Abstract
Maqui-berry is characterised by presenting a high concentration of (poly)phenols, accounting anthocyanins (cyanidin and delphinidin) for over 85% of the total. These coloured flavonoids have demonstrated potential neurological activity, but the evidence of their antinociceptive properties is scarce. In order to cover this gap, different doses (suitable for human administration) of a maqui-berry powder (1.6% anthocyanin), using enteral and parenteral routes of administration, were compared at central and peripheral levels using a nociceptive pain model (formalin test) in mice. Gastric damage analysis as possible adverse effects of analgesic and anti-inflammatory drugs was also explored. Dose-antinociceptive response was confirmed using both routes of administration and in both neurogenic and inflammatory phases of the formalin test, without gastric damage. In conclusion, these preliminary data provide evidence of pharmacological properties of maqui-berry to alleviate nociceptive pain.
Introduction
Pain has a protective function, but in many cases, it is a major symptom of a disease (Ortega et al. Citation2002). In the pharmaceutical armament, there are several efficacious analgesic drugs to treat pain, but adverse effects are an obstacle that reduces this optional resource to relieve it (Bacchi et al. Citation2012), and nowadays society demands more natural treatments.
Aristotelia chilensis (Mol.) Stuntz (Elaeocarpaceae), known commonly as maqui, is a native plant, from southern Chile and part of neighbouring Argentina, widely used in the native traditional herbal medicine. The use of maqui, in folk medicine, was preliminary demonstrated in a study regarding the analgesic and anti-inflammatory properties of the plant leaves (Muñoz et al. Citation2011). Nowadays, the berries have increased their uses and are broadly selected to develop healthy or potential functional foods, because of their biological attributions (high antioxidant capacity, cardio-protection and inhibition of adipogenesis and diabetes symptoms) (Yang and Kortesniemi Citation2015; Foito et al. Citation2018). This activity is mainly due to the (poly)phenolic fraction, specifically anthocyanin-based compounds, that account for over 80% of total (poly)phenols (Gironés-Vilaplana et al. Citation2012; Girones-Vilaplana et al. Citation2014). In addition to those health benefits, it is worth to mention that Mapuche, the most numerous indigenous nation in southern South America living in Chile and Argentina, produces this species as one of the most important used plants for healing, nervous system disorders, pain and inflammation, among others (Molares and Ladio Citation2009; Schmeda-Hirschmann et al. Citation2019). Even so, although it has been described that the ingestion of this berry produces central actions that improve memory and decreases oxidative stress (Bribiesca-Cruz et al. Citation2019), further studies are needed to fill the gap of knowledge about the pharmacological properties on the central nervous system (CNS) of maqui. This neurological activity is mainly related to its high anthocyanin concentration, as they are able to pass through blood-brain barrier (BBB) in their intact form or as derived metabolites (Henriques et al. Citation2020) and, e.g., modulate the Nrf2 pathway to mitigate oxidative stress or neurodegeneration (Ali et al. Citation2018) or enhance memory (Andres-Lacueva et al. Citation2005; Ali et al. Citation2018). Also, its properties against depressive disease, by promoting normal mouse behaviour in both despair swimming and tail suspension tests, have been described (Di Lorenzo et al. Citation2019). Due to these health benefits, when compared to other berries, the expansion and growth of agricultural and business sectors, related to this fruit, is promoting and reaching foreign markets such as the USA, Asia and Europe (Vega-Galvez et al. Citation2021).
On the other hand, natural medicine using fruits is fundamental in the therapeutic since they constitute an arsenal of biologically active substances with pharmacological activity, but in most of the cases without proved scientific evidence. In fact, functional foods are recently one of the interesting options to prevent diseases, but the scientific evidence of their potential is still lacking. Our aim is to obtain preclinical evidence of the pharmacological properties of maqui-berry, as a natural source, to explore its capacity for relief pain, in order to include this ingredient in future food formulations, as a complement or alternative to common over-the-counter (OTC) medicines good for many types of pain (as acetaminophen or non-steroidal anti-inflammatory drugs a.k.a. NSAIDs).
