Abstract
Previous studies on diurnal photosynthesis of macroalgal species have shown that at similar levels of photosynthetically active radiation (PAR, 400–700nm) the photosynthetic rate is lower in the afternoon than in the morning. However, the impacts of solar ultraviolet radiation (UVR, 280–400nm) have been little considered. We investigated the diurnal photosynthetic behaviour of the economically significant red alga Gracilaria lemaneiformis in the absence or presence of UV-A+B or UV-B with a flow-through system. While UV-A and UV-B, respectively, inhibited noontime Pmax by 22% and 14% on the sunny days, UV-A during sunrise (PAR below about 50Wm−2) increased the net photosynthesis by about 8% when compared with PAR alone. UV-A + PAR also resulted in higher apparent photosynthetic efficiency in the morning than in the afternoon period than PAR alone. Nevertheless, integrated daytime photosynthetic production under solar PAR alone was higher than with either PAR + UV-A+B or PAR + UV-A. Relative growth rate in the long term (9 days) matched the integrated photosynthetic production in that UV-A led to 9–15% and UV-B to 19–22% reduction, respectively. UV-absorbing compounds were found to be higher in the thalli exposed to PAR+UV-A+B than under PAR alone, reflecting a protective response to UVR.
Introduction
Macroalgae, living mainly in the intertidal and subtidal zones, remediate CO2 and nutrients in coastal waters and provide shelter for animals in addition to direct or indirect food supplies for humans. They experience dramatic changes in temperature and solar radiation associated with diurnal changes of weather and superimposed tidal rhythms (Davison & Pearson, Citation1996). Solar ultraviolet radiation (UVR, 280–400nm) is a permanently existing environmental factor that macroalgae are usually exposed to. UV-exposure may cause direct damage to key components, such as the D1 protein of PS II (Vass, Citation1997), photosynthetic pigments (Aguilera et al., Citation1999a), key enzymes (Bischof et al., Citation2002) and even DNA (Buma et al., Citation2001). Therefore, understanding the physiological and ecological responses of macroalgae to solar UVR is of potential importance in explaining their in situ physiological behaviour and predicting the ecological consequences in coastal ecosystems as a result of increased UV-B (280–315nm) irradiance due to global stratospheric ozone depletion (Kerr & McElroy, Citation1993; den Outer et al., Citation2005).
Species of macroalgae are distributed to different depths in the intertidal zone and exposed to different levels of photosynthetically active radiation (PAR, 400–700nm) and UVR because of the attenuation effects of seawater. Their vertical distribution is closely related to their sensitivity to UV-B (Hanelt et al., Citation1997; Bischof et al., Citation1998) and their recovery capacity after being damaged by UVR (Gómez & Figueroa, Citation1998). UV-B radiation impairs growth (Grobe & Murphy, Citation1994; Pang et al., Citation2001; Michler et al., Citation2002), photosynthesis (Cordi et al., Citation1997; Aguilera et al., Citation1999b; Han et al., Citation2003), early development (Huovinen et al., Citation2000; Henry & Van Alstyne, Citation2004) and spore germination (Wiencke et al., Citation2000; Han et al., Citation2004) of macroalgae. It can even affect macroalgal community structure (Bischof et al., Citation2006). Different life stages of some macroalgal species showed different abilities to endure UVR, with increased tolerance as individuals differentiate and develop repair and defence capacity (Dring et al., Citation1996; Henry & Van Alstyne, Citation2004).
Macroalgae can protect themselves from being harmed by UVR, or diminish the damage, by avoidance, repair and screening mechanisms (Karentz, Citation1994; Franklin & Forster, Citation1997; Karentz, Citation2001). UV-screening compounds, such as mycosporine-like amino acids (Karentz et al., Citation1991; Dunlap & Shick, Citation1998), scytonemin (Garcia-Pichel & Castenholz, Citation1991; Dillon et al., Citation2002) and phlorotannins (Pavia et al., Citation1997; Pavia & Brock, Citation2000) have been found in many photosynthetic organisms. Cellular content of these compounds increase with increased UV exposure (Pavia et al., Citation1997; Brenowitz & Castenholz, Citation1997; Han & Han, Citation2005) thereby reducing UV-related photoinhibition and damage.
Despite a number of studies demonstrating the deleterious effects of UV-B on macroalgae, researches also show that solar UV-B radiation could play a role in the recovery process of inhibited photosynthesis in a brown alga, Dictyota dichotoma (Flores-Moya et al., Citation1999), and some freshwater plants (Hanelt et al., Citation2006), and that UV-A (315–400nm) radiation could aid in DNA repair (Pakker et al., Citation2000a,b) and enhance growth (Henry & Van Alstyne, Citation2004) in macroalgae. Dring et al. (Citation2001) reported higher maximum quantum yields in several subtidal red algae during recovery after exposure to solar UV-A or UV-A + UV-B in the absence of PAR as compared with the exposures to PAR alone or PAR + UVR, suggesting that UVR was less damaging to photosynthesis than high PAR of natural sunlight. On the other hand, in contrast to the impacts of UVR in short-term experiments, hardly any lasting difference was found in growth between samples exposed to solar radiation with or without UV-B in long-term experiments on Ulva rigida (Altamirano et al., Citation2000) and Fucus serratus (Michler et al., Citation2002). Nevertheless, UVR was found to affect the growth in long-term and field experiments on Laminaria spp (Michler et al., Citation2002; Roleda et al., Citation2006). Despite a number of studies on the negative and positive effects of UVR, little has been documented on the diurnal photosynthetic performance associated with fluctuating solar UVR and PAR. Information about diurnal physiological behaviour under the full spectrum of solar radiation is essential for us to understand the daytime UVR impacts associated with changing solar radiation and to explain the difference in macroalgal responses to UVR between short and long-term experiments.
The aim of the present study was to elucidate the impacts of solar UV-B, UV-A and PAR on the diurnal photosynthetic performance and growth of the economically important red alga, Gracilaria lemaneiformis Bory, which is commercially cultivated in surface water for food and agar around the coast of China.
