacterium longum on allergen-induced lung inflammation in the mouse. Pulmon Pharmacol Ther. 25:325–334.
- Malo JL, Cartier A, Pineau L, L’archevêque J, Ghezzo H, Martin RR. 1986. Lack of acute effects of ascorbic acid on spirometry and airway responsiveness to histamine in subjects with asthma. J Allergy Clin Immunol. 78:1153–1158.
- Matsuyama W, Mitsuyama H, Watanabe M, Oonakahara K, Higashimoto I, Osame M, Arimura K. 2005. Effects of omega-3 polyunsaturated fatty acids on inflammatory markers in COPD. Chest. 128:3817–3827.
- McKeever TM, Lewis SA, Cassano PA, Ocké M, Burney P, Britton J, Smit HA. 2008. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in Dutch adults. Thorax. 63:208–214.
- McKeever TM, Scrivener S, Broadfield E, Jones Z, Britton J, Lewis SA. 2002. Prospective study of diet and decline in lung function in a general population. Am J Respir Crit Care Med. 165:1299–1303.
- Mickleborough TD, Lindley MR, Ionescu AA, Fly AD. 2006. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 129:39–49.
- Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. 2003. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 168:1181–1189.
- Milan SJ, Hart A, Wilkinson M. 2013. Vitamin C for asthma and exercise-induced bronchoconstriction. Cochrane Database Syst Rev. 10:CD010391.
- Miraglia Del Giudice M, Maiello N, Decimo F, Fusco N, D’Agostino B, Sullo N, Capasso M, Salpietro V, Gitto E, Ciprandi G, et al. 2012. Airways allergic inflammation and L. reuterii treatment in asthmatic children. J Biol Reg Homeos Ag. 26:S35–S40.
- Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, Saad M, Yu J. 2009. Inflammatory mechanisms in the lung. J Inflamm Res. 2:1–11.
- Moreira A, Kekkonen R, Korpela R, Delgado L, Haahtela T. 2007. Allergy in marathon runners and effect of Lactobacillus GG supplementation on allergic inflammatory markers. Respir Med. 101:1123–1131.
- Moreira A, Moreira P, Delgado L, Fonseca J, Teixeira V, Padrão P, Castel-Branco G. 2007. Pilot study of the effects of n-3 polyunsaturated fatty acids on exhaled nitric oxide in patients with stable asthma. J Investig Allergol Clin Immunol. 17:309–313.
- Morris A, Noakes M, Clifton PM. 2001. The role of n-6 polyunsaturated fat in stable asthmatics. J Asthma. 38:311–319.
- Muehlmann LA, Zanatta AL, Farias CLA, Bieberbach EW, Mazzonetto AC, Michellotto PV, Fernandes L, Nishiyama A. 2010. Dietary supplementation with soybean lecithin increases pulmonary PAF bioactivity in asthmatic rats. J Nutr Biochem. 21:532–537.
- Nadeem A, Raj HG, Chhabra SK. 2008. Effect of vitamin E supplementation with standard treatment on oxidant–antioxidant status in chronic obstructive pulmonary disease. Indian J Med Res. 128:705–711.
- Nadi E, Tavakoli F, Zeraati F, Goodarzi MT, Hashemi SH. 2012. Effect of vitamin C administration on leukocyte vitamin C level and severity of bronchial asthma. Acta Med Iran. 50:233–238.
- Neuman I, Nahum H, Ben-Amotz A. 2000. Reduction of exercise-induced asthma oxidative stress by lycopene, a natural antioxidant. Allergy. 55:1184–1189.
- Ng TP, Niti M, Yap KB, Tan WC. 2014. Dietary and supplemental antioxidant and anti-inflammatory nutrient intakes and pulmonary function. Public Health Nutr. 17:2081–2086.
- Nomura A, Zhang M, Sakamoto T, Ishii Y, Morishima Y, Mochizuki M, Kimura T, Uchida Y, Sekizawa K. 2003. Anti-inflammatory activity of creatine supplementation in endothelial cells in vitro. Br J Pharmacol. 139:715–720.
