Abstract
Purpose
Spasticity is common in multiple sclerosis (MS), often leading to functional limitations and disability. We developed a conceptual model of spasticity in MS integrating expert opinion, recent literature, and experiences of clinicians and people with MS spasticity.
Methods
A conceptual model was developed based on a targeted literature review of articles published between 2014 and 2019, followed by input from clinicians, then input from participants with MS spasticity. Multidisciplinary experts on spasticity provided guidance at each step.
Results
Key concepts of the integrated spasticity conceptual model included: moderators; triggers; modifiers; treatment; objective manifestations; subjective experience; physical, functional, social, and emotional/psychological impacts; and long-term consequences. Participants with MS spasticity most frequently endorsed spasms, tightness, and pain as descriptors of spasticity. Some participants with MS spasticity had difficulty distinguishing spasticity from other MS symptoms (e.g. muscle weakness). Some triggers, emotional/psychological impacts, and long-term consequences of spasticity reported by participants with MS spasticity were not previously identified in the published literature.
Conclusions
This conceptual model of spasticity, integrating published literature with the experience of clinicians, people with MS spasticity, and experts, demonstrates the complex, multidimensional nature of MS spasticity. This model may be used to improve clinician–patient dialogue, research, and patient care.
IMPLICATIONS FOR REHABILITATION
Many people with multiple sclerosis (MS) have spasticity, generally in the lower limbs, but this symptom is complex and multidimensional and therefore difficult to characterize.
MS spasticity may be influenced by moderators, triggers, modifiers, and treatment, all of which can affect objective measures and the subjective experience of spasticity.
MS spasticity can have physical, functional, social, and emotional/psychological impacts as well as long-term consequences that can affect rehabilitation and ultimately reduce health-related quality of life for people with MS.
Given that people with MS may view spasticity differently than their rehabilitation providers, providers should ask patients about their spasticity, including their moderators, triggers, modifiers, experience, impacts, long-term consequences, and effects on quality of life.
This conceptual model provides a framework to improve clinician-patient dialogue, research, and rehabilitation for MS spasticity.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disorder of the central nervous system resulting in a variety of symptoms and often leading to functional impairment [Citation1, Citation2]. In 2017, it was estimated that there were over 900,000 people with MS (PwMS) in the United States [Citation3]. Up to 80% of PwMS experience spasticity, a complex and multidimensional symptom [Citation4]. Spasticity is challenging to characterize. Physiologic and environmental variables can trigger spasticity, and PwMS may experience spasticity in various ways [Citation5, Citation6]. Published studies that highlight the perspectives of PwMS on spasticity and its impact on health-related quality of life are limited [Citation7]. Since input from both PwMS and clinicians is needed to ultimately improve dialogue between PwMS and their healthcare providers, research is needed to better understand how PwMS and clinicians view MS spasticity based on their respective experiences.
The purpose of this study was to develop a conceptual model of MS spasticity that integrates perspectives from recent peer-reviewed literature with those of clinicians, people with MS spasticity, and multidisciplinary experts in the field of spasticity from academia and industry. This integrated conceptual model provides a framework for describing and understanding spasticity in PwMS.
Methods
Overview of study design
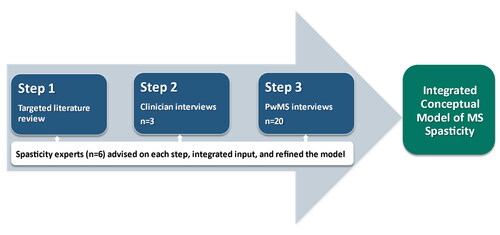
This study used qualitative methods to develop a conceptual model of MS spasticity using a multistep iterative process (). Relevant peer-reviewed literature was reviewed to produce an initial conceptual model. Clinician feedback was then used to revise this initial conceptual model and to develop a guide for interviews of PwMS with spasticity. Semi-structured interviews with PwMS and spasticity were conducted to further refine and finalize the conceptual model. At each step, input was provided by the six authors who are experts on spasticity from different disciplines, including neurology, physiatry, and physical therapy (JB, JRS, JMW, FB, MC, and EFF; ).
