Abstract
The phylogenetic affinities of the brown alga Herpodiscus durvillaeae, an obligate parasite of Durvillaea antarctica (Fucales, Phaeophyceae) endemic to New Zealand, were analysed using combined partial nuclear encoded ribosomal DNA (26S) and plastid encoded RuBisCO (rbcL) gene sequences. Results from phylogenetic analyses place this species within the order Sphacelariales. Molecular data were supported by two morphological features characteristic for the Sphacelariales sensu stricto: the presence of apical cells and the transitory blackening of the cell wall with sodium hypochlorite solution (‘Eau de Javel’). However, the strongly heteromorphic life history distinguishes H. durvillaeae from all other members of the Sphacelariales of which the life cycle is known. This variability in life history provides a new systematic character for the order and should be taken into account in any comprehensive systematic revision of the Sphacelariales.
Introduction
Herpodiscus durvillaeae (Lindauer) South is an obligate endophyte of the southern bull kelp, D. antarctica (Chamisso) Hariot (Fucales, Phaeophyceae). It is endemic to host populations in New Zealand (Lindauer et al., Citation1961; South, Citation1974). The cells of H. durvillaeae contain numerous small discoid plastids, which are grey in colour suggesting a parasitic life style (South, Citation1974; Peters, Citation1990). The sporophyte of the parasite is filamentous, consisting of a perennial endophytic portion and seasonal epiphytic filaments emerging from the internal hyphae, which form characteristic velvety red-brown patches on the host surface. Branching of the filaments occurs only in the internal portion or near the base of the external filaments, just below the host surface. Reproductive structures are limited to the emergent part, with unilocular sporangia borne terminally on short erect filaments between longer vegetative filaments. Spores released from the unilocular sporangia settle on filaments nearby or on/in the sporangia, and transform entirely into so-called ‘secondary unilocular sporangia’ (South, Citation1974). Peters (Citation1990) showed that these features are minute gametophytes entirely turned into gametangia, suggesting a heteromorphic life cycle with a macroscopic sporophyte and a strongly reduced gametophyte.
The combination of its morphological characters has made the classification of H. durvillaeae problematical (Lindauer, Citation1947, Citation1949; South, Citation1974; John & Lawson, Citation1974; Peters, Citation1990). Originally, the parasite was assigned to the family Ralfsiaceae Farlow, first as Herponema sp., later as the new species Hapalospongidion durvilleae Lindauer (Lindauer, Citation1947, Citation1949). However, the type species of the genus Hapalospongidion, H. gelatinosum Saunders, has only a single plate-like chloroplast (John & Lawson, Citation1974). Therefore, the latter authors accommodated the parasite in the genus Basispora, even though the parasitic life style and the basal penetrating filaments of B. durvillaeae (Lindauer) John & Lawson comb. nov. differed markedly from the other two, epilithic and crustose, Basispora species (John & Lawson, Citation1974).
Unlike Basispora, the parasite of Durvillaea superficially resembles the morphology of some members of the Elachistaceae (South, Citation1974), showing features such as the absence of branching in the external phase of the parasite and the position of the primary unilocular sporangia at a distinct height on the filaments, forming a row above the host surface (Fletcher, Citation1987; Womersley, Citation1987; Lee et al., Citation2002). Therefore, South (Citation1974) erected the monotypic Herpodiscus gen. nov. within the Elachistaceae to accommodate this species as H. durvilleae (Lindauer) South. However, in the Elachistaceae, as in other Ectocarpales s.l., plastids are equipped with pedunculate pyrenoids (Womersley, Citation1987; Lee et al., Citation2002), and terminal unilocular sporangia and sexuality are also unknown (Uwai et al., Citation2000, Citation2001, and references therein).
The heteromorphic life history of H. durvilleae with a reduced gametophyte also separates it from the Ralfsiaceae in which gametophytes and sporophytes are isomorphic (Womersley, Citation1987). Instead, the life history of H. durvillaeae resembles that of Syringoderma floridana Henry, a member of the Syringodermatales. Syringoderma floridana has a macroscopic sporophyte and a reduced gametophyte, which settles on the sporophyte and turns entirely into a gametangium (Henry, Citation1984). Consequently, Henry (Peters, Citation1990; E.C. Henry, personal communication) suggested H. durvillaeae might have affinities with the Syringodermatales. The morphology of the Herpodiscus sporophyte, however, is different from the sporophytes of the Syringodermatales, which are fan-shaped and composed of appressed filaments. Peters (Citation1990) concluded that the morphology of H. durvillaeae is too distinct from Syringoderma species for a close relationship.
Its parasitic lifestyle makes H. durvillaeae unique, but it also generates problems for studying its morphology. Attempts to cultivate this species have been unsuccessful to date (E.C. Henry, personal communication; unpublished results), and there is no information available on developmental characters, such as growth patterns and the formation of filaments. Additionally, the perennial part of the thallus is hidden within the tissue of its host D. antarctica: The apparent lack of phaeophycean pigments in the Herpodiscus cells makes it difficult to distinguish them from internal host cells, which are also colourless (South, Citation1974). The present study combined rbcL and nrDNA sequence comparisons with morphological observations using light and electron microscopy to examine the systematic relationships of H. durvillaeae.
Materials and methods
Sampling
Samples of D. antarctica infected with H. durvillaeae were collected at Brighton, Otago, South Island, New Zealand (45°57′11′S; 170°20′9″E), between March 1997 and March 2001. Samples were transported to the laboratory within 30 minutes. Epiphytic patches of H. durvillaeae collected on 3 June 1998 and 20 June 2000 were checked microscopically for the presence of other brown algae. To test for potential contamination, some epiphytic material was removed, inoculated with Provasoli-enriched seawater (PES; Starr & Zeikus, Citation1993) and cultivated at 15°C, 10:14 h light–dark cycle (25µmol photons m−2s−1; Philips TLD 36W/33 cool white fluorescent bulbs) in a Contherm Phytotron Climate Simulator (Contherm, Wellington, New Zealand). Host thallus fragments with parasite patches were freeze-dried, after which external parasite filaments were harvested. Herbarium vouchers were deposited at the Museum of New Zealand, Wellington, New Zealand (WELT; Holmgren et al. Citation1990: WELT A027895, WELT A027896).