Materials and methods
Plant materials
Gracilaria lemaneiformis, is naturally distributed in the sublittoral zone in northern part of China, and has been farmed for years in coastal surface water from November to May in southern China. Plants were collected from their cultivation area where they were grown at a depth of 0.5–1m at Nanao (116.6°E, 23.3°N), Shantou, China, in March, April and May, 2004, or from a farmed population in the coastal water near Qingdao (120°E, 36°N) in September, 2006. Seawater temperature ranged from 16 to 28°C during the periods of March, April and May, 2004, and was controlled at 22±0.5°C for the experiments during September, 2006. The nitrate and phosphate concentrations ranged from 41–43µM and 0.56–0.62µM respectively during this season. The maximal incident irradiances received by the thalli in the farmed area were about 1.2Wm−2 for UV-B, 40Wm−2 for UV-A and 400Wm−2 for PAR. Young plants (about 20cm long) were selected and maintained in sand-filtered flowing seawater for a night before their diurnal photosynthesis was measured next day. For experiments in the September, the plants were transported (in a cooler) from Qingdao and cultured indoors at 15Wm−2 PAR and 22°C for 7 days, and then exposed to solar radiation at the same temperature (to simulate that in April) for another 7 days prior to measuring of diurnal photosynthesis.
Solar radiation measurements and treatments
Incident solar irradiance was continuously monitored using an ELDONET filter radiometer (Real Time Computer, Möhrendorf, Germany), which has three channels for photosynthetically active radiation (PAR, 400–700nm), UV-A (UV-A, 315–400nm) and UV-B radiation (UV-B, 280–315nm). The reliability of this instrument has been internationally recognised (Häder et al., Citation1999; Korbee-Peinado et al., Citation2004), and certificated with a correspondence error less than 0.5% in comparison with the most accurate instrument (certificate No. 2006/BB14/1). The device was installed on the roof of the Nan’ao Marine Biological Station of Shantou University where the experiments were carried out. Three different radiation treatments were implemented as follows: thalli receiving full solar radiation (PAB treatment) in uncovered quartz tubes; thalli receiving UV-A and PAR (PA treatment) in quartz tubes covered with Folex 320 (Montagefolie, Nr 10155099, Folex, Dreieich, Germany); and thalli receiving only PAR (P treatment) in quartz tubes covered with Ultraphan film 395 (UV Opak, Digefra, Munich, Germany). The transmission spectra of the cut-off filters with negligible reflection have been published elsewhere (Korbee-Peinado et al., Citation2004). The uncovered quartz tubes received 4% higher PAR radiation (measured by inserting a PAR sensor inside the quartz container) compared with the covered tubes with the 395 or 320 filters due to the reflection (in water) caused by these filters.
Diurnal photosynthesis measurement
A flow-through system for measuring photosynthesis in running water was set up according to Gao & Umezaki (Citation1989) (). Quartz tubes (4cm inner diameter and 35cm long, 0.44l) were used as assimilation chambers to allow the full spectrum of solar radiation to reach the thalli inside. The flow rate of seawater was controlled at 0.2lmin−1 by a flow meter. Although current speed may affect photosynthetic O2 evolution (Wheeler, Citation1980; Gao et al., Citation1992), this flow rate resulted in the best resolution and avoided the bottle effects incurred with still sealed seawater. It took about 5min for the O2 concentration in the outlet seawater to reach a constant level when the thalli photosynthesized under constant levels of PAR or sunlight. Oxygen concentrations in the inlet and outlet seawater were monitored simultaneously and continuously with a Clark-type oxygen electrode probe (YSI model 5300, Yellow Spring, OH, USA), which was calibrated every day at the early morning and at midday, when the seawater temperature changed by about 1°C. Between six and 10 branches from different individuals were placed in the quartz tube by fixing them on stainless steel wires with the biomass density (fresh weight [FW] of about 270gm−2, which did not result in self-shading [biomass density was 100–5,000gm−2 in the farmed areas]. The rate of photosynthetic O2 evolution [P, µmol O2g−1h−1] was determined as follows: P=(A–B)*F*60*W−1, where A and B represent dissolved O2 concentrations (µmolO2l−1) in the outlet and inlet seawater, respectively; F, the flow rate (L min−1) of seawater; W, fresh weight (g) of the samples used. Parameters for photosynthesis–light (P–E) curves were analysed according to Jassby & Platt (Citation1976): P=Pmax*tanh(α*E/Pmax)+Rd, where P represents photosynthetic rate; E, irradiance, Pmax, the light-saturated photosynthetic rate; α, initial slope at limiting irradiances and, Rd, the dark respiration rate.
Fig. 1. Outline of the flow-through system used for measuring the diurnal photosynthesis of Gracilaria lemaneiformis. Quartz tubes were used as the assimilation chamber. Thalli in the tubes were exposed to different solar radiation treatments by using UV-cutting off filters.
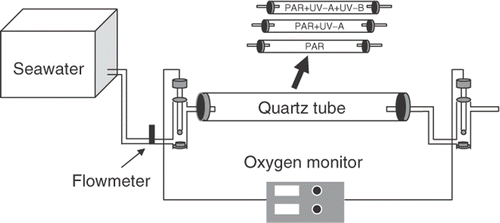
Since the uncovered quartz containers received 4% higher PAR than those covered with the cut-off filters either under low or high levels of solar radiation and this higher portion of PAR might affect the comparison of irradiance-limiting photosynthetic rate with or without UVR, PAR values were calibrated by multiplying 0.96 for the P-E curves obtained without UVR. In order to estimate the daily net photosynthetic production, daytime net photosynthesis expressed (µmol O2) was converted to dry matter using the ratio of 1g O2 to 0.84g dry matter (Ikusima, Citation1965).
To estimate the diurnal photosynthetic performance on a cloudy day in April, we simulated the weather conditions for April 24, 2004 on September 29, 2006 (unexpected high photosynthetic rates were obtained in the presence of UV-A) by reducing the diurnal solar radiation with black plastic neutral screens and controlling the temperature at 22±0.5°C using a cooling unit (CAP-3000, Rikakikai, Tokyo, Japan).
Measurements of growth
Thalli of about 2g FW were maintained in the flow-through seawater in each of the quartz tubes with a biomass density of about 100gm−2 FW (without self-shading) at the same flow rate (0.2lmin−1) as mentioned above for photosynthesis measurements. The growth experiments were run separately from the photosynthetic experiments (with different samples) for 9 days: April 9–17 and May 13–20, 2004. FW of the thalli was measured every 2 days after blotting with tissue paper, and relative growth rate (RGR,% day−1) was estimated as follows: RGR=100*(lnNt –lnN 0)/t, where N 0 is the initial FW and Nt that after t number of days.