Targeted literature review
A targeted literature search of PubMed of English language papers on MS spasticity was performed. Only randomized controlled trials, other interventional studies, observational studies, and review articles were included. Abstracts were included if they were published within the five years prior to when the project was initiated (January 1, 2014 through September 3, 2019). The complete search strategy for this targeted literature review is available in Supplemental Table 1. This resulted in 282 abstracts. Two authors reviewed these abstracts independently for inclusion in the full-text literature review. Abstracts were excluded if they described animal studies, did not mention symptoms or impacts of spasticity, or did not mention spasticity. Of the 282 abstracts retrieved, 29 met these criteria and were reviewed as full-text articles. Of the 29 reviewed articles, only 19 described spasticity in terms of symptoms, impacts, and other concepts of interest and provided insights across MS and other key indications. The spasticity experts were asked to identify additional key references, including seminal papers published outside the screening publication time window and papers referred to by the identified publications. This yielded an additional four articles for a total of 23 articles. Additionally, a desk review of patient organization websites (National Multiple Sclerosis Society [www.nationalmssociety.org]) and the Multiple Sclerosis Trust [www.mstrust.org.uk]) was conducted to identify any additional patient-friendly spasticity descriptors and impacts. These were used to develop the initial conceptual model of MS spasticity.
Interviews with MS clinicians
Three clinicians who treat PwMS at different institutions were individually interviewed via telephone using a semi-structured interview guide. Their identities were not disclosed to the sponsor. Each interview lasted approximately one hour. The interviews focused on the key characteristics of spasticity and how, in their experience, PwMS describe symptoms of spasticity. These clinicians also gave feedback on the relevance and completeness of the initial conceptual model based on the literature review. The interviews were audio recorded. These clinician interviews and subsequent discussions with the experts in spasticity were the basis for initial refinements to the conceptual model which was used to develop a semi-structured patient interview guide.
Interviews with people with spasticity due to MS
Twenty partients with clinician-diagnosed MS of any subtype with MS-related spasticity were individually interviewed via telephone using a patient interview guide. Their identities were not disclosed to the sponsor. The interview took approximately 90 min. The interviews focused on the patients’ experience of spasticity and its impacts. The interviews were audio recorded and transcribed.
Study enrollment
Potential participants with clinician-diagnosed MS-related spasticity were referred by their clinician to a third-party recruitment vendor independent of the study sponsor. The referring clinician confirmed the diagnosis of MS and spasticity and provided additional clinical details. Alternatively, participants could share further details on their diagnosis via screenshots of medical records, confirmation letter from the clinician, or other confirmatory documentation. Potential participants were screened for eligibility prior to the interview. Participants were eligible if they were ≥ 18 years old, could read and write English, were diagnosed with MS of any subtype for ≥ 12 months, and had spasticity due to MS for ≥ 6 months prior to the study as determined by the referring clinician. Exclusion criteria included any comorbid disorder of the central nervous system, a diagnosis of clinically isolated syndrome, or an impairment (e.g. visual) that could interfere with study participation.
Potential participants were recruited during regularly scheduled patient clinical visits (in person or via telehealth) or by phone. Interested PwMS were invited to contact the recruitment vendor to schedule an interview. Written consent was provided by participants at the time of the interview; participants confirmed consent verbally to be audio recorded. Participants were remunerated for their time. This study received ethics approval from an independent institutional review board (Advarra Pro00045192).
Interviews
The interviews were conducted by four trained research staff with experience in qualitative methods. The semi-structured interview guide was used to draw out the participants’ experience of spasticity. Participants were first asked how long they had been diagnosed with MS, what symptoms they typically experienced in relation to spasticity, where those symptoms occurred in their body, and what spasticity meant to them. Additional questions focused on understanding the triggers of spasticity, how long the symptoms lasted, and how daily functioning was affected. Participants were also asked to comment on whether spasticity caused or worsened other symptoms, such as pain, fatigue, or sexual dysfunction. Participants were then asked to describe their spasticity experience and were probed using the key concepts identified in the literature or during the clinician interviews as necessary for further elucidation or clarification. Interviews were continued until saturation was reached. Saturation was defined as the point at which no substantially new themes, descriptions of a concept, or terms were introduced as additional interviews were conducted [Citation8].