- Nyanhanda T, Gould EM, McGhie T, Shaw OM, Harper JL, Hurst RD. 2014. Blackcurrant cultivar polyphenolic extracts suppress CCL26 secretion from alveolar epithelial cells. Food Funct. 5:671–677.
- O’Byrne PM, Postma DS. 1999. The many faces of airway inflammation: asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care. 159:S1–S63.
- Okamoto M, Mitsunobu F, Ashida K, Mifune T, Hosaki Y, Tsugeno H, Harada S, Tanizaki Y. 2000. Effects of dietary supplementation with n-3 fatty acids compared with n-6 fatty acids on bronchial asthma. Intern Med. 39:107–111.
- Okamoto N, Murata T, Tamai H, Tanaka H, Nagai H. 2006. Effects of alpha tocopherol and probucol supplements on allergen-induced airway inflammation and hyperresponsiveness in a mouse model of allergic asthma. Int Arch Allergy Immunol. 141:172–180.
- Osborn DA, Sinn JK. 2007. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev. 4:CD006475.
- Park S-J, Shin W-H, Seo J-W, Kim E-J. 2007. Anthocyanins inhibit airway inflammation and hyperresponsiveness in a murine asthma model. Food Chem Toxicol. 45:1459–1467.
- Patel A, Liebner F, Netscher T, Mereiter K, Rosenau T. 2007. Vitamin E chemistry. Nitration of non-alpha-tocopherols: products and mechanistic considerations. J Org Chem. 72:6504–6512.
- Patel RB, Kotha SR, Sauers LA, Malireddy S, Gurney TO, Gupta NN, Elton TS, Magalang UJ, Marsh CB, Haley BE, Parinandi NL. 2012. Thiol-redox antioxidants protect against lung vascular endothelial cytoskeletal alterations caused by pulmonary fibrosis inducer, bleomycin: comparison between classical thiol-protectant, N-acetyl-l-cysteine, and novel thiol antioxidant, N, N′-bis-2-merca. Toxicol Mech Method. 22:383–396.
- Pawelec G, Goldeck D, Derhovanessian E. 2014. Inflammation, ageing and chronic disease. Curr Opin Immunol. 29:23–28.
- Payan DG, Wong MY, Chernov-Rogan T, Valone FH, Pickett WC, Blake VA, Gold WM, Goetzl EJ. 1986. Alterations in human leukocyte function induced by ingestion of eicosapentaenoic acid. J Clin Immunol. 6:402–410.
- Pearson PJK, Lewis SA, Britton J, Fogarty A. 2004. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 59:652–656.
- Pistelli R, Forastiere F, Corbo GM, Dell’orco V, Brancato G, Agabiti N, Pizzabiocca A, Perucci CA. 1993. Respiratory symptoms and bronchial responsiveness are related to dietary salt intake and urinary potassium excretion in male children. Eur Respir J. 6:517–522.
- Qin S, Alcorn JF, Craigo JK, Tjoeng C, Tarwater PM, Kolls JK, Reinhart TA. 2011. Epigallocatechin-3-gallate reduces airway inflammation in mice through binding to proinflammatory chemokines and inhibiting inflammatory cell recruitment. J Immunol. 186:3693–3700.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. 2007. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care. 176:532–555.
- Romieu I, Barraza-Villarreal A, Escamilla-Núñez C, Texcalac-Sangrador JL, Hernandez-Cadena L, Díaz-Sánchez D, De Batlle J, Del Rio-Navarro BE. 2009. Dietary intake, lung function and airway inflammation in Mexico City school children exposed to air pollutants. Resp Res. 10:122.
- Romieu I. 2005. Nutrition and lung health. Int J Tuberc Lung Dis. 9:362–374.
- Rothkrantz-Kos S, van Dieijen-Visser MP, Mulder PGH, Drent M. 2003. Potential usefulness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem. 49:1510–1517.
- Ruijters EJB, Haenen GRMM, Weseler AR, Bast A. 2014a. The anti-inflammatory efficacy of dexamethasone is protected by (−)-epicatechin. PharmaNutrition. 2:47–52.
