Abstract
Dense stands of the invasive species Sargassum muticum (Yendo) Fensholt develop in tidal pools close to its southern distributional limit in Europe, the southwest coast of Portugal. Along this coast, sheltered tidal pools form a specific habitat in which colonization occurs. Invaded pools are originally inhabited by Cystoseira humilis Kützing. Differences in gamete release between the competing native and alien species might be important for the initial settlement and further spread of the invader. Therefore, we tested whether egg expulsion and embryo settlement in both species had the same timing with respect to lunar and tidal cycles. For more than 2 months during the reproductive season egg expulsion and embryo release were monitored daily for each species. Egg expulsion in S. muticum showed a broadly semilunar periodicity peaking around full and new moon (spring tides), when low tides take place in the morning/evening. In contrast, C. humilis egg expulsion showed an asymmetric semilunar-to-lunar periodicity peaking around waning quarter moon, when low tides occur around midday. Embryo settlement detected in pools was low for both species and less periodic. Phase differences in expulsion events between the two species with respect to the semilunar cycle suggest that cues other than the moon are involved in their timing. Our observations suggest that variations in physiological mechanisms and/or environmental conditions result in different patterns of egg expulsion between the two species. This might have consequences for fertilization success, gamete dispersal and survival. It was further found that peaks in egg expulsion and embryo release (i.e. settlement) in S. muticum were much more synchronous at a site in northern Portugal compared with a site close to the current southern distributional limit in south-west Portugal, possibly as a consequence of thermal stress experienced in the south.
Introduction
Invasions by exotic species are occurring at unprecedented rates as a result of human activities that have increased the number of introductions and the rate of spread of many species (Lodge, Citation1993, Chapin et al., Citation2000). Invasive species can have serious consequences for native biodiversity (Heywood, Citation1989), disturbance regimes (D'Antonio & Visousek, Citation1992), and ecosystem structure and functioning (Vitousek et al., Citation1997a, Citationb; Chapin et al., Citation2000) and are considered a significant component of global change (Vitousek et al., Citation1997a, Citationb). Thus, identifying the factors that influence invasions by exotic species is of critical importance.
The fitness of both invaders and their native competitors is influenced by abiotic environments and interactions with resources, competitors, predators, diseases, etc. in the local environment. The shallow marine environment fluctuates physically on a variety of time-scales, but principally with the light–dark, tidal, semilunar and seasonal cycles (Yamahira, Citation2004). These cycles affect many aspects of the life history of marine organisms, but in particular reproduction is synchronized with these environmental cycles. Organisms release gametes or offspring during a particular time with respect to each cycle, producing diel, tidal, (semi)lunar and seasonal patterns according to the periods of cycles. In part, the outcome of competitive interactions between invasive and native species may lie in differences in the timing of reproductive cycles.
One of the most impressive invaders in the marine habitat and certainly the best studied invasive seaweed is Sargassum muticum (Yendo) Fensholt. Sargassum muticum originates from northwestern Pacific shores of Japan, Russia, Korea and China (Yendo, Citation1907; Critchley et al., Citation1990), but has invaded the American west coast from Alaska to Mexico in about 50 years (Scagel, Citation1956; Deysher & Norton, Citation1982; Critchley et al., Citation1983) and European coasts from Norway to Portugal in about 30 years (Rueness, Citation1989; Critchley et al., Citation1990; Stæhr et al., Citation2000).
Algal species from the family Cystoseiraceae are common natives of the habitats invaded by S. muticum throughout Europe (Fletcher & Fletcher, Citation1975; Jephson & Gray, Citation1977; Gunnill, Citation1985; Arenas et al., Citation1995; Viejo, Citation1997; Andrew & Viejo, Citation1998; Wernberg et al., Citation2000). Members of the Cystoseiraceae and Sargassaceae are closely related (Rousseau & De Reviers, 1999), and some researchers have suggested that indigenous Cystoseiraceae are displaced by S. muticum (Fletcher & Fletcher, Citation1975a; Viejo, Citation1997; Stæhr et al., Citation2000). Arenas et al. (Citation1995) and Viejo (Citation1997) suggested this could be due to differences in life history strategies. This hypothesis was supported by Wernberg et al. (Citation2000) in a comparative study of Halidrys siliquosa and S. muticum in Limfjorden, Denmark. However, many studies on exotic plant invasions have indicated that no single trait or group of traits can completely explain the success of exotic species in new environments (Smith & Knapp, Citation2001).