Molecular biology
Total DNA was extracted from around 20mg freeze-dried external parasite filaments ground in liquid nitrogen following a modified method after van Oppen et al. (Citation1993; Peters & Burkhardt, Citation1998), or by using a DNeasy Plant Mini Kit (Qiagen, Germany), according to the manufacturer's instructions. The extracted genomic DNA was diluted (1:10 and 1:100) with TrisEDTA buffer (0.1% 1M Tris, 0.02% 0.5M EDTA, pH 8.0) for PCR amplifications.
Nuclear ribosomal DNA and rbcL gene regions were amplified in PCR reactions of 10µl volume containing 1µl diluted genomic DNA, 1µl 10 x PCR reaction buffer, 2.5mM MgCl2, 20µM of each dNTP, 1 pmol µl−1 of each primer (primers LSU-16(F), LSU1046(R), rbcL95F, rbcL1087SR, TW5SF, 5.8S1R, 5.8S3F LSU602R and LSU58R according to Peters & Ramirez, Citation2001; primers JO3CSR and ITS1R according to Peters & Burkhardt, Citation1998; new primer AFP4F: ATTATTGATCTTGAACGAGG) and 0.025µl−1 recombinant Taq-polymerase. Chemicals were supplied by MBI Fermentas, Germany.
Amplifications were performed in a thermocycler (PTC 100, MJ Research, USA) or a robocycler (Robocycler Gradient 96, Stratagene Corporation, La Jolla, California). The PCR protocol for both nrDNA and rbcL genes comprised an initial denaturation step (94°C, 3min), followed by 32–35 cycles of denaturation (94°C, 1min), annealing (55°C, 1min), and extension (72°C, 1.5min; final extension 3.5min). PCR products were purified either using a QIAquick Purification Kit (Qiagen, Germany) or by polyethylene glycol precipitation, and were sequenced using an ABI PRISM™ automatic DNA Sequencer (Applied Biosystems, USA) following standard methods.
The rbcL gene was analysed separately and in combination with 26S nrDNA sequences. Outgroup species were Tribonema aequale and Phaeothamnion confervicola in rbcL analyses, and T. aequale for analyses of the combined data set. An additional rbcL data set used a modified taxon sampling closely following Draisma et al. (Citation2002), containing mainly members of the Sphacelariales sensu lato, using eight species as a polyphyletic outgroup: Choristocarpus tenellus, Discosporangium sp., D. mesarthrocarpum, Dictyota dichotoma, D. cervicornis, Syringoderma phinneyi, Onslowia endophytica and Verosphacela ebrachia. The sequences used in this study with their GenBank accession numbers and taxonomic affiliations are available as supplementary data from the European Journal of Phycology website. Sequences were aligned by eye using the text editor in PAUP* version 4.0b10 (Swofford, Citation2002). Unalignable regions of the 26S nrDNA matrix were removed for phylogenetic analysis and calculations of pair-wise distances.
An appropriate model of sequence evolution for each analysed dataset was estimated with Modeltest V3.0.6 (Posada & Crandall, Citation1998), and this model, GTR+I+G in all cases, was used for all distance and likelihood calculations. All three datasets were analysed using maximum parsimony (MP) and maximum likelihood (ML) optimality criteria, and Bayesian analysis. MP trees were estimated with PAUP*4.0b10. MP analyses were conducted using a heuristic search strategy with 10 replicates of random-order sequence addition followed by tree bisection reconnection (TBR) branch swapping. Bootstrap support was estimated with 1,000 replicates, each of 10 replicates of random order sequence addition followed by TBR branch swapping. Maximum likelihood analyses were conducted using PHYML v2.2.4 (Guindon & Gascuel, Citation2003) under the GTR+I+G model of sequence evolution, with concurrent estimation of parameters for invariant sites and gamma-modeled rate heterogeneity. Support was estimated using 500 bootstrap replicates.
Congruence of the combined dataset (RuBisCO and 26S nrDNA genes) was evaluated with partition homogeneity tests (PHT; Farris et al., Citation1995; Cunningham, Citation1997) in PAUP*4.0b10. The optimality criterion was set to maximum parsimony (1,000 replications and simple addition of taxa), with uninformative positions deleted before analysis. Datasets were considered to be congruent if p>0.05 (Farris et al., Citation1995; Cunningham, Citation1997).
Bayesian analyses were carried out using MrBayes v3.1.1 (Ronquist & Huelsenbeck, Citation2003) to run four Metropolis-coupled Markov chain Monte Carlo (MCMC) iterations (one cold and three incrementally heated, temperature parameter=0.2). Two independent MrBayes analyses were run under the GTR+I+G model of sequence evolution for 1,000,000 generations. For the combined dataset the data were partitioned by gene and parameters were optimized independently for each partition. Model parameters were treated as unknown and were estimated in each analysis. Chains were initiated with random starting trees and trees were sampled every 100 generations. Appropriate burn-in values were determined by inspection of plots of log-likelihood against generation time for each run. Trees obtained before this value were discarded, and the remaining trees were used to calculate 50% majority rule consensus trees, in which each clade posterior probability value is represented by the proportion of trees containing that clade.
Morphology
The morphology of the external phase of H. durvillaeae was observed with light and transmission electron microscopy on material collected in Brighton, Otago (New Zealand) between March 2000 and May 2001. Filaments were examined for the presence of two morphological characters typical of members of the order Sphacelariales: the presence of apical cells and the transitory blackening of cells with ‘Eau de Javel' (Migula, Citation1909).
Eau de Javel (circa 5% sodium hypochlorite solution; Reviers & Rousseau, Citation1999) was prepared from common household bleach diluted 1:10 with water. Freshly harvested filaments of H. durvillaeae from a thallus of D. antarctica collected in March 2001 were exposed to Eau de Javel, either by dripping the solution directly onto the Herpodiscus material, or by slow perfusion under a coverslip. Samples were immediately observed under a light microscope. As a positive control for the reaction, Halopteris sp. (Sphacelariales, Phaeophyceae) collected at the same location and date was likewise treated by exposing cross-sections of the main axis to the solution. Permanent slides of Sphacella subtilissima (prepared by D.G. Müller; field material from Fuerteventura: BM 000659630, culture material: BM 000659629) were examined under a light microscope. Photomicrographs were taken on a slide film (Agfa RSX II Professional daylight slide film, ISO 100, Germany).
