Abstract
Purpose
To describe physical functioning after severe COVID-19-infection.
Materials and method
An explanatory sequential mixed method design was used. Thirty-nine participants performed tests and answered questionnaires measuring physical functioning six months after hospitalisation due to COVID-19. Thirty of these participants participated in semi-structured interviews with questions regarding how they perceived their physical functioning and recovery from COVID-19 at 12 months post-hospitalisation.
Results
At six months, physical functioning measured via chair stand test and hip-worn accelerometers was lower than normal reference values. There was a reduction in breathing muscle strength. Participants estimated their functional status during different activities as lower compared to those before COVID-19-infection, measured with a patient-specific functional scale. At one year after infection, there were descriptions of a rough recovery process and remaining symptoms.
Conclusion
Patients recovering from severe COVID-19 seem to have reduced physical functioning and activity levels, and they perceive their recovery to be slow and difficult. They experienced a lack of clinical support and contradictory advice regarding rehabilitation. Coaching in returning to physical functioning after the infection needs to be better co-ordinated and there is a need for guidelines for health professionals to avoid patients receiving contradictory advice.
Implications for Rehabilitation
Coronavirus infection disease-19 (COVID-19) can have a great impact on a person’s physical functioning.
In the early course of the COVID-19 pandemic there were not any clear guidelines regarding rehabilitation for this group of patients.
As some people with COVID-19 may have impairments in their physical functioning up to one year after leaving hospital there is a need to provide rehabilitation.
Introduction
Since December 2019, the pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has made an unquestionable imprint on the world. One meta-analysis found that 11% of patients with laboratory-verified coronavirus infection disease-19 (COVID-19) were admitted to intensive care units (ICU) [Citation1]. Another study comparing COVID-19-infection with seasonal influenza found that patients with COVID-19 were more likely to need intensive care, and that the mean length of their stay in the ICU was twice as long as for those with seasonal influenza [Citation2]. Studies have suggested that an impairment in neuromuscular function [Citation3] or prolonged periods of immobility [Citation4] can lead to post-infectuous impairments in physical functioning.
Most of the reports on the effect of COVID-19 on physical functioning have a followup time of up to two months after disease onset [Citation5], with only a few, more recent, studies with a longer followup time [Citation6]. One study reported that 30% of healthcare workers discharged after COVID-19-infection had not recovered their functional fitness up to one year following hospital discharge [Citation7]. These findings are in line with patients’ self-reported physical activity, which, according to one study, was significantly decreased up to six months post-COVID-19 [Citation8]. In addition to physical impairments, studies have also shown a lower health-related quality of life (HRQoL) in patients post-COVID-19-infection, [Citation9] compared to reference values for healthy people.
Previous research has shown the positive effects of—and need for—different kinds of rehabilitation after previous corona virus outbreaks, such as the severe acute respiratory syndrome (SARS) infection [Citation10,Citation11]. Considering the findings on impairments in physical functioning post-COVID-19-infection, there is a need to map out the difficulties patients might have after COVID-19-infection, in order to provide necessary rehabilitation and aid recovery.
In this study, we describe physical functioning after severe COVID-19-infection. This explanatory mixed methods study sought out objective measures of physical functioning, and followed up with interviews to explore those results more in depth. In the first quantitative phase, patients’ physical functioning was measured using instruments that assessed different domains of physical functioning. Based on these findings, semi-structured qualitative interviews were conducted in the second phase to further explore the quantitative results.
Materials and methods
This study followed a mixed methods sequential explanatory strategy as described by Creswell and Plano Clark [Citation12] to investigate physical functioning after severe COVID-19. Two data collection phases, one following the other, were used in this approach. The two methods were integrated during the interpretation phase in a side-by-side comparison in the discussion section. By using this approach, we were able to use qualitative results to assist in explaining and interpreting quantitative findings. A model of this approach is presented in .
Figure 1. Explanation of mixed methods analysis using a sequential explanatory strategy [Citation12].
![Figure 1. Explanation of mixed methods analysis using a sequential explanatory strategy [Citation12].](/cms/asset/72e3a095-f467-4092-8059-d0ece2585776/idre_a_2201512_f0001_b.jpg)
Setting and participants
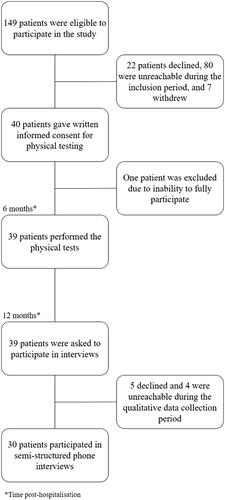
All patients admitted to a University hospital in central Sweden due to a severe COVID-19-infection between March and May 2020 were offered a follow-up visit to a physician at the infection clinic three months after discharge from the hospital. At this visit, the patients were asked to participate in this study. A severe COVID-19 infection was defined as having received treatment with nasal high-flow oxygen therapy (NHF) for more than two days, having received treatment at an ICU, or having experienced thromboembolic complications. Of the 149 patients who had a followup visit between 11 July 2020 and 26 October 2020, 40 patients agreed to participate in the study. One participant was later excluded due to the inability to fully participate (). Of the 39 participants who participated in the physical testing six months post-hospitalisation, 30 agreed to participate in a semi-structured phone interview six months after the quantitative data collection period; i.e., 12 months post-hospitalisation. Five declined and four were unreachable during the qualitative data collection period and therefore excluded ().
