Abstract
Purpose
This study aimed to examine interrater reliability and construct validity of the Montgomery-Asberg Depression Rating Scale (MADRS) semi-structured interview for assessing depression in adults with a primary brain tumour.
Materials and methods
Fifty adults with a primary brain tumour (mean age = 45.86, SD = 12.48) reporting at least mild distress (Distress Thermometer [DT] ≥ 4) were recruited from a multidisciplinary brain tumour clinic and administered a telephone-based cognitive screener, MADRS, Depression Anxiety Stress Scales (DASS) depression subscale and Generalised Anxiety Disorder-7 (GAD-7). Audiotaped interviews were transcribed and then scored by two independent raters.
Results
Interrater reliability for the MADRS total score was excellent (ICC = 0.98) and ranged from good to excellent (ICC = 0.83–0.96) for MADRS items. The MADRS total score was significantly associated with the DT, DASS depression, and GAD-7 (r = 0.50–0.76, p < 0.001), thus providing evidence of construct validity. Individuals with poorer cognitive function reported higher levels of depression.
Conclusions
The findings provide psychometric support for the MADRS as a semi-structured interview for assessing depression after brain tumour. Further research investigating the sensitivity and specificity of the MADRS is recommended.
Implications for Rehabilitation
The Montgomery Asberg Depression Rating Scale can be used to reliably assess depression in individuals with primary brain tumour.
Individuals with poorer cognitive function may be at greater risk of developing depression after brain tumour.
Semi-structured interviews such as the Montgomery Asberg Depression Rating Scale may support clinicians to distinguish depressive symptoms from effects of the illness, thus helping to identify individuals who most warrant psychological support.
Introduction
Primary brain tumour is a heterogenous illness associated with diverse functional impairments [Citation1,Citation2]. People with high-grade gliomas have an average five-year survival rate of only 36% [Citation2]. Research indicates that over half (52–62%) of people with lower-grade gliomas experience tumour recurrence within five years and most of these tumours (80%) progress to malignant or high grade within 10 years [Citation3,Citation4]. Benign brain tumours are the most common type of primary brain tumour, and although not typically life-threatening, can be associated with significant functional impairments that negatively impact quality of survivorship [Citation5].
People with a primary brain tumour can experience multiple sensory, motor, cognitive, and behavioural impairments, which affect their independence and social participation [Citation6–9]. Many individuals develop depression, which may arise from neurobiological mechanisms (e.g., disruption to fronto-limbic regions underlying emotion regulation), existential distress associated with the threat to life and functional losses, or a combination of these factors [Citation9,Citation10]. More severe depression is found to be associated with greater functional impairment, poorer quality of life, elevated risk of suicide, and poorer survival [Citation11,Citation12].
Early detection and management of depression and psychological distress more generally is a key focus of supportive care [Citation13]. However, assessment is complicated by the overlap of mood symptoms with physical, somatic, and neurocognitive symptoms resulting directly from the brain tumour or treatment [Citation14,Citation15]. For example, fatigue, sleep disturbance, concentration, and memory difficulties can be symptoms associated with depression, brain tumour, and treatment [Citation14,Citation15]. Assessment approaches that help to distinguish depressive symptoms from neurocognitive and physical effects of the illness may have greater utility in clinical practice to determine the need for psychological support.
In a survey of psycho-oncology professionals [Citation13], the most common measures used to screen for distress in people with brain tumour included the Distress Thermometer (DT)[Citation16], Hospital Anxiety and Depression Scales (HADS) [Citation17], Depression Anxiety Stress Scales (DASS) [Citation18], Patient Health Questionnaire-9 (PHQ-9) [Citation19], and Generalised Anxiety Disorder-7 Scale (GAD-7) [Citation20]. Although adequate psychometric properties have been demonstrated for these self-report measures in brain tumour research [Citation14,Citation21], due to the constrained items and response options, there is limited scope to clarify the onset, context and nature of mood symptoms.
Clinical interviews are commonly viewed as the “gold standard” for comprehensively assessing mood symptoms and diagnosing mood disorders [Citation22]. In contrast to structured interviews such as the Structured Clinical Interview for DSM-5-Clinician Version (SCID-5-CV) [Citation23], semi-structured interviews provide scope for clarification questions [Citation24]. Despite the potential for greater variability in clinician judgement for scoring, rates of diagnosis and classification of depression have been found to be comparable between semi-structured and structured interviews [Citation24]. Semi-structured interviews may have utility for assessing depression in individuals with cognitive-communication impairments due to the opportunity to reframe questions and use prompts to clarify the onset, frequency, timing, and intensity of mood symptoms.