Materials and methods
The maqui-berry powder was provided by Maqui New Life S.A. (Santiago de Chile, Chile) and consisted of a freeze-dried whole fruit soluble ground, rich in anthocyanins. These anthocyanins (over 85% of the total phenolic compounds) represented 1.6% of powder total weight, while the rest of the composition consisted of dietary fibre (60.4%), carbohydrates (18.1%), proteins (6.5%), fats (5%), minerals (1.4%), other phenolic compounds (0.3%), vitamins (0.1%), ash (2.5%) and moisture (4.1%).
Anthocyanins analysis
Cyanidin (Cy) and Delphinidin (Dp) 3-O-glucoside chloride were purchased from TransMIT (Geiben, Germany), formic acid from Fisher Scientific (Loughborough, UK) and methanol (LC-MS Chromasolv) from Honeywell/Riedel-de-Haen (Seelze, Germany). All solutions were prepared with ultrapure water from a Milli-Q Advantage A10 ultrapure water purification system (Merck Millipore, Darmstadt, Germany).
The maqui powder (100 mg) was diluted in a methanol/Milli-Q water/formic acid (70:29:1, v/v) solvent (10 mL) and centrifuged at 10,500 rpm, during 5 min (Sigma 1-13, B. Braun Biotech International Centrifuge, Osterode, Germany). The supernatants were filtered through a 0.45 µm PVDF membrane (Millex HV13, Millipore, Bedford, MA, USA).
The identification and quantification of anthocyanins were performed by applying the high performance liquid chromatography coupled with diode array detection (HPLC-DAD) method previously reported (Gironés-Vilaplana et al. Citation2012). Briefly, chromatographic analysis of samples (10 µL) was carried out on a 5 µm Luna C18 100 Å (250 × 4.6 mm) column, using Security Guard Cartridges PFD 4 × 3.0 mm, supplied both by Phenomenex (Torrance, CA, USA). Chromatographic separation was achieved with 5% formic acid (solvent A) and methanol (solvent B), upon the linear gradient starting with 15%B, reaching 30%B at 20 min, 40%B at 30 min, 60%B at 35 min, 90%B at 40 min, maintained for 5 min (cleaning), back to initial conditions, and kept for 7 min under those conditions (15%B), previous to next injection, at a flow rate 0.9 mL/min. The equipment was an Agilent Technologies 1220 Infinity Liquid Chromatograph, equipped with a G1313 autoinjector and a 1260 Diode Array Detector (Agilent Technologies, Santa Clara, CA, USA). Chromatograms were recorded and processed on an Agilent ChemStation for LC 3D systems (Agilent Technologies, Santa Clara, CA, USA). Anthocyanins were quantified as cyanidin 3-O-glucoside (detected at 520 nm) and expressed as mg per gram of maqui powder.
Pharmacological study
Animals
Swiss Webster male mice (25–30 g) were used in the pharmacological evaluation. Animals were provided by Instituto Nacional de Psiquiatría “Ramón de la Fuente Muñiz” (Mexico City, Mexico), they were kept at a controlled temperature of (22 ± 1 °C) with light/dark cycle of 12 h and fed ad libitum with standard water and food. Experiments were carried out following the specifications issued by the Committee of Ethics and Research with the approval number NC-123280.0 and NC-17073.0 (CONBIOETICA-09-CEI-010-20170316), as well as according to the Official Mexican Norm for the care and handling animal (NOM-062-ZOO-1999) and the international rules of care and use for laboratory animals.
Reagents and drugs
Tramadol (TR) was acquired from Grünenthal México S.A. C.V. (Mexico City, Mexico). Indomethacin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and formaldehyde at 37% solution from J.T. Baker (Phillipsburg, USA). The maqui-berry powder was used in fresh preparation. Pharmacological evaluation was done using both parenteral (i.p.) and enteral (p.o.) administration. All the treatments were injected in a volume of 0.1 mL/10 g body weight. Vehicle consisted of distilled water.