- Ruijters EJB, Haenen GRMM, Weseler AR, Bast A. 2014b. The cocoa flavanol (−)-epicatechin protects the cortisol response. Pharmacol Res. 79:28–33.
- Sauer JM, Hooser SB, Sipes IG. 1995. All-trans-retinol alteration of 1-nitronaphthalene-induced pulmonary and hepatic injury by modulation of associated inflammatory responses in the male Sprague–Dawley rat. Toxicol Appl Pharmacol. 133:139–149.
- Schachter EN, Schlesinger A. 1982. The attenuation of exercise-induced bronchospasm by ascorbic acid. Ann Allergy. 49:146–151.
- Schertling M. 1989. Einfluss von Ascorbinsäure auf den klinischen Verlauf des infektbedingten Asthma bronchiale und die Bildung von reaktiven Sauerstoffmetaboliten durch BAL-Zellen [unofficial translation]. Z Klin Med. 45:1770–1774.
- Schuster GU, Bratt JM, Jiang X, Pedersen TL, Grapov D, Adkins Y, Kelley DS, Newman JW, Kenyon NJ, Stephensen CB. 2014. Dietary Long-chain omega-3 fatty acids do not diminish eosinophilic pulmonary inflammation in mice. Am J Respir Cell Mol Biol. 50:626–636.
- Schuster GU, Kenyon NJ, Stephensen CB. 2008. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol. 180:1834–1842.
- Schwartz J, Weiss ST. 1994. The relationship of dietary fish intake to level of pulmonary function in the first National Health and Nutrition Survey (NHANES I). Eur Respir J. 7:1821–1824.
- Selman M, King JTE, Pardo A. 2001. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 134:136–151.
- Sexton P, Black P, Metcalf P, Wall CR, Ley S, Wu L, Sommerville F, Brodie S, Kolbe J. 2013. Influence of Mediterranean diet on asthma symptoms, lung function, and systemic inflammation: a randomized controlled trial. J Asthma. 50:75–81.
- Shaheen SO, Jameson KA, Robinson SM, Boucher BJ, Syddall HE, Sayer AA, Cooper C, Holloway JW, Dennison EM. 2011. Relationship of vitamin D status to adult lung function and COPD. Thorax. 66:692–698.
- Shaheen SO, Newson RB, Rayman MP, Wong AP-L, Tumilty MK, Philips JM, Potts JF, Kelly FJ, White PT, Burney PGJ. 2007. Randomised, double blind, placebo-controlled trial of selenium supplementation in adult asthma. Thorax. 62:483–490.
- Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. 2014. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 17:1689–1696.
- Siedlinski M, Boer JMA, Smit HA, Postma DS, Boezen HM. 2012. Dietary factors and lung function in the general population: wine and resveratrol intake. Eur Respir J. 39:385–391.
- Sienra-Monge JJ, Ramirez-Aguilar M, Moreno-Macias H, Reyes-Ruiz NI, Del Rio-Navarro BE, Ruiz-Navarro MX, Hatch G, Crissman K, Slade R, Devlin RB, Romieu I. 2004. Antioxidant supplementation and nasal inflammatory responses among young asthmatics exposed to high levels of ozone. Clin Exp Med. 138:317–322.
- Steinke JW, Borish L. 2001. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2:66–70.
- Stickford JL, Mickleborough TD, Fly AD, Stager JM. 2011. Conjugated linoleic acid’s lack of attenuation of hyperpnea-induced bronchoconstriction in asthmatic individuals in the short term. Int J Sport Nutr Exerc. 21:40–47.
- Strachan DP, Cox BD, Erzinclioglu SW, Walters DE, Whichelow MJ. 1991. Ventilatory function and winter fresh fruit consumption in a random sample of British adults. Thorax. 46:624–629.
- Strickland DH, Holt PG. 2011. T regulatory cells in childhood asthma. Trends Immunol. 32:420–427.
- Surette ME, Koumenis IL, Edens MB, Tramposch KM, Clayton B, Bowton D, Chilton FH. 2003. Inhibition of leukotriene biosynthesis by a novel dietary fatty acid formulation in patients with atopic asthma: a randomized, placebo-controlled, parallel-group, prospective trial. Clin Ther. 25:972–979.