A semi-structured clinical interview commonly used to assess depression is the Montgomery-Asberg Depression Rating Scale (MADRS) [Citation25]. Originally developed as a questionnaire, the MADRS assesses 10 core symptoms (e.g., sadness, pessimistic thoughts, suicidal ideation). Several studies support the utility of the MADRS for assessing depression in clinical samples, including cancer [Citation26] and neurological disorders [Citation27]. High concordance has been reported between face-to-face and telephone, and videoconference and telephone-based administration [Citation28]. In a stroke sample, Laska et al. [Citation29] reported a very high agreement (95%) between raters on the MADRS in terms of assigning the absence or presence of depression based on 22 videotaped administrations. In another stroke sample (n = 150), Sagan et al. [Citation27] reported good internal consistency (α = 0.85), interrater reliability (intra-class correlation coefficient [ICC] = 0.83), convergent validity with the HADS (r = 0.70) and sensitivity of >0.70 and specificity of >0.87 for the MADRS when compared to the Structured Clinical Interview for DSM-IV (SCID).
The MADRS also has promising clinical applications for individuals with brain tumours to determine the onset, context, and nature of mood symptoms. Ownsworth et al. [Citation30] used the MADRS to evaluate the efficacy of a 10-week psychotherapy program relative to a waitlist condition (n = 50). They reported adequate internal consistency (α =0.71) and test-retest reliability (r = 0.85) based on waitlist control data [Citation31]. Participants receiving psychotherapy reported significantly lower depression than waitlist controls, thus supporting the responsiveness of the MADRS. However, interrater reliability was not examined, which is an important consideration for tools reliant on clinical judgement [Citation27,Citation29]. Due to the negative impacts of depression on functional outcomes and survival [Citation11, Citation12, Citation15], it is a priority to evaluate the psychometric properties of assessment approaches that support clinicians in comprehensively assessing depression in the context of tumour and treatment-related effects.
The two overarching psychometric properties of measurement tools are reliability and validity. Reliability refers to the consistency of the measuring method [Citation32]. The current study focuses on interrater reliability or the extent of agreement in scoring judgements between independent raters using the same instrument. Validity refers to how well the measure assesses the construct it is supposed to measure and consists of three main types: content validity (how well items assess the theoretical construct), criterion validity (how well the measure relates to or predicts relevant outcomes or a “gold standard”) and construct validity [Citation32]. Construct validity [Citation33] refers to how well a measure reflects a given construct and is determined through hypothesis testing, which may relate to the relationship of the measure to scores on other instruments assessing the same construct (i.e., convergent validity), or differences between relevant groups [Citation33]. Within this study, we examined construct validity by assessing the extent to which scores on the MADRS correlate with other measures assessing depression and psychological distress [Citation33,Citation34].
Aims and hypotheses
This main study aims were to examine interrater reliability and construct validity of the MADRS. In relation to these aims, the following hypotheses were developed:
H1) Interrater reliability of the MADRS would be at least “good” (ICC ≥0.75) based on two independent raters.
H2) Scores on the MADRS would be significantly and positively correlated (large effect: r ≥ 0.50) with a self-report measure of depression (DASS-21).
H3) Scores on the MADRS would be significantly and positively correlated (with a moderate to large effect size: r = ≥0.40) with other self-report measures of psychological distress (DT & GAD-7).
A further aim was to identify demographic and illness-related characteristics related to depression on the MADRS.
Method
Participants
A convenience sample of individuals with primary brain tumour were referred by treating professionals from a multi-disciplinary brain tumour clinic at a large metropolitan-based hospital during 2018–2019 as part of a telehealth psychological intervention study [Citation31,Citation35]. Individuals aged 18–75 years reporting at least mild distress (DT ≥4/10) with adequate cognitive and English language skills were eligible. Participants who performed in the very impaired range (Z score < −3) on a validated telephone-based measure of cognitive status and/or those with severe receptive and/or expressive aphasia as documented by treating professionals were excluded. Sample size was guided by the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) [Citation36] which recommends a minimum of 50 participants for reliability analyses.
Measures
Demographic and health background information was obtained from participants. Tumour type, grade, and treatment were confirmed by referring treating professionals. Participants were administered the following measures by a psychologist via telephone.
Distress Thermometer (DT)
The DT is a screening tool with demonstrated sensitivity for detecting distress in people with cancer [Citation16]. Individuals rate their distress from 0–10 on a visual analogue scale, adapted for telephone administration. A cut-off score of 4 is considered optimal for detecting clinically significant distress [Citation16].
