Abstract
Ecophysiological characteristics of a sexual population of Chara canescens from Austria (NS) were compared with those from a parthenogenetical propagating population of the Baltic Sea (BS) to find out whether differences in salinity or irradiance acclimation abilities are responsible for the absence of male organisms in the Baltic Sea. Growth, photosynthesis main characteristics and pigmentation were examined at two irradiance levels and four salinities. Shoot elongations were highest for all specimens from both populations at low salinities. Reduction of growth at 12.4 PSU was less pronounced for NS female algae, whereas BS female and NS male algae showed strong growth reduction. Algae from NS had highest Pmax (maximum photosynthesis rate) and α-values (efficiency of light utilization at limiting irradiance) at 1.9 PSU, strongly decreasing at higher salinities. Both parameters were independent of salinity for BS algae. No significant differences between the experimental groups were found with respect to irradiance acclimation. Our results indicate that the absence of male C. canescens in the Baltic Sea is not related to sex-specific ecophysiological characteristics.
Introduction
The dioecious green alga Chara canescens (Desvaux & Lois in Loiseleur, 1810) is widespread in shallow brackish waters of the Northern Hemisphere. Chara canescens is an important primary producer and refuge for animals in shallow coastal areas and inland brackish water sites of Southern and Central Europe (Krause, Citation1997). In the northern parts of Europe (e.g. northern Atlantic, Baltic Sea) and at the Greek and French Mediterranean coast, only female plants, reproducing by means of germinating oospores, can be found. These populations propagate by parthenogenesis only. Interestingly, male plants have been found in a few places (e.g. Austria, Italy and Hungary). This phenomenon was first described by Brown (Citation1857) as ‘geographic sex separation’, later named ‘geographic parthenogenesis’ by Vandel (Citation1928). A general survey about the geographic distribution of male C. canescens has not been carried out recently. Records from Greece turned out to be misidentifications (see Corillion, Citation1972); the origin of herbarium specimens from the surroundings of Montpellier (France) could not be traced to a given location because of insufficient description. Several places marked in the distribution maps of Corillion (France) cannot be supported by literature and herbarium records. Thus, the only European location where the occurrence of a recent population of male C. canescens has been proven, is the border area between Austria and Hungary, where the original specimens came from. Most of the Hungarian ponds described by Filarszky (Citation1924) do not exist anymore. A first survey in 2000 showed that many shallow ponds with female and male individuals of C. canescens still exist in Austria in the National Park Neusiedler See–Seewinkel.
It is well documented that asexual organisms more often occupy environments that are classified as marginal, stressful, transient or disturbed (Bierzychudek, Citation1987; Asker & Jerling, Citation1992; Hörandl, Citation2006). Following the premise that asexual clones arise from sexual ancestors (e.g. Bell, Citation1982; Asker & Jerling, Citation1992; Simon et al., Citation2003) a specific stressor or combination of stressors results in decreased fitness of sexually reproducing organisms and, finally, parthenogenesis. Therefore it is important to consider the fitness of asexually and sexually reproducing organisms under respective environmental conditions in order to assess factors, that potentially lead to geographic parthenogenesis of C. canescens. Possible explanations are (i) higher probability for asexuals to propagate in pioneer habitats, e.g. the Baltic Sea after deglaciation, (ii) higher energetic costs of sexual reproduction, because given that all other things are equal, in a species with two separate sexes, the asexual females produce twice as many daughters as the sexual females (two-fold cost of sex; Maynard Smith, Citation1978), and (iii) differences in ecophysiological acclimation abilities between male and female organisms in general, or between sexually and asexually propagating populations. No detailed study has tackled any of the above-mentioned explanations. The present study deals with the ecophysiological differences, in order to make a first step towards obtaining further insight into the mechanisms, that lead to evolution of parthenogenetic populations.