Antinociceptive activity experimental design
Groups of at least 6 mice received the vehicle, reference drug (TR, a partial opioid agonist and monoamine modulator) or the A. chilensis powder diluted in water, in a volume of 0.1 ml per 10 g body weight (12.5, 25, 62.5, 125 and 250 mg/kg, i.p. and 250, 500 and 1000 mg/kg, p.o.). These doses were considered taking into account our preliminary acute studies in healthy volunteers (Agulló, Domínguez-Perles, et al. Citation2021; Agulló, Villaño, et al. Citation2020), where the anthocyanin bioavailability was analysed after the intake of 330 mL of a juice containing 3.3 g of A. chilensis (54.75 mg of anthocyanins) (Salar et al. Citation2020). In accordance with Nair and Jacob (Citation2016), when extrapolated to average weigh human of 70 kg b.w., this amount would be similar to 500 mg/kg, p.o. in mice, as it would be equivalent to 2.84 g of maqui (47.02 mg of anthocyanins). In order to find the most suitable dose, double or half concentrations (250 and 1000 mg/kg), were studied, for oral intake. In case of parenteral administration, the starting dosage referred to the lowest enteral active dosage studied (250 mg/kg, p.o.), and successive dilutions, as parenteral route has better bioavailability than enteral, requiring lower doses (Thomson Citation2008). After 30 min of treatment, the nociceptive agent (1% formalin intraplantar) was injected to induce licking behaviour, as follows:
Formalin test
After a habituation of 30 min, animals were administered with several doses of the maqui solutions and the reference drug TR (30 mg/kg, i.p. or 50 mg/kg, p.o.). Thirty minutes later, a 20 µl injection was done in the subplantar area of the right hind paw with 1% formalin using a 30-gauge needle. Each mouse was placed into a glass cylinder provided with mirrors to enable a total panorama of the nociceptive response. The time spent in licking the injected paw was taken as nociceptive behaviour. Two periods of high nociceptive activity were considered: the first one was shown immediately after injection, it was considered from 0 to 10 min counted as the time spent licking the first minute (0–1 min), 5–6 min and 10–11 min. This was taken as the early or neurogenic phase. A second period was observed from 10 to 30 min after 1% formalin injection counted for one min in times 15–16 min, 20–21 min, 25–26 min and 30–31 min denominated as the late or inflammatory phase. Control animals received vehicle by the same route and time of administration.
Data were obtained as temporal course curves of the licking behaviour induced in mice. Then, a dose–response plot was built with the area under the curve (AUC) using the trapezoidal rule to determine the significant antinociceptive dose of the maqui powder tested using the enteral and parenteral route of administration.
Gastric damage
Once the formalin test was concluded, animals were euthanized to obtain their stomachs to gastric damage observation. Each stomach was dissected, and they were filled with 10 mL of 10% formalin and fixed for 10 min. Then, each stomach was opened by the greater curvature and washed to remove residues. Finally, the stomachs were scanned to observe possible gastric lesions and compared to those produced by indomethacin (Whittle Citation1977).
Statistical data analysis
Data are expressed as the mean ± standard error of the mean (S.E.M.) of 6 repetitions. Temporal course curves were analysed by two-way ANOVA followed by Bonferroni’s post-hoc test. Dose-response data were analysed by one-way ANOVA followed by Dunnett’s post-hoc test. For this, it was used GraphPad Prism software, version 8.0.2. (GraphPad Software Inc., La Jolla, CA, USA). p < 0.05 was considered statistically significant.
Results
Anthocyanin content of maqui-berry powder
The anthocyanins composition of the maqui powder used in the study was characterised by the presence of 8 anthocyanins (total concentration: 16.59 mg/g maqui powder), being the most abundant delphinidin (Dp) derivates (≈ 83%), followed by cyanidin (Cy) ones: Dp 3-O-glucoside > Dp 3-O-sambubioside-5-O-glucoside > Dp 3,5-O-di-glucoside > Dp 3-O-sambubioside > co-eluting Cy 3-O-sambubioside-5-O-glucoside and Cy 3,5-O-di-glucoside > Cy 3-O-glucoside > Cy 3-O-sambubioside (, ).
Figure 1. HPLC anthocyanin profile and quantification of A. chilensis powder recorded at 520 nm. Peaks are identified as shown in .
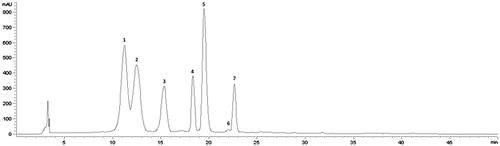
Table 1. Anthocyanins composition of maqui-berry powder.