The first report of S. muticum in Portugal dates from 1991 (Farnham, Citation1997; R. Melo pers communication). In about eight years the species spread to the south-western coast of Portugal, which, at the time of this study, was the southern distributional limit in Europe (S. muticum has subsequently been observed on the southern Portuguese coast). Along this highly exposed coast, S. muticum develops in sheltered tide pools that are originally inhabited by Cystoseira humilis Kützing. In addition to possible differences in life-history strategies (Viejo, Citation1997; Wernberg et al., Citation2000), differences in propagule release between the competing native and alien species might be important for the initial settlement and subsequent spread of the invader (Lonsdale, Citation1999). In the marine environment, particularly in the intertidal zone, the periodicity, timing in relation to tidal cycles, and sensitivity to environmental variables of propagule release may affect the availability, the dispersal patterns and the survival of embryos.
Sargassum muticum expels eggs in synchronized pulses, which occur on average every 13 days, just after spring tides in England, Japan and Southern California (Fletcher, Citation1980; Norton, Citation1981). However, Norton (Citation1981) also reported expulsion occurring at about 7-day intervals, approximately 2 days after every quarter, full or new moon during incubation under laboratory conditions. Eggs are not released from the thallus immediately after extrusion, but are retained on the surface of the receptacle for between 1 and 3 days (Fletcher, Citation1980; Norton, Citation1981). After this period the dissolution of their anchoring mechanism facilitates embryo release (Fletcher, Citation1980; Norton, Citation1981). The retention of eggs probably allows for more efficient fertilization, possibly promoting selfing (both S. muticum and C. humilis are monoecious), and may protect embryos against environmental and mechanical stresses during early development. The subsequent release of the developing embryos with initiating primary rhizoids is thought to enhance survival (Fletcher, Citation1980; Norton, Citation1981). In contrast to S. muticum, the biology of C. humilis has been poorly studied. To our knowledge the periodicity of gamete expulsion and zygote release in C. humilis has not been studied previously. Based on the close relatedness of the species we hypothesized that the periodicities of these reproductive cycles in the two species are similar. These hypotheses were tested in this study by observing patterns of gamete (egg) expulsion and zygote settlement in natural populations near the (then) southern limit of S. muticum in the Iberian peninsular.
Material and methods
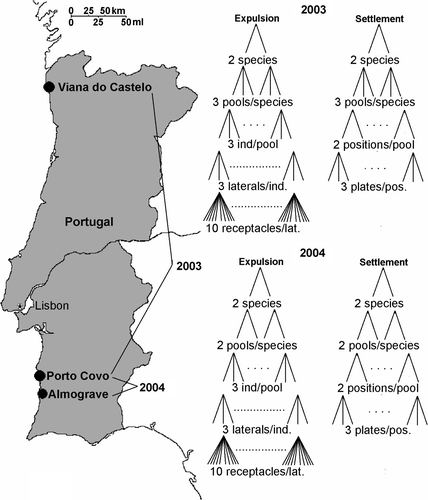
From June 24 to August 31, 2003, the propagule expulsion and settlement of S. muticum and C. humilis were monitored in three pools for each species, located at Praia de Queimado close to the village of Porto Covo. This was repeated in 2004 from July 7 to August 19 at Praia de Queimado and also at Praia de Almograve in 2 pools for each species at both locations (). In each pool, the studied species was the most dominant macrophyte (80–100% coverage). Sampling was performed daily at the end of daytime low water and procedures were identical for both species and during both years.
To study the periodicity of propagule expulsion, two fertile secondary laterals were collected from three plants randomly selected in each pool. Laterals were transported to the laboratory in seawater filled film canisters. Under a dissecting microscope 10 ripe receptacles per lateral were randomly selected and checked for the presence or absence of propagules on their surface. From this data propagule expulsion estimates were calculated for each lateral.
Propagule settlement was studied using artificial substrata (5.96cm2) with high rugosity (described in Ladah et al., Citation2003). In each pool, two bolts were fixed at haphazardly chosen positions under the canopy of the dominant species. Each bolt carried a PVC holder containing three settlement discs. The distance between discs in a holder was approximately 1 cm. Discs were kept in seawater at all times when carefully moved from the bolt to film canisters and transported to the lab. The number of propagules on each disc and in each film canister was counted in the lab.