As for other submerged macrophytes, the distribution of charophytes is mainly dependent on environmental conditions like salinity (e.g. Blindow, Citation2000; Bisson & Kirst, Citation1995) and light availability (e.g. Blindow, Citation2000; Küster et al., Citation2000, Citation2004). Among charophyte species, there is a high variability in salinity preferences (e.g. Olsen, Citation1944; Blindow, Citation2000), which could be referred to different mechanisms of regulating turgor pressure, osmotic potential and ion composition (for review see Bisson & Kirst, Citation1995). Winter & Kirst (Citation1991) depict in detail the osmo- and turgor regulation of asexually propagating female C. canescens, which belongs to the ‘mesohaline brackish water species cluster’ (Bisson & Kirst, Citation1995). These species have a salinity preference of 0.5–20 PSU (Olsen, Citation1944) and regulate turgor pressure mainly via K+, Na+ and Cl− accumulation, but they seem to be unable to support turgor regulation by sucrose accumulation as found for e.g. Chara aspera (Winter & Kirst, Citation1992). Winter & Kirst (Citation1991) showed that the intracellular quotient of K+/Na+ is crucial for survival under hyperosmotic salinity stress conditions, since plants were unable to survive if this quotient falls below 1. With respect to the extracellular K+/Na+ quotient, habitats of asexuals in the Baltic Sea have a quotient of 0.025 (Nessim, 1987), and those of sexuals in Neusiedler See–Seewinkel are about 0.10 (Metz & Forró, Citation1991). These clear differences indicated ion composition as a potential basic stressor. Blindow et al. (Citation2003) showed that salinity-dependent reduction of fertility can restrict charophyte before hyperosmotic salinities reach lethal levels with respect to growth. Winter et al. (Citation1996) reported similar results, showing that high salinities (12 PSU) inhibited the formation of ripe oospores for Tolypella nidifica. For C. canescens, Bonis et al. (Citation1993) described at least salinity-dependent differences in the reproductive effort for asexual organisms, which could support the above assumption, that salinity and/or ion composition are crucial factors. Moreover, Ernst (Citation1921) hypothesized on an influence of Mg2+ ions on the formation of the sexes. The results of Winter & Kirst (Citation1991) suggested that Mg2+ ions play no role in turgor regulation, since their intracellular level was kept constant over the whole range of salinities tested. However, Mg2+ might play a role in sex allocation; the above-cited experiments were performed with parthenogenetic organisms.
Another difference between the habitats of the two populations is the availability of light. In addition to the difference in latitude, the Neusiedler See–Seewinkel population is located in the pannonian climate zone with, compared to the Baltic Sea area, high irradiance during the whole vegetation period. Light availability is of crucial importance for autotrophic organisms, and it is proposed as one of the major factors controlling the distribution of charophytes (Yousef et al., Citation1997; Blindow Citation1992, Citation2000; Schwarz et al., Citation1996, Citation1999; Küster et al., Citation2004). Charophytes acclimate to various light climates in different ways, e.g. by pigmentation changes, changes in morphology and modulation of photosynthetic efficiency (e.g. Küster et al., Citation2004, Blindow et al., Citation2003). For C. canescens, Küster et al. (Citation2004) reported significant differences in high-light acclimation abilities between parthenogenetic plants from the Baltic Sea and the Mediterranean Sea (Greece, Gulf of Corinth), indicating site-specific adaptation of these populations. With respect to sex-specific differences of the bisexual population, Küster et al. (Citation2005) stated that the observed differences do not explain the lack of sexual organisms of C. canescens in the Baltic Sea. However, photosynthetic performance of charophytes is dependent on both salinity and irradiance (Blindow et al., Citation2003). So, to make further progress, a bifactorial analysis is necessary.
To compare the physiological performances of asexually and sexually reproducing C. canescens, the combined effects of light and salinity on plants from the Neusiedler See–Seewinkel and the Baltic Sea were measured, including cross-incubations in water from the two habitats.
Materials and methods
Site description
Chara canescens plants from two locations were used for the laboratory experiments. The first population, originating from Austria (NS population) contains female and male organisms. The area of their origin – Seewinkel – is situated in the National Park system between Neusiedler See and the Hungarian border (47°45′N; 22°20′E). This National Park includes more than 50 shallow ponds, the so-called ‘Lacken’. Organisms were taken from a dense mono-specific stand of female and male C. canescens in the Weißsee Lacke.