For transmission electron microscopy, thallus fragments of D. antarctica infected with H. durvillaeae collected in March 2000, were fixed over-night at 4°C in a modified Karnovsky mixture containing 2% glutardialdehyde, 1% paraformaldehyde, and 1% caffeine in cacodylate buffer (0.1M sodium cacodylate, 2% NaCl, 0.1% CaCl2, pH 7.2; Clayton & Ashburner, Citation1994; Schoenwaelder & Clayton, Citation1998a,b). Samples were rinsed in cacodylate buffer with 1% caffeine and were post-fixed in 1% osmium tetroxide (in cacodylate buffer) for 2 hours at room temperature, before rinsing in cacodylate buffer and water. Samples were dehydrated in an automatic tissue processor (Lynx, Biomedical Corporation Europe Ltd.) in a graded series of ethanol in distilled water. Dehydrated samples were infiltrated in a graded series with the epoxy resin Quetol 651 (Kushida, Citation1974) employing the automatic tissue processor. The resin was polymerized at 60°C for 48–60h.
Ultra-thin sections (70–80nm thick) were cut with a diamond knife (Microstar, 2mm, 45°) mounted on an ultramicrotome (Reichert-Jung, Germany). Sections were collected on Formvar-coated slot grids, and were stained with lead citrate and uranyl acetate (Watson, Citation1958) in an automatic stainer (Ultrostainer 2168, Carlsberg System, LKB Bromma). Stained sections were examined using either a Philips EM 410 transmission electron microscope or an Akashi EM 002A transmission electron microscope. Photographs were taken on sheet or 35mm print negative films (Agfa or Kodak black and white film).
Slides and print negatives were scanned with a slide scanner (Microtek ScanMaker 35t plus) operated with ScanWizard™ Microtek 3.0.7. Digitized photographs were processed and assembled using Adobe Photoshop® version 5.0.
Results
Sequences and alignments
The partial rbcL sequence obtained for H. durvillaeae was 981bp long and did not contain stop codons, nor was it necessary to insert gaps for alignment with rbcL sequences from other brown algae. The translated sequence was similar to rbcL protein sequences from other brown algae. The 5′-partial 26S sequence was 687bp long. (GenBank accession numbers: rbcL: EF460467 [858bp]; 26S: EF460466 [552bp]).
Further Herpodiscus sequences determined were 612bp from the 3′-end of the 18S gene (GenBank accession number EF460465), and a stretch containing 137bp of the 5.8S subunit gene and the entire ITS2 of 431bp length (GenBank accession number EF488952). These sequences were not used in phylogenetic analyses because the 18S gene was not variable enough for analyses, while ITS2 sequences of Herpodiscus were too variable to be unambiguously alignable with sequences of other brown algae.
The partial rbcL alignment of Phaeophyceae contained 45 taxa (TreeBase accession no. M3693), while 28 entities were joined in the partial rbcL alignment concentrating on the Sphacelariales s.l..The former had a length of 1,100bp, an average base composition of (A: 0.31; C: 0.13; G: 0.17; T: 0.39) and contained 510 variable sites of which 409 were parsimony informative. The alignment comprised circa 75% of the complete phaeophycean RuBisCO large subunit gene sequence of 1,467bp (Siemer et al., Citation1998; Draisma et al., Citation2002). The alignment of the Sphacelariales s.l. contained 1,234bp, covering 84.1% of the complete phaeophycean rbcL gene sequence. Its average base composition was (A: 0.30; C: 0.13; G: 0.21; T: 0.36), and it comprised 476 variable positions. Of these 343 were parsimony informative. The sequence of H. durvillaeae as well as those of other reference brown algae did not cover the whole length of the alignment.
The alignment of partial 26S sequences comprised 37 phaeophycean taxa. Difficulties in aligning the sequences within the variable D1 and D2 regions required a reduction of this data set to 300bp (circa 9% of the complete 26S sequence of Scytosiphon lomentaria, length: 3,245bp; Kawai et al., Citation1995). The average base composition of the reduced dataset was (A: 0.27; C: 0.19; G: 0.32; T: 0.22). Of its 77 variable positions, 46 were parsimony informative. The PHT resulted in p=0.113, meaning rbcL and 26S data sets were congruent (Farris et al., Citation1995; Cunningham, Citation1997).
Phylogenetic analyses
Phaeophyceae
Within the data sets containing sequences of Phaeophyceae from various orders, analyses of rbcL alone and in combination with 26S data resulted in similar overall tree topologies (). As in previous analyses (e.g. Reviers & Rousseau, Citation1999; Draisma et al., Citation2001, Cho et al., Citation2004), most established orders within the Phaeophyceae received strong support in all phylogenetic analyses, while relationships between orders were less well resolved. Differences between both ML trees (rbcL+26S: ; rbcL: not shown, bootstrap consensus tree under TreeBase accession no. 51984) were apparent in the branching order of some monophyletic groups, for example the Sporochnales and Scytothamnales, while the backbones of both trees did not receive significant bootstrap support. In all analyses, the most basal brown algae were the Discosporangiales, followed by the Dictyotales. In the combined rbcL+26S analyses, the Syringodermatales together with the Sphacelariales and Onslowiaceae formed a non-supported clade, while all other brown algal orders were part of a strongly supported monophyletic group, the so-called ‘crown’ of the Phaeophyceae (Reviers & Rousseau, Citation1999).
Fig. 1. Phylogenetic tree of the Phaeophyceae based on the maximum likelihood analysis of combined partial rbcL and 26S data (GTR+I+G model, –ln likelihood=14013.8779). Numbers above lines indicate bootstrap values (left: ML; right: MP), numbers below Bayesian posterior probabilities. Dashes indicate that branches received a bootstrap support of 50% or less, or less than 70% Bayesian probability. The scale bar indicates substitutions per site.
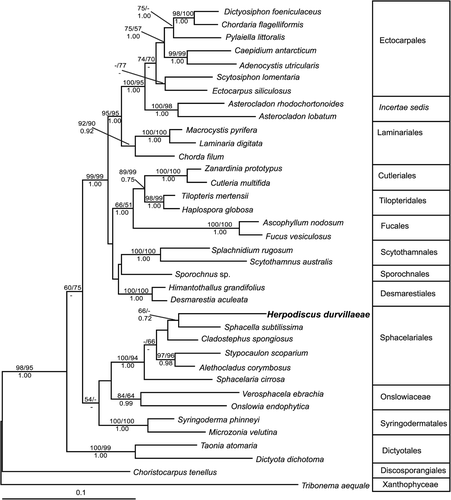
In all trees, H. durvillaeae was unambiguously resolved as a member of the monophyletic Sphacelariales s.s. receiving high bootstrap support in all analyses (95% in the ML analysis of rbcL data alone). However, apart from the Stypocaulaceae, clades within the Sphacelariales were not well supported in any tree based on this data set, including the cluster that H. durvillaeae formed with Sphacella subtilissima.