In 2004 at Praia de Almograve, we increased sampling to twice a day (mornings and evenings) on 6 days with two low waters during daylight hours, and four times during daylight on 4 days (July 16/17 and August 14/15, 2004) during a full tidal cycle between early morning, and early evening. In both cases sampling consisted of a single lateral from four different individuals (for egg expulsion) and three independent artificial substrata (for zygote settlement) in each of two pools, which were the only two still accessible during high tides, and in which expulsion and settlement had previously been observed.
To compare the periodicity of propagule expulsion and settlement of S. muticum near the current southern limit with that further north, from June 24 to July 25, 2003, we also studied a population at Praia Norte in Viana do Castelo in Northern Portugal (). At this location S. muticum has been established since the early 1990s and it is the dominant macrophyte in intertidal pools and the shallow subtidal. Propagule expulsion was monitored daily from a 100 receptacles subsampled from five branches originating from five randomly selected plants. Propagule settlement was studied in two other pools close by, each with five artificial substrata, placed underneath the S. muticum canopy. Expulsion and settlement data were compared with tidal amplitude data provided by the Instituto Hidrografico Lisboa (www.hidrografico.pt).
Differences in egg release and embryo settlement of Sargassum muticum between morning and evening sampling as well as among the four daily samples representing a tidal cycle were tested with ANOVA. For the analysis of propagule expulsion data the factors lateral and individual were treated as random factors and daytime (morning or evening) or tidal stage as fixed. For the analysis of propagule settlement bolts and pools were considered random factors and daytime a fixed factor. The assumption of homogeneity of variances in the ANOVA was analysed with Cochran's test (Winer et al., 1991). In case of heterogeneity of variances settlement data were natural log-transformed.
In order to test for lunar or semilunar cycles in the propagule expulsion we performed linear regression analyses on the expulsion and the lunar day as described in deBruyn & Meeuwig (Citation2001). The lunar month was divided into 2Π radians to give each day an angular equivalent, θ. The data are then analysed by simple linear regression, using the model:
Results
Egg expulsion in both species from southern Portugal was clearly periodic, although with considerable ‘noise’ resulting in broad events covering a period of about a week (). Days with no expulsed eggs present were relatively rare especially for S. muticum in 2003. Standard deviations of the percentage of receptacles with expulsed eggs were large. For both species embryo settlement was low (especially in 2003) and roughly followed the pattern of egg expulsion although it was even more irregular (). Despite the high variance in the data, egg expulsion of both species showed a semi-lunar periodicity (). The broad egg expulsion events in S. muticum from southern Portugal, occurring over several days against a considerable background, clearly peaked around both the full and new moons, with similar amplitudes (, ). Egg expulsion in C. humilis also showed a semilunar periodicity in 2003, but in contrast to S. muticum, the peak amplitudes were asymmetric, larger around the waning quarter moon and smaller around the waxing quarter moon (). In 2004 the apparent periodicity of egg expulsion in C. humilis was lunar, rather than semi-lunar. The sampling period was shorter in 2004, and possible reasons for the observed pattern might have been related to gaps in sampling, or a failed expulsion event during the single waning quarter moon, as observed in 2003 ().
Fig. 2. Mean daily egg expulsion (black bars) and embryo settlement (white bars) of Sargassum muticum and Cystoseira humilis. Error bars show standard deviations between pools (n=3 in 2003 and n=2 in 2004). Year mean and upper 95% confidence limits (based on 10,000 replicates) are indicated by the solid and dashed lines respectively.
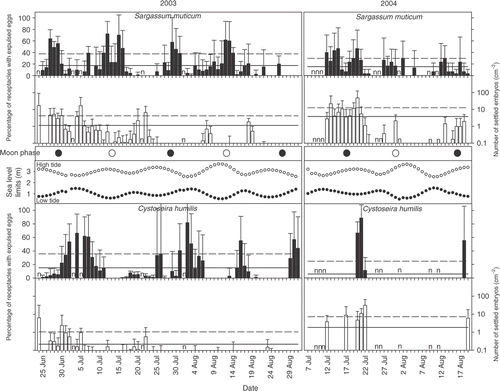
Embryo settlement was low for both species and showed little evidence of periodicity; settlement events were observed coincident with, or up to a week following, peaks in egg expulsion (, ). Daily embryo settlement densities of S. muticum in the S. muticum dominated pools were more than twice as high compared to C. humilis in C. humilis dominated pools (p<0.001, Mann-Whitney Rank Sum Test), with 2.02 (±5.93 SD) and 0.78 (±3.47 SD) embryos cm−2 day−1, respectively.
