Abstract
Fucus vesiculosus from the northern Baltic Sea (5 psu) and from the Irish Sea (35 psu) were cultivated at different temperatures, salinities and dissolved inorganic carbon (DIC) concentrations with the addition of different nutrient concentrations. The influence of these abiotic factors was assessed by measuring photosynthesis as electron transport rate (ETR) and growth as relative growth rate (RGR). The maximal ETR and the RGR of the Irish Sea plants in their natural seawater (50.8 µmol electrons m−2 s−1; 0.024 g g−1 day−1) were significantly higher than those of the Baltic plants in their natural seawater (21.9 µmol electrons m−2 s−1; 0.007 g g−1 day−1). When Baltic F. vesiculosus was cultivated at a DIC concentration similar to that of the Irish Sea, the ETR as well as RGR increased, but never equalled the rates of the marine F. vesiculosus from the Irish Sea. Cultivation at different salinities showed that F. vesiculosus from the Baltic has a higher ETRmax and RGR at low salinities (5–10 psu) than F. vesiculosus from the Irish Sea, whose ETR and RGR decreased sharply in salinities below 20 psu. Plants from both sites grown at high nutrient concentrations, however, performed better at low salinities than those grown under low nutrient conditions. Salinity had the greatest impact on differences in ETR and RGR between the two populations, followed by differences in DIC and nutrient concentrations. There was a highly significant correlation between ETRmax and RGR in plants from both sites and across the full range of culture conditions, indicating that the same amount of energy from photosynthesis is used for growth in both varieties of the species at different salinities. The photosynthesis of F. vesiculosus in the northern Baltic is close to the minimum demand for growth, which may account for their small size. The temperature optimum for F. vesiculosus from the Baltic was 4–10°C, while that for F. vesiculosus from the Irish Sea was 15–20°C. The photosynthesis of Irish Sea plants was less strongly affected by exposure to high irradiances than that of plants from the Baltic.
Introduction
Fucus vesiculosus is a common intertidal brown seaweed on the rocky shores of the North Atlantic (van den Hoek et al., Citation1995). On the European shores F. vesiculosus occurs from Northern Russia to Morocco (Powell, Citation1963), but it is also one of the few perennial marine macroalgae that have colonized the Baltic Sea (Ignatius, Citation1981). Bergström et al. (Citation2005) have recently suggested that a dwarf morphotype of F. vesiculosus, with a shorter thallus, thinner and more richly branched fronds, which grows in mixed populations with F. vesiculosus in the northern Baltic, should be recognized as a new species, Fucus radicans. The Baltic is a young sea, and most organisms are believed to have entered the area within the last 8,000 years (Snoeijs, Citation1999). The species diversity in the area is low, mainly due to the young age of the Baltic Sea and the low salinity of the water, which decreases from approximately 20 psu in the southwest, at its entrance from the Skagerrak, to 1 psu in the north (Rönnberg & Bonsdorff, Citation2004). The lowered salinity is caused by the high inflow of fresh water from many rivers, especially in the north, and the weak exchange of saltwater with the Atlantic (Omstedt & Axell, Citation2003). The geographical distribution of F. vesiculosus in the Baltic appears to be limited by salinity (Russell, Citation1988), and plants can be found from the southern Baltic Sea to the Quark (63°50′N) in the Gulf of Bothnia at salinities down to 4 psu (Kautsky, Citation1992; Raven & Samuelsson, Citation1988) and in the Gulf of Finland down to 3–6 psu (Bäck & Ruuskanen, Citation2000). Whilst the most obvious difference between the Baltic and the Atlantic is salinity, this is correlated with other major environmental variables. For example, the concentration of dissolved inorganic carbon (DIC, i.e.) in fully marine waters (approximately 2.0 mol m−3; Surif & Raven, Citation1989) is substantially higher than in brackish waters like the Baltic Sea (approximately 1.0 mol m−3; Raven & Samuelsson, Citation1988). In contrast, the nutrient concentration in the Baltic Sea has increased several-fold during the last century, which has resulted in an increase in pelagic primary production by 30–70% (Elmgren, Citation1989) but also other significant ecological effects, such as the decrease in the depth limit of F. vesiculosus (e.g. Kangas et al., Citation1985; Eriksson et al., Citation1998). The nutrient concentrations in the Irish Sea are generally not elevated and the present anthropogenic nutrient input is not thought to have caused eutrophication in the coastal and offshore waters of the Irish Sea (McGovern et al., Citation2002). Eutrophication in the Gulf of Bothnia is not as severe as in the central Baltic Sea (Swedish Environmental Protection Agency, Citation2001). The sea water temperature in the Baltic shows large variations between summer and winter, while the temperature range in Atlantic water is less pronounced. During the summer the surface water temperatures are similar in the Irish Sea and the Baltic Sea (approximately 20°C), but during the winter the temperature in the northern Baltic might drop below zero due to ice (Andersson et al., Citation1992; Swedish Environmental Protection Agency, Citation2001).