Determination of photosynthetic pigments and UV-absorbing compounds
The samples for pigment analyses were stored at –20°C. About 200mg (FW) thalli were extracted in 10ml absolute methanol for 24h at 4°C in darkness, which resulted in complete extraction. After centrifugation at 5,000g for 10min this extract was used to determine the amounts of UV-absorbing compounds (UVAC) and photosynthetic pigments using a scanning spectrophotometer (UV 530, Beckman Coulter, Fullerton, CA, USA). The UVAC content was estimated by determining the ratio of UVAC to chlorophyll a (Chl a) according to Dunlap et al. (Citation1995) and converting the ratio to content per FW. Chl a concentration was estimated according to Wellburn (Citation1994).
Data treatment and statistic analysis
Diurnal photosynthetic rates were related to solar irradiance to generate photosynthesis versus light curves, and were integrated to estimate daily net production. UVR, UV-A or UV-B-induced inhibition was obtained as follows: (PPAR–PPAB)/PPAR*100%, (PPAR–PPA)/PPAR*100% or (PPA–PPAB)/PPAR*100%, where PPAB, PPA and PPAR represent the net photosynthetic rates under the treatments of PAR + UV-A+B, PAR + UV-A or PAR alone, respectively. ANOVA and t-test were used to establish differences among the different treatments. A confidence level of 95% was used in all analyses.
Results
Diurnal photosynthetic performance of Gracilaria lemaneiformis in March, April (1-day simulated in September and May showed that daytime photosynthesis almost followed the pattern of sunlight regardless of the solar radiation treatment, increasing and becoming saturated with increased solar irradiance in the morning, and declining with decreasing solar radiation in the afternoon (). On April 20, which was cloudless, net photosynthetic rate decreased continuously from morning to afternoon, showing clear photoinhibition during the diurnal course. Photosynthetic O2 evolution was inhibited in thalli receiving the full spectrum of sunlight (PAB treatment) or PAR + UV-A (PA treatment) compared with samples treated with PAR (P) alone from 8:00 am to 4:00pm for all days in March, April or May (). The inhibition induced by UVR on the sunny days was higher than on the cloudy days (). UV-A and UV-B inhibited noontime Pmax by 22% and 14% on the sunny days and by 19% and 2% on the cloudy days. During the sunrise periods (PAR below about 50Wm−2), the ratio of net photosynthetic rate under UV-A + PAR to that under PAR alone significantly (p<0.05) increased by up to 36% (mean percent 8%), indicating that low UV-A levels, during the early morning, enhanced the net photosynthetic rate. However, such a UV-A-related significant enhancement was not found in the late afternoon.
Fig. 2. Diurnal photosynthetic O2 evolution of Gracilaria lemaneiformis thalli exposed to PAR alone (P), PAR+UV-A (PA) and PAR+UVR (PAB) associated with solar radiations (PAR, UV-A and UV-B) on March 16 (A), March 21 (B), April 20 (C), May 23 (E) and May 28 (F), 2004, and on September 29, 2006 (D, under controlled temperature at 21.5–22.5°C and reduced solar radiation) to simulate the cloudy day, April 24, 2004. The temperature of the flowing-through seawater ranged 16–18°C on March 16, 17–19°C on March 21, 21–23°C on April 20, 26–28°C on May 23, 26–28°C on May 28.
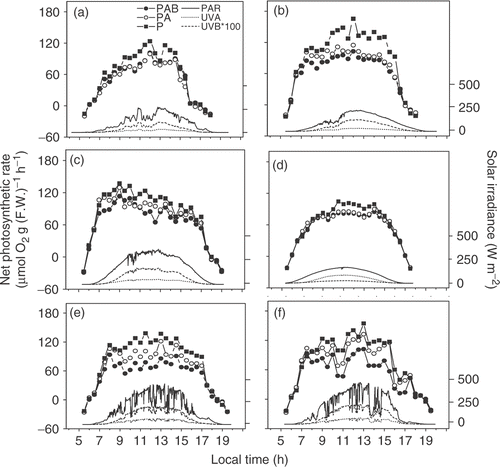
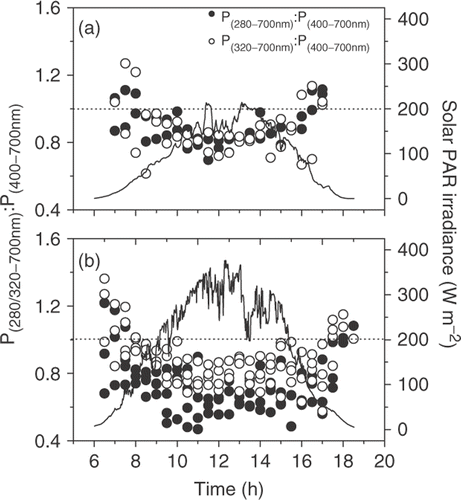
Fig. 3. Diurnal photosynthetic O2 evolution under PAR+UVR (280–700nm) and PAR+UV-A (320–700nm) as compared with PAR (400–700nm) in relation to mean solar irradiance (PAR), on cloudy (A) and sunny days (B).
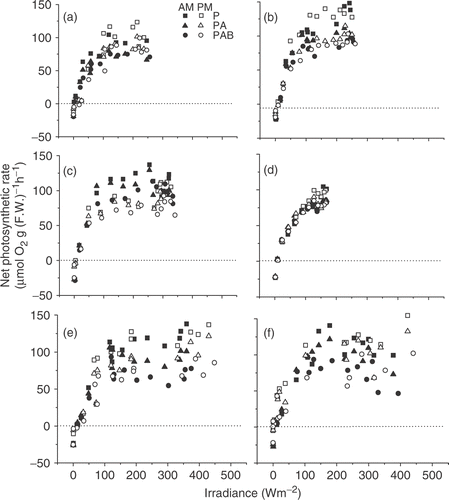
When the net photosynthetic rates () were plotted against solar PAR irradiances, the relationship of net photosynthesis and solar irradiance (P–E curves) was obtained for the thalli under different radiation treatments (). In the morning the apparent photosynthetic efficiency (α) was 1.90±0.35, 2.38±0.31 and 2.04±0.20 under PAR, PAR + UV-A and PAR + UV-A+B, respectively (). The presence of UV-A significantly enhanced the efficiency by 25% (p<0.05). However, both UV-A and UV-B reduced efficiency in the afternoon (p<0.05). When morning and afternoon were compared, it appeared that α was higher (p<0.05) in the morning than in the afternoon under solar radiation with UV-A (PA) or UVR (PAB) (). In the absence of UVR, no significant (p>0.1) difference was found in α between the morning and afternoon. The presence of UVR did not result in significant difference in the light-saturating point (Ik), no difference between morning and afternoon. Pmax showed a significant increase (p<0.05) when UVR was screened off, but did not demonstrate any significant (p>0.1) difference between the morning and afternoon ().