- Tabak C, Arts IC, Smit HA, Heederik D, Kromhout D. 2001a. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: the MORGEN Study. Am J Respir Crit Care. 164:61–64.
- Tabak C, Smit HA, Heederik D, Ocké MC, Kromhout D. 2001b. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy. 31:747–755.
- Tabak C, Smit HA, Räsänen L, Fidanza F, Menotti A, Nissinen A, Feskens EJM, Heederik D, Kromhout D. 1999. Dietary factors and pulmonary function: a cross sectional study in middle aged men from three European countries. Thorax. 54:1021–1026.
- Takamura K, Nasuhara Y, Kobayashi M, Betsuyaku T, Tanino Y, Kinoshita I, Yamaguchi E, Matsukura S, Schleimer RP, Nishimura M. 2004. Retinoic acid inhibits interleukin-4-induced eotaxin production in a human bronchial epithelial cell line. Am J Physiol-Lung C. 286:L777–L785.
- Tazzeo T, Bates G, Roman HN, Lauzon A-M, Khasnis MD, Eto M, Janssen LJ. 2012. Caffeine relaxes smooth muscle through actin depolymerization. Am J Physiol-Lung C. 303:L334–L342.
- Tecklenburg SL, Mickleborough TD, Fly AD, Bai Y, Stager JM. 2007. Ascorbic acid supplementation attenuates exercise-induced bronchoconstriction in patients with asthma. Respir Med. 101:1770–1778.
- Thien FC, Mencia-Huerta JM, Lee TH. 1993. Dietary fish oil effects on seasonal hay fever and asthma in pollen-sensitive subjects. Am Rev Respir Dis. 147:1138–1143.
- Ting S, Mansfield LE, Yarbrough J. 1983. Effects of ascorbic acid on pulmonary functions in mild asthma. J Asthma. 20:39–42.
- Trenga CA, Koenig JQ, Williams PV. 2001. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Arch Environ Health. 56:242–249.
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 20:159–166.
- U.S. Food and Drug Administration. 2014. Guidance for industry: evidence-based review system for the scientific evaluation of health claims. Available from: http://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/ucm073332.htm
- van Acker SABE, Koymans LMH, Bast A. 1993. Molecular pharmacology of vitamin E: structural aspects of antioxidant activity. Free Radic Biol Med. 15:311–328.
- van de Pol MA, Lutter R, Smids BS, Weersink EJM, van der Zee JS. 2011. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy. 66:39–47.
- Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. 2007. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 62:786–791.
- Varraso R, Willett WC, Camargo CA. 2010. Prospective study of dietary fiber and risk of chronic obstructive pulmonary disease among US women and men. Am J Epidemiol. 171:776–784.
- Verma R, Kushwah L, Gohel D, Patel M, Marvania T, Balakrishnan S. 2013. Evaluating the ameliorative potential of quercetin against the bleomycin-induced pulmonary fibrosis in Wistar rats. Pulm Med. 2013:921724.
- Vieira RP, Duarte ACS, Claudino RC, Perini A, Santos ABG, Moriya HT, Arantes-Costa FM, Martins MA, Carvalho CRF, Dolhnikoff M. 2007. Creatine supplementation exacerbates allergic lung inflammation and airway remodeling in mice. Am J Respir Cell Mol. 37:660–667.
- Villani F, Comazzi R, De Maria P, Galimberti M. 1998. Effect of dietary supplementation with polyunsaturated fatty acids on bronchial hyperreactivity in subjects with seasonal asthma. Respiration. 65:265–269.
- Vos AP, van Esch BC, Stahl B, M’Rabet L, Folkerts G, Nijkamp FP, Garssen J. 2007. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int Immunopharmacol. 7:1582–1587.
- Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. 2008. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 38:501–511.
- Wagner JG, Jiang Q, Harkema JR, Illek B, Patel DD, Ames BN, Peden DB. 2007. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free Radic Biol Med. 43:1176–1188.