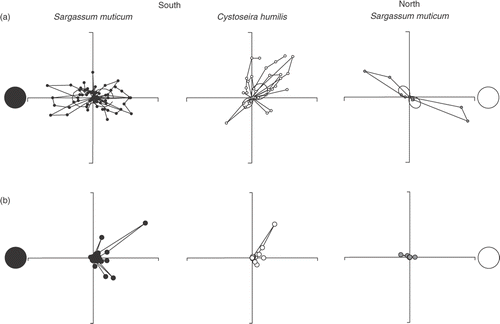
Fig. 3. Polar plot of the semi-lunar pattern of egg expulsion (a) and embryo settlement (b) of Sargassum muticum (South Portugal: black dots; North Portugal: dark grey dots) and Cystoseira humilis (white dots). Thin solid lines connect data points of the same lunar cycle, thick solid lines are the fitted periodic model (percentage of egg expulsion=b0+b1×sin θ+b2×cos 2θ).
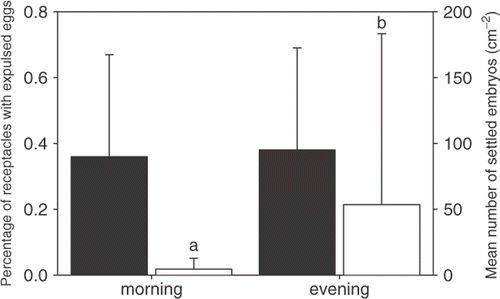
In S. muticum, expulsion mainly took place on days when the low tide occurred in the morning or in the late afternoon, whereas in C. humilis it was concentrated around midday. We found no differences in egg expulsion between mornings and evenings (p=0.770), but settlement of S. muticum embryos was about 12 times lower in mornings compared with evenings (p=0.013), indicating that embryos were mainly released from the receptacles during midday high tide (). Four samplings during daytime representing a tidal cycle sampling showed no differences over time in the percentage of receptacles with expulsed eggs (p=0.303) and settlement of embryos (p=0.660).
Fig. 4. Mean egg expulsion (black bars) and number of settled embryos (white bars) of Sargassum muticum during morning and evening samplings on the same day. Error bars show standard deviations (n=6), lower case letters indicate grouping of means with significant differences (α=0.05).
In northern Portugal, at Viana do Castelo, expulsion of S. muticum eggs showed a much more synchronized semi-lunar periodicity than at the site in southern Portugal. Discrete peaks of egg expulsion occurred 2–3 days following new and full moons (), which indicated a slight phase shift compared with southern Portugal. Embryo settlement was also synchronous and observed a few days after the initiation of egg expulsion. Settlement densities were as low as in Praia de Queimado (southern Portugal) in 2003, but the temporal pattern of settlement was less chaotic, presumably reflecting the greater synchrony of egg expulsion ().
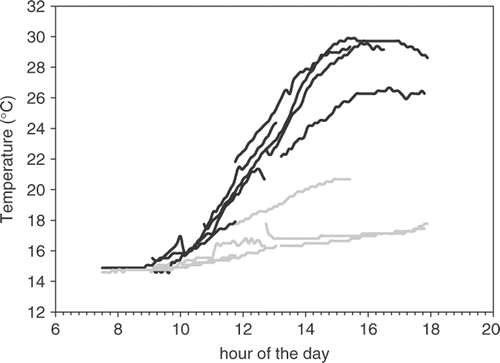
Temperature profiles of S. muticum dominated pools on the Atlantic coast of southern Portugal during various low tides demonstrated strong thermal clines between the S. muticum covered surface layer and the shaded understorey at the bottom of the pools (). Surface layer temperatures reached a maximum of 30°C and maximum temperature difference between the top and bottom was more than 10°C.