After the interviews, participants were asked to complete the following forms and assessments: a sociodemographic and clinical information form, the Patient Determined Disease Steps (PDDS) [Citation9], and Spasticity Numeric Rating Scale (NRS-S). The NRS-S was a single question asking, “On a scale of 0 to 10, please indicate your level of spasticity over the last 24 h, considering 0 as ‘no spasticity’ and 10 as ‘worst possible spasticity’”.
Data analysis
The patient participant interview transcripts were reviewed and the content coded for concepts by trained coders. The codes were then entered into a saturation grid to track and tally identified concepts. Content analysis was performed using thematic analysis in ATLAS.ti version 8.0. (ATLAS.ti, Bergmannstraße 68, Berlin, Germany). Demographic and self-reported clinical data were analyzed using descriptive statistics, such as frequencies, means, and percentages.
Results
Literature review
The 23 full-text articles reviewed yielded an initial conceptual model that included moderators, triggers, modifiers, treatment, objective manifestations, subjective experiences, and impacts of MS spasticity (, Column 2; Supplemental Table 2) [Citation10, Citation11].
Table 1. Multistep process for developing the integrated conceptual model of multiple sclerosis spasticityTable Footnotea.
Interviews with MS clinicians
The clinicians were two physicians and a physical therapist. They had an average of 22 years of experience treating PwMS. They reported PwMS using a wide variety of spasticity descriptors, including stiffness, spasms, muscle contractions, weakness, heaviness, tightness, and cramping. All clinicians described PwMS reporting pain as a part of the spasticity experience. The key revisions of the conceptual model based on the clinician interviews and the input of the multi-disciplinary experts on spasticity were the addition of: the triggers of physical dysfunction (e.g. skin lesions) and other environmental factors (e.g. tight clothing); altered voluntary movement pattern, which is an objective manifestation of spasticity in the final model; both slowness of movement and muscle jerks as subjective experiences in the final model; and the impact of loss of leisure and physical activities as a long-term consequence. Other key revisions include the removal of the descriptors of rigidity, limited range of motion, and orthopedic symptoms and the impact of disability (, Column 3; Supplemental Table 3).
Interviews with people with spasticity due to MS
Participant characteristics
Participants (n = 20) had a mean age of 47.3 ± 9.6 years and were mostly female (n = 17, 85%) and white (n = 16, 80%) (). Sixty percent of participants reported their employment status as disabled, and 80% had at least some college education. Participants were diagnosed with MS for 11.6 ± 8.8 years. They reported experiencing spasticity for 12.3 ± 9.5 years, and their average spasticity NRS-S score was 5.4 (standard deviation = 1.6). Participants experienced spasticity in the legs (n = 19/20, 95%), arms (n = 16/20, 80%), feet or toes (n = 13/20, 65%), fingers or hands (n = 10/20, 50%), hips (n = 7/20, 35%), and back (n = 4/20, 20%). The most common current antispasticity treatments included stretching (n = 16/20, 80%), oral baclofen (n = 12/20, 60%), and massage (n = 11/20, 55%).
Table 2. Demographic and self-reported clinical characteristics.
The quantitative results and representative qualitative quotes from the interviews with PwMS are provided in . A summary of the concepts identified during this research at each step is listed in Supplemental Table 3. In this table, key changes are noted from each step of the model development.
Table 3. Summary of interview information from PwMS.
Final conceptual model
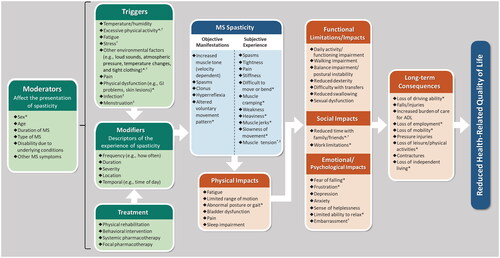
The draft conceptual model based on the targeted literature review was modified by findings from the interviews of clinicians and people with spasticity due to MS and was finalized by the multidisciplinary experts on spasticity ( Column 4; ). The final conceptual model describes moderators (variables that affect the presentation of spasticity for PwMS), triggers, modifiers (descriptors of the experience of spasticity), and treatment of spasticity. These affect a range of objective manifestations and subjective experiences of MS spasticity. These subsequently result in physical impacts that can lead to functional limitations, social impacts, and emotional/psychological impacts. The conceptual model then describes the long-term consequences of spasticity, which may ultimately reduce the health-related quality of life of PwMS.