Antinociceptive activity of maqui-berry
Temporal course curves of the antinociceptive effects of maqui-berry, evaluated after parenteral administration at different doses (12.5, 25, 62.5, 125 and 250 mg/kg, i.p.), allowed observing antinociceptive response in the first minute of the test. All doses significantly inhibited nociception at central stage reaching the effect of the reference drug TR (30 mg/kg, i.p.) (). This significant inhibition was also observed in the inflammatory stage, where maqui, at doses of 62.5, 125 and 250 mg/kg, inhibited in a total manner the nociceptive behaviour in all the time period evaluated, being better than the response induced by the reference drug () (Treatment F6,35 = 11.37, p < 0.0001; Time F3.654,127.9 = 75.48, p < 0.0001; Interaction F36,210 = 2.484, p < 0.0001).
Figure 2. Temporal course curves (A) and dose-response (AUC) antinociceptive effects of A. chilensis powder (AC) and tramadol (TRA, reference drug) after parenteral administration on the neurogenic (B) and inflammatory (C) phases of the 1% formalin intraplantar in mice compared to the vehicle. A two-way ANOVA followed by Bonferroni’s post-hoc test for temporal course curves and a one-way ANOVA followed by Dunnett’s post-hoc test for dose-response data. *p < 0.05, **p < 0.01 and ***p < 0.001, n = 6 repetitions.
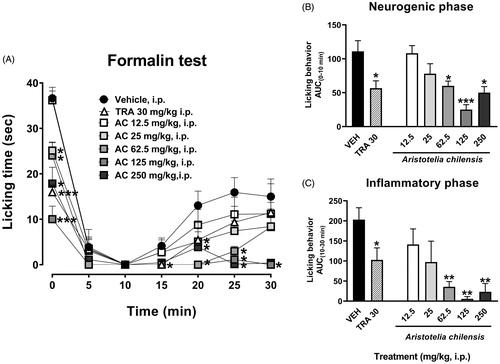
Integration of the nociceptive response as area under the curve allowed observing that maqui powder produced a dose-dependent and significant antinociceptive response in the neurogenic phase (F6,35 = 7.73, p < 0.0001), reaching a maximal effect at dosage of 125 mg/kg (). A dosage of 62.5 mg/kg of this powder induced an equivalent effect than the reference drug TR (30 mg/kg, i.p.) ().
In a similar manner, significant diminution in the time spent licking in mice was observed in the inflammatory phase (F6,35 = 5.29, p = 0.0006) (). It began from a dosage of 62.5 mg/kg, which produced better response than the reference drug, and remained when dose was increased at 125 or 250 mg/kg, i.p. ().
In case of the enteral administration of the maqui powder, the nociceptive response induced by the allogenic formalin remained inhibited in the first minute of the neurogenic stage from a dosage of 500 mg/kg, p.o. This response was intensified in the fifth minute after formalin since a dosage of 250 mg/kg showed similar effect than 500 or even 1000 mg/kg, p.o. (). In a similar manner than the parenteral administration, maqui (500 and 1000 mg/kg, p.o.) produced an almost total inhibition in the nociception in all the period evaluated for the inflammatory stage () (Treatment F4,24 = 16.55, p < 0.0001; Time F3.651,87.63 = 61.84, p < 0.0001; Interaction F24,144 = 5.14, p < 0.0001).
Figure 3. Temporal course curves (A) and dose-response (AUC) antinociceptive effects of A. chilensis freeze-dried (AC) and tramadol (TRA, reference drug) after enteral administration on the neurogenic (B) and inflammatory (C) phases of the 1% formalin intraplantar in mice compared to the vehicle. A two-way ANOVA followed by Bonferroni’s post-hoc test for temporal course curves and a one-way ANOVA followed by Dunnett’s post-hoc test for dose-response data. *p < 0.05, **p < 0.01 and ***p < 0.001, n = 6 repetitions.
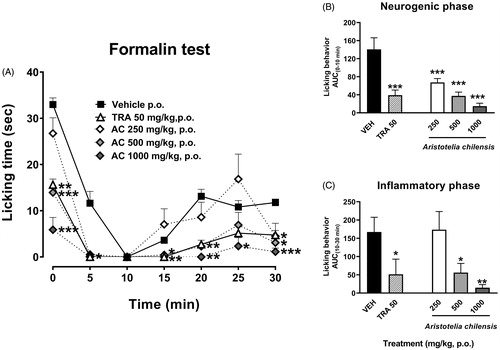
The antinociceptive effect using enteral administration was also significant and a dose-response manner was observed in both the neurogenic () (F4,24 = 38.15, p < 0.0001) and inflammatory phases () (F4,24 = 7.17, p = 0.0006) resembling the effect of TR (50 mg/kg, p.o.).