Figure 2. Patient inclusion flowchart.
Quantitative methods
Data collection
Six months after hospitalisation due to COVID-19 participants’ physical functioning was tested at the University hospital. At the same time, they responded to the questionnaires. All participants performed the same tests and answered the same questionnaires assessing different aspects of physical functioning and HRQoL. All tests are psychometrically tested and described below [Citation13–18]. The tests were conducted by two of the authors (AB.Z. and E.NS.). An interpretor was needed for three of the participants since they did not speak Swedish. Each visit lasted for approximately 90 min.
Information on demographics such as sex, age, body mass index (BMI), smoking habits and marital status was obtained from the participants themselves at the time of the physical testing, as was information on previous comorbidities and length of hospital admission. Data was collected using SMART-TRIAL, an electronic case report form, where results from the physical tests and questionnaires also were stored.
Functional capacity and leg strength were assessed using 6-min walk test (6MWT) [Citation13] and the chair stand test [Citation14]. 6MWT measures the distance the participant can walk in six minutes. The chair stands test measures the number of times the participant can rise from a chair during 30 or 60 s, respectively. Percutaneous oxygen saturation and heart rate were assessed (Wristox2, Nonin Medical Inc, Plymouth, MN, USA) both at rest as well as at set intervals during the tests. Perceived exertion was also assessed, using the Borg scale. The Borg scale measures a patient’s perceived shortness of breath and fatigue on a scale from zero to ten, with a higher number indicating more discomfort [Citation13].
Grip strength was assessed using a hand dynamometer (JAMAR) [Citation19]. Pulmonary function (FEV%, FEV1, percent of predicted, and peak expiratory flow (PEF)) was assessed using routine spirometry (MicroLab, Vyaire Medical, Chicago, IL, USA) [Citation20]. Breathing muscle strength was assessed with maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) (MicroRPM, Vyaire Medical, Chicago, IL, USA) [Citation21–23].
Physical activity (PA) level was assessed using a hip-worn accelerometer with a sampling rate of 30 Hz (GT3X, Actigraph), which was worn during waking hours. To be included in the analysis the accelerometer had to be worn for at least 10 h/day for four days. Non-wear time was defined as >60 min of consecutive zero counts, with the allowance of 2 min of non-zero counts within those 60 min [Citation24]. Counts per minute (CPM) was used as a measure of mean PA level.
Experienced breathlessness was assessed using the modified Medical Research Council (mMRC) dyspnoea scale. The questionnaire consists of five statements about perceived breathlessness during different physical activities measured on a scale from zero to four, a lower number indicating less breathlessness [Citation15].
Self-reported activity limitations were assessed via the Patient-Specific Functional Scale (PSFS) and Disability Rating Index (DRI). The PSFS allows the participant to choose up to five activities that he/she cannot perform to the same extent as they could before the infection, and then rate on a scale from zero to ten to what extent they can perform that activity compared to their performance before COVID-19. Participants were asked to choose three different activities related to work, leisure, and PA. A score of zero indicates that they cannot perform the activity, and ten means that they can perform it at the same level as they could before the infection [Citation16]. The DRI is a questionnaire containing 12 items related to physical functioning where the participant marks on a 100 mm visual analogue scale to which extent they can perform the activities, a lower score connotating fewer difficulties [Citation17].
Health-Related Quality of Life (HRQoL) was assessed using EuroQol 5 Dimension 5 Level (EQ-5D-5L), which contains five questions regarding different aspects of HRQoL, each divided into five levels of severity. The dimensions assessed are mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The responses can then be converted into a value set ranging from −1 to 1, where a lower value indicates a lower HRQoL, in order to compare it with other studies or norms [Citation18]. To calculate a value set, participants’ answers need to be converted into a health state code consisting of five numbers; where 11111 is the highest value, and connotes the fewest issues, and 55555 is the lowest value and connotes the highest number of issues. The health state code was then inserted into an EQ-5D-5L index value calculator, which measured a value set based on a predetermined value set obtained from a population norm. In this study, the Spanish value set was used to calculate a population-specific value set [Citation25].