Fig. 4. Net photosynthetic rate of Gracilaria lemaneiformis as a function of irradiance in the morning (black symbols) and afternoon (white symbols) under different radiation treatments on March 16 (A), March 21 (B), April 20 (C), May 23 (E) and May 28 (F), 2004, and on September 29, 2006 (D, temperature controlled at 21.5–22.5°C and solar radiation reduced) to simulate the cloudy day, April 24, 2004.
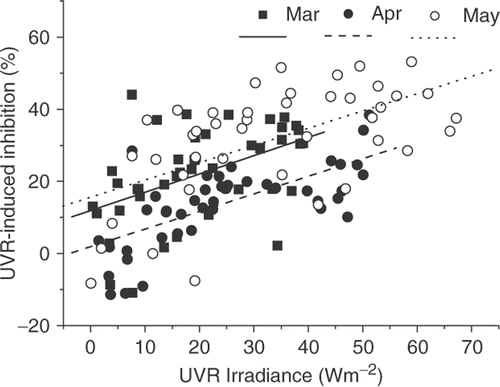
When UVR-induced inhibition was plotted as a function of UV irradiance for all the diurnal photosynthetic measurements, it appeared that inhibition increased with increasing UVR (). The highest inhibition was found in May, while the lowest was in April. A significant (p<0.05) difference was found in the inhibition between May and April at the highest UV radiation levels of about 55Wm−2.
Fig. 5. UVR-induced photosynthetic inhibition (from early morning to late afternoon) in relation to UVR irradiance in March, April (including September 29, 2006, simulated conditions for the cloudy day, April 24, 2004) and May, 2004. The lines represent a linear fit of the data (p<0.001).
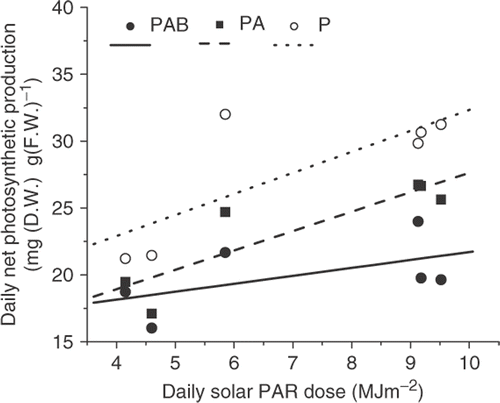
Integration of daytime net photosynthesis provides the daytime photosynthetic production under different radiation treatments (). The daily net production was highest under PAR alone when UVR was screened off. The lowest daily net production was found in thalli receiving the full spectrum of sunlight. Nevertheless, the daily net production increased with increased PAR dose per day regardless of the treatments.
Fig. 6. Daily net photosynthetic production of Gracilaria lemaneiformis when exposed to PAR alone (P), PAR+UV-A (PA) and PAR+UVR (PAB) as a function of daily solar PAR dose in March, April (including September 29, 2006, simulated conditions for the cloudy day, April 24, 2004) and May, 2004. The daily net production was based on the integrated daytime photosynthesis shown in . R 2 values were 0.61, 0.77 and 0.31 for PAR alone (P), PAR+UV-A (PA) and PAR+UVR (PAB), respectively.
When the FW of the thalli receiving different solar radiations was monitored in April () or May (), it increased faster without UV-B. Over a period of 9 days biomass increased by about 53% (PAB), 58% (PA) and 82% (P) in April, and by about 100% (PAB), 174% (PA) and 175% (P) in May, indicating that screening off UVR enhanced the growth. Relative growth rate (RGR) derived from the changes in fresh biomass, was significantly (p<0.01) higher in the thalli receiving PAR alone (P) than those exposed to UV-A + PAR (PA) or UVR + PAR (PAB) () in April. However, no significant (p>0.1) difference was found between PA and PAB treatments in this month. In May RGR increased significantly (p<0.001) when UV-B was filtered out (P, PA) but showed insignificant (p>0.5) change when UV-A was also removed (PA) compared to P treatment, indicating the negative impact of UV-B alone. Comparison of the RGR values between April and May demonstrates that RGR was higher (p<0.01) in May than in April under any of the solar radiation exposures irrespective of the presence of UV-B or UVR. The RGR values derived from daytime net photosynthesis and night dark respiration were about 9% (PAB), 12% (PA) and 14% (P) per day, while that based on measured biomass change was abut 7% (PAB), 9% (PA) and 10% (P) (). Although photosynthesis-based RGR values (RGR-Pd) were higher, they matched the measured RGR (RGR-Bm, relative growth rate determined by biomass increase) in the order of P > PA > PAB, with a 19–22% reduction with UV-B and 9–15% with UV-A.
Fig. 7. Change in fresh mass (A,B) of Gracilaria lemaneiformis thalli under different solar radiation treatments (PAR alone [P], PAR+UV-A [PA] and PAR+UVR [PAB]) with time and changes of PAR, UV-A and UV-B from April 9–17 (A) or from May 12–20 (B). The corresponding relative growth rates were compared among the treatments in the two months (C). Vertical bars represent±SD of the means (three individuals in each of three tubes). Different letters show significant (p<0.05) differences among the treatments.
![Fig. 7. Change in fresh mass (A,B) of Gracilaria lemaneiformis thalli under different solar radiation treatments (PAR alone [P], PAR+UV-A [PA] and PAR+UVR [PAB]) with time and changes of PAR, UV-A and UV-B from April 9–17 (A) or from May 12–20 (B). The corresponding relative growth rates were compared among the treatments in the two months (C). Vertical bars represent±SD of the means (three individuals in each of three tubes). Different letters show significant (p<0.05) differences among the treatments.](/cms/asset/b717c5a1-c031-4eb8-9103-541a2ed95f73/tejp_a_298849_o_f0007g.gif)
Absorption spectra of the methanol extracts from the thalli showed high amounts of UV-absorbing compounds (UVAC) at 325nm (). UVAC content by FW was higher under full spectrum of solar irradiance than with PAR alone in April (p<0.05) (). Chl a content decreased significantly (p<0.01) in thalli exposed to the full spectrum of solar radiation (PAB), however, it changed little (p>0.1) when P and PA treatments were compared (). The UVAC/Chl a ratio was higher (p<0.05) under PAB than P treatment in April and May cultures (). Exposure to UV-A + PAR (PA) did not lead (p>0.1) to any increase in UVAC/Chl a ratio compared with PAR alone (P) ().