Gastric damage
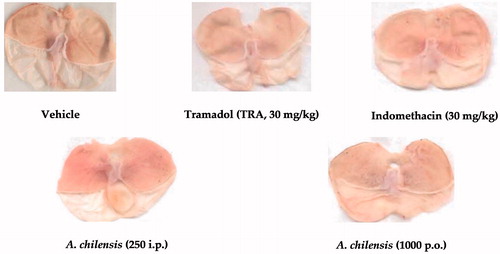
Gastric damage, as a possible adverse effect produced by anti-inflammatory analgesic drugs, was not observed at any dose or route of administration tested of the A. chilensis powder in comparison to a slightly but positive response in a reference drug like indomethacin ().
Figure 4. Representative photos of the gastric damage evaluation of dissected stomachs from mice receiving treatment of vehicle, positive anti-inflammatory drug (indomethacin) and the highest dosage of A. chilensis using parenteral (250 mg/kg, i.p.) or. enteral (1000 mg/kg, p.o.) route of administration and after the formalin test in mice.
Discussion
Maqui-berry is a source of several anthocyanidin constituents, many of them derived from delphinidin compound (≈83% of total anthocyanins − 13.71 mg/g). Moreover, previous studies have demonstrated that these anthocyanins are bioavailable for humans (Agulló, Domínguez-Perles, et al. Citation2021; Agulló, Domínguez-Perles, Moreno, et al. Citation2020; Agulló, Villaño, et al. Citation2020), and they could be detected intact in brain, among other tissues (Kalt et al. Citation2008). It has also been reported that a stabilisation of delphinidin, in a formulation for system administration, reversed mechanical and thermal hyperalgesia, as well as local inflammation, in part, because of its capacity to scavenge superoxide anion radicals with and inhibitory concentration of 70 ± 5 µM (Sauer et al. Citation2020). In our work, the complete maqui-berry powder demonstrated antinociceptive activity by enteral and parenteral administration in a chemical pain model reinforcing the analgesic spectrum of this natural product.
In preclinical studies, antinociceptive effects of some vegetables (Baenas et al. Citation2017) and fruits (González-Trujano et al. Citation2015) have been observed in a similarly range of doses assayed using analgesic drugs with anti-inflammatory activity (González-Trujano et al. Citation2007). Antinociceptive response depends on the species and kind of painful stimulus, but also on the sensitivity based in part on the metabolic action. In this respect, humans might be 6-fold more sensitive than rats and 12-fold more than mice. Experimental data in animals are usually found in a range of doses with a major magnitude in comparison to humans in clinic. For this reason, allometric approach considers the differences in body surface area, which is associated with animal weight while extrapolating the doses of therapeutic agents among the species. This approach assumes that there are some unique characteristics on anatomical, physiological and biochemical processes among species, and the possible difference in pharmacokinetics/physiological time is accounted by allometric scaling (Nair and Jacob Citation2016). Due to this, dosages of 62.5 mg/kg, i.p. or 500 mg/kg, p.o., that presented the best activity, would suggest doses of 5.06 mg/kg or 40.50 mg/kg, respectively, indicating an ingestion of 3.2 g of maqui, with a total anthocyanin concentration of approx. 50 mg for an average humans weight (70 kg) (Walpole et al. Citation2012).
