Abstract
Purpose
To identify, and classify, according to International Classification of Functioning, Disability and Health (ICF), clinically applicable outcome measures that have been used to evaluate lower limb orthotic management post-stroke and to investigate which outcome measures recorded the largest effect sizes.
Materials and methods
Electronic searches were performed in Pubmed, Cochrane, Web of Science, Cinahl, Scopus and Embase databases from inception to May 2020. Articles were included if they investigated clinical outcomes in people post-stroke who had received a lower-limb orthotic intervention.
Results
88 articles underwent full-text review and 54 were included in the review, which was performed in accordance with the Preferred Reporting Items for Systematic Review (PRISMA) principles. 48 different outcome measures were identified; effect sizes were able to be calculated from 39 studies. The most frequently applied outcome measures were the 10-metre Walk Test and the timed-up-and-go test. Outcome measures that recorded large effect sizes in two or more studies were the 10-metre Walk Test, Functional Reach Test, and Physiological Cost Index. When coded according to the ICF, the most frequently represented codes were d450 (Walking) and d455 (moving around).
Conclusions
Results suggest that outcome measures related to mobility (ICF chapter d4) are most often applied to evaluate orthotic management post-stroke. Effect sizes appear to be greatest in outcome measures related to velocity, balance, and energy expenditure.
The 10-meter Walk Test appears to have the greatest effect size when evaluating orthotic management post-stroke.
While outcome measures related to mobility are commonly applied when evaluating orthotic management post-stroke, rehabilitation professionals should consider complementing these with measures representing the participation domain of the ICF.
IMPLICATIONS FOR REHABILITATION
Globally, stroke is the second major cause of death and disability, with an incidence of 203 per 100 000 people [Citation1] and a worldwide average of 13 million new cases yearly [Citation2]. Loss or difficulty with ambulation has been described as one of the most devastating results of a stroke and, gait restoration is often a primary goal of rehabilitation [Citation3]. Clinical practice guidelines from a number of organisations from around the world recommend the use of an orthosis as an effective method of compensating for impaired functional performance following a stroke [Citation4–7].
Within the orthotics field, recovery assessment following a stroke often focuses on measurements of functional status, with an emphasis on gait, transfers, and balance. The choice of appropriate outcome measures is essential for good practice and a reflection of how the clinician has operationalised “success” [Citation8]. With increasing promotion of the biopsychosocial model of health, it has been suggested that the assessment of stroke recovery in the rehabilitation setting is often too narrow and should be broadened to include a more comprehensive assessment [Citation9].
The International Classification of Functioning, Disability and Health (ICF) provides a useful framework for monitoring and describing health outcomes and changes in health status from a broad biopsychosocial perspective. Within the ICF, human functioning is classified on three levels; the level of the body, the whole person, and the whole person in a social context [Citation10]. As such, components of health and outcome measures addressing health st tus can be classified under three domains; body structure and function, activities, and participation.
Several systematic reviews have been conducted to identify outcome measures that have been used in various aspects of stroke rehabilitation. Ashford et al. [Citation11] focused specifically on outcome measures that assess day to day performance. Eight specific measures were identified, all of which the authors classified as being within the activity domain of the ICF. Galeoto et al. [Citation12] reviewed studies that used outcome measures to assess the loss of functionality as a result of a stroke. They identified 41 measures of which 6 were validated in multiple studies. While these authors did not attempt to classify outcome measures within the ICF, they reported that the most used outcome measures were the Frenchay Activities Index and the Activity Card sort. A similar study, focusing on paediatric stroke, identified 38 unique outcome measures, with the most frequently applied being the Wechsler Intelligence scales, paediatric stroke outcome, and Bayley scales of infant development [Citation13]. Interestingly, this review reported that the same outcome measure was not used in more than 2 studies, reflecting the general lack of consensus in which outcome measures are relevant to apply.
In the only review with a specific focus on lower-limb orthotic management of stroke, Figueiredo et al. [Citation14] reviewed outcome measures and motion capture systems which had been used in randomised trials evaluating orthotic interventions for gait rehabilitation. 39 outcome measures were identified with gait speed, step length, cadence, and stride duration being the most frequently reported measures. Most outcome measures identified in this study were recorded using laboratory-based motion capture systems that are not routinely accessible in the clinical setting. The authors classified most measures under the activity domain of the ICF.
The aim of this systematic review was to identify and classify according to ICF, clinical outcome measures that have been used to evaluate results of lower limb orthotic management post-stroke and to investigate which outcome measures recorded the largest effect sizes. Specifically, we aimed to identify which outcome measures have been used most often and which are typically used in combination with each other. We were also interested in determining which outcome measures recorded the largest effect sizes when they were used to evaluate orthotic interventions. Finally, we aimed to identify which domains, chapters, and codes of the ICF are most commonly addressed in outcome measures used to evaluate orthotic interventions.
For this study, a clinical outcome measure was defined as a functional performance measure, patient-reported outcome measure, or proxy measure, suitable for routine application in a clinical setting. Notably, measures more germane to controlled laboratory environments, such as observations based on three-dimensional gait analysis and related measures of kinetics and kinematics were intentionally excluded from consideration.