Fig. 8. Absorption spectra for April (A) and May (B), UVAC (C), Chl a content (D) and UVAC:Chl a ratio (E) of Gracilaria lemaneiformis thalli after exposed to the radiation treatments (PAR alone [P], PAR+UV-A [PA] and PAR+UVR [PAB]) in April and May for 9 days, respectively. Different letters show significant (p<0.05) differences among the treatments.
![Fig. 8. Absorption spectra for April (A) and May (B), UVAC (C), Chl a content (D) and UVAC:Chl a ratio (E) of Gracilaria lemaneiformis thalli after exposed to the radiation treatments (PAR alone [P], PAR+UV-A [PA] and PAR+UVR [PAB]) in April and May for 9 days, respectively. Different letters show significant (p<0.05) differences among the treatments.](/cms/asset/75cdc7b7-8c76-4dc4-8c09-80c1066a29a0/tejp_a_298849_o_f0008g.gif)
Discussion
In this study we found that UVR induced photosynthetic inhibition at high levels of solar radiation around noontime, but the presence of UV-A enhanced the apparent photosynthetic efficiency by up to 25% and resulted in higher photosynthetic rates during the sunrise period compared to PAR alone. While the presence of UV-A had negligible effect on the growth of the alga, filtering UV-B enhanced in long-term (9 days) exposures. The enhancement caused by UV-A in the morning could hardly be associated with the radiation treatments, since cutting-off 320 and 395 films reflected 4% of the solar PAR.
Table 1. Maximal net photosynthetic rate, Pm (µmol O2g−1h−1), apparent photosynthetic efficiency, α (µmol O2g−1h−1)/(Wm−2), light-saturating irradiance, Ik (W m−2), derived from the photosynthesis-light relationships (P–E curves) for all the diurnal measurements in under different radiation treatments. The values in parentheses are for the PM in contrast to the AM values. Data are means±SD for six P–E curves ().
Diurnal photosynthesis of macroalgae has been previously found to be depressed in the afternoon on sunny days in Macrocystis pyrifera surface canopy (Gerard, Citation1986), Sargassum spp. (Gao & Umezaki, Citation1989; Gao, Citation1990), Ulva curvata, Codium decorticatum, Dictyota dichotoma, Petalonia fascia and Gracilaria foliifera (Ramus & Rosenberg, Citation1980). The photosynthetic efficiency of O2 evolution was found to be higher in the morning than in the afternoon in Ulva rotundata (Henley et al., Citation1991) and Sargassum horneri (Gao, Citation1990) under solar PAR. Such an afternoon photosynthetic depression was not found on rainy or highly cloudy days (Gao & Umezaki, Citation1989) and may be largely removed by superimposing a light fluctuation on the diurnal regime as demonstrated in phytoplankton (Marra, Citation1978). Contrarily, the red alga Gelidiella acerosa was found to photosynthesize inefficiently in the morning compared to midday and afternoon (Ganzon-Fortes, Citation1997). Due to the light-transmission characteristics of the incubation vessels used (glass, polyethylene and polycarbonate materials) which do not allow UV-B and part of UV-A to penetrate (Van Donk et al., Citation2001), these previous findings only demonstrated the asymmetrical diurnal photosynthesis under PAR, without UV-B or UVR being considered. On the other hand, the highest photoinhibition at noon was observed in macroalgae under the full spectrum of solar radiation (Huppertz et al., Citation1990; Hanelt, Citation1992), however, the effects caused by UVR have only infrequently been differentiated from those of PAR (Hanelt et al., Citation1997; Flores-Moya et al., Citation1999). This study shows that UVR further depressed the apparent photosynthetic efficiency in the afternoon on sunny days. In addition, UV-A enhanced the apparent photosynthetic efficiency and increased net photosynthetic rate during the sunrise period. However, such enhancement was not significant during the sunset period. Accumulated damage caused by UVR at noontime and slow recovery until sunset may be accountable for this discrepancy. Diurnal photosynthetic inhibition and recovery in G. lemaneiformis displayed different patterns according to overcast conditions, reflecting the differences in the balance between damage and repair. Diurnal rhythms or feedback effect of UVR on Calvin-cycle enzymes may also contribute to the observed reduction. The result that UV-B did not bring any difference in the Pmax between the afternoon and morning in G. lemaneiformis could be attributed to fluctuating solar irradiance, which occurred during most of the experimental days (). It is possible that highly fluctuating solar irradiance, especially that of UV-A, could refurbish damaged photosynthetic apparatus and ameliorate the afternoon Pmax depression, based on the balance between damage and repair.
Table 2. Relative growth rates (RGR) derived from daily photosynthetic production (daytime photosynthesis minus night respiration) (Pd) and those measured directly from biomass changes (Bm) in April and May, 2004 under different radiation treatments.
The present study, on the other hand, showed that UV-A enhanced the photosynthetic O2 evolution and apparent quantum yield in G. lemaneiformis under low levels of solar radiation, which could be associated with UVR-energizing or UV-A-stimulated key enzyme activity for photosynthetic CO2 fixation, as recently found for phytoplankton (Gao et al., Citation2007). Although high levels of UV-A radiation at midday caused photosynthetic inhibition of some macroalgae (Häder et al., Citation2001), low levels of UV-A radiation has been found to enhance the growth of brown algae, Fucus gardneri, embryos (Henry & Van Alstyne, Citation2004) as well as photosynthetic CO2 fixation by phytoplankton (Helbling et al., Citation2003). UV-A has also been found to aid in DNA repair (Pakker et al., Citation2000a,b). Recently, absorption of UV-A energy has been found to be transferred to chl a, which then emitted red fluorescence in a diatom (Orellana et al., Citation2004).
In the present work, UV-B reduced the growth rate of G. lemaneiformis in both April and May, however, UV-A only affected the growth rate negatively in the April experiment. Different fluctuating patterns of incident solar radiation between the April and May experimental periods () may account for this difference. Frequently changing levels of solar radiation due to cloud movement, or low levels of UVR on cloudy days in May (), could lead to UV-A enhanced photosynthesis and subsequently improve growth.