The second population originated from a shallow estuarine lagoon, Darß-Zingster Bodden Kette (DZBK), on the south-western coast of the Baltic Sea (BS population). The populations contain female plants only. The DZBK is characterized by a large gradient in salinity (1–12 PSU). Organisms used in the experiments, were taken from the Bodstedter Bodden (54°35′N; 12°60′E), a basin with an average salinity of 3 PSU. Detailed information about the field sites are given by Schiewer (Citation1998) for the Baltic Sea site and Löffler (Citation1957) and Metz & Forró (Citation1991) for Neusiedler See–Seewinkel.
Experimental setup
Light and salinity growth dependency was measured by incubating plant apices of about 3cm length at two irradiances and four salinities in an outdoor setup. Salinities were: (i) 1.9 PSU, obtained from the original habitat of the NS-population; (ii) 2.3 PSU, obtained from the original habitat of the BS-population; (iii) 5.8 PSU, obtained from the Baltic Sea at a location close to the BS population; and (iv) 12.4 PSU, also obtained from the Baltic Sea at a location near Wismar (Salzhaff), where C. canescens populations also occur. All plants were incubated in 1.5l vessels with sediments taken from the same sites. No additions of nutrients were made. Low-light conditions were obtained by wrapping the glass cylinders with neutral density foils, reducing the incident irradiance (photosynthetically active radiation, PAR) to 50% of the 100% level. The compartments for 100% levels were covered by a corresponding highly PAR-transparent foil, to prevent significant differences in incident ultraviolet irradiance, although the latter was suppressed. After pre-incubation for 3 days in the laboratory, the vessels were placed in the outdoor experimental set-up for 10–12 days. The size of each individual was measured every day with a ruler. The position of the vessels in the outdoor setup was changed every day in order to avoid permanent-shading effects for single vessels and individuals, respectively. Each glass cylinder was covered by cling-film to reduce evaporation. Unavoidable water loss was replaced daily using distilled water.
Photosynthesis measurements
Net oxygen exchange at increasing irradiances from 0 to approximately 2,400µmol photons m−2s−1 was measured by means of a computer-controlled light dispenser system (light pipette system MK2, Illuminova, Sweden), equipped with a Clark-type electrode. The apices were placed in a 2.5-ml Plexiglas chamber, and temperature was kept constant by means of a thermostat. The photosynthetic parameters, maximum photosynthetic rate in the absence of photoinhibition (P max) and light dependency of photosynthesis at limiting irradiances (α), both expressed per unit Chla, were calculated by a fitting procedure using an iterative exponential regression of the equation given in Walsby (Citation1997). E k was calculated by dividing P max by α (Talling, Citation1957). Each sample was extracted overnight in 3ml N,N-Dimethylformamide (DMF) at 4C for chlorophyll determination. The absorption values of the extracts were read at 647nm and 663nm in a spectrophotometer (Specord M40, Carl Zeiss Jena, Germany), and the chlorophyll content was calculated using formula and extinction coefficients of Porra et al. (Citation1989).
Statistical analysis
SPSS software was used for statistical analysis of the data obtained. Effects of origin and treatments (irradiances and salinity) and their interactions were tested by three-way ANOVA. Analysis of variances was followed by Tukey post-hoc comparisons to test for differences between the three experimental groups (NS male, NS female, BS female) with respect to both salinity and irradiance.
Results
A brief summary of the results from three-way ANOVA is given in . These results in the context of the respective experiments will be presented in the following section.
Table 1. F-values and significancies for three-way ANOVA of the effects of location, irradiance, salinity and their interactions on photosynthetic parameter, chlorophyll concentration and growth rate.