Methods
A systematic review of the current literature was performed to identify clinical outcome measures used to evaluate orthotic management in individuals who have had a stroke. Outcome measures identified in the review were then coded according to the ICF. The review was performed in accordance with Preferred Reporting Items for Systematic Review (PRISMA) principles [Citation15] and an a priori registration of the protocol was completed in the PROSPERO register (CRD42018091172).
Search strategy
With support from an academic librarian (information specialist), a search for relevant literature was conducted by NR and PS in May 2018, with no limits on the year of publication. An additional search using the same search strings was conducted by NR for the period of May 2018 until May 2020. Searches were conducted in Pubmed, Cochrane, Web of Science, Cinahl, Scopus, and Embase databases. The search string was developed in accordance with the recommendations of Bramer et al. [Citation16]. To develop the search string, we first identified index terms (e.g., Medical Subject Headings – MeSH terms) representing (1) stroke, (2) orthotic devices, and (3) the lower extremities. We then identified the entry terms (synonyms) that were listed under each index term and included these as free text search terms within the title and abstract fields. An example of the search string used in the Pubmed database is included as Supplementary Table I.
Eligibility criteria
Eligibility criteria for studies included in this review are presented in . Only English language, peer-reviewed articles were included. Types of studies eligible for inclusion were randomised controlled trials, case-control studies, cohort studies, case series studies, and qualitative studies. The population of interest was adults who had experienced a stroke. If studies also investigated individuals with other conditions, they were only included if the group with stroke was reported independently of the other groups. Articles were only included if they investigated lower-limb orthotic devices as an intervention. Studies comparing orthotic devices to no device were included as well as studies comparing different device designs. Types of orthotic devices were limited to the lower extremities and could include foot orthoses, ankle-foot orthoses (AFOs), knee orthoses, knee-ankle-foot orthoses (KAFOs), or functional electrical stimulation (FES) with surface stimulation. Articles were excluded if they were editorials, commentaries, dissertations, single case studies or reviews, or if they only used laboratory-based outcome measures that were considered by the authors as not being readily available for use in the clinical setting. This included two- and three-dimensional motion analysis and force plate data.
Table 1. Eligibility criteria for included studies.
Study selection and data extraction
After removing duplicates, all studies identified from the database search were imported into the web application Rayyan QCRI [Citation17] and the two authors performed independent reviews of titles and abstracts, indicating whether each abstract should be included or excluded. Their response and comments were recorded in Rayyan but not visible to the other author. When this review was complete the results of both authors were compared and articles in which the authors disagreed were discussed in relation to the inclusion/exclusion criteria until consensus was reached. Both authors then reviewed the full text of the remaining articles and those that did not meet the inclusion criteria were removed. Once again disagreements were discussed until a consensus was reached. If consensus was not reached at any stage a third reviewer would have been consulted.
Data extracted from the remaining articles included citation (author/year), study design, sample characteristics (age, time post-stroke, sample size, drop-outs), description of the orthotic intervention, accommodation time, testing procedures, clinical outcome measures used, and results of the study. One author initially extracted all the data and the second author confirmed the accuracy of the retrieved information.
Assessment of methodological quality
The studies remaining in the review were assessed for methodological quality by both authors using the relevant standardised critical appraisal tool from the Joanna Briggs Institute [Citation18]. Specific questions can be seen in Supplementary Tables II and III. Questions that were deemed irrelevant for the subject area were removed from the critical appraisal tools. Given that orthotic interventions were an integral part of this systematic review, an additional question was added to all appraisal tools to assess whether there was an adequate description of the orthotic interventions provided. Criteria for describing the orthosis was based upon those proposed by Lintanf et al. [Citation19] and required the identification of the manufacturer and model of FES systems and at least 3 of the following criteria relative to AFOs: (1) custom versus off-the-shelf fabrication, (2) material, (3) ankle angle, or (4) joint restrictions. When conducting the appraisal, a score was allocated to each question for each article (“+” = low risk of bias, “−” = high risk of bias, and “?” = unclear risk of bias). Case series studies were only included if 5 or more questions were scored as having a low risk of bias while RCTs were included if 7 or more questions were scored as having a low risk of bias. Any disagreement between reviewers was resolved through discussion.
Calculation of effect size
When possible, data available from the selected studies were used to calculate Cohen’s d which provides an indication of the effect of orthotic interventions on specific outcomes. Effect sizes were calculated using the Psychometrica effect size calculators and formulas described by Lenhard and Lenhard [Citation20]. When the standard deviations (sd) data that is required for calculating effect sizes were not reported, but data related to standard error (SE) was available, standard deviations were calculated using the formula SD = SE√n. Effect sizes were interpreted as follows; d ≤ 0.2 = trivial; d > 0.2 = small; s > 0.5 = moderate; d > 0.8 = large and d > 1.3 = very large [Citation21].
Analysis and coding of outcome measures
Outcome measures identified in the review were coded according to the ICF by first identifying the appropriate domain and chapter and then the appropriate category within each chapter [Citation22]. Within the activity and participation domain, chapters d1–d4 were considered to represent the activity domain, defined as actions and tasks executed by the individual, while chapters d5–d9 represented participation, defined as involvement in a life situation [Citation23]. Specific items that were related to personal factors were listed separately as these are not currently classified within the ICF.