Photosynthetic inhibition over the short term (<1 day), reflecting reduced photosynthetic efficiency and/or carboxylation, may not agree with growth induced by UVR over the long-term (>1 week), though algal growth is based on photosynthesis. Daily production of G. lemaneiformis was inhibited by UVR to a greater extent compared with RGR estimated from biomass change (). Amelioration of UVR-related harm by accumulated UVAC could contribute to the decreased photo-inhibition on growth. On the other hand, excretion of dissolved organic matter or loss of algal tissue might be responsible for the difference between the RGR and photosynthetic production. Despite the difference, however, photosynthetic response to solar UVR agreed well with that of growth, in that both UV-A and UV-B had an inhibitory effect in G. lemaneiformis, with a decrease of 19–22% by UV-B and 9–15% by UV-A. Natural levels of UV-B radiation have been found to damage DNA in macroalgae (Pakker et al., Citation2000b) and reduce the growth rate of Ulva spp (Altamirano et al., Citation2000; Han & Han, Citation2005) and Fucus gardneri embryos (Henry & Van Alstyne, Citation2004). Enhanced levels of UV-B can further inhibit macroalgal growth as found in the brown algae Ectocarpus rhodochondroides (Santas et al., Citation1998) and Dictyota dichotoma (Kuhlenkamp et al., Citation2001). The bilateral (positive at low and negative at high levels) effects of UV-A could magnify the discrepancy in UV-related inhibition between the integrated photosynthetic production and growth according to the weather conditions.
Macroalgae in nature often exhibit high levels of UV-absorbing compounds, such as MAAs in the red alga Porphyra columbina (Korbee-Peinado et al., Citation2004), an unknown UV-B absorbing substance in the green alga Ulva pertusa (Han & Han, Citation2005) and phlorotannin in the brown algae Ascophyllum nodosum and Fucus gardneri (Pavia et al., Citation1997; Henry & Van Alstyne, Citation2004). These compounds have been suggested to play a protective role against solar UVR (Oren & Gunde-Cimerman, Citation2007). In the present study, the levels of UVAC were higher in the thalli of G. lemaneiformis under the full spectrum of solar radiation than the UVR-free treatments, reflecting an induction responsive to UVR. Synthesis of UVAC has been found to be induced by UV-B in Chondrus crispus (Karsten et al., Citation1998), P. columbina (Korbee-Peinado et al., Citation2004) and U. pertusa (Han & Han, Citation2005). Such stimulation is dependent on both dose and wavelength, with higher accumulation of UVAC under high daily doses (Karsten et al., Citation1998, Franklin et al., Citation2001). Accumulation of UVAC or the increased ratio of UVAC to Chl a in G. lemaneiformis could have diminished the harmful effects of solar UVR, which may lead to decreased inhibition of growth over a longer time period (). On the other hand, thallus morphological differences and related optical characteristics can also affect macroalgal response to UVR (Roleda et al., Citation2006).
Based on the present study on G. lemaneiformis, diurnal photosynthetic performance of macroalgae can differ when solar UVR is considered. Both UV-A and B negatively affect the photosynthesis at midday, while UV-A could act positively, either enhancing photosynthesis or photo-repairing under reduced solar radiation, and therefore have a insignificant effect on growth over the long term. Diurnal photosynthetic production of G. lemaneiformis appeared to depend not only on levels of PAR and UVR but also on fluctuating patterns of solar radiation, reflecting the difficulty in comparing outdoor short- and long-term experiments, because of cloud movement or overcast conditions on a daily basis. UV-protective mechanisms during acclimation, such as accumulation of UVAC and related optical characteristics of the tissue, can also reduce the UVR-related inhibition and benefit growth. Temperature changes may also affect the diurnal photosynthetic performance since the enzymatic activity responsible for repairing UVR-damage changes with temperature. Further study is needed to test the temperature-dependency of UVR-related diurnal photosynthetic behaviour.
Acknowledgements
We thank the anonymous reviewers for their constructive comments. This study was supported by “863” project (2006AA10A413) from Ministry of Science and Technology, National Natural Science Foundation of China (Key Project No. 90411018) and Ministry of Education (No.308015). We thank Professors Donat-P. Häder and E. Walter Helbling for helpful comments during their research visits to Shantou University.
References
- Aguilera , J , Jiménez , C , Figueroa , FL , Lebert , M and Häder , DP . 1999a . Effect of ultraviolet radiation on thallus absorption and photosynthetic pigments in the red alga Porphyra umbilicalis . J. Photochem. Photobiol. B Biol. , 48 : 75 – 82 .
- Aguilera , J , Karsten , U , Lippert , H , Vögele , B , Philipp , E , Hanelt , D and Wiencke , C . 1999b . Effects of solar radiation on growth, photosynthesis and respiration of marine macroalgae from the Arctic . Mar. Ecol. Prog. Ser. , 191 : 109 – 119 .
- Altamirano , M , Flores-Moya , A and Figueroa , FL . 2000 . Long-term effects of natural sunlight under various ultraviolet radiation conditions on growth and photosynthesis of intertidal Ulva rigida (Chlorophyceae) cultivated in situ . Bot. Mar. , 43 : 119 – 126 .
- Bischof , K , Hanelt , D and Wiencke , C . 1998 . UV-radiation can affect depth zonation of Antarctic macroalgae . Mar. Biol. , 131 : 597 – 605 .
- Bischof , K , Kräbs , G , Wiencke , C and Hanelt , D . 2002 . Solar ultraviolet radiation affects the activity of ribulose-1,5-bisphosphate carboxylase-oxygenase and the composition of photosynthetic and xanthophylls cycle pigments in the intertidal green alga Ulva lactuca L . Planta , 215 : 502 – 509 .
- Bischof , K , Gómez , I , Molis , M , Hanelt , D , Karsten , U , Lüder , U , Roleda , MY , Zacher , K and Wiencke , C . 2006 . Ultraviolet radiation shapes seaweed communities . Rev. Environ. Sci. Biotechnol. , 5 : 141 – 166 .
- Brenowitz , S and Castenholz , RW . 1997 . Long-term effects of UV and visible irradiance on natural populations of a scytonemin-containing cyanobacterium (Calothrix sp.) . FEMS Microbiol. Ecol. , 24 : 343 – 352 .
- Buma , AGJ , De Boer , MK and Boelen , P . 2001 . Depth distributions of DNA damage in Antarctic marine phyto- and bacterioplankton exposed to summertime UV radiation . J. Phycol. , 37 : 200 – 208 .
- Cordi , B , Depledge , MH , Price , DN , Salter , LF and Donkin , ME . 1997 . Evaluation of chlorophyll fluorescence, in vivo spectrophotometric pigment absorption and ion leakage as biomarkers of UV-B exposure in marine macroalgae . Mar. Biol. , 130 : 41 – 49 .
- Davison , JR and Pearson , GA . 1996 . Stress tolerance in intertidal seaweeds . J. Phycol. , 32 : 197 – 211 .
- Den Outer , PN , Slaper , H and Tax , RB . 2005 . UV radiation in the Netherlands: Assessing long-term variability and trends in relation to ozone and clouds . J. Geophys. Res. D: Atmospheres , 110 : 1 – 11 .