Figure 2. Integrated conceptual model of multiple sclerosis spasticity.
*Variable not identified in literature review.
†Variable not identified in clinician interviews.
‡Variable not identified in patient interviews.
Abbreviations: ADL = activities of daily living; GI = gastrointestinal; MS = multiple sclerosis.
Triggers, MS Spasticity - Subjective Experience, Functional Limitations/Impacts, Social Impacts, Emotional/Psychological Impacts, and Long-term Consequences are listed in order of decreasing percentage of patients reporting the variable, as in .
Discussion
Spasticity is common in PwMS, although difficult to characterize because it is complex and multidimensional. Our conceptual model, derived from this qualitative study, integrates a targeted literature review, clinician opinions, and insights from people with spasticity due to MS with opinions from experts in the field of MS spasticity, to provide a comprehensive framework to better describe and understand spasticity in PwMS.
The literature provided the information necessary to develop the initial draft of the conceptual model of MS spasticity. The key concepts obtained from the literature review included moderators of MS spasticity (duration of MS, age, sex, type of MS), triggers of spasticity, descriptors of spasticity, and impacts and consequences of spasticity (Supplemental Table 2 and Supplemental Table 3) [Citation4–6, Citation11–30]. The spasticity experts reviewed this information, and then the draft model was used to develop the interview guide used for clinician interviews.
The clinicians and multi-disciplinary experts gave us information that further refined the model. Two additional triggers were added: skin lesions and tight clothing. Their feedback also led us to remove rigidity and limited range of motion from the model. The following descriptors of spasticity were added based upon their feedback: weakness, slowness of movement, altered voluntary movement pattern, and muscle jerks. Disability was removed from impacts and consequences. Frustration, loss of mobility, difficulty with transfers, and reduced life expectancy were added to impacts and long-term consequences.
The PwMS confirmed many items, identified some new concepts, and recommended removal of some triggers. Specifically, the new concepts were social impacts of reduced time with family/friends and embarrassment. They also recommended adding triggers of stress, physical activity (e.g. movement daily activities, exertion, sitting/standing too long) and other environmental factors, and removing triggers of urinary tract infection, full bladder, and sleep.
In addition, spasticity can occur alongside other symptoms of MS [Citation12], making it difficult for PwMS to distinguish between the variety of sensorimotor dysfunctions they experience and spasticity. For instance, a few participants mentioned tremor or numbness as part of their spasticity experience; however, this descriptor was not confirmed in the literature or by the multidisciplinary experts on spasticity as being associated with spasticity. The authors believe that participants were using “tremor” in a nontechnical way to describe an aspect of their experience. For instance, it is possible that they were experiencing clonus, which is a rhythmic movement commonly associated with spasticity. Weakness (subjective experience), bladder dysfunction (physical impact), and reduced swallowing (functional impact) were included in the conceptual model, as these items were associated with spasticity according to participants and the literature. The conceptual model includes moderators such as MS duration, type of MS, and other MS symptoms to acknowledge that there are external factors, both MS-related and non-related, that may influence the experience of spasticity.
This conceptual model presents pain as a prevalent and complicated component of spasticity; the recent literature [Citation6, Citation28], opinions from clinicians and multidisciplinary experts on spasticity, and participant interviews confirmed pain as a subjective experience, a trigger, a physical impact, and a moderator of MS spasticity. Almost all participants reported pain as part of their spasticity experience. Clearly, understanding the relationship between pain and spasticity is important for optimizing the management of MS spasticity. Some patient participants felt that fatigue, which is one of the most common and debilitating symptoms of MS [Citation31, Citation32], could be caused by pain (subjective experience and physical impact) and soreness from spasticity. Thus, fatigue was included as both a trigger and physical impact of spasticity.