Tides are almost absent in the Baltic Sea with only small and irregular water level changes of up to 0.5 m from the mean water level, which are related to meteorological conditions (Ignatius et al., Citation1981). Consequently, F. vesiculosus grows permanently submerged in the Baltic and experiences relatively stable abiotic conditions. In the Atlantic, F. vesiculosus grows in the intertidal zone and is exposed to regular variations in abiotic factors (e.g. salinity, temperature, nutrient concentration, carbon concentration and light exposure) during the tidal cycle (Dring & Brown, Citation1982).
These differences between the Baltic and the Irish Sea are likely to affect the physiology of F. vesiculosus in several ways. The aim of the present study was to examine the ability of F. vesiculosus from the Baltic and the Irish Seas to grow and photosynthesize in different conditions. The environmental conditions used in the experiments covered the range of temperatures, salinities, nutrient and DIC concentrations experienced by plants in the two seas. In addition, the tolerance to high irradiance (photosynthetically active radiation, 400–700 nm, PAR) was studied for both populations. The main questions to be answered were: (i) Are photosynthesis and growth rates different for Baltic and marine F. vesiculosus? (ii) If so, which of the abiotic factors contribute most to the difference in photosynthesis and growth rate? (iii) Is the optimal temperature for photosynthesis different for the Baltic and the Irish Sea plants? (iv) Are the tolerances to high irradiance of PAR different for Baltic and Irish Sea plants?
Material and methods
Experiments were carried out in February 2004. Fucus vesiculosus was collected in the middle of the intertidal zone in the Irish Sea (Portaferry, Northern Ireland; 54°26′N, 05°26′W) at a salinity of 35 psu, and from a depth of 1 m in the non-tidal Gulf of Bothnia, northern Baltic (Åstön, Sweden; 62°24′N, 17°45′E) at a salinity of 5 psu. The water temperatures measured at collection of the algae were 5°C in the Baltic and 8°C in the Irish Sea. The Baltic plants were transported moist in polythene bags inside an insulated container to the laboratory in Northern Ireland, where they were kept for recovery for 3 days at 8°C and 50 µmol m−2 s−1 (10 : 14-h light–dark cycle), with daily measurements of the maximal quantum yield of electron transport through photosystem II (F v/F m). Only plants that regained their initial F v/F m were used in the following experiments.
Photosynthesis:irradiance (P:E) curves
Photosynthesis was estimated from chlorophyll fluorescence measurements using a pulse amplitude modulated fluorometer (Diving PAM, Walz, Effeltrich, Germany). The absolute electron transport rate (Abs ETR) was calculated as:
Optimal temperature
The effects of different temperatures on the photosynthesis of F. vesiculosus from the Baltic and the Irish Sea were examined by cultivating 10 thallus tips, each with a fresh weight (FW) of about 10 g, in their natural seawater at different temperatures (0, 4, 10, 15, 20 or 25°C) for a period of 1 week at 100 µmol m−2 s−1 under a 10 : 14-h light–dark cycle. The water was changed every second day. Photosynthesis: irradiance curves were measured as described above. Measurements were performed initially and after 1 week of cultivation at the different temperatures.