- Dillon , JI , Tatsumi , CI , Tandingan , PI and Castenholz , RI . 2002 . Effect of environmental factors on the synthesis of scytonemin, a UV-screening pigment, in a cyanobacterium (Chroococcidiopsis sp.) . Arch. Microbiol. , 177 : 322 – 331 .
- Dring , M , Makarov , V , Schoschina , E , Lorenz , M and Lüning , K . 1996 . Influence of ultraviolet radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta) . Mar. Biol. , 126 : 183 – 191 .
- Dring , MJ , Wagner , A and Lüning , K . 2001 . Contribution of the UV component of natural sunlight to photoinhibition of photosynthesis in six species of subtidal brown and red seaweeds . Plant Cell Environ. , 24 : 1153 – 1164 .
- Dunlap , WC and Shick , JM . 1998 . UV radiation absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective . J. Phycol. , 34 : 418 – 430 .
- Dunlap , WC , Rae , GA , Helbling , EW , Villafañe , VE and Holm-Hansen , O . 1995 . Ultraviolet-absorbing compounds in natural assemblages of Antarctic phytoplankton . Antarct. J. , 30 : 323 – 326 .
- Flores-Moya , A , Hanelt , D , Figueroa , F. Altamirano , M , Vinegla , B and Salles , S . 1999 . Involvement of solar UV-B radiation in recovery of inhibited photosynthesis in the brown alga Dictyota dichotoma (Hudson) Lamouroux . J. Photochem. Photobiol. B Biol. , 49 : 129 – 135 .
- Franklin , LA and Forster , RM . 1997 . The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology . Eur. J. Phycol. , 32 : 207 – 232 .
- Franklin , LA , Kräbs , G and Kuhlenkamp , R . 2001 . Blue light and UV-A radiation control the synthesis of mycosporine-like amino acids in Chondrus crispus (Florideophyceae) . J. Phycol. , 37 : 257 – 270 .
- Ganzon-Fortes , ET . 1997 . Diurnal and diel patterns in the photosynthetic performance of the Agarophyte Gelidiella acerosa . Bot. Mar. , 40 : 93 – 100 .
- Gao , K . 1990 . Diurnal photosynthetic performance of Sargassum horneri . Jpn J. Phycol. , 38 : 163 – 165. . (In Japanese with English summary.)
- Gao , K and Umezaki , I . 1989 . Studies on diurnal photosynthetic performance of Sargassum thunbergii I. Changes in photosynthesis under natural sunlight . Jpn J. Phycol. , 37 : 89 – 98 .
- Gao , K , Aruga , Y , Asada , K , Ishihara , T , Akano , T and Kiyohara , M . 1992 . Enhancement of photosynthetic CO2 fixation of the red alga Porphyra yezoensis Ueda in flowing seawater . Jpn J. Phycol. , 40 : 397 – 400 . (In Japanese with English summary.)
- Gao , K , Wu , Y , Li , G , Wu , H , Villafañe , VE and Helbling , EW . 2007 . Solar UV radiation drives CO2 fixation in marine phytoplankton: A double-edged sword . Plant Physiol. , 144 : 54 – 59 .
- Garcia-Pichel , F and Castenholz , RW . 1991 . Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment . J. Phycol. , 27 : 395 – 409 .
- Gerard , VA . 1986 . Photosynthetic characteristics of giant kelp (Macrocystis pyrifera) determined in situ . Mar. Biol. , 90 : 473 – 482 .
- Gómez , I and Figueroa , FL . 1998 . Effects of solar UV stress on chlorophyll fluorescence kinetics of intertidal macroalgae from southern Spain: a case study in Gelidium species . J. Appl. Phycol. , 10 : 285 – 294 .
- Grobe , CW and Murphy , TM . 1994 . Inhibition of growth of Ulva expansa (Chlorophyta) by ultraviolet-B radiation . J. Phycol. , 30 : 783 – 790 .
- Häder , DP , Lebert , M , Marangoni , R and Colombetti , G . 1999 . ELDONET – European light dosimeter network hardware and software . J. Photochem. Photobiol. B Biol. , 52 : 51 – 58 .
- Häder , DP , Porst , M and Lebert , M . 2001 . Photoinhibition in common Atlantic macroalgae measured on site in Gran Canaria . Helgol. Mar. Res. , 55 : 67 – 76 .
- Han , T , Han , YS , Kain , JM and Häder , DP . 2003 . Thallus differentiation of photosynthesis, growth, reproduction, and UV-B sensitivity in the green alga Ulva pertusa (Chlorophyceae) . J. Phycol. , 39 : 712 – 721 .
- Han , T , Kong , JA , Han , YS , Kang , SH and Häder , DP . 2004 . UV-A/blue light-induced reactivation of spore germination in UV-B irradiated Ulva pertusa (Chlorophyta) . J. Phycol. , 40 : 315 – 322 .
- Han , YS and Han , T . 2005 . UV-B induction of UV-B protection in Ulva pertusa (Chlorophyta) . J. Phycol. , 41 : 523 – 530 .
- Hanelt , D . 1992 . Photoinhibition of photosynthesis in marine macrophytes of the South Chinese Sea . Mar. Ecol. Prog. Ser. , 82 : 199 – 206 .
- Hanelt , D , Wiencke , C and Nultsch , W . 1997 . Influence of UV radiation on the photosynthesis of arctic macroalgae in the field . J. Photochem. Photobiol. B Biol. , 38 : 40 – 47 .
- Hanelt , D , Hawes , I and Rae , R . 2006 . Reduction of UV-B radiation causes an enhancement of photoinhibition in high light stressed aquatic plants from New Zealand lakes . J. Photochem. Photobiol. B Biol. , 84 : 89 – 102 .
- Helbling , EW , Gao , K , Goncalves , RJ , Wu , H and Villafañe , VE . 2003 . Utilization of solar ultraviolet radiation by phytoplankton assemblages from the Southern China Sea when exposed to fast mixing conditions . Mar. Ecol. Prog. Ser. , 259 : 59 – 66 .
- Henley , WJ , Levavasseur , G , Franklin , LA , Lindley , ST , Ramus , J and Osmond , CB . 1991 . Diurnal responses of photosynthesis and fluorescence in Ulva rotundata acclimated to sun and shade in outdoor culture . Mar. Ecol. Prog. Ser. , 75 : 19 – 28 .