The participants also estimated their current health quality (HQ) on a visual analogue scale (VAS) from 0 to 100, where a higher score indicates greater perceived health [Citation18].
Statistical analysis
Descriptive statistics were calculated for all physical tests and questionnaires, as well as for demographic data. After visual assessment of histograms for normality, results were presented either as means (M) and standard deviations (SD) if the data was normally distributed, or, if not, as medians and interquartile ranges (IQR). Analyses were performed using IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, N.Y., USA). Regarding the different physical tests, each participant’s individual results on the tests were compared to normal values for their corresponding age group and sex [Citation19,Citation22,Citation26–28], using the non-parametric one-tailed sign test, with the null hypothesis that there was no difference in performance from reference values.
Qualitative methods
Data collection
A semi-structured interview guide was drafted based on the aim of the study and proofread by the research group, which reached a consensus regarding which questions to include. The interview guide was then tested in two pilot interviews with two individuals not involved in this study. The main questions dealt with the individual’s experiences regarding their physical functioning and improvements, or lack thereof, during the year following discharge from the hospital. The three main questions were “How do you experience your recovery after COVID?”, “How do you experience your physical activity level and exercise?” and “How do you experience your health today?” which were followed up with what came up in the dialogue. For example “Can you give an example?” or “Can you tell me more?”.
Telephone interviews were held individually and by the same researcher (A.T.). An interpreter was present during three interviews where the participants did not speak Swedish. The interviews were audio-recorded with the consent of the interviewee, and transcribed verbatim by one of the researchers (A.T.) or by a professional transcriber. Each interview lasted 18–67 min. The difference in interview length was mainly due to how much the participants had to describe regarding their recovery and remaining disabilities.
Data analysis
All transcripts were read through for accuracy by the interviewer. The data were analysed using qualitative content analysis, as described by Graneheim and Lundman [Citation29]. The transcripts were entered into Nvivo 11 (QSR International 2017) software package for qualitative data management. A.T. read through each transcript several times to gain a sense of the transcript as a whole, in order to then extract important statements (i.e., meaning units) related to the study objectives, which were each labelled with a code. A.T., E.NS. and AB.Z. organized the codes into categories and subcategories based on similarities in their manifest content. The categories were then gathered under one overarching theme based on their latent content. A.T., E.NS., AB.Z and M.EC. met regularly to discuss the analytical process of categorizing and subcategorizing the codes, and topics were modified where necessary. Each subcategory was finally paired with quotes from the interviews to illustrate the experiences of the participants, as well as to add transparency and trustworthiness to our findings and interpretations of the data [Citation30].
Ethical considerations
The study protocol was approved by the Swedish Ethical Review Authority (2020-04498 and 2021-00835) and written informed consent was provided by all participants. The possibility of identifying specific individuals in the current study is very low since the participants were pseudonymised, all records were encoded during data collection, and all results are presented at the aggregated group level. The test results, audio files, and transcripts were saved on a password-protected server. The code key and other relevant materials were stored in a safe locker. Only authorised personnel have access to the data.
Citations from the interviews increase the risk that specific individuals might be recognised, but at the same time, they strengthen the trustworthiness of the results. To reduce the risk of identification, all the names of places, people, and other factors that could be used to identify an individual have been censored from the citations.
The study complies with the Helsinki Declaration [Citation31] and follows the COREQ (COnsolidated criteria for REporting Qualitative research) checklist when reporting findings from the qualitative data [Citation32].
Results
Quantitative results
A summary of the demographic data of the participants is presented in . Regarding the EQ-5D-5L at 6 months after COVID-19-infection, the participants’ value set resulted in a median value of 0.83 (IQR 0.28). The participants rated their HQ VAS at the time of the data collection at an average of 80 out of 100 (), meaning that they felt quite well but not fully recovered six months after COVID-19-infection.
Table 1. General characteristics of participants.
Table 2. Tests measuring physical functioning six months after hospitalisation due to COVID-19.
The results of all the physical tests are presented in . For some tests, the participants in this study performed worse than could be expected for their corresponding age group. This was true for the chair stand test and the MIP test. There were no differences between the values for 6MWT, grip strength, and PA-level when compared to reference values for the respective individuals’ age and sex.
Regarding the mMRC scale, participants rated, on average, one on a scale from zero to four; which on the questionnaire correlates to “not troubled by breathlessness except during strenuous exercise”. The scores on the DRI were low (median 284, IQR 361), which indicates non-extensive problems performing different activities related to physical functioning. Looking at the results of the PSFS, participants scored on average seven, seven, and six on leisure activity, work, and PA respectively, indicating that they can perform the activities but not to the same extent as they could pre-COVID-19 (). Examples of activities the participants could not perform to the same extent as they could before they contracted COVID-19 included desk work or work activities including concentration; walking or household chores as leisure activities; and running or biking as a PA.