Salinity, carbon and nutrients
Artificial seawater (Instant Ocean Sea salt, Eco-systems Inc.) was used to obtain different salinities. The controls were kept in their natural seawaters. The artificial water did not contain any nutrients, which were added separately. Samples of the artificial seawater as well as the natural seawaters were deep frozen and sent for analysis of ion and nutrient contents, which was performed by LMI AB, Helsingborg, Sweden. The composition of the media is shown in .
Table 1. The content of the major elements (B, Ca, Cl, K, Mg, Na and S) and nutrients (NH4, NO3 and PO4) in artificial seawater (ASW) of different salinities, and for natural seawater (NSW) from the Irish Sea (37.4 psu) and the Bothnian Sea (4.6 psu). The concentrations of B, Ca, Cl, K, Mg, Na and S, are expressed in mmol l−1, whereas the concentrations of the NH4, NO3 and PO4 are expressed in μmol l−1. The analysis was performed by LMI AB, Helsingborg, Sweden.
In comparable studies of different salinity levels, it is quite common to dilute natural seawater with distilled water to obtain lower salinities and to evaporate seawater to obtain higher salinities. The problem with this approach is that the content of DIC and nutrients changes with dilution or evaporation, and that is why artificial water was used in this study. Vegetative tips (5 g fresh weight, FW, n = 10) from healthy looking plants of F. vesiculosus from the Baltic and the Irish Sea were tied with strings to the bottom of aquaria, so that the plants floated at a water depth of approximately 30 cm. They were kept initially in seawater from their natural site under outdoor conditions with aeration for 24 h before onset of the experiment. Natural conditions of light (maximum irradiance 500 µmol m−2 s−1) and temperature (8–10°C) were used during cultivation and experiments. After 24 h the tips were blotted, weighed (to obtain initial FW) and transferred to culture media of different salinities (5, 10, 20, 35 or 45 psu), with two different nutrient levels (high or low concentration) and two different carbon concentrations (high or low concentration).
Nutrients were added from stock solutions of NaH2PO4, NH4Cl and NaNO3 to obtain final concentrations of 12 µmol l−1, 0.75 µmol
l−1 and 0.2 µmol
l−1 (‘high’) or 3 µmol
l−1, 0.185 µmol
l−1 and 0.05 µmol
l−1 (‘low’). At the ‘high’ concentrations used, both NO3 and PO4 were present in higher concentrations than normally found in the Gulf of Bothnia and the Irish Sea (SMHI, Citation2005; DEFRA, Citation2005). These concentrations of NO3 were higher than those found in the Baltic Sea proper (where eutrophication is a problem), but the PO4 concentration was equal to that in the Baltic Sea proper (SMHI, Citation2005). At the ‘low’ concentrations used, all three nutrients were lower than the concentrations in the Gulf of Bothnia and the Irish Sea.
Bicarbonate (NaHCO3) was added to obtain a low DIC concentration of 1.0 mol m−3 (similar to that found in the Baltic) or a high DIC concentration of 2.0 mol m−3 (similar to that found in oceanic seawater with a salinity of 35 psu). The pH was kept constant at 8.2 with Tris Biological buffer (Sigma-Aldrich Química SA, Madrid, Spain). At a natural pH of about 8, the major part of the DIC in seawater is present as bicarbonate (, Stumm & Morgan, Citation1996). Earlier work has shown that F. vesiculosus is able to use
as a source of inorganic carbon (Surif & Raven, Citation1989, Citation1990).
Cultivation time was 5 weeks. Every culture treatment consisted of two replicate aquaria, with five replicate plants in each. No significant differences were observed between the replicate aquaria, and the results were therefore pooled in the analysis and presentation. The water was changed every second day. Controls, with untreated natural seawater from the respective collection sites, were used. To make sure that the process of separating the tips from the rest of the plant did not affect photosynthetic performance, whole plants (n = 5) as well as tips (n = 5) were used as controls for photosynthetic measurements.