Some outcome measures include multiple items representing different codes within the ICF. For example, the Functional Independence Measure (FIM) includes one item related to eating which is represented in the ICF under the Activities and Participation (Self-Care, code d550) and also includes an item related to bladder control which is classified under body functions, (functions of the digestive, metabolic and endocrine systems, b610). To ensure accurate coding of outcome measures, the individual components of each measure were carefully considered, and when appropriate, outcome measures were assigned multiple codes.
To explore the frequency of use of outcome measures, as well as how common they were used in combination, a network analysis was performed. This analysis included only outcome measures that were identified in two or more studies. Nodes in the network were created to represent the number of studies that included each specific outcome measure, with the size of the node reflecting the number of times each specific outcome measure was represented. Links between the nodes were used to indicate when two or more outcome measures were applied in the same study. The thickness of the link between nodes was used to represent the number of studies in which outcomes measures appeared together in the same study.
Results
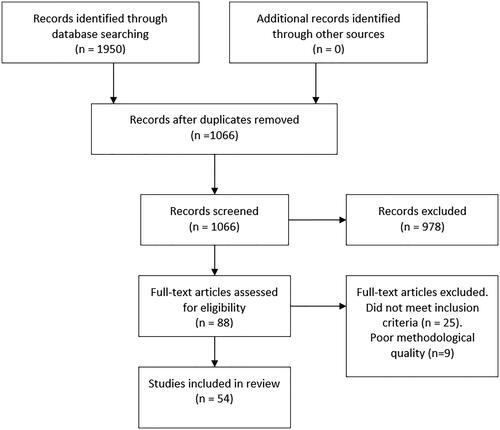
presents a PRISMA diagram depicting the flow of information through different phases of the review. The initial database search identified 1950 articles. After the removal of duplicates, the titles and abstracts of 1066 articles were screened independently by both authors. Agreement to include or exclude articles based on title and abstract was 94%. The remaining articles were discussed between the two authors until an agreement was reached. 88 articles were included for full-text review. A further 25 articles were removed after full-text review. Reasons for excluding articles included inappropriate outcome measures, a sample with a mix of pathological conditions in which the data for subjects who had a stroke could not be separated, and studies that did not focus on outcomes related to orthotic use.
Risk of bias
Nine articles did not meet the criteria for inclusion on the basis of methodological quality and were subsequently removed. Methodological quality for the remaining articles is presented in Supplementary Tables II and III. The most poorly addressed categories were “adequate description of the orthotic intervention (case series studies)” and “blinding outcomes assessors to the treatment (RCTs).”
A number of studies did not report accommodation times with the orthosis or the accommodation time was unclear. However, given that there is currently no consensus regarding appropriate accommodation times we did not include this as a point of consideration when assessing methodological quality.
Article summary
The final selection of 54 articles in the review included 39 case-series studies and 15 randomised controlled trials. summarises the general characteristics of the studies. The sample size ranged from 3 to 495 with a mean of 47.6 (SD = 87.8). The mean age of participants represented in the studies was 57.7 years (SD = 9.6; the range of means reported in studies = 42.5–71). Studies included both male and female participants with the average number of males being 49.5 and females 26.4. One study did not report the sex of participants [Citation24]. Post-stroke duration ranged from 0.3–1043 months (mean = 52.6; SD = 143.1).
Table 2. Summary of articles included in the systematic review.
The studies can be divided into those comparing outcomes related to (1) the use of an orthotic device versus no device (30 studies) (2) evaluating the effects of a device over time (21 studies), (3) comparing two different devices within-subjects (9 studies) or between groups (12 studies), and (4) comparison of early versus delayed orthotic provision (1 study). A number of studies included two or three of these aims.
Outcome measures and effect size
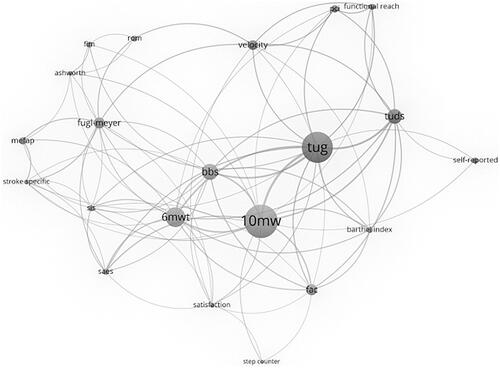
48 different clinical outcome measures were identified in the articles included in the review. For a detailed description of each outcome measure and how to apply them, we recommend the Physiopedia web site [Citation25] and the Shirly Ryan Ability Lab Rehabilitation Measures Database [Citation26]. Results of the network analysis, performed to explore the relationship between specific outcome measures, are presented as . The 10-meter Walk Test (10MWT) (used in 25 studies) the Timed Up and Go Test (TUG) (used in 19 studies) and the 6-meter Walk Test (6MWT) (used in13 studies) were applied most often while the 10MWT and the TUG were the two most frequently paired outcome measures.