Table 3. The questionnaires mMRC, PSFS and DRI, measuring physical functioning six months after hospitalisation due to COVID-19.
Qualitative results
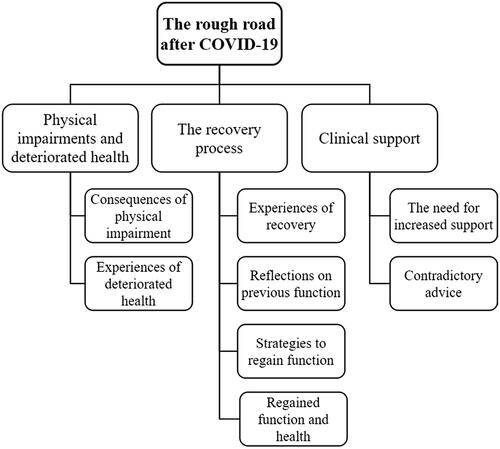
One overarching theme was identified; the rough road after COVID-19. This theme refers to the journey to recovery; it deals with health issues and impairments which have had an impact on participants’ lives and their daily activities. It also includes descriptions of managing one’s own recovery, and the feelings that arise when things are rough and do not go as planned. The theme contains three categories related to the participants’ experience of their physical functioning, physical activity level, and health: physical impairments and deteriorated health, the process of recovery and clinical support (). Each category includes several subcategories and illustrative quotes are provided for each subcategory.
Figure 3. Displaying the overarching theme, three categories, and the sub-categories identified from semi-structured interviews exploring individuals’ experiences of COVID-19 and their perceived impact on physical activity.
Physical impairments and a deteriorated health
This category () is related to different aspects of participants’ physical functioning and health. The subcategory consequences of physical impairment includes descriptions of how various impairments in physical functioning have had an impact on participants’ ability to work and pursue their interests in their everyday lives. Some felt that while they were able to manage their work they were completely exhausted by the end of the day and unable to continue with their day as they had previously done. Some participants had to rely on walking aids and home-help services.
“What’s more, I’m over 55 years old and, as I said, I’ve worked a lot, more than 80 per cent [of full-time] and extra hours. But that’s done with. I work very seldom - only if someone asks me […] I don’t work any extra hours. I’m happy if I can manage to work my 80 per cent.” (Participant 8).
There were also descriptions of increased passiveness and less interest in certain activities; not only strenuous activities but also lighter house work. The participants sounded dispirited, as the feelings of passivity and disinterest stemmed from lower stamina. Because of these physical impairments, most participants stated that they had been forced to quit some of their previous hobbies and interests, which was disheartening for many.
“Well, I would like more, I would like to go to the gym but I don’t feel that I have the stamina yet. I used to go, but I don’t really have the stamina yet, to go to the gym.” (Participant 12).
The subcategory experiences of deteriorated health deals with how newly-acquired diseases or symptoms were a reason why the participant felt their health had deteriorated. This occurred both because of a worry as to why they had been stricken with these diseases, but also whether this meant that there was a risk that they would also be affected by other diseases. Many also felt an increased burden on their health from these new ailments, since they had already been so affected by COVID-19. In addition to the physical impairments and somatic symptoms, psychological symptoms were also mentioned. There were descriptions of nightmares and flashbacks about the acute phase of the disease and the time spent in-hospital, as well as increased anxiety and depression.
“Now it’s every once in a while, when I make too much of an effort, that I feel a burning feeling in my lungs the day after. And that feeling is tough. Because everything comes back from the time I was sick, at the beginning. Is this how it’s going to be, will it start all over again? And then, after a day, it passes.” (Participant 13).
Sleeping disorders were not uncommon in our group. The one thing that the participants described to have the greatest impact on their health and described as being the most crippling was increased tiredness. Some had an increased need to sleep, and some needed more rest throughout the day and between activities.
“Then I usually think, well, I am 67 years old after all, I might just be tired. But it’s not fatigue, because I can sit inside, reading, knitting, doing whatever and suddenly I feel like, uh oh, I need to close my eyes. Then my eyelids go down and I take a – we can call it a mini nap for thirty minutes or an hour.” (Participant 25).
The process of recovery
In this category, consisting of four subcategories (), the participants described their experiences with their recovery from COVID-19 from the time of discharge from the hospital to the time of the interview, one year following discharge. The subcategory experiences with recovery describes how the participants experienced their recovery and how well they tolerated exercise. This generated a great variety of answers, ranging from experiences of a good recovery with no problems exercising to quite a tough journey with difficulties performing even non-strenuous activities.