The formalin test used in this study as acute and tonic pain model induces nociception by injured tissue generating moderate and continuous pain behaviour in two phases. The early phase seems to be caused predominantly by C-fibre activation due to the peripheral stimulus, while the late phase appears to be dependent on the combination of an inflammatory reaction in the peripheral tissue and functional changes in the dorsal horn of the spinal cord (Tjølsen et al. Citation1992). It is well known that centrally acting drugs, like opioids, inhibit neurogenic and inflammatory phases, while peripherally acting drugs, like NSAIDs, inhibit only the inflammatory phase (Sani et al. Citation2012). TR was used as positive analgesic drug in this study since it is a well-known partial opioid agonist, but also a monoamine modulator, that inhibits nociception in both phases of formalin test (Tlacomulco-Flores et al. Citation2020). Significant antinociceptive effect was produced in the presence of A. chilensis in both phases of formalin test suggesting actions at central and peripheral levels. These results agree with a study of Muntingia calabura, a species also from Elaeocarpaceae family, which demonstrated both central and peripheral antinociceptive activity associated with its antioxidant and anti-inflammatory effects (Sani et al. Citation2012; Mahmood et al. Citation2014; Zakaria et al. Citation2016). These results suggest that the high anthocyanin content in A. chilensis (Cespedes et al. Citation2017; Céspedes-Acuña et al. Citation2018; Agulló, Villaño, et al. Citation2020), like the abundant derived from delphinidin compound, might influence the antinociceptive activity of this species. Delphinidin and cyanidin are dominant anthocyanidins in some berries, which possess potential antioxidant activity (Sauer et al. Citation2020) and strongly inhibited mediators of inflammation and pain like tumour necrosis factor alpha (TNF-α) induced by cyclooxygenase 2 (COX-2) expression (Hwang et al. Citation2009).
The antioxidant activity of berry polyphenols has been related to their protective effects against cellular oxidative stress and inflammation (Vega-Galvez et al. Citation2021). The formalin test damages the cells producing radioactive oxygen species by macrophage action and the phenolic compounds, mainly anthocyanins, present in this berry might reduce the negative effect of formalin due to the scavenging of oxygen-free radicals and the inhibition of oxidative enzymes (Ortiz et al. Citation2020; Romero-González et al. Citation2020). Due to this, it can be established that the maqui-berry anthocyanins or their bioactive metabolites could cross the BBB (Andres-Lacueva et al. Citation2005; Agulló, Villaño, et al. Citation2020) and act as antinociceptive, without gastric damage, after oral ingestion. Due to this, this preliminary study enforces maqui-berry as promising healthy food, at neurological level.
On the other hand, no gastric damage was noticed in mice after administration and significant antinociceptive response. Data agree to previous studies, where it has been demonstrated anti-ulcer activity of anthocyanins (Alvarez-Suarez et al. Citation2011; Kim et al. Citation2011) increasing the benefits of this species consume in pain therapy against non-steroidal drugs, such as indomethacin or aspirin, that induce this frequent and common kind of tissue injury.
Conclusions
In conclusion, the results obtained from this study, together with literature, give evidence of properties of maqui-berry for pain therapy and suggest a small ingestion of this berry could act as a potential natural alternative or complement to the use of usual drugs for the analgesic therapy. Moreover, future researches focussed on the mechanisms of action could be helpful in order to confirm the peripheral and central antinociceptive activities of maqui-berry. All this together makes this berry a promising ingredient for healthy beverages or other processed foods.
Author contributions
M.E.G.-T., A.H.-L., E.E.-C. and F.P.: Experimental design; V.A. and C.G.-V.: experimental chromatographic analysis; V.A., M.E.G.-T. and A.H.-L.: pharmacological experiments; V.A., M.E.G.-T., A.H.-L. and C.G.-V.: data analysis; V.A., M.E.G.-T., A.H.-L., E.E.-C., F.P. and C.G.-V.: manuscript preparation,; M.E.G.-T., F.P. and C.G.-V.: financial support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Agulló V, Domínguez-Perles R, García-Viguera C. 2021. Sweetener influences plasma concentration of flavonoids in humans after an acute intake of a new (poly)phenol-rich beverage. Nutr Metab Cardiovasc Dis. 31(3):930–938.
- Agulló V, Domínguez-Perles R, Moreno DA, Zafrilla P, García-Viguera C. 2020. Alternative sweeteners modify the urinary excretion of flavanones metabolites ingested through a new maqui-berry beverage. Foods. 9(1):41.
- Agulló V, Villaño D, García-Viguera C, Domínguez-Perles R. 2020. Anthocyanin metabolites in human urine after the intake of new functional beverages. Molecules. 25(2):371.
- Ali T, Kim T, Rehman SU, Khan MS, Amin FU, Khan M, Ikram M, Kim MO. 2018. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol Neurobiol. 55(7):6076–6093.