The results from the P : E-curves revealed that the photosynthesis of both populations was saturated at 400 µmol m−2 s−1 (see ), so that Abs ETR was measured with dark adapted algae (10 min dark adaptation) after 5 min exposure to this irradiance. These measurements were performed initially and after 5 week's cultivation in the different seawater treatments, using the initial AF and the AF measured after 5 week's exposure to the different treatments. All measurements were performed around midday (12 : 00–14 : 00 h) to avoid possible diurnal variations in photosynthesis. At the end of the experiment, new P : E-curves were measured (as described above) for the control algae. No significant differences from initial values were observed ().
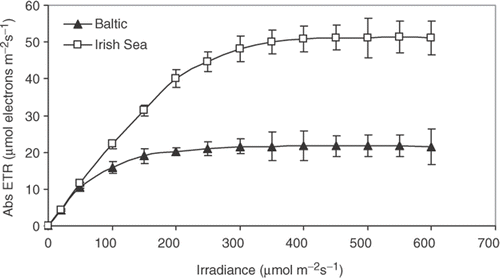
Fig. 1. Photosynthesis, expressed as absolute electron transport rate (Abs ETR), as a function of irradiance for Fucus vesiculosus from the Gulf of Bothnia (northern Baltic, 5 psu) and from the Irish Sea (35 psu). Measurements were performed with thallus tips (n = 10). Irradiance was increased stepwise from 20 to 600 µmol m−2 s−1, with 5 min at each irradiance and 5 min darkness in between. Temperature = 10°C. Error bars are 95% confidence intervals.
The growth was calculated as relative growth rate (RGR), based on changes in dry weight (DW):
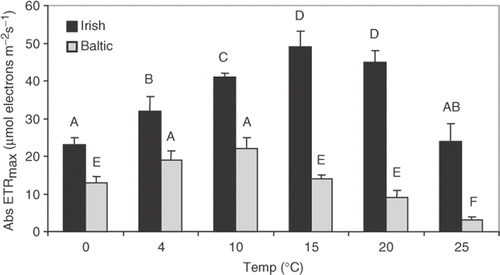
Fig. 2. Saturated photosynthetic rate (Abs ETRmax) of Fucus vesiculosus from the Gulf of Bothnia (northern Baltic, 5 psu; grey) and the Irish Sea (35 psu; black) at different temperatures. Plant tips (10 g FW each, n = 10) were cultivated in their natural seawater for 1 week at 100 µmol m−2 s−1 under a 10 : 14-h light–dark cycle. Means with the same letter are not significantly different at p = 0.05 (Tukey tests).
Tolerance to high PAR
The tolerance of plants to high irradiance (1,500 µmol m−2 s−1 PAR was measured after the 5-week culture treatment (described above). Samples were fastened in the leaf clip (used for all fluorescence measurements), placed in a beaker filled with seawater (with the same composition as used in the 5-week treatment) in a constant temperature water bath at 10°C. Initial values of F v/F m were measured after 10 min dark adaptation. The plants were exposed to high PAR for 1 h and allowed to recover in dim light (30 µmol m−2 s−1) for 1 h. F v/F m was measured again after 10 min dark adaptation after the recovery period.
Statistics
Paired t-test (comparison of values before and after treatment) and two-way ANOVA (between treatments) were used for statistical analysis with a 95% significance level. The data were analysed by multivariate linear regression with photosynthesis, RGR or recovery in F v/F m after exposure to high PAR, as dependent variables and salinity, nutrients and carbon as covariates. The data was classified into Baltic or Atlantic origin of the alga. The data on F v/F m recovery were subjected to angular transformation before analysis.
Results
P:E-curves
ETRmax of F. vesiculosus from the Irish Sea was significantly higher (ANOVA, p < 0.001) than that of F. vesiculosus from the Baltic (50.8 vs 21.9 µmol electrons m−2 s−1; ). Plants from the Baltic reached light saturation at lower irradiances than plants from the Irish Sea, but plants from both populations had reached the ETRmax value at 400 µmol m−2 s−1, the irradiance that was used to measure ETR in the 5-week experiment. There was no difference between the populations in the ETRs recorded at the two lowest irradiances tested ().