“At the start I was very tired, horribly tired, the day after. So, on that day I maybe didn’t take a long walk but strolled around at home, went to the car or out on the lawn, things like that. But the next day I felt fine again and it didn’t seem so bad. I could take a walk, and then got back and felt really bad the day after. But it’s easing up gradually and eventually it wasn’t that often. After a few months it wasn’t nearly as bad. It was that every-other-day thing I guess.” (Participant 13).
Many also expressed a feeling of sadness when thinking about how slow their recovery had been and their inability to keep up the same intensity in physical activities as they could before COVID-19. Others described that their recovery had been a tough journey but that it had gone well, expressing pride in being able to get themselves through something so difficult and feelings of relief that it had gone well in the end. When the participants talked about their views on further recovery, two perspectives emerged from the group; one with a positive view and a belief that everything would go well, and one expressing worry, fear, and uncertainty as to what the future held for them.
In the subcategory reflections on previous function, participants reflected on their previous level of physical activity and how that affected the course of events during the acute phase of COVID-19. Some initially felt that it was unjust that they had to go through this tough journey when they were in such good shape, but then suggested that maybe they were able to handle the disease and survive because they were in good shape at the time they fell ill.
“I wondered about it when I talked to somebody at the hospital, when I woke up, and they said that I didn’t spend more than a couple of days in the respirator because I was in good physical shape. Then I thought that, no, at first, I thought it was unfair that this happened to me at all. Because, based on what I read, only those with some pre-existing condition or who were overweight or old or so on - and I thought it wasn’t fair. But then I thought it might be fair that, since I was in decent shape, I didn’t have to spend more time here. So it’s a bit of both.” (Participant 14).
The subcategory strategies to regain function contains descriptions regarding strategies to attempt to regain physical functioning throughout the year following their discharge from the hospital. Most started with slow, short walks around their house and neighbourhood, some with walking aids, and gradually increased these walks in length and intensity. Many then went on and added more strenuous activities and exercises to challenge themselves and increase their strength and endurance. The participants were surprised that they had been so affected, and felt lost regarding how to best regain full functioning. One thing that seemed to spur many participants to come back to a normal state of function was their own toughness and stubbornness. They felt that it was their own drive more than anything else that helped them in their journey to recovery. Apart from physical activity as a means of regaining function, some made life style changes such as a switch to a healthier diet, a minimized alcohol intake, and active efforts to lose weight. Falling ill with COVID-19 became an eye-opener regarding their health, and participants felt lucky to have survived.
“Yes, from the start it was – it was really very simple. It was simply about just learning how to walk, since at that time it was the walking frame on walks. And then it went from short distances to trying to increase to longer and longer walks. In the beginning, I walked 3 or 4 times a day. And when I came home, indeed, I believe I could manage to walk from one property boundary to the next in my neighbourhood, 35 or 40 meters, before I had sit down on the walking frame’s seat. But around the block, a park, the neighbourhood, increasing all the time to challenge myself. Then, different kinds of workouts with a little dumbbell and very, very light weights, just to get some movement.” (Participant 15).
The subcategory regained function and health contains reflections regarding the fact that despite difficulties and struggles in coming back to work, exercising, and even in activities of daily living, the majority of participants still perceived that they had an overall good health and felt content that they had regained the physical functioning that they had lost while they were sick. They had returned to work and did not experience any difficulties working. They had also either not perceived any cognitive dysfunctions or recovered from any difficulties with impaired memory, poor concentration or brain fog, and felt relieved that they did not suffer from such symptoms anymore. When talking about specific situations, such as leisure activities or household chores, there were descriptions of different things that participants could not do after COVID-19 because of impairments to their strength, endurance, or functioning. It could be activities such as cleaning, or gardening. But when talking about their overall health they still perceived it as “good”.
Clinical support
The category “clinical support” includes two subcategories describing the support the participants had received or had needed during their recovery following discharge from the hospital ().
The subcategory a need for increased support showed that there was a need for more support and advice regarding rehabilitation and prognosis post-discharge. Participants felt that they were left to their own devices regarding how to plan their rehabilitation and what to do in order to regain their physical functioning, leaving them feeling frustrated and uncertain about what to do in this new situation. There were expressions of worry about coming home, since they felt that they did not know what awaited them. Many also expressed a need to talk about what they had been through and help processing the experience of being so sick and hospitalised.
The subcategory contradictory advice contains statements regarding the contradictory advice participants received regarding how best to regain their function. The advice and recommendations differed, so that the same patient received contradictory advice and different patients received different general advice. Some of them received instructions that they should take it slow and not overexert themselves, and others that they needed to be up and move about as much as possible, while some received different advice from different health care workers. This created confusion and worry, since they did not know what could be helpful and what could be harmful when it came to exercise and excertion.