The long-term consequences of spasticity are difficult to assess with traditional clinical research studies, which usually last a maximum of two years; therefore, consequences beyond two years may not be well represented in the literature. However, PwMS described several long-term consequences of spasticity, including contractures, fear of falling, difficulty with transfers, loss of employment, loss of driving ability, loss of independent living, and increased burden of care. Given that PwMS may live decades with spasticity [Citation33], additional research is needed to elucidate the long-term consequences of spasticity in order to develop and implement strategies to minimize their occurrence and improve the quality of life of PwMS.
This study had a number of strengths. Our model included multiple perspectives, including those of clinicians who treat many patients with MS spasticity and the expertise of people with the lived experience in an iterative process to develop this conceptual model of MS spasticity. During the iterative development process, experts from academia and industry further informed the development of the model. Importantly, the interviews with PwMS were repeated until saturation was reached. Another strength is that the project team utilized experts in conceptual models and qualitative research to conduct this project. This model confirms that MS spasticity is complex and multifaceted. The model takes a very complex experience and breaks it down into moderators, triggers, modifiers, and treatment of spasticity as well as describing spasticity (both objective and subjective experiences). In addition, this model describes the impacts and long-term consequences of spasticity that PwMS experience. The most comprehensive scale to date used to assess MS spasticity, the MSSS-88, focuses on the impacts of spasticity in PwMS but does not address moderators, modifiers, treatment, triggers, or long-term consequences of MS spasticity. [Citation34].
The development of this conceptual model is not without limitations. Our initial literature review was not systematic and was time limited. However, this only served as a starting place for the iterative multistep model-building process. Some of the symptoms and consequences of spasticity described in our final model could also be explained by other symptoms of MS (e.g. difficulty moving that a PwMS attributes to spasticity may actually be related to muscle weakness). However, our approach balanced input from PwMS and clinicians to mitigate this limitation. Bias could have been introduced by financial compensation to the PwMS, although it was provided as reimbursement for their time at fair market value, as is standard in clinical research. Finally, all study participants being US based, and the sample of clinicians and PwMS was small. However, the demographics of our sample of 20 people with MS is similar to that of other studies [Citation33, Citation35], and is in line with recent literature on qualitative concept elicitation, which indicates that a sample of 20 people should capture 97% of symptom concepts [Citation36].
Conclusion
Spasticity in PwMS is common, complex, and has a wide range of moderators, triggers, modifiers, treatment, objective manifestations, and subjective experiences. MS spasticity can have impacts that are physical, functional, social, and psychological as well as long-term consequences that can ultimately reduce health-related quality of life. This integrated conceptual model of MS spasticity includes the perspectives of PwMS, clinicians who treat PwMS, and multidisciplinary spasticity experts. The model may have several practical applications at the point of care, including improving clinician-patient dialogue, allowing treatment decisions to be personalized, assisting PwMS to set goals, and facilitating spasticity education. Furthermore, the model may be used to select and validate clinical research endpoints and to inform regulatory decision making.
Author contributions
Francois Bethoux, MD has received honoraria from GW Pharma, now a part of Jazz Pharmaceuticals, Inc. for participation in advisory board meetings; honoraria from Osmotica for consulting; and royalties from Springer International Publishing for being co-editor of a book.
Edelle Field-Fote, PT, PhD, FAPTA, FASIA serves as a paid consultant for Greenwich Biosciences, Inc., now a part of Jazz Pharmaceuticals, Inc.
William R. Lenderking, PhD, Katelyn N. Cutts, MS, and Erica Zaiser, PhD are employees of Evidera, which was contracted by Greenwich Biosciences, Inc., now a part of Jazz Pharmaceuticals, Inc. to perform this study.
Joanne Wagner, PT, PhD and Joris Berwaerts, MD are former employees of Greenwich Biosciences, Inc., now a part of Jazz Pharmaceuticals, Inc. Carlsbad, CA, USA.