Figure 2. Network analysis. Circles (nodes) represent each outcome measure, the larger the circle the more publications that used this specific measure. Lines represent outcomes measures which appear together in the same article, the thicker the line the more times the measures are used together. The more central the nodes are placed, the more coupling they have with other nodes. fim: Functional Independence Measure; rom: range of motion; mefap: Modified Emory Functional Ambulation Profile; stroke specific: stroke specific quality of life; sis: Stroke Impact Scale; saes: serious adverse events; 6mwt: 6-minute Walk Test; 10mwt: 10-minute Walk Test; bbs: Berg Balance Scale; tug: timed up and go; pci: Physiological Cost Index; Tuds: timed up and down stairs; self-reported: self-report satisfaction; fac: functional ambulation categories).
The vast majority of studies investigated ankle-foot orthoses (AFOs) of varying designs (36 studies) or Functional Electrical Stimulation (FES)(8 studies). Eight studies compared an AFO to FES, one study investigated the effects of knee orthosis provision [Citation27] and three studies included evaluations of knee-ankle-foot-orthoses (KAFOs) [Citation28–30]. summarises outcome measures, indicates which measures detected a statistically significant difference between independent variables, and presents the effect size of intervention outcomes that were calculated from data presented in the study. Detailed results of all studies, including effect sizes, are presented in Supplementary Table IV.
Table 3. Summary of statistical analysis and effect size for each outcome measure.
Effect sizes were able to be calculated for 39 studies. Effect sizes were generally trivial (d < 0.2) or small (d = 0.2–0.49) for most outcome measures. Outcome measures in which two or more studies recorded large (d = 0.8–1.29) or very large (d > 1.3) effect sizes were the 10MWT, functional reach test, physiological cost index (PCI) and the Functional Independence Measure (FIM). When comparing an orthosis condition to a no orthosis condition, 6 studies reported large or very large effects on at least one outcome measure. 10 studies reported large or very large effect sizes when investigating the effects of orthoses over time, 2 studies reported large or very large effect sizes when comparing two different orthoses within-subjects, and 3 when comparing two different orthoses between subjects. Only one study investigated the effects of early versus delayed orthotic provision [Citation31] and data was not available to calculate effect sizes for this study.
presents all outcome measures coded in accordance with the ICF. The vast majority of outcome measures included items/components representing the activity domain and specifically chapter d4, which is related to mobility. 26 outcome measures included items coded under d450 (walking), 16 outcome measures were coded under d455 (moving around) and 12 coded as d410 (changing basic body position). ICF chapters related to body functions generally addressed muscle tone (b735 = 5 outcome measures) or exercise tolerance (b455 4 outcome measures). Few outcome measures addressed issues related to participation (chapters d5–d9) but of these, most were coded under self-care (d5). Only 1 to 2 outcome measures were coded under domestic life (d6), interpersonal interactions and relations (d7), major life areas (d8), and community, social and civic life (d9).
Table 4. Coding of outcome measures according to ICF.
Discussion
Appropriate use of outcome measures in stroke rehabilitation is central to good clinical practice. The use of standardized outcome measures is recognized as a means of monitoring patient status, assessing the effectiveness of an intervention, and contributing to the quality of care provided to patients [Citation32]. With so many outcome measures available, it can be difficult to determine the most appropriate measure to apply in the clinical setting. This paper provides clinicians with an overview of outcome measures that have been used in original research studies and summarises effect sizes to provide an indication of the meaningfulness of results related to each measure. We have also categorised all outcome measures according to the ICF to provide clinicians with a clearer indication of the health-related concepts contained within each instrument.
In examining the aggregated data, the preponderance of outcome measures identified in this review was related to measuring aspects of mobility, coded within the activity domain of the ICF. This finding is consistent with previous reviews related to outcome measures in stroke rehabilitation, which have also identified measures within the activity domain as being most prevalent [Citation11,Citation33]. Previous studies, however, only classified measures in relation to major ICF categories and did not consider second-level classifications which provide considerably more detail.
A large number of outcome measures within the activity domain is not a surprising result given the expectation that the use of a lower-limb orthosis will compensate for impaired gait performance following a stroke [Citation4]. It should be noted however that the limited number of studies using outcome measures representing other ICF domains, and in particular the participation domain, may reflect a rather narrow perspective of the effects that orthoses may have on health and functioning. It may also reflect difficulties in attributing participation-related outcomes specifically to an orthotic device given that many variables other than the orthosis itself may influence results [Citation32].
In the only other review of outcome measures assessing lower-limb orthotic management of stroke Figueiredo et al. [Citation33] reported the most commonly used outcome measures as being; temporospatial measures (e.g., gait speed, step length, and cadence), kinematics (e.g., range of motion) and functional measures (e.g., TUG, BBS, and 10MWT). Given that the present study excluded laboratory-based measures that were not considered to be readily accessible in the clinical environment, results were slightly different to those of Figueiredo et al. however, similarities were observed in relation to functional measures with the most frequently used measures being the 10MWT, TUG, and 6MWT. All three of these outcome measures investigate walking in a controlled environment, defined in the ICF as moving along a surface on foot for short or long distances and including walking on different surfaces and around obstacles (d450) [Citation23]. The outcome measures coded under d450 can be contrasted to mobility measures, coded in the ICF as d455 (moving around), and addressing walking and moving around in various places and situations. While measures related to moving around were certainly represented in studies included in this review, their frequency of use was much lower, suggesting that results of orthotic management are most often evaluated in controlled environments rather than naturalistic settings. Additional outcome measures coded under mobility addressed the ability to change and maintain body position (d410–d429). This included sitting to stand maneuvers as well as reaching tasks.