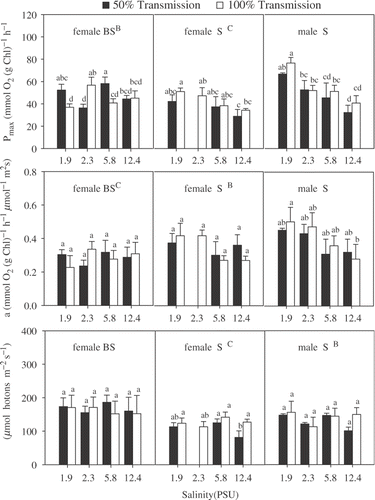
Summarizing the results of the photosynthesis measurements (), all photosynthetic parameters showed no irradiance dependency (non-significant irradiance effect; ). With respect to salinity, P max of male and female NS plants showed a stronger decrease with increasing salinity at both irradiances, resulting in significant differences between e.g. 1.9 PSU and 12.4 PSU (significant salinity effect; ; ), whereas P max values of BS female algae did not show any significant salinity dependency at either irradiance. Analysing the whole dataset, all three groups (female BS and NS and male NS) were significantly different with respect to P max (, ). Photosynthetic efficiency (α) showed a similar trend in all experiments, but a significant salinity effect on α was observed for the male NS plants only (). The trends observed for female NS and BS plants were insignificant. Differences with respect to individual sexes and locations were significant in between all three groups (significant location effect; , ). As a result of this pattern, calculated E k values (P max/α) showed no salinity-dependent trends in any of the groups. Corresponding to P max and α, the three groups were significantly different with respect to E k ().
Fig. 1. Photosynthetic characteristics for female and male Chara canescens originating from NS and female plants collected from BS populations incubated in different irradiance – salinity regimes. The effect of location, irradiance and salinity was tested by a three-way ANOVA. Different capital letters indicate significant differences between clones and different lower case letters significant treatment effect within the locations (one-way ANOVA, Tukey post-hoc test, p<0.05).
Due to pronounced differences in photosynthetic parameters, differences in pigmentation could be assumed. shows chlorophyll concentrations and ratios measured after the acclimation period. In all three groups Chla and Chlb concentration increased with decreasing irradiance (). Chla and Chlb concentration of NS male plants growing at their original salinity increased significantly in comparison to all other salinities in the experimental set with low irradiance. BS algae exhibited significantly higher chlorophyll concentrations than the other two groups under all experimental treatments (significant location effect; , ). NS male plants usually exhibited the lowest chlorophyll concentrations, but the differences were insignificant in a few cases ().
Fig. 2. Pigment characteristics for female and male Chara canescens originating from NS and female plants collected from BS populations incubated in different irradiance – salinity regimes. The effect of location, irradiance and salinity was tested by a three-way ANOVA. Different capital letters indicate significant differences between clones and different lower case letters significant treatment effect within the locations (one-way ANOVA, Tukey post-hoc test, p<0.05).
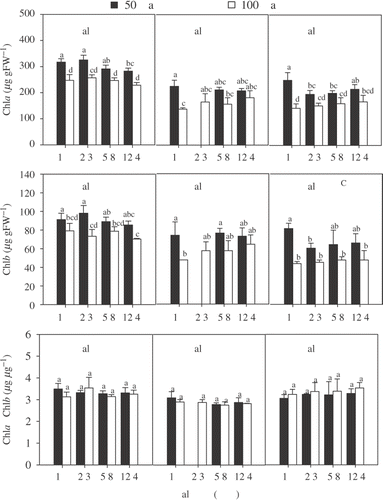
No significant differences between the experimental treatments were observed for the Chla/b ratio, indicating only negligible changes in the relative antennae size under salinity and/or irradiance acclimation (non-significant irradiance and salinity effect; ). NS female algae provided the lowest Chla/b ratio (significant location effect; ; ) under all conditions, but NS male plants and BS plants were not significantly different. Although inclusion of carotenoid content would be helpful to characterize irradiance acclimation, the values are not shown. All experimental groups formed antheridia or oogonia during the experiment and their high carotenoid content dominated the results measured. Thus, they could not be used for analysis. Unfortunately, attempts to remove reproductive organs from the algae resulted in handling stress, which influenced values for chlorophyll content to an unacceptable extent.