Discussion
Our results clearly showed differences in the timing of egg expulsion and zygote settlement events between the invading brown seaweed Sargassum muticum and the native C. humilis. S. muticum displayed a semilunar egg expulsion pattern that was concentrated around new and full moon coinciding with or just before spring tide, whereas expulsion by the native competitor C. humilis took place around waning moon, just before neap tide. This difference in timing suggests variability in the physiological mechanisms that regulate these events (see below). Phase differences between consecutive expulsion events occurred between the species, suggesting that tidal and diurnal cues are more important than semilunar cues in entraining the response (Yamahira, Citation2004).
Expulsion in C. humilis was concentrated more around neap tides after new moon compared to after full moon. Environmental conditions involved in the physiological expulsion mechanisms might vary between these two types of neap tide. During neap tides, following new moon, tide pools are exposed longer both in the number of days in a row as well as in hours per day and this increases with the elevation level of the pool. To what extent pool elevation level/emergence time influences egg expulsion and embryo settlement is uncertain and remains to be determined.
The pattern of egg expulsion around spring tides in S. muticum corresponds to the field descriptions of Fletcher (Citation1980) and Norton (Citation1981). The expulsion periodicity we observed in the field differed from the weekly pattern described by Norton (Citation1981) in laboratory cultures. Incubation of receptacles by us in a climate room (17°C, 14:10 h light–dark cycle) also generated a weekly release pattern (Engelen, unpublished data). This presumably reflects differences in lab versus field conditions (e.g., absence of tidal cues in the laboratory) and might be of interest in understanding the expulsion mechanism and cues involved. Although the periodicity of egg expulsion was the same at the northern site (Viana do Castelo), synchrony was much higher resulting in sharp expulsion peaks. This might be caused by differences in local conditions/cues that affect the timing of egg expulsion and embryo release in S. muticum, or it could reflect the physiological status of the algae, and perhaps indicate some level of stress affecting the capacity to time reproductive events.
In general, Sargassum species display a periodicity of egg release that is loosely based around the new moon (May & Clayton, Citation1991; Inoh, Citation1930) or both new and full moon (Tahara, Citation1909, 1913). As mentioned by May & Clayton (Citation1991) S. muticum seems to belong to the second category, with egg releases twice each lunar cycle.
One hypothesis is that differences in pattern detected between Praia de Queimado (southern Portugal) and Viana do Castelo (northern Portugal) could be caused by environmental stress. Although seawater temperatures are quite similar (sea surface temperatures differ by 1–2°C; Instituto Hidrografico Lisboa), there are large differences in air temperature and the number of sun hours. During low tide this can cause large temperature differences between tide pools in the north and south of Portugal. During the hot July months of 2003 and 2004 senescence of reproductive laterals in the South of Portugal occurred much earlier than in 2002, when a few fertile laterals remained in August (Engelen et al., unpublished data). We hypothesize that wave exposure and/or temperature stress might have been the reasons for higher variation in both egg expulsion and low embryo settlement at this location. In Viana do Castelo, tidepool temperatures were slightly lower (data not shown) and the S. muticum individuals are less exposed to wave action, overall, conditions more favourable for S. muticum. May & Clayton (Citation1991) also noted differences in synchronization of egg expulsion in Sargassum vestitum. They found that release of eggs usually took place simultaneously in individuals at the same location, but could occur at different times in more distant populations, suggesting that local environmental factors are important in controlling the timing of reproductive events. In addition, S. vestitum showed variation in the timing of egg expulsion in a single population between decaying old plants and healthy young plants as well as between pools: in some, release took place on the morning tide, whereas in others, it took place at the evening tide. Mobile marine intertidal molluscs can dominate rocky shores and play a major role in the structuring of intertidal communities through their grazing activities (Hawkins & Hartnoll, Citation1983), this has prompted numerous studies on their activity and foraging behaviour (Gray & Hodgson, Citation1998; and references therein). Since the foraging patterns of these grazers are related to seasonal, tidal and day/night cycles (Hawkins & Hartnoll, Citation1983), differences in timing of expulsion and settlement events between seaweeds might have large consequences for the survival of their embryos and their competition.