- Alvarez-Suarez JM, Dekanski D, Ristić S, Radonjić NV, Petronijević ND, Giampieri F, Astolfi P, González-Paramás AM, Santos-Buelga C, Tulipani S, et al. 2011. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS One. 6(10):e25878.
- Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. 2005. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 8(2):111–120.
- Bacchi S, Palumbo P, Sponta A, Coppolino MF. 2012. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 11(1):52–64.
- Baenas N, González-Trujano ME, Guadarrama-Enríquez O, Pellicer F, García-Viguera C, Moreno DA. 2017. Broccoli sprouts in analgesia - preclinical in vivo studies. Food Funct. 8(1):167–176.
- Bribiesca-Cruz I, Moreno DA, García-Viguera C, Gallardo JM, Segura-Uribe JJ, Pinto-Almazán R, Guerra-Araiza C. 2019. Maqui berry (Aristotelia chilensis) extract improves memory and decreases oxidative stress in male rat brain exposed to ozone. Nutr Neurosci. 0(0):1–13. Online ahead of print..
- Cespedes CL, Pavon N, Dominguez M, Alarcon J, Balbontin C, Kubo I, El-Hafidi M, Avila JG. 2017. The Chilean superfruit black-berry Aristotelia chilensis (Elaeocarpaceae), Maqui as mediator in inflammation-associated disorders. Food Chem Toxicol. 108(Pt B):438–450.
- Céspedes-Acuña CL, Xiao J, Wei ZJ, Chen L, Bastias JM, Avila JG, Alarcon-Enos J, Werner-Navarrete E, Kubo I. 2018. Antioxidant and anti-inflammatory effects of extracts from Maqui berry Aristotelia chilensis in human colon cancer cells. J Berry Res. 8(4):275–296.
- Di Lorenzo A, Sobolev AP, Nabavi SF, Sureda A, Moghaddam AH, Khanjani S, Di Giovanni C, Xiao J, Shirooie S, Tsetegho Sokeng AJ, et al. 2019. Antidepressive effects of a chemically characterized maqui berry extract (Aristotelia chilensis (Molina) Stuntz) in a mouse model of post-stroke depression. Food Chem Toxicol. 129(January):434–443.
- Foito A, McDougall GJ, Stewart D. 2018. Evidence for health benefits of berries. Annu. plant rev. online. 1(1). doi:10.1002/9781119312994.apr0600
- Gironés-Vilaplana A, Mena P, García-Viguera C, Moreno DA. 2012. A novel beverage rich in antioxidant phenolics: maqui berry (Aristotelia chilensis) and lemon juice. LWT. 47(2):279–286.
- Girones-Vilaplana A, Mena P, Moreno DA, Garcia-Viguera C. 2014. Evaluation of sensorial, phytochemical and biological properties of new isotonic beverages enriched with lemon and berries during shelf life. J Sci Food Agric. 94(6):1090–1100.
- González-Trujano ME, Pellicer F, Mena P, Moreno DA, García-Viguera C. 2015. Antinociceptive and anti-inflammatory activities of a pomegranate (Punica granatum L.) extract rich in ellagitannins. Int J Food Sci Nutr. 66(4):395–399.
- González-Trujano ME, Peña EI, Martínez AL, Moreno J, Guevara-Fefer P, Déciga-Campos M, López-Muñoz FJ. 2007. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J Ethnopharmacol. 111(3):476–482.
- Henriques JF, Serra D, Dinis TCP, Almeida LM. 2020. The anti-neuroinflammatory role of anthocyanins and their metabolites for the prevention and treatment of brain disorders. Int J Mol Sci. 21(22):8653.
- Hwang MK, Kang NJ, Heo YS, Lee KW, Lee HJ. 2009. Fyn kinase is a direct molecular target of delphinidin for the inhibition of cyclooxygenase-2 expression induced by tumor necrosis factor-α. Biochem Pharmacol. 77(7):1213–1222.
- Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SAE, Graf BA, O’Leary JM, Milbury PE. 2008. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 56(3):705–712.
- Kim SJ, Park YS, Paik HD, Chang HI. 2011. Effect of anthocyanins on expression of matrix metalloproteinase-2 in naproxen-induced gastric ulcers. Br J Nutr. 106(12):1792–1801.