Optimal temperature
Fucus vesiculosus from the Irish Sea had the highest ETRmax at 15–20°C, while plants from the Baltic showed the highest ETRmax at 4–10°C (). ETRmax decreased at 25°C for plants from both populations, with a more pronounced drop for Baltic plants. At all temperatures, ETRmax for Irish Sea plants was higher than for Baltic plants. The temperature during the 5-week culture experiment was 8–10°C, which is slightly below the optimal temperature for F. vesiculosus from the Irish Sea, but falls within the optimal temperature range for F. vesiculosus from the Baltic.
Salinity, carbon and nutrients
After cultivation for 5 weeks in high nutrients and DIC concentrations, F. vesiculosus from the Irish Sea exhibited higher ETRmax than the Baltic plants at salinities from 20 to 45 psu (b), whereas the ETRmax of Baltic plants was higher at salinities of 5 to 10 psu (a). The optimal salinity for F. vesiculosus from the Baltic was 10 to 20 psu, while that for plants from the Irish Sea was 20 to 35 psu (a,b). The salinities at which the ETR-values were lowest were 45 psu for F. vesiculosus from the Baltic and 5 psu for the Irish Sea plants (a,b).
Fig. 3. Saturated photosynthetic rate (Abs ETR) of Fucus vesiculosus from (a) the Gulf of Bothnia (northern Baltic, 5 psu) and (b) the Irish Sea (35 psu), cultivated at different salinities for 5 weeks. Two concentrations of dissolved inorganic carbon (DIC) were used (Low C = 1.0 mol DIC m−3; High C = 2.0 mol DIC m−3) as well as two concentrations of nutrients (Low = 3 µmol NO3 l−1, 0.185 µmol PO4 l−1, 0.05 µmol NH4 l−1; High = 12 µmol NO3 l−1, 0.75 µmol PO4 l−1, 0.2 µmol NH4 l−1). Abs ETR measured after 5-min exposure to 400 µmol m−2 s−1, after a 10 min dark adaptation. Histograms show means of 10 replicates and error bars are 95% confidence limits.
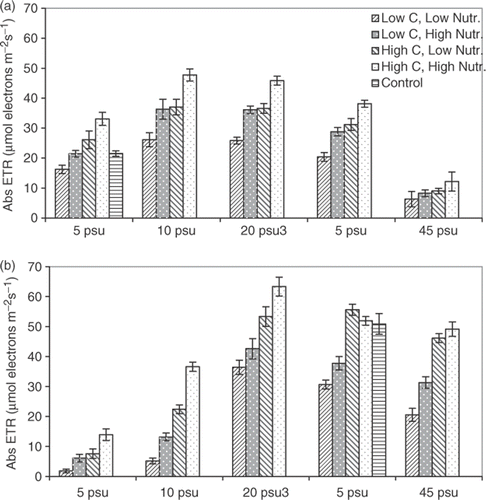
Cultivation at the higher nutrient concentration resulted in higher ETRmax than in the lower nutrient concentration over almost all salinities for F. vesiculosus from both sites (a,b). ETRmax was also higher following cultivation in the higher concentration of DIC at most salinities, and the effects of high nutrients and high DIC were additive, so that the highest ETR-values were obtained when both nutrients and DIC were high, and the lowest when both nutrients and DIC were low (a,b).