“The whole time I have had to experiment with myself and the only thing the doctor said was don’t do too much, don’t overexert yourself. Yes, but at what point is that? I don’t know. I don’t know until I fall over. That made it very, very hard when I was discharged from the hospital because then it was also like, don’t lie down, try to sit up and move as much as you can. But don’t overexert yourself.” (Participant 20).
Discussion
In this study, we described physical functioning after severe COVID-19-infection, and the process of recovery. At six months, we found a poorer physical functioning compared to reference values for some tests, but for several measures, such as 6MWT, no differences to reference values were detected (). However, many participants estimated their physical capacity to be lower compared to what it was before COVID-19, even 12 months after the infection. This is in line with other reports [Citation33–35].
As physical tests were performed only once post-infection we compared them to previously-published reference values. Some, but not all, participants’ results were poor compared to the reference value. The chair stand test, which measures lower body muscular function [Citation36], showed a median of 28 repetitions in 60 s compared to the reference of over 30 repetitions [Citation26]. In contrast, no differences in the reference values were noted regarding the 6MWT, which assesses general functional performance and endurance [Citation36]. However, in the interviews, the participants expressed feeling limited when it came to more strenuous activities, but not activities of lower intensity. This could explain why they have good results on some tests and not on others.
The measures of breathing muscle strength is overall below reference values in this study. The median MIP was 69 cmH2O for the participants, and thus lower than the values for all ages for both men and women, except for women between ages 70–83 years [Citation22]. This shows impairment in the breathing muscles of the participants in the present study, which is in line with previously-described impairments related to dyspnoea and reduced work capacity [Citation37]. Based on the mMRC, the patients experienced some trouble with breathlessness during more strenuous activities. In the interviews, the patients describe that they have nearly the same physical functioning level as they did before COVID-19, except for breathlessness; for example when climbing stairs or walking uphill. This means that the impairment seen on the test results seems to have an actual impact on the participants, and is not only something seen on the test.
The PA level of the participants in our study was lower than that of a similar cohort of healthy adults in Norway. The cohort in this study was divided into two age groups; <65 years and ≥65 years, to be able to compare the study’s results to the reference values. PA levels were lower in both age groups. In the <65 years group in the present study, the PA level was 325 CPM () compared to a mean value of 369 CPM in healthy men and women [Citation28]. In the age group ≥65 years, the PA level in the present study was 235 CPM () compared to 309 CPM [Citation28]. These findings are in line with the participants’ statements in the interviews six months later, where a common statement is that they are not as physically active as they had been before COVID-19, and that they feel that they are more sedentary than they used to be. In the interviews, we also receive a more detailed explanation as to why they are not as physically active as before, where problems such as impairments in strength, endurance, function, and lower stamina are mentioned. This could suggest that patients with severe COVID-19 might still have physical impairments even one year after the infection. Previous reports of self-reported PA of patients following COVID-19 show similar findings, with a decreased walking time six months after the infection [Citation8]. The participants also described remaining fatigued 12 months after COVID-19, which has been shown to be common following COVID-19 [Citation38]. The mechanisms behind post-infectious impairments in physical functioning are still unclear. Studies have suggested that it might be because of an impairment in neuromuscular function [Citation3], or because of prolonged periods of immobility [Citation4].
Because of the small cohort in this study, all analyses except for measurements of PA were made on the group as a whole and not stratified based on age or sex, making it difficult to compare some of our results to reference values. In the instances where a fitting reference value matched the current participants’ age and sex, a sign test was used to explore differences. But in some instances, such comparisons were not possible. One example of this is the reference values for grip strength, which are generally stratified based on age, sex and dominant/non-dominant hand. But the grip strength of the right hand in this study, which was 36 kg (), is comparable to the grip strength of the dominant hand of a man aged between 65–69 years [Citation19], which is a slightly older age group than the mean age of this cohort. Similarly, the grip strength of the left hand, which in this study was 34 kg (), is comparable to the grip strength of the non-dominant hand of a man aged between 70–74 years [Citation19]. This could be explained by the fact that our result is based on the grip strength of both men and women, but it could also be an expression of impairment in neuromuscular function.
One of the questions asked during the interviews was whether the participants felt that they had received the help that they needed from health services regarding post-hospitalisation rehabilitation, to which many answered that they had not or that they had received contradictory advice regarding strategies for regaining function. Considering that this was during the beginning of the pandemic, there were not any clear guidelines regarding rehabilitation. Since then, studies have been published highlighting the need for different kinds of rehabilitation to aid patients in their recovery, such as pulmonary rehabilitation, exercise rehabilitation and musculoskeletal rehabilitation [Citation10]. Still, there is a need for tailored rehabilitation based on the symptoms and impairments the patients perceive after hospitalisation for COVID-19.