The concentration of expulsion in S. muticum in the morning and late afternoon might be explained by photo-inhibition and/or temperature stress around midday. During a midday low tide, typical summer light levels are around 2000 µmol photons m−2s−1 and the temperature of the surface layer of a tide pool can almost double in the course of a low tide rising up to 30°C (). However, the lunar cycle, tidal cycle and time of day are interacting factors. In Portugal, spring tides are always in the (early) morning and neap tides are concentrated around midday, in contrast to Northern parts of the species range, e.g. France, UK. Experiments in the lab and/or monitoring at different locations will be needed to separate these factors.
Temporal patterns of gamete release in natural populations of brown algae have been most intensively studied for the Fucaceae (Brawley, Citation1992; Serrão et al., Citation1996; Pearson & Brawley, Citation1996; Berndt et al., Citation2002, reviewed by Pearson & Serrão, Citation2006). Peaks of release generally coincide with periods of low water motion in the environment (e.g., slack high tide in intertidal/estuarine populations, low-tide exposure of tidal pool populations), which the algae detect as variations in photosynthetic carbon supply across the boundary layer (Pearson et al., Citation1998). This and other environmental signals, which might include light quality (Pearson & Brawley, 1998; Pearson et al., Citation2004), function to coordinate and synchronize the turgor-driven expulsion of gametes in fucoids (Speransky et al., Citation2001).
It seems reasonable to assume that other Fucales, like C. humilis and S. muticum, have similar physiological mechanisms and signalling pathways that coordinate gamete expulsion. Our data would then suggest that egg expulsion in C. humilis occurs during midday low-tide series, (similar to another upper intertidal tidepool alga, Fucus distichus [Pearson & Brawley, Citation1996), but during morning or evening low tides in S. muticum. Therefore, the environmental and/or physiological conditions under which, e.g., carbon limitation occurs may be different for the two species.
In conclusion, egg expulsion in S. muticum took place during spring tides (morning/evening lows), whereas in C. humilis expulsion occurred just before neap tides (lows concentrated around midday). This suggests variability in the physiological mechanisms that regulate egg expulsion and might cause differences in fertilization success, gamete dispersal and survival between the two species. Whether or not this influences the competition between the species remains unknown. Spatial variation in expulsion and settlement of S. muticum among pools, within pools and between north and south Portugal suggest that local conditions play an important role in the exact timing and synchronization of propagule expulsion and settlement.
Acknowledgements
We would like to thank the director and staff of the CIEMAR laboratory in Sines for their hospitality and support. This study was funded by the EU project ALIENS EVK3-CT-2001-00062. AHE was supported by scholarship SFRH/BPD/7153/2001 of the Portuguese Science Foundation (FCT).
References
- Andrew , NL and Viejo , RM . 1998 . Ecological limits to the invasion of Sargassum muticum in northern Spain . Aquatic Bot. , 60 : 251 – 263 .
- Arenas , F , Fernández , C , Rico , JM , Fernández , E and Haya , D . 1995 . Growth and reproductive strategies of Sargassum muticum (Yendo) Fensholt and Cystoseira nodicaulis (Whit.) Roberts . Sci. Mar. , 59 : 1 – 8 .
- Batchelet , E . 1981 . Circular Statistics in Biology , London, UK : Academic Press .
- Berndt , M-L , Callow , JA and Brawley , SH . 2002 . Gamete concentrations and timing and success of fertilization in a rocky shore seaweed . Mar. Ecol. Prog. Ser. , 226 : 273 – 285 .
- Brawley , SH . 1992 . Fertilization in natural populations of the dioecious brown alga Fucus ceranoides and the importance of the polyspermy block . Mar. Biol. , 113 : 145 – 157 .
- Chapin , FS , Iii Zavaleta , ES , Eviner , VT , Naylor , RL , Vitousek , PM , Reynolds , HL and Hooper , DU . 2000 . Consequences of changing biodiversity . Nature , 405 : 234 – 242 .
- Critchley , AT , Farnham , WF and Morell , SL . 1983 . A chronology of new European sites of attachment for the invasive brown alga, Sargassum muticum, 1973–1981 . Mar. Biol. Ass. UK , 63 : 799 – 811 .
- Critchley , AT , Farnham , WF , Yoshida , T and Norton , TA . 1990 . A bibliography of the invasive alga Sargassum muticum (Yendo) Fensholt (Fucales; Sargassaceae) . Bot. Mar. , 33 : 551 – 562 .