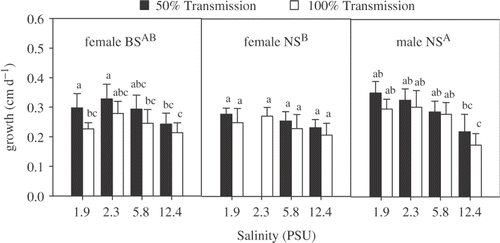
summarizes results of the growth experiments for plants from all three experimental groups, expressed as relative shoot elongation. In all cases, shoot elongation decreased with increasing irradiance (significant irradiances effect; ). This effect is part of the morphological irradiance acclimation of charophytes, described by Blindow et al. (Citation2003) and Küster et al. (Citation2005). shows that BS females had their highest shoot elongation at 2.3 PSU and the NS males at 1.9 PSU (significant salinity effect; ). Interestingly, these salinities match with the respective field salinities. In contrast, NS female plants did not show such a clear salinity growth response. Shoot elongation of NS male plants was significantly higher than that of NS female plants (significant location effect; , ).
Fig. 3. Shoot elongation of female and male Chara canescens originating from NS and female plants collected from BS populations incubated in different irradiance – salinity regimes. The effect of location, irradiance and salinity was tested by a three-way ANOVA. Different capital letters indicate significant differences between clones and different lower case letters significant treatment effect within the locations (one-way ANOVA, Tukey post-hoc test, p<0.05).
Discussion
Geographic parthenogenesis is the geographically distinct distribution of closely related sexual and asexual organisms (Vandel, Citation1928; Bell, Citation1982; Hörandl, Citation2006). Parthenogenetic organisms are more common in marginal, extreme, transient, stressful, or disturbed habitats than their closely related sexual ancestors. Chara canescens is the only species of the genus including both sexually and asexually reproducing populations. Because all bisexual populations described so far originate from inland brackish water sites with climatic conditions that include high irradiance during the main growth period, the question arises whether salinity or a combination of both factor triggers the occurrence or absence of parthenogenesis.
The most intriguing point with respect to irradiance acclimation is the constancy of E k in all C. canescens populations at both irradiances and all salinities tested. This might be interpreted as a general feature of this species, focussing irradiance acclimation on morphological rather than biochemical mechanisms, as indicated by the pronounced shifts in chlorophyll content. This has been shown (Küster et al. Citation2004) in more detail for parthenogenetic C. canescens individuals of different populations (Baltic Sea and Greece), employing a finer graduation of acclimation irradiance. Our results demonstrate that the observed constancy of E k is not restricted to parthenogenetic populations of this species or certain salinity ranges. But it is not a special feature of charophytes, because other charophyte species (e.g. L. papulosum, Küster et al., Citation2000; C. aspera, Blindow et al., Citation2003) follow the classical high-irradiance acclimation mode, with an increase of E k at increasing acclimation irradiance. This is caused by an increase in P max, and is typically accompanied by decreasing α-values (Falkowski & Raven, Citation1997). However, the observed constancy of E k, a parameter that was shown to be a reliable indicator of the irradiance acclimation status in several algal species (e.g. Kroon et al. Citation1993; Menendez & Sanchez, Citation1998; Schubert et al. Citation2006), was the result of quite different changes in P max and α during our investigations. BS algae did not show a distinct pattern in the reaction of both parameters with respect to salinity or irradiance. NS algae of both sexes exhibited clear salinity effects on both parameters, i.e. decreasing values with increasing salinity. The highest values were calculated for plants that grew at the salinity of their original location.
In general, the observed mode of salinity dependence in both NS groups, which led to the best photosynthetic performance in the salinity range of their origin, is in good agreement with results from investigations on other species. The absence of such salinity dependence in BS plants can be interpreted as a result of the high variability of salinity in their original habitat. In coastal lagoons of the western Baltic Sea salinity can change irregularly within less than one hour over the range of <0.5 PSU to >16 PSU. Under such circumstances, a broad salinity tolerance may be more useful than a fine-tuning to minor changes in the external environment.