Sphacelariales
The phylogenetic analyses of the data set limited to basal brown algae led to trees with similar topologies (ML tree presented in ). In the outgroup, the Discosporangiales took the most basal position, followed by the Dictyotales, Onslowiaceae and Syringoderma phinneyi.
Fig. 2. Phylogenetic tree (ML) for basal Phaeophyceae inferred from partial rbcL sequences (GTR+I+G model, –ln likelihood=−8376.330601). Numbers above lines indicate bootstrap values (left: ML; right: MP), numbers below Bayesian posterior probabilities. Dashes indicate that branches received a bootstrap support of 50% or less, or less than 70% Bayesian probability. The scale bar indicates substitutions per site.
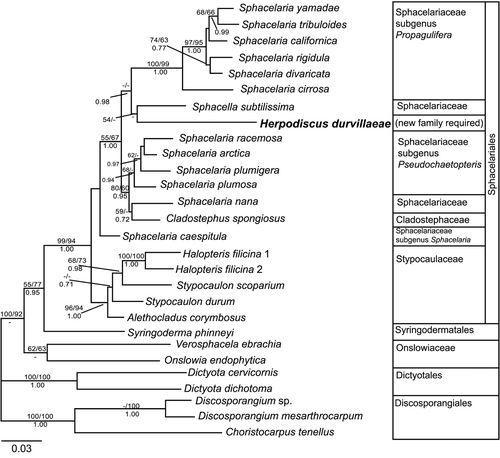
Within the Sphacelariales s.s., the well supported Stypocaulaceae branched off first, followed by Sphacelaria caespitula in an unsupported position. The remaining Sphacelaria species were divided among two clades. Members of the subgenus Propagulifera formed a highly supported clade, while the subgenus Pseudochaetopteris, together with Cladostephus spongiosus and S. nana, formed another clade, which had only moderate bootstrap support. Relationships within both of these clades were not resolved with high bootstrap support.
Herpodiscus durvillaeae and S. subtilissima together took a position between the Propagulifera and Pseudochaetopteris clusters. This branch only received a weak support in ML analyses, leaving the affinities of Herpodiscus and Sphacella with each other and with other Sphacelariales s.s. mostly unsupported.
Morphological observations ()
In autumn and winter, infected thalli of D. antarctica were easily distinguishable from healthy fronds () by the typical brown-reddish patches of the external phase of H. durvillaeae ().
Figs. 3–9. Morphology of the parasitic of Herpodiscus durvillaeae. . Population of the host, Durvillaea antarctica, showing healthy fronds without signs of infection with H. durvillaeae. St. Kilda, 20km north of Brighton, Otago, New Zealand, March 1997. . Phylloids of the host (D. antarctica) with extensive red-brown patches of external filaments of H. durvillaeae. Brighton, August 1997. . Transverse section through external filaments of fresh H. durvillaeae Brighton, August 1997. Filaments above the host surface (arrowhead) show a row of unilocular sporangia (arrow), the tips of the filaments covered with pennate diatoms. , . Transmission electron microscopy of sections of host and parasite. . Longitudinal section through the meristoderm at the end of the host's growth season showing two apical cells of H. durvillaeae (p) among host cells at the surface. Parasite cell contents appear concentrated at their apices. Shrinkage of the host cells and the loss of most of their physodes (holes indicated by arrows) are preparation artefacts. . Longitudinal section through the base of a parasite patch. Filaments of H. durvillaeae showing acroblastic branching (arrow). , . Reaction with Eau de Javel. . Transitory blackening of cell walls of H. durvillaeae immediately after treatment (arrow). Gametangia-turned gametophytes have coloured internal walls (arrowhead). Dried material, scraped from the surface of a parasite patch. (WELT A027896). . Reaction of external terminal cells of fresh H. durvillaeae with Eau de Javel as front moves through the tips of the external filaments. Some terminal cells are completely bleached (double arrowhead), while others display blue bands of colouring just below their apical hemisphere (arrows). Terminal cells have swollen tips and contain large nuclei (arrowheads).
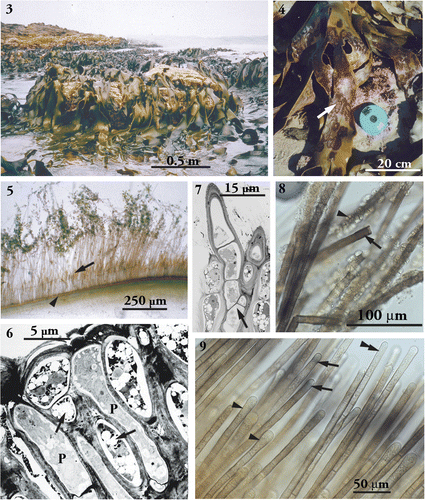
External filaments of H. durvillaeae () were uniseriate, unbranched and consisted of regular, cylindrical cells with uniform diameters within filaments (width up to 15µm). Most external cells were the same length as the apical cells. Only occasionally were shorter cells observed (not shown). In undisturbed filaments, the tips were formed of single cells, which appeared slightly swollen and displayed large nuclei visible in the light microscope (). Gametophytes were frequently observed on filaments close to empty unilocular sporangia.
In transverse transmission electron micrograph (TEM) sections through infected phylloids of the host Durvillaea, internal cells of Herpodiscus were distinguishable from the host cells by their apparent lack of physodes. Herpodiscus cells embedded in the host cortex displayed irregular shapes in contrast to the ordered arrangement of the host cells, while parasite cells growing in the host meristoderm were more or less cylindrical, but narrower than the external cells (width up to 10µm). Close to the surface of the host, parasite cells were observed which showed a concentration of cytoplasm towards their apices (). Branching was limited to the internal phase of Herpodiscus. On one occasion, an acroblastic branching pattern was observed in internal parasite filaments close to the host surface ().
The reaction with Eau de Javel was observed in live and dried material of H. durvillaeae. All external Herpodiscus cells including gametophytes showed a transitory blackening of the cell walls when in contact with Eau de Javel () as did the Halopteris cross-sections (not shown). The blackness disappeared after around 5–10 seconds, and the tissue then bleached completely. In the large terminal cells, a faint blue band was observed just below the apical hemisphere, which persisted slightly longer than the blackening of the rest of the cell (). In contrast, the tissue of the host D. antarctica did not show any blackening before bleaching (not shown). However, the short duration of the transitory blackening of the Herpodiscus filaments did not allow location of the parasite filaments within the host tissue.