Joshua R. Steinerman, MD is an employee of Jazz Pharmaceuticals, Inc., Philadelphia, PA, USA
Supplemental Material
Download MS Word (69 KB)Acknowledgements
Writing support was provided by Katie Crosslin, PhD and Stephen Gilliver, PhD, of Evidera/PPD.
Disclosure statement
Michelle Cameron, MD, PT, MCR serves as a paid consultant for Greenwich Biosciences, Inc., now a part of Jazz Pharmaceuticals, Inc.
Additional information
Funding
References
- Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169–180. doi: 10.1056/NEJMra1401483.
- Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet. 2018;391(10130):1622–1636. doi: 10.1016/S0140-6736(18)30481-1.
- Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. doi: 10.1212/WNL.0000000000007035.
- Bethoux F, Marrie RA. A cross-sectional study of the impact of spasticity on daily activities in multiple sclerosis. Patient. 2016;9(6):537–546. doi: 10.1007/s40271-016-0173-0.
- Barin L, Salmen A, Disanto G, et al. The disease burden of multiple sclerosis from the individual and population perspective: which symptoms matter most? Mult Scler Relat Disord. 2018;25:112–121. doi: 10.1016/j.msard.2018.07.013.
- Norbye AD, Midgard R, Thrane G. Spasticity, gait, and balance in patients with multiple sclerosis: a cross-sectional study. Physiother Res Int. 2020;25(1):e1799. doi: 10.1002/pri.1799.
- Bhimani R, Anderson L. Clinical understanding of spasticity: implications for practice. Rehabil Res Pract. 2014;2014:279175. doi: 10.1155/2014/279175.
- Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant. 2018;52(4):1893–1907. doi: 10.1007/s11135-017-0574-8.
- Learmonth YC, Motl RW, Sandroff BM, et al. Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurol. 2013;13:37. doi: 10.1186/1471-2377-13-37.
- Lance JW. Pathophysiology of spasticity and clinical experience with baclofen. In Lance JW, Feldman, R.G., Young, R.R. and Koella, W.P., editor. Spasticity: disordered motor control. Chicago: Year Book; 1980. p. 185–204.
- Pandyan AD, Gregoric M, Barnes MP, et al. Spasticity: clinical perceptions, neurological realities and meaningful measurement. Disabil Rehabil. 2005;27(1-2):2–6. doi: 10.1080/09638280400014576.
- Paolucci S, Martinuzzi A, Scivoletto G, et al. Assessing and treating pain associated with stroke, multiple sclerosis, cerebral palsy, spinal cord injury and spasticity. Evidence and recommendations from the italian consensus conference on pain in neurorehabilitation. Eur J Phys Rehabil Med. 2016;52(6):827–840.
- Dressler D, Bhidayasiri R, Bohlega S, et al. Defining spasticity: a new approach considering current movement disorders terminology and botulinum toxin therapy. J Neurol. 2018;265(4):856–862. doi: 10.1007/s00415-018-8759-1.
- Balantrapu S, Sosnoff JJ, Pula JH, et al. Leg spasticity and ambulation in multiple sclerosis. Mult Scler Int. 2014;2014:649390. doi: 10.1155/2014/649390.
- Milinis K, Young CA, Trajectories of Outcome in Neurological Conditions (TONiC) study. Systematic review of the influence of spasticity on quality of life in adults with chronic neurological conditions. Disabil Rehabil. 2016;38(15):1431–1441. doi: 10.3109/09638288.2015.1106592.
- Flachenecker P, Henze T, Zettl U. Spasticity in patients with multiple sclerosis–clinical characteristics, treatment and quality of life. Acta Neurol Scand. 2014;129(3):154–162. doi: 10.1111/ane.12202.
- Zettl UK, Henze T, Essner U, et al. Burden of disease in multiple sclerosis patients with spasticity in Germany: mobility improvement study (move I). Eur J Health Econ. 2014;15(9):953–966. doi: 10.1007/s10198-013-0537-5.
- Trojano M, Celius EG, Donze C, et al. Clinical case reviews and poster sessions in multiple sclerosis spasticity: main outcomes and highlights. Eur Neurol. 2014;72(Suppl 1):15–19. doi: 10.1159/000367619.