Since the interviews were carried out 12 months after hospitalisation (i.e., 6 months after the physical tests), the statements do not correspond to the participants’ physical status during the tests. The participants were offered a chance to perform the tests again 12 months following hospitalisation, but most declined. The difference in time between the physical testing and the interviews can explain, for example, why many participants experienced breathlessness during the 6MWT and chair stand test (), but stated that they did not have much problem with breathlessness during different activities at the time of the interviews.
Regarding the HRQoL we found that this group of participants rated their health lower than that of the Spanish population norm (our median value set, at 0.83 (), compared to a mean of 0.90 in the Spanish study). Interestingly the HQ VAS in this study was higher (median 80, ) than that of the norm value (mean 76) [Citation25]. This is in line with how the participants describe their health in the interviews, stating that they do experience some lingering symptoms and impairments in function, but still perceive their health to be good, overall. The participants elaborated on this, saying that compared to how affected they were shortly after COVID-19, they feel much better now. They have been through a life-changing experience, which might be why they rate their health higher than the norm. This might explain the higher HQ VAS score.
One of the strengths of this study is its design, where both quantitative and qualitative data were used. This aided in the interpretation of the results since this is a relatively new field where many questions have yet to be answered. It is also, to our knowledge, one of the few studies at the moment looking at physical functioning with a follow-up time of one year. Most previous studies on physical functioning after COVID-19 focus on the time immediately after disease onset [Citation5], but previous studies on other coronavirus infections have shown impairments for up to two years after infection [Citation39], making it crucial to further investigate the long term effects of COVID-19 on physical functioning.
Another strength is the large number of participants in the interviews. This increases the credibility of the study, and therefore its trustworthiness, since many different perspectives are represented, as are people of different ages and sexes. It may also make the results more applicable to a larger population because of the heterogenous group in this study. Member checking was not performed during the course of this study, but continuous discussions were held during the process of analysis, and the results, including the citations, were approved by the whole research group. This strengthens the confirmability, and thus trustworthiness of the study, because the results are based on the participants’ stories.
A limitation of this study would be the small sample size. This made it difficult to calculate more in-depth statistics with stratifications. But despite this, our results point to the fact that this group of participants continues to have a slight impairment in their physical functioning, which should be further investigated in future studies with larger sample sizes. Another limitation is that the data is only from the one-time point, making it impossible to measure changes in participants’ results over time. To have some comparison we used general reference values [Citation19,Citation22,Citation25–28], which are not specific to our population. Since improvements have been seen when looking at one-year post-infection [Citation7] it would be interesting to know whether this improvement continues after this period, especially since studies on previous coronaviruses have shown impairments up to two years following infection [Citation39].
Lastly, it is important to acknowledge the large group of non-responders. Whether this is due to them being too ill to participate in our study or because they had recovered and felt like they did not have much to contribute is unknown and a limitation. But nevertheless, it could have affected our results, either by accentuating the differences found in the tests or by downplaying them. This would also be a reason why further studies on larger groups are necessary, in order to validate these results.
In conclusion, we found that patients recovering from severe COVID-19 seem to have reduced physical functioning and activity levels and they perceived their recovery to be slow and difficult. They experienced a lack of clinical support and contradictory advice regarding rehabilitation. It is obvious from the interviews that coaching regarding a full return to physical functioning after the infection needs to be better coordinated and that there is a need for guidelines for health professionals to avoid patients receiving contradictory advice.
Acknowledgement
We would like to thank all the participants for taking part in the tests and sharing their experiences with being sick with COVID-19 in the interviews.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated and analysed during the current study are not publicly available, as they contain information that could compromise the integrity of research participants. However, they are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Li J, Huang DQ, Biyao Z, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449–1458.
- Piroth L, Cottenet J, Mariet AS, et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9(3):251–259.
- Stevens RD, Dowdy DW, Michaels RK, et al. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33(11):1876–1891.
- Herridge MS, Moss M, Hough CL, et al. Recovery and outcomes after the acute respiratory distress syndrome (ARDS) in patients and their family caregivers. Intensive Care Med. 2016;42(5):725–738.
- Simonelli C, Paneroni M, Vitacca M, et al. Measures of physical performance in COVID-19 patients: a mapping review. Pulmonology. 2021;27(6):518–528.
- Heiberg KE, Heggestad AKT, Jøranson N, et al. Brain fog’, guilt, and gratitude: experiences of symptoms and life changes in older survivors 6 months after hospitalisation for COVID-19. Eur Geriatr Med. 2022;13(3):695–703.
- Xiong L, Li Q, Cao X, et al. Dynamic changes of functional fitness, antibodies to SARS-CoV-2 and immunological indicators within 1 year after discharge in Chinese health care workers with severe COVID-19: a cohort study. BMC Med. 2021;19(1):163.