Observations on brown algae from the contamination tests
Filamentous brown algae, which grew in the crude cultures of material scraped from the surface of external Herpodiscus patches, did not react with Eau de Javel (not shown). DNA extracted from a brown alga isolated from one of these cultures produced a partial 18S and ITS1nrDNA sequence of 762bp length which, in a BLAST search, aligned well with a sequence of Myrionema strangulans Greville (GenBank accession number AJ439856; 98% identical base pairs in a query coverage of 85%) and other members of the order Ectocarpales. The alga apparently belonged to the Ectocarpales, and sequences were not further analysed.
Discussion
The present study represents the first molecular approach to investigate the phylogenetic relationships of the New Zealand endemic H. durvillaeae, considered to be the only known parasitic brown alga (Lüning, Citation1985). DNA sequence analyses revealed that H. durvillaeae is a member of the Sphacelariales Migula. This is at variance with previously proposed affiliations with the Ralfsiales and Ectocarpales, based on morphology and life history (Lindauer, Citation1949; John & Lawson, Citation1974; South, Citation1974), proposals which had been questioned by Peters (Citation1990) and E.C. Henry (personal communication, and in Peters, Citation1990).
Detection of an apparently functional brown algal rbcL sequence in H.durvillaeae, which is assumed to be fully parasitic, is surprising. Contamination by other brown algae as source of this plastid-encoded sequence can be excluded because extreme care was taken during sampling of H. durvillaeae thalli for DNA extraction to include material of only this species. In addition, material scraped from H. durvillaeae patches on Durvillaea was used to start cultures but only yielded brown algae of the Ectocarpales, i.e. distant from Sphacelariales. As in previous attempts (Peters, Citation1990, E.C. Henry, personal communication) H. durvillaeae did not grow in culture. This suggests that the photosynthetic capacities of H. durvillaeae warrant more detailed investigation.
The placement of H. durvillaeae in the Sphacelariales is backed by two morphological features characteristic for this order: the presence of apical cells and the positive reaction of the parasite with Eau de Javel. The Sphacelariales share apical growth, a plesiomorphic character in brown algae, with several other phaeophycean orders, such as the Syringodermatales, Dictyotales, Scytothamnales, Cutleriales and Fucales (Henry, Citation1984; van den Hoek et al., Citation1995; Reviers & Rousseau, Citation1999), but they are distinguished from all other brown algae by the transitory blackening of their cell walls by Eau de Javel (Reinke, Citation1890; Migula, Citation1909; Prud'homme van Reine, Citation1982; Reviers & Rousseau, Citation1999). Members of the Choristocarpacecae and Onslowiaceae, which were in the past included in the Sphacelariales s.l. (Henry, Citation1987a,Citationb; Womersley, Citation1987), do not react with Eau de Javel (Prud'homme van Reine, Citation1982, Citation1993). Recent molecular systematic studies support the exclusion of both families from the Sphacelariales s.s. (Draisma et al., Citation2001; Draisma & Prud'homme van Reine, Citation2001; Kawai et al., Citation2007) which is confirmed in the present study.
When treated with Eau de Javel, Sphacelarialean cells at first turn dark brown to black, then they bleach and become colourless (Reinke, Citation1890; Migula, Citation1909; Prud'homme van Reine, Citation1982; Reviers & Rousseau, Citation1999). The chemistry underlying this reaction, discovered in the 19th century (Reinke, Citation1890), is still unknown (W. Prud'homme van Reine, personal communication). The substance stained is thought to be localized in the cell walls, in the middle lamella (Reinke, Citation1890). In H. durvillaeae, all cells, even in dried material, reacted with Eau de Javel, suggesting that the reacting substance is a permanent part of the cell wall.
The cell walls of the Sphacelariales are multi-layered (Karyophyllis et al., Citation2000). In the apical cells of Sphacelaria rigidula Kützing, the cell wall is thinnest at the apical hemisphere, i.e. in the area where the cell grows. The deposition of additional layers of wall material appears to be limited to the area just below the apical hemisphere where the cell wall reaches its greatest width (Karyophyllis et al., Citation2000). The bluish band that appeared below the apical hemisphere in the terminal cells of the external phase of Herpodiscus upon treatment with Eau de Javel, suggests that the substance reacting with the bleach may be more concentrated at these sites, probably indicating freshly deposited wall material. Additionally, the swollen, elongated shape of the terminal cells displaying large nuclei, indicate the presence of actively growing cells, i.e. apical cells.
In the internal phase of Herpodiscus, observations of terminal cells were hindered by its endophytic nature. However, cells close to the host surface at the beginning of the external season of the parasite, which were observed with TEM, showed a concentration of compartments and cell plasma in their apical portions. A similar polarization was observed in apical cells of Sphacelaria tribuloides Meneghini. (Katsaros et al., Citation1983), suggesting that the terminal cells of Herpodiscus observed within the host were also apical cells, about to break through the surface and to develop into external filaments. It is likely that apical growth also occurs in those parts of the endophytic phase that are deeply immersed in the host tissue. Lucas (Citation1998, p.30) assumed for parasitic fungi that “The apical mode of growth of most fungi is the key to the success of these organisms…as…parasites.” Likewise, apical growth appears more likely to penetrate the very tough thallus of D. antarctica than intercalary growth.
Position of H. durvillaeae within the Sphacelariales
Within the Sphacelariales s.s., three families comprising eight genera are currently recognized based on morphology (): the Sphacelariaceae, Stypocaulaceae and Cladostephaceae (Oltmanns, Citation1922; Prud'homme van Reine, Citation1982, Citation1993). However, the morphological features separating these families and genera within the Sphacelariales, such as different branching patterns and the presence or absence of secondary growth in the segments have been shown to be unreliable, due to high levels of homoplasy, phenotypic plasticity and/or polymorphisms (Draisma et al., Citation2002). Moreover, molecular systematic analyses reveal that the family Sphacelariaceae is paraphyletic, with the monotypic Cladostephaceae embedded within it (Draisma et al., Citation2002; this study), warranting a re-arrangement of taxa within the order. Draisma et al. (Citation2002) suggest different solutions, of which the most divisive requires keeping the families Stypocaulaceae and Sphacelariaceae, including Cladostephus in the latter, and creating new genera in the Sphacelariaceae for the various clusters within the paraphyletic genus Sphacelaria.