- Vermersch P. MObility ImproVEment with spasticity in multiple sclerosis in Europe: the MOVE 1 EU study. Neurodegener Dis Manag. 2014;4(6):407–415. doi: 10.2217/nmt.14.44.
- Cordeau D, Courtois F. Sexual disorders in women with MS: assessment and management. Ann Phys Rehabil Med. 2014;57(5):337–347. doi: 10.1016/j.rehab.2014.05.008.
- Meuth SG, Vila C, Dechant KL. Effect of sativex on spasticity-associated symptoms in patients with multiple sclerosis. Expert Rev Neurother. 2015;15(8):909–918. doi: 10.1586/14737175.2015.1067607.
- Milinis K, Tennant A, Young C. Spasticity in multiple sclerosis: associations with impairments and overall quality of life. Mult Scler Relat Disord. 2016;5:34–39. doi: 10.1016/j.msard.2015.10.007.
- Flachenecker P. Evolution of multiple sclerosis spasticity-associated symptoms: latest data. Neurodegener Dis Manag. 2016;6(6s):9–12. doi: 10.2217/nmt-2016-0047.
- Shaikh A, Phadke CP, Ismail F, et al. Relationship between botulinum toxin, spasticity, and pain: a survey of patient perception. Can J Neurol Sci. 2016;43(2):311–315. doi: 10.1017/cjn.2015.321.
- Giacoppo S, Bramanti P, Mazzon E. Sativex in the management of multiple sclerosis-related spasticity: an overview of the last decade of clinical evaluation. Mult Scler Relat Disord. 2017;17:22–31. doi: 10.1016/j.msard.2017.06.015.
- Maitin IB, Cruz E. Special considerations and assessment in patients with multiple sclerosis. Phys Med Rehabil Clin N Am. 2018;29(3):473–481. doi: 10.1016/j.pmr.2018.03.003.
- Cheung J, Rancourt A, Di Poce S, et al. Patient-identified factors that influence spasticity in people with stroke and multiple sclerosis receiving botulinum toxin injection treatments. Physiother Can. 2015;67(2):157–166. doi: 10.3138/ptc.2014-07.
- Rizzo M, Hadjimichael O, Preiningerova J, et al. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004;10(5):589–595. doi: 10.1191/1352458504ms1085oa.
- Sweatman WM, Heinemann AW, Furbish CL, et al. Modified PRISM and SCI-SET spasticity measures for persons with traumatic spinal cord injury: results of a rasch analyses. Arch Phys Med Rehabil. 2020;101(9):1570–1579. doi: 10.1016/j.apmr.2020.05.012.
- Fernández Ó, Costa-Frossard L, Martínez-Ginés M, et al. The broad concept of "spasticity-plus syndrome" in multiple sclerosis: a possible new concept in the management of multiple sclerosis symptoms. Front Neurol. 2020;11:152. doi: 10.3389/fneur.2020.00152.
- Fisk JD, Pontefract A, Ritvo PG, et al. The impact of fatigue on patients with multiple sclerosis. Can j Neurol Sci. 1994;21(1):9–14. doi: 10.1017/S0317167100048691.
- Kobelt G, Thompson A, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–1136. doi: 10.1177/1352458517694432.
- Kister I, Bacon TE, Chamot E, et al. Natural history of multiple sclerosis symptoms. Int J MS Care. 2013;15(3):146–158. doi: 10.7224/1537-2073.2012-053.
- Hobart JC, Riazi A, Thompson AJ, et al. Getting the measure of spasticity in multiple sclerosis: the multiple sclerosis spasticity scale (MSSS-88). Brain. 2006;129(Pt 1):224–234. doi: 10.1093/brain/awh675.
- Langer-Gould AM, Gonzales EG, Smith JB, et al. Racial and ethnic disparities in multiple sclerosis prevalence. Neurology. 2022;98(18):e1818–e1827. doi: 10.1212/WNL.0000000000200151.
- Turner-Bowker DM, Lamoureux RE, Stokes J, et al. Informing a priori sample size estimation in qualitative concept elicitation interview studies for clinical outcome assessment instrument development. Value Health. 2018;21(7):839–842. doi: 10.1016/j.jval.2017.11.014.