- D'antonio , CM and Visousek , PM . 1992 . Biological invasions by exotic grasses, the grass-fire cycle, and global change . Ann. Rev. Ecol. Syst. , 23 : 63 – 87 .
- De Bruyn , AMH and Meeuwig , JJ . 2001 . Detecting lunar cycles in marine ecology: periodic regression versus categorical Anova . Mar. Ecol Prog. Ser. , 214 : 307 – 310 .
- Deysher , LE and Norton , TA . 1982 . Dispersal and colonization in Sargassum muticum (Yendo) Fensholt . J. Exp. Mar. Biol. Ecol. , 56 : 179 – 195 .
- Farnham , WF . 1997 . “ Espèces invasives sur les côtes de la Manche et de l’Atlantique. ” . In Dynamique d’Espèces Marines Invasives: Application à l’Expansion de Caulerpa taxifolia en Méditerranée. (Séminaire Organisé Avec le Concours du Ministère de l’Environment et du Programme “Environment, Vie, Sociétés” du CNRS les 13–14–15 Mars 1997) , 15 – 35 . Paris, , France : Lavoisier . (In English with French summary.)
- Fletcher , RL . 1980 . Studies on the recently introduced brown alga Sargassum muticum (Yendo) Fensholt. III. Periodicity in gamete release and 'incubation' of early embryo stages . Bot. Mar. , 23 : 425 – 432 .
- Fletcher , RL and Fletcher , SM . 1975 . Studies on the recently introduced brown alga Sargassum muticum (Yendo) Fensholt. I. Ecology and reproduction . Bot. Mar. , 18 : 149 – 156 .
- Gray , DR and Hodgson , AN . 1998 . Foraging and homing behaviour in the high-shore, crevice-dwelling limpet Helcion pectunculus (Prosobranchia: Patellidae) . Mar. Biol. , 132 : 283 – 294 .
- Gunnill , FC . 1985 . Population fluctuations of seven macroalgae in southern California during 1981-1983 including effects of severe storms and an El Niño . J. Exp. Mar. Biol. Ecol. , 85 : 149 – 164 .
- Hawkins , SJ and Hartnoll , RG . 1983 . Grazing of intertidal algae by marine invertebrates . Oceanogr. Mar. Biol. A Rev. , 21 : 195 – 282 .
- Heywood , VH . 1989 . “ Patterns, extents and modes of invasions by terrestrial plants ” . In Biological Invasions: A Global Perspective , Edited by: Drake , JA , Mooney , HA , di Castri , F , Groves , RH , Kruger , FJ , Rejmanek , M and Williamson , M . 31 – 60 . Chichester, UK : Wiley .
- Inoh , S . 1930 . Embryological studies on Sargassum . Science Reports Tohoku Imperial University , 5 : 423 – 438 .
- Jephson , NA and Gray , PWG . 1977 . “ Aspects of the ecology of Sargassum muticum (Yendo) Fensholt in the Solent region of the British Isles. I. The growth cycle and epiphytes. ” . In Biology of Benthic Organisms. Proceedings of the 11th European Symposium on Marine Biology , Edited by: Keegan , BF , Ceidigh , PO and Boaden , PJS . 367 – 375 . Oxford, , UK : Pergamon Press .
- Ladah , L , Bermudez , R , Pearson , G and Serrão , E . 2003 . Fertilization success and recruitment of dioecious and hermaphroditic fucoid seaweeds with contrasting distributions near their southern limit . Mar. Ecol. Progr. Ser. , 262 : 173 – 183 .
- Lodge , DM . 1993 . Biological invasions: lessons for ecology . Trends Ecol. Evol. , 8 : 133 – 136 .
- Lonsdale , WM . 1999 . Global patterns of plant invasions and the concept of invasibility . Ecology , 80 : 1522 – 1535 .
- May , DI and Clayton , MN . 1991 . Oogenesis, the formation of oogonial stalks and fertilization in Sargassum vestitum (Fucales, Phaeophyta) from southern Australia . Phycologia , 30 : 243 – 256 .
- Norton , TA . 1981 . Gamete expulsion and release in Sargassum muticum . Bot. Mar. , 14 : 465 – 470 .