Table 1. Comparison of the morphology of Herpodiscus durvillaeae and of members of families of the Sphacelariales s.s. currently recognized (Prud'homme van Reine, Citation1982, Citation1993; Womersley, Citation1987; Peters, Citation1990; D.G. Müller, personal communication; present study).
In this scenario, based on its phylogenetic position between the two Sphacelaria subgenera, Pseudochaetopteris and Propagulifera, H. durvillaeae would have to be included in the Sphacelariaceae. Indeed, Herpodiscus lacks cortication and axillary zoidangia, and has isogamous reproduction, separating it from the Stypocaulaceae (South, Citation1974). And at least in external filaments, its mode of growth appeared to be leptocaulous, as cells did not enlarge further. The difference in diameter noticeable between internal and external filament cells was presumably caused by the tight structure of the host cortex. Herpodiscus moreover shares some characters with the morphologically simplest member of the Sphacelariaceae, S. subtilissima, which distinguish them from the other members of the Sphacelariaceae: For example, the absence of longitudinal walls, and possibly also the lack of the regular formation of secondary segments by the subdivision of subapical cells. Sphacella cells, like those of other Sphacelariaceae, do not grow secondarily after cell division, thus the occasional presence of secondary transverse cell walls is indicated by cells half the usual length (Prud'homme van Reine, Citation1982). A similar pattern appears to be present in Herpodiscus as shorter cells were observed by South (Citation1974), possibly indicating rare events of secondary segment formation. The irregular shape of the endophytic cells of Herpodiscus did not allow any conclusions on growth patterns of the internal phase.
Another character Herpodiscus shares with Sphacella is its obligate epi-endophytism. Sphacella thalli are anchored with their basal parts in the tissue of their host (Capromitra costata (Stackhouse) Batters, Sporochnus sp., Bellotia sp.; Reinke, Citation1890; Womersley, Citation1987). This is not an unusual feature for the Sphacelariaceae (Womersley, Citation1987). Reinke (Citation1890, Citation1891) even separated Sphacelaria species with endophytic bases (‘Sphacelariae parasiticae') from those without (‘Sphacelariae autonomae'), using the terms 'endophytic' and 'parasitic' as synonyms. Sauvageau (1900, cited in Prud'homme van Reine, Citation1982) considered the formation of endophytic filaments not necessarily an indication for parasitism. Indeed, partly endophytic specimens of Sphacelaria cirrosa (Roth) C. Agardh do not depend on photosynthetic products from their host (as Sphacelaria bipinnata (Kützing) Sauvageau; Goodband, Citation1973). Likewise, Sphacelaria caespitula Lyngbye has an endophytic base, but is also capable of growing either epiphytically or epilithically (Prud'homme van Reine, Citation1982).
Sphacella and Herpodiscus, on the other hand, are only found as epi-endophytes. Their extensive endophytic bases consist of filaments that deeply penetrate the host tissue (Reinke, Citation1891; South, Citation1974; Prud'homme van Reine, Citation1982; Womersley, Citation1987). However, Sphacella is pigmented and can be cultivated without its host (D.G. Müller, personal communication; Draisma et al., Citation2002), while Herpodiscus shows evidence for genuine parasitism. So far it has not been successfully cultivated under laboratory conditions without its host (South, Citation1974; Peters, Citation1990; E.C. Henry, personal communication). Moreover, ultrastructural studies show the presence of a symplastic contact between cells of Herpodiscus and of its host D. antarctica (unpublished results), although a transfer of assimilates from the host to the parasite has yet to be proven.
In conclusion, H. durvillaeae shares some features with S. subtilissima. Nevertheless, its heteromorphic life history distinguishes H. durvillaeae from Sphacella and all other members of the Sphacelariales. In the Phaeophyceae, an isomorphic versus heteromorphic life history is generally considered a reliable taxonomic character (Clayton, Citation1981, Citation1990; van den Hoek et al., Citation1995; Graham & Wilcox, Citation2000). For example, in the Ectocarpales s.l., an order comprising taxa with various diplontic-haplontic life histories, it is one of the characters used to separate families (Peters & Ramirez, Citation2001). As long as their sexuality is not reduced or unknown, members of the Sphacelariales usually have a diplontic-haplontic life history with isomorphic or only slightly heteromorphic generations (Clayton, Citation1981; Prud'homme van Reine, Citation1982, Citation1993; Womersley, Citation1987; Draisma et al., Citation2002). Even S. subtilissima produces plurilocular zoidangia of a Sphacelaria-like type, which were previously unknown and have so far only been observed in culture (Reinke Citation1891; Prud'homme van Reine, Citation1982; D.G. Müller, personal communication).
In Herpodiscus, in contrast, gametophytes and gametangia are extremely reduced (Peters, Citation1990). And even though the original description of the Sphacelariales does not include the life history of its members (Migula, Citation1909), rendering placement of Herpodiscus in this order unproblematical, this feature nevertheless separates H. durvillaeae from all other known members of the Sphacelariales. Consequently, the accommodation of H. durvillaeae within this order would warrant a placement in its own monotypic family, thus requiring an even more divisive rearrangement of taxa within the Sphacelariales s.s. than suggested by Draisma et al. (Citation2002), i.e. splitting the subgenera of Sphacelaria into separate families.
Consequently, a new family would also have to be created for Sphacella subtilissima as it is not more closely related to Herpodiscus than to some Sphacelaria species. This raises the question whether the simple morphologies of both Herpodiscus and Sphacella are ancient or the result of reduction, i.e. are they derived from a (possibly common) parenchymatous ancestor with typical sphacelarialean morphology? The phylogenetic affinities of Herpodiscus and Sphacella may be solved by including sequences of other 'simple' Sphacelariaceae in the analyses, such as Sphacelaria pulvinata Hooker & Harvey. This species grows as an epiphyte of Carpophyllum maschalocarpum (Turner) Greville and is also endemic in New Zealand. It has mostly uniseriate and rarely branched erect filaments and thus appears to be very similar to S. subtilissima (Adams, Citation1994). However, so far no molecular data are available from S. pulvinata or any other members of the Sphacelariales from New Zealand or Australia.
In general, there is no comprehensive study of Southern Hemisphere Sphacelariales (Draisma et al., Citation2002), even though the Australasian region is considered one of the main centres of distribution of this order and is rich in endemic taxa (Prud'homme van Reine, Citation1982). For example, of the seven species of Sphacelaria present in New Zealand, only two are cosmopolitan, one occurs only in New Zealand and South Australia, and the other four are endemic to New Zealand (Adams, Citation1994). The only sphacelarialean genus not present in Australasia is nevertheless from the Southern Hemisphere: Alethocladus (Stypocaulaceae) is restricted to Antarctic and sub-Antarctic waters (Prud'homme van Reine, Citation1993).