The outcome measure with the most promising results in terms of effect size was the 10MWT with 6 of 18 studies in which effect size data was available recording large or very large effects. None of the 7 studies with available effect size data using the 6MWT achieved results that were classified as large or very large, while only one of the 16 studies with effect size data using the TUG test recorded a large or very large effect size. Based upon this result, and recognizing the limited data available, we would recommend the use of the 10MWT to evaluate the relative effects of orthotic management on outcomes related to walking. An additional measure of mobility that warrants further investigation in future studies is the functional reach test. Two of three studies with effect size data recorded large or very large effect sizes associated with this measure.
While outcome measures within the activity domain are well represented in this review, we recommend that future research should evaluate outcome measures related to self-care and other aspects of participation that may be affected by the use of an AFO. In this study, the patient-reported Stroke Impact Scale (SIS) was the most commonly applied outcome measure classified within the participation domain of the ICF. Unfortunately, effect size could only be calculated for two of five studies utilizing the SIS. One of these recorded a very large effect size.
In addition to prioritizing factors related to activity and participation, the ICF core sets for stroke indicate that environmental factors are highly relevant to investigate [Citation34]. The core sets for stroke specifically include environmental issues related to support and relationships from immediate family (e310), health professionals (e355) and Health services, systems, and policies (e580). It is interesting to note that these issues were not addressed in any of the outcome measures included in this review.
Given the focus of this review on measures with high clinical utility, the sole measure of metabolic functions (b540) was the PCI. While trials have been somewhat limited, 2 studies in this review recorded large [Citation35] or very large [Citation36] effect sizes warranting further investigations of this measure in future research studies.
Most studies with large or very large effect sizes compared either an orthosis condition to a no orthosis condition or investigated the relative effects of an orthosis over time. Very few studies in which two orthosis conditions were compared recorded large or very large effect sizes associated with specific outcome measures. This is likely because relative differences between the conditions are smaller. Results are consistent with those reported in a systematic review by Ferreira et al. [Citation37]. These authors noted significant improvements in gait velocity in studies comparing the use of an AFO to no AFO but not when comparing rigid versus articulated AFOs.
Study limitations
This review should not be considered as an exhaustive list including all the outcome measures that could be used to assess health and health-related domains in individuals prescribed with orthotic devices post-stroke. The authors limited their search to English language publications and chose not to include gray literature. It does however provide an indication of measures that would be of interest to investigate further and highlights ICF domains in which application of outcomes measures for evaluation of orthotic interventions are lacking.
A number of studies did not report sufficient information to calculate effect size which is considered important when interpreting results of orthotic interventions related to each outcome measure. While hypothesis testing with associated p-values was presented in most studies, the p-value only indicates the likelihood that the results differ from chance and is dependent upon the sample size. Effect sizes provide an indication of how meaningful the result is in terms of the magnitude of the difference in mean scores or the strength of the relationship [Citation38].
It should be recognized that classifying measures according to the ICF is not a straightforward process and there is currently no consensus on how this should be done appropriately. We have done our best to review the specific items within each outcome measure and to classify them according to the appropriate chapter and code within the ICF. While the reliability of our coding has not been established, we believe that the classification of outcome measures can still be useful in guiding clinicians in the selection of outcome measures representing different domains within the ICF.
Conclusion
The need to choose appropriate outcome measures in clinical practice has become increasingly important and there is currently no consensus on specific outcome measures that should be used in clinical practice to evaluate the effects of orthotic management post-stroke. Our review presents a comprehensive summary of outcome measures that have been used in original research studies, reports effect sizes associated with outcomes reported in each study, and classifies each outcome measure according to the ICF. Results suggest that outcome measures related to mobility have been prioritized by studies included in this review and that effect sizes were most promising when using the 10-meter Walk Test to evaluate orthotic management post-stroke. Other outcome measures in which large effect sizes were reported were the functional reach test and physiological cost index.
Supplementry Material
Download MS Word (256.7 KB)Acknowledgements
The authors wish to thank Stefan Carlstein, Librarian at Jönköping University for generating the network analysis that was presented in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- GBD. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:459–480.
- Lindsay MP, Norrving B, Sacco RL, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2019. Int J Stroke. 2019;14(8):806–817.
- Winstein CJ, Stein J, Arena R, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47(6):e98–e169.
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
- The Department of Vetran Affairs and The Department of Defense. VA/DoD Clinical practice guidelines for the management of stroke rehabilitation. Version 4. Washington (DC): VA/DoD; 2019.
- Scottish Intercollegiate Guidelines Network. Management of patients with stroke: Rehabilitation, prevention and management of complications, and discharge planning. Edinburgh (UK): Scottish Intercollegiate Guidelines Network; 2010.
- Bowen A, James M, Young G, editors. National clinical guidelines for stroke. London (UK): Royal College of Physicians; 2016.
- Coster WJ. Making the best match: selecting outcome measures for clinical trials and outcome studies. Am J Occup Ther. 2013;67(2):162–170.