Beside the differences between the two locations, there were differences between the two NS groups as well. NS male plants showed higher P max and α-values than NS female plants at the salinity of their origin, but at higher salinities both groups showed comparable values. The latter was the result of a more pronounced decrease in both parameters in NS male plants under increasing salinity. Moreover, the observed loss of photosynthetic capacity and efficiency of NS male plants with increasing salinity was accompanied by a slight increase in the Chla/b ratio, which was not observed for NS female plants. One might hypothesize that these differences could explain the absence of male plants at BS locations, presuming that male plants have higher requirements for photosynthetic performance than female ones. In such a case the stronger decrease in P max at low salinities could restrict the occurrence of male plants, irrespective of the fact that the absolute level of photosynthetic performance at higher salinities is still comparable to female plants. Then, growth rates should be indicative.
As observed during our investigations, increasing shoot elongation as a response to low light conditions has been reported for several higher plants (e.g. Pilon & Santamaria, 2002) as well as in charophytes (Blindow et al. Citation2003). All three C. canescens groups showed a growth optimum in the range of the three lowest salinities (1.9–5.8 PSU) at both irradiance levels. This is in good agreement with field observations (e.g. Olsen Citation1944; Blindow Citation1992; and Langangen Citation1993). Winter & Kirst (Citation1991) studied turgor regulation of C. canescens in detail, but found no turgor regulation below 4 PSU, and in this salinity range, K+ concentration remained stable. Salinity values above 4 PSU led to K+ and Na+ (and Cl−) turgor pressure regulation, while above 13 PSU Na+ inflow (with constant K+) provided increasing Na+/K+ quotients, which gradually poisoned the algae (Winter & Kirst, Citation1991). The drop in growth rates at 12.4 PSU by all three groups in our experiments might therefore be interpreted as a result of decreasing turgor pressure. Similar results were reported by Winter & Kirst (Citation1990) for C. vulgaris, where shoot elongation was reduced if salinity increased to about 6 PSU, and by Blindow et al. (Citation2003) for C. aspera, where shoot elongation started to decrease at 20 PSU.
The ion composition in the waterbodies of the two investigated areas is quite different and the K+/Na+ ratio (NS: 0.10; BS: 0.025) is particularly relevant. As a result of the higher Na+ concentration and consequently more disadvantageous K+/Na+ ratio, BS plants must spend more energy for ion regulation than NS plants in their original habitat (Winter & Kirst, Citation1991, see above). A comparison of the resulting growth rates gave no indication of a significant sex- or location-specific difference between NS male and BS female plants. Both groups showed similar growth at all salinities. In addition, shoot elongation of NS female plants was not influenced in the respective range of salinities, and all three groups formed oogonia or antheridia during the experiments. The latter indicated no inhibition of fertility under any of the experimental conditions. Thus, the above-mentioned hypothesis, that NS male plants are more susceptible to salinity or ionic stress, must be discarded.
Summarizing, the three groups of C. canescens plants from the two European locations showed clear and significant differences in their regulation of photosynthetic parameters with changing salinity, and in their pigmentation with changing irradiance flux. Regarding the differences between the original habitats, these differences seemed to be correlated with adaptive processes of the plants in the respective habitats, and cannot explain the absence of sexual specimens in the Baltic Sea. Moreover, growth rates were influenced by irradiance and salinity, but the influences of the locations were minor. Further studies in the field and in the laboratory, targeting specifically the mechanism of parthenogenesis and the quantification their relative benefits, are required to make the next step in searching for the mechanism, that led and leads to geographic parthenogenesis of C. canescens.
Acknowledgements
This study was funded by the Deutsche Forschungsgemeinschaft (FKZSchu983/8-1/2). Thanks go to Prof A. Herzig and all members of the Limnologische Station Illmitz and to the administration for the National Park Neusiedler See–Seewinkel (Austria) and A. Küster, who first found that there are still males in this region in 1999.
References
- Asker , SE and Jerling , L . 1992 . Apomixis in Plants , London, UK : CRC Press .
- Bell , G . 1982 . The Masterpiece of Nature: The Evolution and Genetics of Sexuality , London, UK : CroomHelm .