Considering this general lack of phylogenetic information for Southern Hemisphere Sphacelariales, we refrain from proposing any re-arrangements (including new families and genera) for the order at present. A detailed (morphological and molecular genetic) study of New Zealand and Australian species may not only solve the phylogenetic affinites of Herpodiscus (and S. subtilissima) to other members of the Sphacelariales, but by including representatives from the whole Southern Hemisphere, i.e. species from South Africa and South America, new concepts may arise regarding the phylogenetic affinities within the order and its geographical origin may be discovered.
SUPPLEMENTARY MATERIAL
Download Zip (56.5 KB)Acknowledgements
We would like to thank Eric C. Henry, Willem Prud’homme van Reine, Stefano Draisma and Dieter G. Müller for personal communications. Dieter G. Müller kindly provided the slides with preserved material of Sphacella subtilissima. Wendy A. Nelson and two anonymous reviewers greatly improved the manuscript with their valuable comments. Staff from the South Campus Electron Microscopy Unit, University of Otago, Dunedin, kindly assisted in the preparation of the EM samples. We thank Johannes Imhoff, Kiel, for lending us laboratory equipment. PhD. scholarships by the German Academical Exchange Service (DAAD) and by the University of Otago to S.H., and funding from the Division of Sciences, University of Otago, to C.L.H. are gratefully acknowledged.
References
- Adams , NM . 1994 . Seaweeds of New Zealand , Christchurch, , New Zealand : Canterbury University Press .
- Cho , GY , Lee , SH and Boo , SM . 2004 . A new brown algal order, Ishigeales (Phaeophyceae), established on the basis of plastid protein-coding rbcL, psaA, and psbA region comparisons . J. Phycol , 40 : 921 – 936 .
- Clayton , MN . 1981 . “ Phaeophyta ” . In Marine Botany: An Australasian Perspective , Edited by: Clayton , MN and King , RJ . Melbourne, , Australia : Longman Cheshire .
- Clayton , MN . 1990 . “ Phylum Phaeophyta. ” . In Handbook of Protoctista , Edited by: Margulis , L , Corliss , JO , Melkonian , M , Chapman , DJ and McKhann , HI editorial coordinator . Vol. 35 , 698 – 714 . Boston, , USA : Jones & Bartlett Publ. .
- Clayton , MN and Ashburner , CM . 1994 . Secretion of phenolic bodies following fertilisation in Durvillaea potatorum (Durvillaeales, Phaeophyta) . Eur. J. Phycol , 29 : 1 – 9 .
- Cunningham , CW . 1997 . Can three incongruence tests predict when data should be combined? . Mol. Biol. Evol , 14 : 733 – 740 .
- Draisma , SGA and Prud'homme Van Reine , WF . 2001 . Onslowiaceae fam. nov. (Phaeophyceae) . J. Phycol , 37 : 647 – 649 .
- Draisma , SGA , Prud'homme Van Reine , WF , Stam , WT and Olsen , JL . 2001 . A reassessment of phylogenetic relationships within the Phaeophyceae based on RUBISCO large subunit and ribosomal DNA sequences . J. Phycol , 37 : 586 – 603 .
- Draisma , SGA , Olsen , JL , Stam , WT and Prud'homme Van Reine , WF . 2002 . Phylogenetic relationships within the Sphacelariales (Phaeophyceae): rbcL, RUBISCO spacer and morphology . Eur. J. Phycol , 38 : 385 – 401 .
- Farris , JS , Källersjö , M , Kluge , AG and Bult , C . 1995 . Testing significance of incongruence . Cladistics , 10 : 315 – 319 .
- Fletcher , RL . 1987 . “ Volume 3, Part 1 Fucophyceae (Phaeophyceae) ” . In Seaweeds of the British Isles , London, UK : The Natural History Museum .
- Goodband , SJ . 1973 . Observations on the development of endophytic filaments of Sphacelaria bipinnata (Kütz.) Sauv. on Halidrys siliquosa (L.) Lyngb . Br. J. Phycol , 8 : 175 – 179 .
- Graham , LE and Wilcox , LW . 2000 . Algae , Sydney, , Australia : Prentice Hall .
- Guindon , S and Gascuel , O . 2003 . A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood . Syst. Biol , 15 : 696 – 704 .
- Henry , EC . 1984 . Syringodermatales ord. nov. and Syringoderma floridana sp. nov. (Phaeophyceae) . Phycologia , 23 : 419 – 426 .
- Henry , EC . 1987a . The life history of Onslowia endophytica (Sphacelariales, Phaeophyceae) in culture . Phycologia , 26 : 175 – 181 .
- Henry , EC . 1987b . Morphology and life history of Onslowia bahamensis sp. nov. and Verosphacela ebrachia gen. et. sp. nov., with a reassessment of the Choristocarpaceae (Sphacelariales, Phaeophyceae) in culture . Phycologia , 26 : 182 – 191 .
- Holmgren , PK , Holmgren , NH and Barnett , LC . 1990 . Index Herbariorum. Part. I: The Herbaria of the World. , 8th ed. , Vol. 120 , Regnum Vegetabile .
- John , DM and Lawson , GW . 1974 . Basispora, a new genus of the Ralfsiaceae . Br. Phycol. J , 9 : 285 – 290 .
- Karyophyllis , D , Katsaros , C and Galatis , B . 2000 . F-Actin involvement in apical cell morphogenesis of Sphacelaria rigidula (Phaeophyceae): mutual alignment between cortical actin filaments and cellulose microfibrils . Eur. J. Phycol , 35 : 195 – 203 .
- Katsaros , C , Galatis , B and Mitrakos , K . 1983 . Fine structural studies on the interphase and dividing apical cells of Sphacelaria tribuloides (Phaeophyta) . J. Phycol , 19 : 16 – 30 .
- Kawai , H , Muto , H , Fujii , T and Kato , A . 1995 . A linked 5S rRNA gene in Scytosiphon lomentaria (Scytosiphonales, Phaeophyceae) . J. Phycol , 31 : 306 – 311 .
- Kawai , H , Hanyuda , T , Draisma , SGA and Müller , DG . 2007 . Molecular phylogeny of Discosporangium mesarthrocarpum (Phaeophyceae) with reinstatement of the order Discosporangiales . J. Phycol , 43 : 186 – 194 .