- Mahmood ND, Nasir NLM, Rofiee MS, Tohid SFM, Ching SM, Teh LK, Salleh MZ, Zakaria ZA. 2014. Muntingia calabura: a review of its traditional uses, chemical properties, and pharmacological observations. Pharm Biol. 52(12):1598–1623.
- Molares S, Ladio A. 2009. Ethnobotanical review of the Mapuche medicinal flora: use patterns on a regional scale. J Ethnopharmacol. 122(2):251–260.
- Muñoz O, Christen P, Cretton S, Backhouse N, Torres V, Correa O, Costa E, Miranda H, Delporte C. 2011. Chemical study and anti-inflammatory, analgesic and antioxidant activities of the leaves of Aristotelia chilensis (Mol.) Stuntz, Elaeocarpaceae. J Pharm Pharmacol. 63(6):849–859.
- Nair A, Jacob S. 2016. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 7(2):27–31.
- Ortega A, Roca A, Micó JA. 2002. Modelos animales de dolor. Una visión crítica. Rev la Soc Esp Del Dolor. 9(7):447–453.
- Ortiz T, Argüelles-Arias F, Illanes M, García-Montes JM, Talero E, Macías-García L, Alcudia A, Vázquez-Román V, Motilva V, De-Miguel M. 2020. Polyphenolic maqui extract as a potential nutraceutical to treat TNBS-induced Crohn’s disease by the regulation of antioxidant and anti-inflammatory pathways. Nutrients. 12(6):1718–1752.
- Romero-González J, Shun Ah-Hen K, Lemus-Mondaca R, Muñoz-Fariña O. 2020. Total phenolics, anthocyanin profile and antioxidant activity of maqui, Aristotelia chilensis (Mol.) Stuntz, berries extract in freeze-dried polysaccharides microcapsules. Food Chem. 313(November 2019):126115.
- Salar FJ, Agulló V, García-Viguera C, Domínguez-Perles R. 2020. Stevia vs. sucrose: influence on the phytochemical content of a citrus–maqui beverage—a shelf life study. Foods. 9(2):219.
- Sani MHM, Zakaria ZA, Balan T, Teh LK, Salleh MZ. 2012. Antinociceptive activity of methanol extract of Muntingia calabura leaves and the mechanisms of action involved. Evid Based Complement Alternat Med. 2012:1–10.
- Sauer R-S, Krummenacher I, Bankoglu EE, Yang S, Oehler B, Schöppler F, Mohammadi M, Güntzel P, Ben-Kraeim A, Holzgrabe U. 2020. Stabilization of delphinidin in a complex with sulfobutylether-β-cyclodextrin allows for antinociception in inflammatory pain. Antioxid Redox Signal. doi:https://doi.org/10.1089/ars.2019.7957
- Schmeda-Hirschmann G, Jiménez-Aspee F, Theoduloz C, Ladio A. 2019. Patagonian berries as native food and medicine. J Ethnopharmacol. 241(May):111979.
- Thomson A. 2008. The enteral vs parenteral nutrition debate revisited. J Parenter Enter Nutr. 32(4):474–481.
- Tjølsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. 1992. The formalin test: an evaluation of the method. Pain. 51(1):5–17.
- Tlacomulco-Flores LL, Déciga-Campos M, González-Trujano ME, Carballo-Villalobos AI, Pellicer F. 2020. Antinociceptive effects of Salvia divinorum and bioactive salvinorins in experimental pain models in mice. J Ethnopharmacol. 248:112276.
- Vega-Galvez A, Rodríguez A, Stucken K. 2021. Antioxidant, functional properties and health-promoting potential of native South American berries: a review. J Sci Food Agric. 101(2):364–378.
- Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I. 2012. The weight of nations: an estimation of adult human biomass. BMC Public Health. 12(1):439.
- Whittle BJR. 1977. Mechanisms underlying gastric mucosal damage induced by indomethacin and bile-salts, and the actions of prostaglandins. Br J Pharmacol. 60(3):455–460.
- Yang B, Kortesniemi M. 2015. Clinical evidence on potential health benefits of berries. Curr Opin Food Sci. 2:36–42.
- Zakaria ZA, Mohd Sani MH, Kadir AA, Kek TL, Salleh MZ. 2016. Antinociceptive effect of semi-purified petroleum ether partition of Muntingia calabura leaves. Rev Bras Farmacogn. 26(4):408–419.