Brief test of adult cognition by telephone (BTACT)
The BTACT [Citation37] is a telephone-based measure of cognitive status. In the current study, the BTACT provided an index of global cognitive status based on five subtests measuring working memory, processing speed, episodic verbal memory (immediate and delayed) and executive function. Two subtests (Attention Switching/Reaction Time and Reasoning) were not administered, due to our prior research indicating that people with brain tumour experience difficulties completing these tasks over the telephone [Citation38]. A composite score was derived, by summing and averaging age-adjusted Z scores for each subtest.
Montgomery-Asberg depression rating scale (MADRS)
The MADRS [Citation39] is a semi-structured interview (25–30 min) that assesses the presence and severity of depressive symptoms over the past week. The 10 items include Reported Sadness, Apparent Sadness, Tension, Sleep, Appetite, Concentration, Lassitude, Inability to feel, Pessimistic thoughts , and Suicidal Thoughts. Example questions and prompts include: “In the past week, have you been feeling sad or unhappy?,” “Can you describe what that’s been like for you?,” “How long have you been feeling this way?” [Citation39] (p. 56). As outlined in the interview guidelines [Citation39] follow-up questions regarding the onset and context of symptoms (e.g., appetite changes during chemotherapy) were included to help distinguish depressive symptoms from brain tumour and treatment-related effects. The 10 items are rated from 0 (minimal or no symptoms) to 6 (maximum symptoms) to yield a total score of 0–60. Scores ≥12 signify clinical levels of depression [Citation39]. Internal consistency of the MADRS for the current sample was good (α = 0.80; inter-item correlations = 0.18–0.54).
Depression, anxiety and Stress Scales (DASS-21)
The DASS-21 [Citation18] is a self-report measure assessing symptoms of depression, anxiety, and stress over the past seven days. Items are rated on a 4-point Likert scale (0 = "did not apply to me at all" to 4 = "applied to me very much, or most of the time"), with higher scores indicating greater symptomology [Citation18]. Total scores are multiplied by two to enable clinical cut-offs to be applied based on the DASS-42 (depression scores ≥ 10 are clinically elevated). Only the depression scale from the DASS was used for the current study, which assesses affective and behavioural symptoms (e.g., "I felt that I had nothing to look forward to"). Internal consistency was excellent for this sample (α = 0.93).
Generalised anxiety disorder (GAD-7) scale
The GAD-7 [Citation20] is a 7-item self-report measure assessing symptoms of generalised anxiety disorder over the past 14 days. Items are rated on a 4-point Likert scale (0 = "not at all" to 3 = "nearly every day”), with higher scores indicating the greater frequency of anxiety symptoms (e.g., "I felt I was close to panic"). The scale is scored by summing the seven items, where scores of ≥ 5 are considered clinically elevated [Citation20]. The GAD-7 has been validated in primary care settings and the general population; and is commonly used to assess anxiety after brain tumour [Citation40]. Internal consistency of the GAD-7 was good for the current sample (α = 0.89).
Procedure
Ethical clearance was obtained from the Metro South Health (HREC/QMS/43843) and Griffith University (HREC 2018/808) Human Research Ethics Committees. After providing informed consent via an ethically approved verbal consent procedure, participants were administered the DT, BTACT, MADRS, GAD-7, and DASS-21 over the telephone by a psychologist. Audio recordings of the MADRS were transcribed verbatim and scored by the psychologist and an independent rater (a post-graduate clinical psychology trainee). Comprehensive training in administering and scoring the MADRS was provided by an experienced clinical neuropsychologist (TO). Training included roleplay exercises and scoring of eight previously recorded and transcribed interviews (not included in the data) with feedback and discussion on scoring discrepancies.
Data analysis and statistical methods
Statistical analyses were conducted using the IBM Statistical Package for the Social Sciences (SPSS), version 25. There were no missing data for any variables. Data were screened for errors of data entry and were found to meet assumptions for parametric analysis. Time since injury was positively skewed; however, the transformation of this variable did not alter the significance of the findings and therefore results are based on the non-transformed variable.
Interrater reliability of the MADRS
Interrater reliability was investigated using the ICC for the MADRS items and total score. In accordance with Hallgren [Citation41], an ICC two-way mixed model was used to examine the absolute agreement between raters, with average measures reported. The ICC value ranges from 0 to 1, with values below 0.50 indicating poor reliability; 0.50 to <0.75 indicating moderate reliability; 0.75 to <0.90 indicating good reliability; and ≥0.90 indicating excellent reliability [Citation41].
Construct validity (hypotheses testing)
Pearson’s correlation was used to test the second and third hypotheses. Pearson’s correlation coefficients range between −1 and 1 and indicate the direction (- negative; + positive) and magnitude of the relationship between two variables. Effect sizes for the correlation are interpreted as follows: r = 0.10–0.29 is small; r = 0.30–0.49 is moderate and r ≥ 0.50 is large [Citation42].