- Henry , BE and Van Alstyne , KL . 2004 . Effects of UV radiation on growth and phlorotannins in Fucus gardneri (Phaeophyceae) juveniles and embryos . J. Phycol. , 40 : 527 – 533 .
- Huppertz , K , Hanelt , D and Nultsch , W . 1990 . Photoinhibition of photosynthesis in the marine brown alga Fucus serratus as studied in field experiments . Mar. Ecol. Prog. Ser. , 66 : 175 – 182 .
- Huovinen , PS , Oikari , AOJ , Soimasuo , MR and Cherr , GN . 2000 . Impact of UV radiation on the early development of the giant kelp (Macrocystis pyrifera) gametophytes . Photochem. Photobiol. , 72 : 308 – 313 .
- Ikusima , I . 1965 . Ecological studies on the productivity of aquatic plant communities І Measurement of photosynthetic activity . Bot. Mag. Tokyo , 78 : 202 – 211 .
- Jassby , AD and Platt , T . 1976 . Mathematical formulation of the relationship between photosynthesis and light for phytoplankton . Limnol. Oceanogr. , 21 : 540 – 547 .
- Karentz , D . 1994 . Ultraviolet tolerance mechanisms in Antarctic marine organisms . Antarctic Res. Ser. , 62 : 93 – 110 .
- Karentz , D . 2001 . “ Chemical defenses of marine organisms against solar radiation exposure: UV-absorbing mycosporine-like amino acids and scyotnemin ” . In Marine Chemical Ecology , Edited by: McClintock , JB and Baker , BJ . 481 – 486 . Birmingham, , USA : CRC Press .
- Karentz , D , Mceuen , FS , Land , MC and Dunlap , WC . 1991 . Survey of mycosporine-like amino acid compounds in Antarctic organisms: potential protection from ultraviolet exposure . Mar. Biol. , 108 : 157 – 166 .
- Karsten , U , Franklin , LA , Lüning , K and Wiencke , C . 1998 . Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta) . Planta , 205 : 257 – 262 .
- Kerr , JB and Mcelroy , CT . 1993 . Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion . Science , 262 : 1032 – 1034 .
- Korbee-Peinado , N , Abdala-Díaz , RT , Figueroa , FL and Helbling , EW . 2004 . Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids in Porphyra columbina (Rhodophyta) from Patagonia, Argentina . J. Phycol. , 40 : 248 – 259 .
- Kuhlenkamp , R , Franklin , LA and Lüning , K . 2001 . Effect of solar ultraviolet radiation on growth in the marine macroalga Dictyota dichotoma (Phaeophyceae) at Helgoland and its ecological consequences . Helgol. Mar. Res. , 55 : 77 – 86 .
- Marra , J . 1978 . Effect of short-term variation in light intensity on photosynthesis of a marine phytoplankter: A laboratory simulation study . Mar. Biol. , 46 : 191 – 202 .
- Michler , T , Aguilera , J , Hanelt , D , Bischof , K and Wiencke , C . 2002 . Long-term effects of ultraviolet radiation on growth and photosynthetic performance of polar and cold-temperate macroalgae . Mar. Biol. , 140 : 1117 – 1127 .
- Orellana , MV , Petersen , TW and Van Den Engh , G . 2004 . UV-excited blue auto- fluorescence of Pseudo-nitzschia multiseries (Bacillariophyceae) . J. Phycol. , 40 : 705 – 710 .
- Oren , A and Gunde-Cimerman , N . 2007 . Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? . FEMS. Microbiol. Lett. , 269 : 1 – 10 .
- Pakker , H , Beekman , CA C and Breeman , AM . 2000a . Efficient photoreactivation of UVB-induced DNA damage in the sublittoral macroalga Rhodymenia pseudpalmata (Rhodophyta) in relation to ultraviolet-B-exposure . Eur. J. Phcol. , 35 : 109 – 114 .
- Pakker , H , Martins , R , Boelen , P , Buma , AGJ , Nikaido , O and Breeman , AM . 2000b . Effects of temperature in the photoreactivation of ultraviolet-B induced DNA damage in Palmaria palmata (Rhodophyta) . J. Phycol. , 36 : 334 – 341 .
- Pang , S , Gomez , I and Lüning , K . 2001 . The red macroalga Delesseria sanguinea as a UVB-sensitive model organism: selective growth reduction by UVB in outdoor experiments and rapid recording of growth rate during and after UV pulse . Eur. J. Phycol. , 36 : 207 – 216 .
- Pavia , H and Brock , E . 2000 . Extrinsic factors influencing phlorotannin production in the brown alga Ascophyllum nodosum. . Mar. Ecol. Prog. Ser. , 193 : 285 – 294 .
- Pavia , H , Cervin , G , Lindgren , A and Åberg , P . 1997 . Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum . Mar. Ecol. Prog. Ser. , 157 : 139 – 146 .
- Ramus , J and Rosenberg , G . 1980 . Diurnal photosynthetic performance of seaweeds measured under natural conditions . Mar. Biol. , 56 : 21 – 28 .
- Roleda , MY , Wiencke , C and Hanelt , D . 2006 . Thallus morphology and optical characteristics affect growth and DNA damage by UV radiation in juvenile Arctic Laminaria sporophytes . Planta , 223 : 407 – 417 .
- Santas , R , Korda , A , Lianou , C and Santas , P . 1998 . Community responses to UV radiation: I. Enhanced UVB effects on biomass and community structure of filamentous algal assemblages growing in a coral reef mesocosm . Mar. Biol. , 131 : 153 – 162 .
- Vass , I . 1997 . “ Adverse effects of UV-B light on the structure and functions of the photosynthetic apparatus ” . In Handbook of Photosynthesis , Edited by: Pessarakali , M . 931 – 946 . New York, , USA : Marcel Dekker .
- Van Donk , E , Faafeng , BA , De Lange , HJ and Hessen , DO . 2001 . Differential sensitivity to natural ultraviolet radiation among phytoplankton species in Arctic lakes (Spitsbergen, Norway) . Plant Ecol. , 154 : 249 – 259 .
- Wellburn , AR . 1994 . The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution . J. Plant. Physiol. , 144 : 307 – 313 .
- Wheeler , WN . 1980 . Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera . Mar. Biol. , 56 : 103 – 110 .
- Wiencke , C , Gómez , I , Pakker , H , Flores-Moya , A , Altamirano , M , Hanelt , D , Bischof , K and Figueroa , F . 2000 . Impact of UV radiation on viability, photosynthetic characteristics and DNA of brown algal zoospores: implications for depth zonation . Mar. Ecol. Prog. Ser. , 197 : 217 – 229 .