- Welsh EJ, Bara A, Barley E, Cates CJ. 2010. Caffeine for asthma. Cochrane Database Syst Rev. 1:CD001112.
- West CE, Hammarström M-L, Hernell O. 2013. Probiotics in primary prevention of allergic disease-follow-up at 8–9 years of age. Allergy. 68:1015–1020.
- Wheeler DS, Catravas JD, Odoms K, Denenberg A, Malhotra V, Wong HR. 2004. Epigallocatechin-3-gallate, a green tea-derived polyphenol, inhibits IL-1 beta-dependent proinflammatory signal transduction in cultured respiratory epithelial cells. J Nutr. 134:1039–1044.
- Wiser J, Alexis NE, Jiang Q, Wu W, Robinette C, Roubey R, Peden DB. 2008. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic Biol Med. 45:40–49.
- Wood LG, Hazlewood LC, Foster PS, Hansbro PM. 2010. Lyprinol reduces inflammation and improves lung function in a mouse model of allergic airways disease. Clin Exp Allergy. 40:1785–1793.
- Woodward EA, Prêle CM, Nicholson SE, Kolesnik TB, Hart PH. 2010. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology. 131:118–127.
- Wu M-Y, Hung S-K, Fu S-L. 2011. Immunosuppressive effects of fisetin in ovalbumin-induced asthma through inhibition of NF-κB activity. J Agric Food Chem. 59:10496–10504.
- Xie Y-C, Dong X-W, Wu X-M, Yan X-F, Xie Q-M. 2009. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int Immunopharmacol. 9:194–200.
- Yasuda A, Inoue K-I, Sanbongi C, Yanagisawa R, Ichinose T, Yoshikawa T, Takano H. 2010. Dietary supplementation with fructooligosaccharides attenuates airway inflammation related to house dust mite allergen in mice. Int J Immunopathol Pharmacol. 23:727–735.
- Yates CM, Calder PC, Ed Rainger G. 2014. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Therapeut. 141:272–282.
- Yazdanpanah L, Shidfar F, Moosavi AJ, Heidarnazhad H, Haghani H. 2010. Energy and protein intake and its relationship with pulmonary function in chronic obstructive pulmonary disease (COPD) patients. Acta Med Iran. 48:374–379.
- Yin H, Liu W, Goleniewska K, Porter NA, Morrow JD, Peebles RS. 2009. Dietary supplementation of omega-3 fatty acid-containing fish oil suppresses F2-isoprostanes but enhances inflammatory cytokine response in a mouse model of ovalbumin-induced allergic lung inflammation. Free Radic Biol Med. 47:622–628.
- Yoneda J, Chin K, Torii K, Sakai R. 2011. Effects of oral monosodium glutamate in mouse models of asthma. Food Chem Toxicol. 49:299–304.
- You H, Wei L, Sun W-L, Wang L, Yang Z-L, Liu Y, Zheng K, Wang Y, Zhang W-J. 2014. The green tea extract epigallocatechin-3-gallate inhibits irradiation-induced pulmonary fibrosis in adult rats. Int J Mol Med. 34:92–102.
- Yurach MT, Davis BE, Cockcroft DW. 2011. The effect of caffeinated coffee on airway response to methacholine and exhaled nitric oxide. Respir Med. 105:1606–1610.
- Zhang B, An J, Shimada T, Liu S, Maeyama K. 2012. Oral administration of Enterococcus faecalis FK-23 suppresses Th17 cell development and attenuates allergic airway responses in mice. Int J Mol Med. 30:248–254.
- Zhong H, Zhou XJ, Hong JG. 2013. The effects of vitamin D on allergen-induced expression of interleukin-13 and interleukin-17 in cord blood CD4 + T cells. Iran J Allergy Asthm. 13:93–97.
- Zhou E, Fu Y, Wei Z, Yang Z. 2014. Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 5:2106–2112.
- Zoia MC, Fanfulla F, Bruschi C, Basso O, De Marco R, Casali L, Cerveri I. 1995. Chronic respiratory symptoms, bronchial responsiveness and dietary sodium and potassium: a population-based study. Monaldi Arch Chest Dis. 50:104–108.