Associations between demographic and illness characteristics and depression
Due to the small subgroup sizes for demographic and illness-related variables, the non-parametric equivalent of independent samples t-tests (Mann-Whitney U test) or ANOVAs (Kruskal-Wallis One-Way Analysis of Variance by ranks test) were used. Pearson’s correlation was used to examine associations between the level of depression on the MADRS and continuous variables (e.g., age, time since diagnosis, global cognitive status).
Results
Sample characteristics
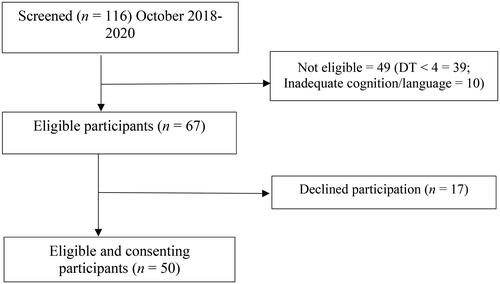
A total of 116 individuals with primary brain tumour were referred for potential participation over a two-year period between October 2018 and 2020. As shown in , the screening process indicated that 49 individuals were not eligible (DT < 4, inadequate cognition based on the BTACT [Z-score <-3] or severe aphasia as documented by treating professionals) and 17 declined. Fifty participants with a primary brain tumour (28 male; 22 female) aged 22 – 81 years (M = 47.90, SD = 1) were recruited. The mean age for females was 44.50 (SD = 13.49) and the mean age for males was 48.21 (SD = 13.28). As shown in , tumour types included benign (n = 16, 32%), lower-grade glioma (n = 12, 24%), and high-grade glioma (n = 22, 44%). The most common tumour types were glioblastoma (n = 12, 24%) and meningioma (n = 10, 20%). The mean time since diagnosis was 41.54 months (SD = 42.2) ranging between 1 and 183 months.
Table 1. Summary of Participants’ Demographic and Tumour-related Characteristics (n = 50).
Descriptive data
Levels of distress on the DT ranged from 4–10 (M = 6.48, SD = 2.1). The mean composite on the BTACT was in the average range (M = −0.24, SD = 0.92), with scores ranging from below average (Z = −2.72) to above average (Z = 1.50). Descriptive data for MADRS items and total scores are presented in . As shown, the average MADRS score (M = 19.38, SD = 7.50) was in the clinical range for depression and 88% of the sample had scores in the clinical range (≥ 12). On the DASS depression (M = 12.7, SD = 10.1) and GAD-7 (M = 9.42, SD = 5.6), 51% and 81% of the sample were in the clinical range, respectively.
Table 2. Descriptive Data and Intraclass Correlation Coefficients for the MADRS.
Interrater reliability of the MADRS
MADRS total scores were consistent across independent raters, with a range of 0–38 (M = 19.38, SD = 7.50) for rater 1, and 0–37 (M = 19.08, SD = 8.07) for rater 2. As shown in , interrater reliability for the MADRS total score was excellent (ICC = 0.98) and the standard error of measurement (SEM) was one point. Agreement between raters on the MADRS items ranged from good (ICC = 0.83, Pessimism) to excellent (ICC = 0.96, Concentration), and SEM ranged from 0.25 to 0.40.
Construct validity of the MADRS
As reported in , level of depression on the MADRS was significantly and positively correlated with the DASS-21 depression subscale (r = 0.76, p < 0.001, 95% CI [0.61–0.86]), GAD-7 (r = 0.65, p < 0.001, 95% CI [0.47–0.79]) and DT score (r = 0.50, p < 0.001, 95% CI [0.26–0.68]). Further, the DASS-21 depression and GAD-7 were significantly associated (r = 0.68, p < 0.001, 95% CI [0.49–0.81]), and were correlated with the DT (r = 0.38, p < 0.01, 95% CI [0.11–0.59], r = 0.50, p < 0.001, 95% CI [0.26–0.69], respectively).
Table 3. Pearson’s Correlations Assessing Associations between the MADRS and Other Measures of Depression and Psychological Distress (95th percentile confidence intervals are shown in brackets).