- Delbressine JM, Machado FVC, Goërtz YMJ, et al. The impact of post-covid-19 syndrome on self-reported physical activity. IJERPH. 2021;18(11):6017.
- Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93(2):1013–1022.
- Barker-Davies RM, O’Sullivan O, Senaratne KPP, et al. The Stanford hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54(16):949–959.
- Demeco A, Marotta N, Barletta M, et al. Rehabilitation of patients post-COVID-19 infection: a literature review. J Int Med Res. 2020;48(8):030006052094838.
- Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. 2nd ed. Thousand Oaks, CA: SAGE Publications; 2011.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117.
- Bohannon RW. Sit-to-stand test for measuring performance of lower extremity muscles. Percept Mot Skills. 1995;80(1):163–166.
- Bestall JC, Paul EA, Garrod R, et al. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586.
- Kowalchuk Horn K, Jennings S, Richardson G, et al. The patient-specific functional scale: psychometrics, clinimetrics, and application as a clinical outcome measure. J Orthop Sports Phys Ther. 2012;42(1):30–42.
- Salén BA, Spangfort EV, Nygren ÅL, et al. The disability rating index: an instrument for the assessment of disability in clinical settings. J Clin Epidemiol. 1994;47(12):1423–1435.
- Feng Y-S, Kohlmann T, Janssen MF, et al. Psychometric properties of the EQ-5D-5L: a systematic review of the literature. Qual Life Res. 2021;30(3):647–673.
- Wang Y-C, Bohannon RW, Li X, et al. Hand-grip strength: normative reference values and equations for individuals 18 to 85 years of age residing in the United States. J Orthop Sports Phys Ther. 2018;48(9):685–693.
- Hedenström H. Värt att veta om spirometri. 3rd ed. Skärholmen: Boehringer Ingelheim; 2003.
- American Thoracic Society/European Respiratory Society American thoracic society/European respiratory society ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624.
- Sclauser Pessoa IMB, Parreira VF, Fregonezi GAF, et al. Reference values for maximal inspiratory pressure: a systematic review. Can Respir J. 2014;21(1):43–50.
- Evans JA, Whitelaw WA. The assessment of maximal respiratory mouth pressures In adults. Respir Care. 2009;54:1348–1359.
- Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188.
- Garcia-Gordillo MA, Adsuar JC, Olivares PR. Normative values of EQ-5D-5L: in a spanish representative population sample from Spanish health survey, 2011. Qual Life Res. 2016;25(5):1313–1321.
- Strassmann A, Steurer-Stey C, Lana KD, et al. Population-based reference values for the 1-min sit-to-stand test. Int J Public Health. 2013;58(6):949–953.
- Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37(1):150–156.
- Hansen BH, Kolle E, Steene-Johannessen J, et al. Monitoring population levels of physical activity and sedentary time in Norway across the lifespan. Scand J Med Sci Sports. 2019;29(1):105–112.
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: concepts, procedures and measures to achieve trustworthiness. Nurse Educ Today. 2004;24(2):105–112.
- Eldh AC, Årestedt L, Berterö C. Quotations in qualitative studies: reflections on constituents, custom, and purpose. Int J Qual Methods. 2020;19:1–6.
- World Medical Association World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357.
- Faghy MA, Maden-Wilkinson T, Arena R, et al. COVID-19 patients require multi-disciplinary rehabilitation approaches to address persisting symptom profiles and restore pre-COVID quality of life. Expert Rev Respir Med. 2022;16(5):595–600.
- Ashton R, Ansdell P, Hume E, et al. COVID-19 and the long-term cardio-respiratory and metabolic health complications. Rev Cardiovasc Med. 2022;23(2):053.
- Schandl A, Hedman A, Lyngå P, et al. Long-term consequences in critically ill COVID-19 patients: a prospective cohort study. Acta Anaesthesiol Scand. 2021;65(9):1285–1292.
- Bennell K, Dobson F, Hinman R. Measures of physical performance assessments: self-Paced walk test (SPWT), stair climb test (SCT), six-minute walk test (6MWT), chair stand test (CST), timed up & go (TUG), sock test, lift and carry test (LCT), and car task. Arthritis Care Res. 2011;63(S11):S350–S370.
- Gigliotti F. Mechanisms of dyspnea in healthy subjects. Multidiscip Respir Med. 2010;5(3):195–201.
- Schouborg LB, Molsted S, Lendorf ME, et al. Risk factors for fatigue and impaired function eight months after hospital admission with COVID-19. Dan Med J. 2022;69:8210633.
- Rooney S, Webster A, Paul L. Systematic review of changes and recovery in physical function and fitness After severe acute respiratory syndrome–related coronavirus infection: implications for COVID-19 rehabilitation. Phys Ther. 2020;100(10):1717–1729.