- Pearson , GA and Brawley , SH . 1996 . Reproductive ecology, of Fucus distichus (Phaeophyceae): an intertidal alga with successful external fertilization . Mar. Ecol. Prog. Ser. , 143 : 211 – 223 .
- Pearson , GA and Brawley , SH . 1998 . A model for signal transduction during gamete release in the fucoid alga Pelvetia compressa . Plant Physiol. , 118 : 305 – 313 .
- Pearson , GA and Serrão , EA . 2006 . Revisiting synchronous gamete release by fucoid algae in the intertidal zone: fertilization success and beyond? Integr . Comp. Biol. , 46 : 587 – 597 .
- Pearson , GA , Serrão , EA and Brawley , SH . 1998 . Control of gamete release in fucoid algae: sensing hydrodynamic conditions via carbon acquisition . Ecology , 79 : 1725 – 1739 .
- Pearson , GA , Serrão , EA , Dring , MJ and Schmid , R . 2004 . Blue- and green-light signals for gamete release in the brown alga, Silvetia compressa . Oecologia , 138 : 193 – 201 .
- Rousseau , F and Reviers , De B . 1999 . Phylogenetic relationships within the Fucales (Phaeophyceae) based on combined partial SSU+LSU rDNA sequence data. . Eur. J. Phycol , 34 : 53 – 64 .
- Rueness , J . 1989 . Sargassum muticum and other introduced Japanese macroalgae: biological pollution of European coasts . Mar. Poll. Bull. , 20 : 173 – 176 .
- Scagel , RF . 1956 . Introduction of a Japanese alga, Sargassum muticum into the north-east Pacific . Fish Res. Pap. Wash. Dep. Fish , 1 : 49 – 59 .
- Serrão , EA , Pearson , G , Kautsky , L and Brawley , SH . 1996 . Successful external fertilization in turbulent environments . Proc. Natl. Acad. Sci. USA , 93 : 5286 – 5290 .
- Smith , MD and Knapp , AK . 2001 . Physiological and morphological traits of exotic, invasive exotic, and native plant species in tallgrass prairie . Int. J. Plant Sci. , 162 : 785 – 792 .
- Speransky , VV , Brawley , SH and Mccully , ME . 2001 . Ion fluxes and modification of the extracellular matrix during gamete release in fucoid algae . J. Phycol. , 37 : 555 – 573 .
- Staehr , PA , Pedersen , MF , Thomsen , MS , Wernberg , T and Krause-Jensen , D . 2000 . The invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community . Mar. Ecol. Prog. Ser. , 207 : 79 – 88 .
- Tahara , M . 1909 . On the periodical liberation of oospheres in Sargassum . Bot. Mag. Tokyo , 23 : 151 – 153 .
- Tahara , M . 1913 . Oogonium liberation and the embryogeny of some Fucaceous algae . Journal of the College of Science, Imperial University of Tokyo , 23 : 1 – 13 .
- Viejo , RM . 1997 . The effects of colonization by Sargassum muticum on tidepool macroalgal assemblages . J. Mar. Biol. Ass. UK , 77 : 325 – 340 .
- Vitousek , PM , D'antonio , CM , Loope , LL , Rejmanek , M and Westbrooks , R . 1997a . Introduced species: a significant component of human-caused global change . NZ J. Ecol. , 21 : 1 – 16 .
- Vitousek , PM , Mooney , HA , Lubchenco , J and Melillo , JM . 1997b . Human domination of Earth's ecosystems . Science , 277 : 494 – 499 .
- Wernberg , T , Thomsen , MS , Stæhr , PA and Pedersen , MF . 2000 . Comparative phenology of Sargassum muticum and Halidrys siliquosa (Phaeophyceae: Fucales) in Limfjorden, Denmark . Bot. Mar. , 43 : 31 – 39 .
- Winer , BJ , Brown , DR and Michels , KM . 1991 . Statistical Principles in Experimental Design , New York, , USA : McGraw-Hill .
- Yamahira , K . 2004 . How do multiple environmental cycles in combination determine reproductive timing in marine organisms? A model and test . Functional Ecology , 18 : 4 – 15 .
- Yendo , K . 1907 . The Fucaceae of Japan. . J. Coll. Sci. Imp. Univ. Tokyo , 21 1–174+18 plates