- Zhang T, Liu L, Xie R, et al. Value of using the international classification of functioning, disability, and health for stroke rehabilitation assessment: a multicenter clinical study. Medicine. 2018;97(42):e12802.
- World Health Organization. Towards a common language for functioning, disability and health. Geneva (Switzerland): ICF; 2002.
- Ashford S, Brown S, Turner-Stokes L. Systematic review of patient-reported outcome measures for functional performance in the lower limb. J Rehabil Med. 2015;47(1):9–17.
- Galeoto G, Iori F, De Santis R, et al. The outcome measures for loss of functionality in the activities of daily living of adults after stroke: a systematic review. Top Stroke Rehabil. 2019;26(3):236–245.
- Engelmann KA, Jordan LC. Outcome measures used in pediatric stroke studies: a systematic review. Arch Neurol. 2012;69(1):23–27.
- Figueiredo J, Moreno JC, Matias AC, et al. Outcome measures and motion capture systems for assessing lower limb orthosis-based interventions after stroke: a systematic review. Disabil Rehab Assist Technol. 2019. DOI:10.1080/17483107.2019.1695966
- Moher D, Altman D, Liberati A, et al. PRISMA statement. Epidemiology. 2011;22:128.
- Bramer WM, de Jonge GB, Rethlefsen ML, et al. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. 2018;106(4):531–541.
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
- The Joanna Briggs Institute. The Joanna Briggs Institute critical appraisal tools [Internet]. Adelaide (Australia): The Joanna Briggs Institute; 2018. Available from: http://joannabriggs.org/research/critical-appraisal-tools.html
- Lintanf M, Bourseul J-S, Houx L, et al. Effect of ankle-foot orthoses on gait, balance and gross motor function in children with cerebral palsy: a systematic review and meta-analysis. Clin Rehabil. 2018;32(9):1175–1188.
- Lenhard W, Lenhard A. Calculation of effect sizes [Internet]. Dettelbach (Germany): Psychometrica; 2016. Available from: https://www.psychometrica.de/effect_size.html
- Crutzen R. Adding effect sizes to a systematic review on interventions for promoting physical activity among European teenagers. Int J Behav Nutr Phys Activity. 2010;7:1–5.
- World Health Organization. How to use the ICF: a practical manual for using the International Classification of Functioning, Disability and Health (ICF). Geneva (Switzerland): WHO; 2013.
- World Health Organization. International classification of functioning, disability and health. Geneva (Switzerland): World Health Organization; 2001.
- Taylor P, Burridge J, Dunkerley A, et al. Clinical audit of 5 years provision of the Odstock dropped foot stimulator. Artif Organs. 1999;23(5):440–442.
- Physiopedia. Physiopedia outcome measures [Internet]. London (UK): Physiopedia; 2020 [28th October 2020]; Available from: https://www.physio-pedia.com/Category:Outcome_Measures
- Shirly Ryan Ability Lab. Rehabilitation Measures Database. Chicago (IL): Shirly Ryan Ability Lab; 2020.
- Portnoy S, Frechtel A, Raveh E, et al. Prevention of genu recurvatum in poststroke patients using a hinged soft knee orthosis. PM R. 2015;7(10):1042–1051.
- Ota T, Hashidate H, Shimizu N, et al. Differences in activities of daily living between people with subacute stroke who received knee-ankle-foot and ankle-foot orthoses at admission. J Phys Ther Sci. 2018a;30(10):1245–1250.
- Ota T, Hashidate H, Shimizu N, et al. Difference in independent mobility improvement from admission to discharge between subacute stroke patients using knee-ankle-foot and those using ankle-foot orthoses. J Phys Ther Sci. 2018b;30(8):1003–1008.
- Ota T, Hashidate H, Shimizu N, et al. Early effects of a knee-ankle-foot orthosis on static standing balance in people with subacute stroke. J Phys Ther Sci. 2019;31(2):127–131.
- Nikamp CDM, Buurke JH, van der Palen J, et al. Six-month effects of early or delayed provision of an ankle-foot orthosis in patients with (sub)acute stroke: a randomized controlled trial. Clin Rehabil. 2017;31(12):1616–1624.
- Salter K, Jutai JW, Teasell R, et al. Issues for selection of outcome measures in stroke rehabilitation: ICF participation. Disabil Rehabil. 2005;27(9):507–528.
- Figueiredo J, Moreno JC, Matias AC, et al. Outcome measures and motion capture systems for assessing lower limb orthosis-based interventions after stroke: a systematic review. Disabil Rehabil Assist Technol. 2019;9:1–10.
- Geyh S, Cieza A, Schouten J, et al. ICF core sets for stroke. J Rehabil Med. 2004;36(0):135–141.
- Erel S, Uygur F, Şimşek IE, et al. The effects of dynamic ankle-foot orthoses in chronic stroke patients at three-month follow-up: a randomized controlled trial. Clin Rehabil. 2011;25(6):515–523.
- Suat E, Fatma U, Nilgun B. The effects of dynamic ankle-foot orthoses on functional ambulation activities, weight bearing and spatio-temporal characteristics of hemiparetic gait. Disabil Rehabil. 2011;33(25–26):2605–2611.