Associations between demographic and illness characteristics and depression
A significant and negative correlation (r = −0.33, p = 0.018) was found between the level of depression and global cognitive status (BTACT), whereby greater symptoms of depression were associated with poorer global cognitive status. There was no linear relationship between level of depression and age (r = −0.04, p = 0.78), time since diagnosis (r = 0.12, p = 0.39), or years of education (r = −0.12, p =0.40). As shown in , the results of the Mann-Whitney U Test or Kruskal-Wallis one-way ANOVA indicated that level of depression did not significantly differ according to gender (U = 339.0, p = 0.54), tumour type (H = 0.57, p = 0.75), tumour grade (H = 2.52, p = 0.47), tumour stage (U = 139.5, p = 0.76), treatment type (see ), previous mental health history (U = 304.5, p =.14), or relationship status (U = 245.5, p = 0.72). In terms of tumour subgroups, there were no significant differences in levels of depression in individuals with the two most common tumour types, namely, glioblastoma (M rank = 10.96) and benign meningioma (M rank = 12.15; U = 53.5, p = 0.67).
Table 4. Between Group Comparisons for Demographic and Tumour-Related Characteristics on Depression Symptoms Measured by the MADRS.
Discussion
This study examined the psychometric properties of the MADRS as a measure of depression for individuals with brain tumour. Overall, the results showed that raters scored items on the MADRS in a highly consistent manner across participants, indicating excellent interrater reliability for the MADRS total score, and good agreement between raters for the MADRS items. The MADRS also demonstrated evidence of convergent validity in relation to other validated self-report measures of psychological distress (DT, DASS-21 depression, GAD-7). The proportion with clinically significant depression was much higher (88%) than previous research [Citation11], potentially due to sampling of individuals seeking psychological support. Individuals with poorer cognitive function had higher levels of depression on the MADRS. Given the negative impact of depression on individuals’ functional status, quality of life and prognosis for survival [Citation11,Citation12,Citation15], these findings have important implications for identifying individuals who most warrant psychological support.
Individuals with brain tumours often face many barriers to accessing timely psychological support [Citation13]. Although we previously found that most professionals routinely screen for distress using tools such as the DT, HADS and DASS-21 [Citation13], accurate and reliable detection of depression can be confounded by the direct effects of brain tumour or related treatments [Citation14]. This may lead to overidentification or under-identification of people with clinically elevated mood symptoms [Citation21]. A particular concern is that clinicians might attribute mood symptoms (e.g., fatigue, loss of motivation) to the brain tumour or its treatment and hence not refer individuals or provide mental health treatment. Assessment approaches that support clinicians to distinguish depressive symptoms from the effects of the illness and associated treatments have greater utility in practice to determine the need for psychological support. There is preliminary evidence that effective treatment of depression through integrated psychotherapy and cognitive rehabilitation intervention is associated with improvement in quality of life and functional well-being [Citation30].
Interview schedules such as the MADRS have several potential advantages over brief screening tools such as the DT, DASS-21 and HADS, as the semi-structured format provides the scope to reframe questions and use prompts to clarify the onset, frequency, timing and intensity of mood symptoms. This may account for the higher rate of clinically elevated symptoms on the MADRS (88%) as compared to the DASS-21 (51%) in the current study. Unlike the DASS which only measures affective and behavioural symptoms, the MADRS assesses physical and somatic symptoms of depression such as lassitude, sleep, and appetite changes. Despite the potential overlap with the effects of the illness and treatment, these symptoms can reflect mood disturbance and should not be overlooked. The semi-structured format of the MADRS supports clinicians to clarify the basis for these symptoms [Citation27]. Clinical interviews are often regarded as the “gold standard” for comprehensively assessing mood symptoms and diagnosing mood disorders [Citation22]. However, administration of the MADRS takes longer than questionnaires (i.e., 25–30 min vs. 3–5 min) and rigorous training is required for administration, scoring, and interpretation. Therefore, the utility of the MADRS is reliant on robust psychometric properties, including consistency of measurement or interrater reliability.
In line with previous research involving clinical samples that reported good to excellent interrater reliability (ICC = 0.83–0.95) of the MADRS [Citation26,Citation28,Citation43], interrater reliability of the MADRS total score was found to be excellent (ICC = 0.98) with very low measurement error (SEM = one point) in the current brain tumour sample. Values in the excellent range (ICC ≥0.90) indicate a high degree of rater agreement [Citation41]. The high level of consistency in scoring may be due to the rigorous approach used in training assessors in scoring the MADRS. Consistent with other studies that reported variability in scoring across MADRS items (e.g., Geijer et al. [Citation43], ICC: 0.87–0.97), the consistency in scoring the Pessimism item (ICC = 0.83) was somewhat lower and had a larger confidence interval (0.68–0.90), although the ICC was still in the “good” range. Notably, the instructions for scoring the MADRS indicate that responses to other items can influence the scoring of a specific item. For example, responses to item 1 (Reported Sadness) might differ from responses to item 9 (Pessimism); but should be considered when scoring both items. Overall, the findings extend on previous evidence of reliability (internal consistency, test-retest reliability) [Citation31] to demonstrate interrater reliability of the MADRS for people with brain tumours when administered via telephone.