- Kushida , H . 1974 . A new method for embedding with a low viscosity epoxy resin "Quetol 651" . J. Electron Microsc , 23 : 197
- Lee , E-Y , Pedersen , PM and Lee , IK . 2002 . Neoleptonema yongpilii E.-Y. Lee & I. K. Lee, gen. et sp. nov. (Phaeophyceae), based on morphological characters and RuBisCo spacer sequences . Eur. J. Phycol , 37 : 237 – 245 .
- Lindauer , VW . 1947 . An annotated List of the Brown Seaweeds, Phaeophyceae, of New Zealand . Trans. R. Soc. New Zealand , 76 : 542 – 566 .
- Lindauer , VW . 1949 . Notes on marine algae of New Zealand. I . Pacific Science , 3 : 340 – 352 .
- Lindauer , VW , Chapman , VJ and Aiken , M . 1961 . The marine algae of New Zealand. II. Phaeophyceae . Nov. Hedwig , 3 : 129 – 350 .
- Lucas , JA . 1998 . Plant Pathology and Plant Pathogens, ed.3 , Oxford, UK : Blackwell Science .
- Lüning , K . 1985 . Meeresbotanik , Stuttgart, , Germany : Georg Thieme Verlag .
- Migula , W . 1909 . Kryptogamen-Flora von Deutschland, Deutsch-Österreich und der Schweiz. Band II. Algen. 2. Teil: Rhodophyceae, Phaeophyceae, Characeae , Gera, , Germany : Verlag Friedrich von Zezschwitz .
- Oltmanns , F . 1922 . Morphologie und Biologie der Algen , Jena, , Germany : Zweiter Band Phaeophyceae: Rhodophyceae 2nd edn. Fischer .
- Peters , AF . 1990 . Taxonomic implications of gamete fusions in the parasitic brown alga Herpodiscus durvilleae . Can. J. Bot , 68 : 1398 – 1401 .
- Peters , AF and Burkhardt , E . 1998 . Systematic position of the kelp endophyte Laminarionema elsbetiae (Ectocarpales sensu lato, Phaeophyceae) inferred from nuclear ribosomal DNA sequences . Phycologia , 37 : 114 – 120 .
- Peters , AF and Ramirez , ME . 2001 . Molecular phylogeny of small brown algae, with special reference to the systematic position of Caepidium antarcticum (Adenocystaceae, Ectocarpales) . Cryptogamie Algol , 22 : 187 – 200 .
- Posada , D and Crandall , K . 1998 . MODELTEST: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Prud’homme Van Reine , WF . 1982 . A taxonomic revision of the European Sphacelariales , Leiden, , The Netherlands : Leiden Botanical Series 6 .
- Prud’homme Van Reine , WF . 1993 . Sphacelariales (Phaeophyceae) of the world, a new synthesis . Korean J. Phycol , 8 : 145 – 160 .
- Reinke , J . 1890 . Uebersicht der bisher bekannten Sphacelariaceen . Ber. Deut. Bot. Gesell , 8 : 201 – 215 .
- Reinke , J . 1891 . Beiträge zur vergleichenden Anatomie und Morphologie der Sphacelariaceen . Bibl. Bot , 23 : 1 – 40 .
- Reviers , BDE and Rousseau , F . 1999 . Towards a new classification of the brown algae . Prog. Phycol. Res , 13 : 107 – 201 .
- Ronquist , F and Huelsenbeck , JP . 2003 . MrBayes 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Schoenwaelder , MEA and Clayton , MN . 1998a . Secretion of phenolic substances into the zygote wall and cell plate in embryos of Hormosira and Acrocarpia (Fucales, Phaeophyceae) . J. Phycol , 34 : 969 – 980 .
- Schoenwaelder , MEA and Clayton , MN . 1998b . The secretion of phenolic compounds following fertilization in Acrocarpia paniculata (Fucales, Phaeophyta) . Phycologia , 37 : 40 – 46 .
- Siemer , BL , Stam , WT , Olsen , JL and Pedersen , PM . 1998 . Phylogenetic relationships of the brown algal orders Ectocarpales, Chordariales, Dictyosiphonales, and Tilopteridales (Phaeophyceae) based on RUBISCO large subunit and spacer sequences . J. Phycol , 34 : 1038 – 1048 .
- South , GR . 1974 . Herpodiscus gen. nov. and Herpodiscus durvilleae (Lindauer) comb. nov., a parasite of Durvillea antarctica (Chamisso) Hariot endemic to New Zealand . J. R. Soc. New Zealand , 4 : 455 – 461 .
- Starr , RC and Zeikus , JA . 1993 . UTEX – The culture collection of algae at the University of Texas at Austin 1993 List of cultures . J. Phycol , 29 ( Suppl ) : 1 – 106 .
- Swofford , DL . 2002 . “ PAUP* (Phylogenetic analysis using parsimony) version 4.0 b10 ” . Sunderland, , USA : Sinauer Associates .
- Uwai , S , Kogame , K and Masuda , M . 2000 . Morphology and life history of four Japanese species of Elachista (Elachistaceae, Phaeophyceae), including a new record of . E. fucicola. Phycol. Res , 48 : 267 – 279 .
- Uwai , S , Kogame , K and Masuda , M . 2001 . A taxonomic study of Elachista taeniaeformis complex and E. vellosa from the western Pacific (Elachistaceae, Phaeophyta) . Phycologia , 40 : 67 – 77 .
- Van Den Hoek , C , Mann , DG and Jahns , HM . 1995 . Algae. An Introduction to Phycology , Cambridge, UK : Cambridge University Press .
- Van Oppen , MJH , Olsen , JL , Stam , WT , Van Den Hoek , C and Wiencke , C . 1993 . Arctic-antarctic disjunctions in the benthic seaweeds Acrosiphonia arcta (Chlorophyta) and Desmarestia viridis/willii (Phaeophyta) are of recent origin . Mar. Biol , 115 : 381 – 386 .
- Watson , ML . 1958 . Staining of tissue sections for electron microscopy with heavy metals . J. Biophys. Biochem. Cytol , 4 : 475 – 485 .
- Womersley , HBS . 1987 . The Marine Benthic Flora of Southern Australia Part II , Adelaide, , Australia : South Australian Government Printing Division .
Supplementary information
Supplementary material for this manuscript can be found online free of charge at www.informaworld.com