- Ferreira LAB, Neto HP, Grecco LAC, et al. Effect of ankle-foot orthosis on gait velocity and cadence of stroke patients: a systematic review. J Phys Ther Sci. 2013;25(11):1503–1508.
- Tomczak M, Tomczak E. The need to report effect size estimates revisited. An overview of some recommended measures of effect. Trends Sport Sci. 2014;1:19–25.
- Barrett C, Taylor P. The effects of the odstock drop foot stimulator on perceived quality of life for people with stroke and multiple sclerosis. Neuromodulation. 2010;13(1):58–64.
- Beckerman H, Becher J, Lankhorst GJ, et al. Walking ability of stroke patients: efficacy of tibial nerve blocking and a polypropylene ankle-foot orthosis. Arch Phys Med Rehabil. 1996;77(11):1144–1151.
- Beckerman H, Becher J, Lankhorst GJ, et al. The efficacy of thermocoagulation of the tibial nerve and a polypropylene ankle-foot orthosis on spasticity of the leg in stroke patients: results of a randomized clinical trial. Clin Rehabil. 1996;10(2):112–120.
- Bethoux F, Rogers H, Nolan K, et al. The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabil Neural Repair. 2014;28(7):688–697.
- Bethoux F, Rogers H, Nolan K, et al. Long-term follow-up to a randomized controlled trial comparing peroneal nerve functional electrical stimulation to an ankle foot orthosis for patients with chronic stroke. Neurorehabil Neural Repair. 2015;29(10):911–922.
- Bouchalova V, Houben ELS, Tancsik D, et al. The influence of an ankle-foot orthosis on the spatiotemporal gait parameters and functional balance in chronic stroke patients. J Phys Ther Sci. 2016;28(5):1621–1628.
- Cakar E, Durmus O, Tekin L, et al. The ankle-foot orthosis improves balance and reduces fall risk of chronic spastic hemiparetic patients. Eur J Phys Rehabil Med. 2010;46:363–368.
- Chen CL, Teng YL, Lou SZ, et al. Effects of an anterior ankle-foot orthosis on walking mobility in stroke patients: get up and go and stair walking. Arch Phys Med Rehabil. 2014;95(11):2167–2171.
- de Sèze MP, Bonhomme C, Daviet JC, et al. Effect of early compensation of distal motor deficiency by the Chignon ankle-foot orthosis on gait in hemiplegic patients: a randomized pilot study. Clin Rehabil. 2011;25(11):989–998.
- de Wit D, Buurke J, Nijlant J, et al. The effect of an ankle-foot orthosis on walking ability in chronic stroke patients: a randomized controlled trial. Clin Rehabil. 2004;18(5):550–557.
- DeMeyer L, Brown M, Adams A. Effectiveness of a night positioning programme on ankle range of motion in patients after hemiparesis: a prospective randomized controlled pilot study. J Rehabil Med. 2015;47(9):873–877.
- Ding XD, Zhang GB, Chen HX, et al. Color Doppler ultrasound-guided botulinum toxin type a injection combined with an ankle foot brace for treating lower limb spasticity after a stroke. Eur Rev Med Pharmacol Sci. 2015;19:406–411.
- Do KH, Song JC, Kim JH, et al. Effect of a hybrid ankle foot orthosis made of polypropylene and fabric in chronic hemiparetic stroke patients. Am J Phys Med Rehabil. 2014;93:130–137.
- Doğan A, Mengüllüoğlu M, Özgirgin N. Evaluation of the effect of ankle-foot orthosis use on balance and mobility in hemiparetic stroke patients. Disabil Rehabil. 2011;33(15–16):1433–1439.
- Embrey DG, Holtz SL, Alon G, et al. Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Arch Phys Med Rehabil. 2010;91(5):687–696.
- Everaert D, Stein R, Abrams G, et al. Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: a multicenter randomized controlled trial. Neurorehabil Neural Repair. 2013;27(7):579–591.
- Farmani FM-B, Pei MA, et al. The effect of rocker bar ankle foot orthosis on functional mobility in post-stroke hemiplegic patients. Iranian Rehabil J. 2015;13(3):109–112.
- Farmani F, Mohseni Bandpei MA, Bahramizadeh M, et al. The effect of different shoes on functional mobility and energy expenditure in post-stroke hemiplegic patients using ankle-foot orthosis. Prosthet Orthot Int. 2016;40(5):591–597.
- Granat MH, Maxwell DJ, Ferguson ACB, et al. Peroneal stimulator: evaluation for the correction of spastic drop foot in hemiplegia. Arch Phys Med Rehabil. 1996;77(1):19–24.
- Grissom SP, Blanton S. Treatment of upper motoneuron plantarflexion contractures by using an adjustable ankle-foot orthosis. Arch Phys Med Rehabil. 2001;82(2):270–273.
- Hale J, Seale J, Jennings J, et al. An advanced ground reaction design ankle-foot orthosis to improve gait and balance in individuals with post-stroke hemiparesis: a case series. J Prosthet Orthot. 2013;25(1):42–47.
- Hung JW, Chen PC, Yu MY, et al. Long-term effect of an anterior ankle-foot orthosis on functional walking ability of chronic stroke patients. Am J Phys Med Rehabil. 2011;90:8–16.