In addition to reliability, construct validity is an essential psychometric property to determine how well a measure reflects a given construct. In previous research, the construct validity of the MADRS has been demonstrated through a strong association with the HADS depression scale (r = 0.70) in stroke but had not previously been examined for brain tumour [Citation27]. As hypothesised, the MADRS was strongly associated with DASS-21 depression (r = 0.76), DT (r = 0.50) and GAD-7 (r = 0.65), providing evidence of construct validity. Apart from sleep, lassitude, appetite, and concentration items, which can be impacted by the direct effects of the tumour or its treatment [Citation14], the MADRS measures affective and behavioural symptoms. Items that assess sadness, inability to feel, and pessimistic and suicidal thoughts are closely related to item content of DASS depression, whereas tension and pessimism overlap with GAD-7 item content. Due to the transdiagnostic nature of depression and anxiety, which share a general affective distress component, it is common for measures of depression and anxiety to be highly related [Citation18].
Consistent with a systematic review of factors related to emotional adjustment after brain tumour [Citation15], demographic and tumour characteristics were not significantly associated with depression in the current study. Previous research has indicated that younger age may be a risk factor for psychological distress in people with glioma [Citation44,Citation45]. The non-significant association between age and depression in the current study may be due to the mixed tumour characteristics of the sample or the different approach to measuring depression compared to other studies. In terms of tumour characteristics, Arnold et al. [Citation40] reported higher levels of psychological distress among individuals with lower tumour grades as compared to higher tumour grades. It is possible that the effect of tumour type on mood symptoms might vary according to various factors, including demographics (e.g., age and gender), cognitive status and psychosocial characteristics [Citation44]. Despite typically having a better prognosis for survival, individuals with a benign brain tumour may still experience significant functional impairments that reduce their quality of survivorship and hence contribute to mood symptoms [Citation5].
In the current study, poorer cognitive status on the BTACT was significantly associated with depression on the MADRS. This reinforces previous research by Ownsworth et al. [Citation46] who found that poorer performance on a global neuropsychological composite derived from a comprehensive test battery was related to greater depression on the DASS. Although directionality cannot be inferred from the cross-sectional data, individuals with poorer cognitive status may experience greater activity limitations (e.g., inability to work and drive) and difficulty employing coping strategies to manage emotional distress. In line with this view, Spitz et al. [Citation47] found that poorer cognition in individuals with brain injury was associated with less use of productive coping strategies and poorer emotional adjustment. Notably, one of the MADRS items relates to concentration, which may partly account for elevated depression scores for individuals with poorer cognitive functioning.
Study limitations
A key methodological limitation of this study relates to the use of a convenience sample of individuals with brain tumour, who were recruited based on having at least mild distress (DT ≥4) and were seeking psychological support in the context of a telehealth psychological intervention [Citation35]. Participants also differed in terms of tumour characteristics, time since diagnosis and treatment type. We did not have access to data concerning time since treatment, other concurrent diagnoses (other than mental health history) and all types of medications used, which may potentially influence depression symptoms. Although the heterogeneity concerning tumour type and treatment is common in clinical practice, the sample may not be representative of the broader brain tumour population, which may affect generalisability of the findings. Further research is needed to evaluate the reliability and validity of the MADRS for assessing depression in individuals presenting with low distress on the DT (< 4), or those not seeking psychological support. As an important caveat, the present findings cannot attest to the value of information gained from the MADRS relative to self-report measures in terms of sensitivity or specificity for detecting depression.
In future brain tumour research, it is recommended that the diagnostic accuracy of the MADRS be examined. Although established in other clinical populations (e.g., stroke) [Citation27], this has yet to be examined in the brain tumour population to support the diagnosis of mood disorder. Such research would support the identification of population-specific cut-off scores, which can differ between populations (e.g., ≥8 for stroke and ≥12 for general clinical population). As used previously to examine the diagnostic accuracy of the DASS after brain injury [Citation48], the SCID-5-CV [Citation23] may have utility for examining diagnostic accuracy of the MADRS in brain tumour. Further research is also needed to examine a broader range of factors that increase individuals’ vulnerability to depression, including illness appraisals, coping strategies, and formal and informal support [Citation15].
Clinical implications
Overall, the findings support the reliability and validity of the MADRS as a clinician-rated, telephone-administered assessment of depression after brain tumour. This is particularly beneficial in the context of the COVID-19 pandemic, where remote psychological assessment and management is often necessary [Citation49]. It is recommended that clinicians receive training and supervision to enhance the uniform administration and scoring of the MADRS. The findings also highlight that poor cognitive functioning may be a risk factor for developing depression. Both cognitive and mental health screenings are essential to identify individuals’ rehabilitation and psychological support needs [Citation13]. Interventions that integrate components of psychotherapy and cognitive rehabilitation show preliminary efficacy for treating depression after brain tumour [Citation30,Citation31].