- Bierzychudek , P . 1987 . “ Patterns in plant parthenogenesis ” . In The Evolution of Sex and its Consequences , Edited by: Stearns , SC . 197 – 217 . Basel, , Switzerland : Birkhäuser-Verlag .
- Bisson , MA and Kirst , GO . 1995 . Osmotic acclimation and turgor pressure regulation in algae . Naturwissenschaften , 82 : 461 – 471 .
- Blindow , I . 1992 . Decline of charophytes during eutrophication: comparison with angiosperms . Freshwat. Biol. , 28 : 9 – 14 .
- Blindow , I . 2000 . Distribution of Charophytes along the Swedish coast in relation to salinity and eutrophication . Int. Revue Hydrobiol. , 85 : 707 – 717 .
- Blindow , I , Dietrich , J , Möllmann , N and Schubert , H . 2003 . Growth, photosynthesis and fertility of Chara aspera under different light and salinity conditions . Aquat. Bot. , 76 : 213 – 234 .
- Bonis , A , Grillas , P , Vanwijck and C. Lepart , J . 1993 . The effect of salinity on the reproduction of coastal submerged macrophytes in experimental communities . J. Veget. Sci. , 4 : 461 – 468 .
- Brown , A . 1857 . Über Parthenogenesis bei Pflanzen . Abhandl. d. k. Akad. d. Wiss. zu Berlin , 1856 : 311 – 376 .
- Corillion , R . 1972 . Les Charophycées de France et d'Europe Occidentale , Koenigstein, , Germany : Koeltz Scientific Books .
- Ernst , A . 1921 . Die Nachkommenschaft aus amphimiktisch und apogam entstandenen Sporen von Chara crinita . Zeitschr. f. indukt. Abstgsl. , 25 : 185 – 197 .
- Falkowski , PG and Raven , JA . 1997 . Aquatic photosynthesis , Oxford, UK : Blackwell .
- Filarszky , F . 1924 . Die Verbreitung der Chara crinita (Wallr.) beiderlei Geschlechts in Ungarn . Mat. Nat. Ber. Ungarn , 33 : 105 – 132 .
- Hörandl , E . 2006 . The complex causality of geographical parthenogenesis . New Phytol. , 171 : 525 – 538 .
- Krause , W . 1997 . “ Charales (Charophyceae) ” . In Süßwasserflora von Mitteleuropa , Edited by: Ettl , H , Gärtner , G , Heynig , H and Mollenhauer , D . Vol. Band 18 , 1 – 202 . Jena, , Germany : Fischer .
- Kroon , B , Prezelin , BB and Schofield , O . 1993 . Chromatic regulation of quantum yields for photosystem II charge separation, oxygen evolution, and carbon fixation in Heterocapsa pygmaea (Pyrrophyta) . J. Phycol. , 29 : 453 – 462 .
- Küster , A , Schaible , R and Schubert , H . 2000 . Light acclimation of the charophyte Lamprothamnium papulosum . Aquat. Bot. , 68 : 205 – 216 .
- Küster , A , Schaible , R and Schubert , H . 2004 . Light acclimation of photosynthesis in three charophyte species . Aquat. Bot. , 79 : 111 – 124 .
- Küster , A , Schaible , R and Schubert , H . 2005 . Sex-specific light acclimation of Chara canescens (Charophyta) . Aquat. Bot. , 83 : 129 – 140 .
- Langangen , A . 1993 . Some morphological and ecological observations on Chara canescens (Charophyte) . Cryptogamie Algologie , 14 : 215 – 220 .
- Löffler , H . 1957 . Vergleichende limnologische Untersuchungen an den Gewässern des Seewinkels (Burgenland). I. Der winterliche Zustand der Gewässer und deren Entomostrakenfauna . Verh. zool.-bot. Ges. Wien , 97 : 27 – 52 .
- Loiseleur-Deslongchamps , JLA . 1810 . Notice sur les plantes a ajouter a la Flore de France (Flora Gallica) , 1 – 196 . Paris, , France : J.B. Sajou .
- Maynard-Smith , J . 1978 . The Evolution of Sex , Cambridge, UK : Cambridge University Press .