- Hyun CW, Kim BR, Han EY, et al. Use of an ankle-foot orthosis improves aerobic capacity in subacute hemiparetic stroke patients. PM R. 2015;7(3):264–269.
- Iwata M, Kondo I, Sato Y, et al. An ankle-foot orthosis with inhibitor bar: effect on hemiplegic gait. Arch Phys Med Rehabil. 2003;84(6):924–927.
- Kazutoshi T, Shuji M, Keiko I, et al. Short-term effects of physiotherapy combining repetitive facilitation exercises and orthotic treatment in chronic post-stroke patients. J Phys Ther Sci. 2017;29:212–215.
- Kluding PM, Dunning K, O’Dell MW, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke. 2013;44(6):1660–1669.
- Laufer Y, Hausdorff JM, Ring H. Effects of a foot drop neuroprosthesis on functional abilities, social participation, and gait velocity. Am J Phys Med Rehabil. 2009;88(1):14–20.
- Maeda N, Kato J, Azuma Y, et al. Energy expenditure and walking ability in stroke patients: their improvement by ankle-foot orthoses. IES. 2009;17(2):57–62.
- McCain KJ, Smith PS, Querry R. Ankle-foot orthosis selection to facilitate gait recovery in adults after stroke: a case series. J Prosthet Orthot. 2012;24(3):111–123.
- Nolan KJ, Savalia KK, Lequerica AH, et al. Objective assessment of functional ambulation in adults with hemiplegia using ankle foot orthotics after stroke. PM R. 2009;1(6):524–529.
- O’Dell MW, Dunning K, Kluding P, et al. Response and prediction of improvement in gait speed from functional electrical stimulation in persons with poststroke drop foot. PM R. 2014;6(7):587–601.
- Pardo V, Galen S, Gahimer JE, et al. Effects of custom-molded and prefabricated hinged ankle-foot orthoses on gait parameters and functional mobility in adults with hemiplegia: a preliminary report. J Prosthet Orthot. 2015;27(1):33–38.
- Park JH, Chun MH, Ahn JS, et al. Comparison of gait analysis between anterior and posterior ankle foot orthosis in hemiplegic patients. Am J Phys Med Rehabil. 2009;88(8):630–634.
- Pavlik AJ. The effect of long-term ankle-foot orthosis use on gait in the poststroke population. J Prosthet Orthot. 2008;20:49–52.
- Prenton S, Kenney LP, Stapleton C, et al. Feasibility study of a take-home array-based functional electrical stimulation system with automated setup for current functional electrical stimulation users with foot-drop. Arch Phys Med Rehabil. 2014;95(10):1870–1877.
- Rao N, Aruin AS. Role of ankle foot orthoses in functional stability of individuals with stroke. Disabil Rehabil Assist Tech. 2016;11(7):595–598.
- Salisbury L, Shiels J, Todd I, et al. A feasibility study to investigate the clinical application of functional electrical stimulation (FES), for dropped foot, during the sub-acute phase of stroke - A randomized controlled trial. Physiother Theory Pract. 2013;29(1):31–40.
- Sheffler L, Hennessey M, Naples G, et al. Peroneal nerve stimulation versus an ankle foot orthosis for correction of footdrop in stroke: impact on functional ambulation. Neurorehabil Neural Repair. 2006;20(3):355–360.
- Sheffler LR, Taylor PN, Gunzler DD, et al. Randomized controlled trial of surface peroneal nerve stimulation for motor relearning in lower limb hemiparesis. Arch Phys Med Rehabil. 2013;94(6):1007–1014.
- Shin Y, Lee D, Kim M. The effect of newly designed multi joint ankle foot orthosis on the gait and dynamic balance of stroke patients with foot drop. J Phys Ther Sci. 2017;29(11):1899–1902.
- Simons C, Asseldonk E, Kooij H, et al. Ankle-foot orthoses in stroke: effects on functional balance, weight-bearing asymmetry and the contribution of each lower limb to balance control. Clin Biomech. 2009;24(9):769–775.
- Street T, Swain I, Taylor P. Training and orthotic effects related to functional electrical stimulation of the peroneal nerve in stroke. J Rehabil Med. 2017;49(2):113–119.
- Tyson SF, Thornton HA. The effect of a hinged ankle foot orthosis on hemiplegic gait: objective measures and users’ opinions. Clin Rehabil. 2001;15(1):53–58.
- van Swigchem R, Vloothuis J, den Boer J, et al. Is transcutaneous peroneal stimulation beneficial to patients with chronic stroke using an ankle-foot orthosis? A within-subjects study of patients’ satisfaction, walking speed and physical activity level. J Rehabil Med. 2010;42(2):117–121.
- Wang R, Yen L, Lee C, et al. Effects of an ankle-foot orthosis on balance performance in patients with hemiparesis of different durations. Clin Rehabil. 2005;19(1):37–44.
- Zissimopoulos A, Fatone S, Gard S. The effect of ankle-foot orthoses on self-reported balance confidence in persons with chronic poststroke hemiplegia. Prosthet Orthot Int. 2014;38(2):148–154.
- Lipsey M, Wilson D. Practical meta-analysis. Thousand Oaks (CA): Sage publications; 2001.