Conclusion
The current findings provide further psychometric support for the use of the MADRS for measuring depression in individuals with brain tumour. Used in conjunction with distress screening tools, the MADRS may have utility for systematically exploring the onset, frequency, timing and intensity of mood symptoms in the context of brain tumours. Our findings indicate that screening and monitoring for depression may be particularly important for individuals with poor cognitive functioning, who may be at heightened risk of developing mood symptoms. It is recommended that future research examine the diagnostic accuracy of the MADRS for identifying mood disorders in this population.
Acknowledgements
The authors would like to thank Giverny Parker for her assistance with recruitment and data collection. This study was funded in part by a National Health and Medical Research Council Partnership Project Grant [ID: 1152217].
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
Raw data were generated at Griffith University. Derived data supporting the findings of this study are available from the corresponding author [TO] on request.
References
- Leece R, Xu J, Ostrom QT, et al. Global incidence of malignant brain and other central nervous system tumours by histology, 2003–2007. Neuro Oncol. 2017;19(11):1553–1564.
- Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019;21(Supplement_5):v1–v100.
- Mucha-Małecka A, Gliński B, Hetnał M, et al. Long-term follow-up in adult patients with low-grade glioma (WHO II) postoperatively irradiated. Analysis of prognostic factors. Rep Pract Oncol Radiother. 2012;17(3):141–145.
- Teng C, Zhu Y, Li Y, et al. Recurrence- and malignant progression-associated biomarkers in low-grade gliomas and their roles in immunotherapy. Front Immunol. 2022; 13:899710.
- Tsay SL, Chang JY, Yates P, et al. Factors influencing quality of life in patients with benign primary brain tumors: prior to and following surgery. Support Care Cancer off J Multinatl Assoc Support Care Cancer. 2012;20(1):57–64.
- Nicol C, Ownsworth T, Cubis L, et al. Subjective cognitive functioning and associations with psychological distress in adult brain tumour survivors. J Cancer Surviv. 2019;13(5):653–662.
- Cubis L, Ownsworth T, Pinkham MB, et al. The social trajectory of brain tumor: a qualitative metasynthesis. Disabil Rehabil. 2018;40(16):1857–1869.
- Cubis L, Ownsworth T, Pinkham MB, et al. The importance of staying connected: mediating and moderating effects of social group memberships on psychological well-being after brain tumor. Psychooncology. 2019;28(7):1537–1543.
- Mellado-Calvo N, Fleminger S. Cerebral tumours. In: David AS, Fleminger S, Kopelman MD, Lovestone S, Mellers JD, editors. Lishman’s organic psychiatry: a textbook of neuropsychiatry., 4th ed. Singapore: Wiley-Blackwell; 2009. p. 281–308.
- Ownsworth T. Coping with the unthinkable: psychosocial advances in the management of primary brain tumour. Brain Impair. 2016;17(3):265–272.
- Huang J, Zeng C, Xiao J, et al. Association between depression and brain tumour: a systematic review and meta-analysis. Oncotarget. 2017;8(55):94932–94943.
- Shi C, Lamba N, Zheng LJ, et al. Depression and survival of glioma patients: a systematic review and meta-analysis. Clin Neurol Neurosurg. 2018;172:8–19.
- Ownsworth T, Lion K, Sansom-Daly UM, et al. Scoping the psychological support practices of Australian health professionals working with people with primary brain tumour and their families. Psychooncology. 2022;31(8):1313–1321.
- Ownsworth T, Little T, Turner B, et al. Assessing emotional status following acquired brain injury: the clinical potential of the depression, anxiety and stress scales. Brain Inj. 2008;22(11):858–869.
- Ownsworth T, Hawkes A, Steginga S, et al. A biopsychosocial perspective on adjustment and quality of life following brain tumour: a systematic evaluation of the literature. Disabil Rehabil. 2009;31(13):1038–1055.
- Goebel S, Mehdorn HM. Measurement of psychological distress in patients with intracranial tumours: the NCCN distress thermometer. J Neurooncol. 2011;104:357–364.
- Snaith RP. The hospital anxiety and depression scale. Health Qual Life Outcomes. 2003;1(1):29.
- Lovibond SH, Lovibond PF. Manual for the depression anxiety stress scales. 2nd ed. Sydney (Australia): psychology Foundation; 1995.
- Löwe B, Unützer J, Callahan CM, et al. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42(12):1194–1201.
- Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097.