- Menendez , M and Sanchez , A . 1998 . Seasonal variations in P-I responses of Chara hispida L. and Potamogeton pectinatus L. from stream Mediterranean ponds . Aquat. Bot. , 61 : 1 – 15 .
- Metz , H and Forrò , L . 1991 . The chemistry and crustacean zooplankton of the seewinkel pans - a review of recent conditions . Hydrobiologia , 210 : 25 – 38 .
- Nessim , RB . 1987 . Untersuchungen zur Verteilung der Hauptkomponenten des Salzgehaltes im Wasser und Sediment der Darss-Zingster Boddengewässer unter besonderer Berücksichtigung der Ionenanomalie sowie erste Erhebungen über den Schwermetallgehalt. , PhD thesis Rostock, , Germany : University of Rostock .
- Olsen , S . 1944 . “ Danish Charophyta. Chorological, Ecological and Biological Investigation. ” . In Biologiske Skrifter, Bind II, Nr.1. , København, , Denmark : Det Kongelige Danske Videskabernes Selskap .
- Pilon , J and Santamaria , L . 2002 . Clonal variation in morphological and physiological responses to irradiance and photoperiod for the aquatic angiosperm Potamogeton pectinatus . J. Ecol. , 90 : 859 – 870 .
- Porra , RJ , Thompson , WA and Kriedemann , PE . 1989 . Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy . Biochim. Biophys. Acta , 975 : 384 – 394 .
- Schiewer , U . 1998 . 30 years’ eutrophication in shallow brackish waters – lessons to be learned . Hydrobiologia , 363 : 73 – 79 .
- Schubert , H , Andersson , M and Snoeijs , P . 2006 . Relationship between photosynthesis and non-photochemical quenching of chlorophyll fluorescence in two red algae with different carotenoid composition . Mar. Biol. , 149 : 1003 – 1014 .
- Schwarz , AM , Hawes , I and Williams , CH . 1996 . The role of photosynthesis light relationships in determining lower depth limits of Characeae in South Island, New Zealand lakes . Freshwat. Biol. , 35 : 69 – 79 .
- Schwarz , AM , Hawes , I and Williams , CH . 1999 . Mechanisms underlying the decline and recovery of a characean community in fluctuating light in a large oligotrophic lake . Aust. J. Bot. , 47 : 325 – 336 .
- Simon , JC , Delmotte , F , Rispe , C and Crease , T . 2003 . Phylogenetic relationships between parthenogens and their sexual relatives: the possible routes to parthenogenesis in animals . Biol. J. Linnean Soc. , 79 : 151 – 163 .
- Talling , JF . 1957 . Some observations on the stratification of Lake Victoria . Limnol. Oceanogr. , 2 : 213 – 221 .
- Vandel , A . 1928 . La parténogenèse géographique. Contribution à l'étude biologique et cytologique de la parténogenèse naturelle . Bull. Biol. France Belg. , 62 : 164 – 281 .
- Walsby , AE . 1997 . Numerical integration of phytoplankton photosynthesis through time and depth in a water column . New Phytol. , 136 : 189 – 209 .
- Winter , U and Kirst , GO . 1990 . Salinity response of freshwater charophyte, Chara vulgaris . Plant Cell Environ. , 13 : 123 – 134 .
- Winter , U and Kirst , GO . 1991 . Partial turgor pressure regulation in Chara canescens and its implications for a generalized hypothesis of salinity response in Charophytes . Bot. Acta , 104 : 37 – 46 .
- Winter , U and Kirst , GO . 1992 . Turgor pressure regulation in Chara aspera (Charophyta): the role of sucrose accumulation in fertile and sterile plants . Phycologia , 31 : 240 – 245 .
- Winter , U , Soulie-Marsche , I and Kirst , GO . 1996 . Effects of salinity on turgor pressure and fertility in Tolypella (Characeae) . Plant Cell Environ. , 19 : 869 – 879 .
- Yousef , M , Küster , A , Schubert , H and Von Nordheim , H . 1997 . Charakterisierung der Characeenbestände an der Küste Mecklenburg-Vorpommerns . Bodden , 5 : 3 – 23 .