- Pranckeviciene A, Bunevicius A. Depression screening in patients with brain tumours: a review. CNS Oncol. 2015;4(2):71–78.
- Davison TE, McCabe MP, Mellor D. An examination of the “gold standard” diagnosis of major depression in aged-care settings. Am J Geriatr Psychiatry. 2009;17(5):359–367.
- First MB, Williams JBW, Karg RS, et al. User’s guide for the SCID-5-CV structured clinical interview for DSM-5® disorders: clinical version. Arlington: American Psychiatric Publishing; 2016.
- Levis B, Benedetti A, Riehm KE, et al. Probability of major depression diagnostic classification using semi-structured versus fully structured diagnostic interviews. Br J Psychiatry. 2018;212(6):377–385.
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389.
- Eskelinen M, Selander T, Ollonen P, et al. Moderate/severe depression (MADRS) can affect the quality of life and outcome among patients admitted to breast cancer diagnosis unit. Anticancer Res. 2017;37(5):2641–2647.
- Sagen U, Vik TG, Moum T, et al. Screening for anxiety and depression after stroke: comparison of the hospital anxiety and depression scale and the Montgomery and Åsberg depression rating scale. J Psychosom Res. 2009;67(4):325–332.
- Kobak KA, Williams JBW, Jeglic E, et al. Face-to-face versus remote administration of the Montgomery-Asberg depression rating scale using videoconference and telephone. Depress Anxiety. 2008;25(11):913–919.
- Laska AC, Mårtensson B, Kahan T, et al. Recognition of depression in aphasic stroke patients. Cerebrovasc Dis. 2007;24(1):74–79. 30.
- Ownsworth T, Chambers S, Damborg E, et al. Evaluation of the making sense of brain tumour program: a randomized controlled trial of a home-based psychosocial intervention. Psychooncology. 2015;24(5):540–547.
- Ownsworth T, Cubis L, Prasad T, et al. Feasibility and acceptability of a telehealth platform for delivering the making sense of brain tumour programme: a mixed-methods pilot study. Neuropsychol Rehabil. 2022;32(3):378–406.
- Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–745.
- Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol. 2010;10(1):22.
- Abma IL, Rovers M, van der Wees PJ. Appraising convergent validity of patient-reported outcome measures in systematic reviews: constructing hypotheses and interpreting outcomes. BMC Res Notes. 2016; 9(1):226.
- Ownsworth T, Chambers S, Aitken JF, et al. Evaluation of a telehealth psychological support intervention for people with primary brain tumour and their family members: study protocol for a randomised controlled trial. Eur J Cancer Care . 2019;28(4):e13132.
- Mokkink LB, Terwee CB, Patrick DL, et al. COSMIN checklist manual. Amsterdam (The Netherlands): University Medical Center; 2012.
- Tun PA, Lachman ME. Telephone assessment of cognitive function in adulthood: the brief test of adult cognition by telephone. Age Ageing. 2006;35(6):629–632.
- Jones S, Ownsworth T, Shum DHK. Feasibility and utility of telephone-based psychological support for people with brain tumour: a single-case experimental study. Front Oncol. 2015;5:71. DOI:10.3389/fonc.2015.00071
- Williams JBW, Kobak KA. Development and reliability of a structured interview guide for the Montgomery-Åsberg depression rating scale (SIGMA). Br J Psychiatry. 2008;192(1):52–58.
- Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro Oncol. 2008;10(2):171–181.
- Hallgren KA. Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34.
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. New York (NY): Routledge; 1988.
- Geijer J, Baigi A, Aiff H. Inter-rater reliability among psychiatrists when assessing depression according to the Montgomery–Åsberg depression rating scale. Nord J Psychiatry. 2021;75(8):607–613.
- Hu Y, Deng F, Zhang L, et al. Depression and quality of life in patients with gliomas: a narrative review. JCM. 2022;11(16):4811.
- Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76.
- Ownsworth T, Dwan T, Chambers S, et al. The moderating effect of estimated pre-morbid IQ on the relationship between neuropsychological status and subjective well-being after brain tumour. J Psychosom Res. 2014;76(3):257–260.
- Spitz G, Schönberger M, Ponsford J. The relations among cognitive impairment, coping style, and emotional adjustment following traumatic brain injury. J Head Trauma Rehabil. 2013;28(2):116–125.
- Dahm J, Wong D, Ponsford J. Validity of the depression anxiety stress scales in assessing depression and anxiety following traumatic brain injury. J Affect Disord. 2013;151(1):392–396.
- Ownsworth T, Chan RJ, Jones S, et al. Use of telehealth platforms for delivering supportive care to adults with primary brain tumours and their family caregivers: a systematic review. Psychooncology. 2021;30(1):16–26.