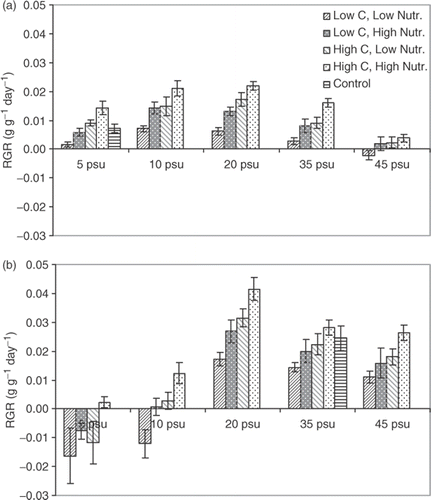
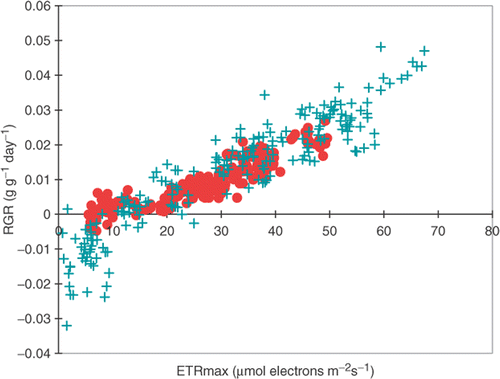
The pattern of variation of RGR for F. vesiculosus from both sites across the full range of salinities, nutrients and DIC was similar to that of ETRmax (). Consequently, there was a highly significant correlation between RGR and ETRmax over the full range of treatments (ANOVA, F 1,398 = 1975.48, r 2 = 0.832; ). One difference between RGR and ETRmax was the absence of significant differences in RGR for the Irish Sea plants at the salinities 20-45 psu with a low DIC concentration (b). At the two lowest salinities (5 and 10 psu), F. vesiculosus from the Irish Sea lost tissue, resulting in negative values of RGR (b). This was probably caused by destruction of the tissue due to a high water uptake.
Fig. 4. Relative growth rate (RGR) of Fucus vesiculosus from (a) the Gulf of Bothnia (northern Baltic, 5 psu) and (b) the Irish Sea (35 psu), cultivated at different salinities for 5 weeks (nutrient and DIC concentrations as in ). RGR estimated from changes in dry weight (DW) and expressed as g (DW) per g (DW) and day. Histograms show means of 10 replicates and error bars are 95% confidence limits.
A multivariate linear regression model with ETRmax or RGR as dependent variable was significant (for ETR: F 8,391 = 75.175; for RGR: F 8,391 = 6.612), and explained 61% and 58% of the variations in photosynthesis and growth, respectively. Salinity had the greatest impact on the model for ETR (F 4,391 = 90.432), followed by nutrients together with DIC (F 3,391 = 74.257), but the sites from which the plants were obtained contributed relatively little to the variation (F 1,391 = 16.899).
Fig. 5. Correlation between photosynthetic rate (ETRmax) and growth rate (RGR) for Fucus vesiculosus from the Gulf of Bothnia (•) and the Irish Sea (+). Both variables measured after 5 weeks’ cultivation at different salinities (5, 10, 20, 35 and 45 psu) with different nutrient concentrations (see ) and inorganic carbon concentrations (1.0 or 2.0 mol m−3).
Tolerance to high PAR
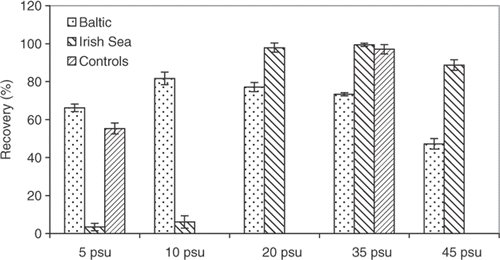
Fucus vesiculosus from the Irish Sea showed over 90% recovery of F v/F m following exposure to high irradiance in salinities from 20 to 45 psu, but almost no recovery was observed in salinities of 5 and 10 psu (). At these two lower salinities, the tolerance was higher for F. vesiculosus from the Baltic but, at all higher salinities, the tolerance of the Baltic plants was lower than those from the Irish Sea (). Variation in nutrient and DIC concentrations had little effect on degree of recovery of F v/F m following exposure to high irradiance (data not shown). A multivariate linear regression model with recovery in F v/F m as dependent variable was significant (ANOVA, F 8,391 = 49.503) and explained 50% of the variation in recovery of F v/F m after exposure to high PAR. Salinity (F 4,391 = 92.277) had the greatest impact on the model, whereas the nutrient and DIC contributed relatively little to the variation (F 3,391 = 7.214).
Fig. 6. Recovery of photosynthesis following exposure to high irradiance (1500 µmol m−2 s−1 PAR) for Fucus vesiculosus from the Gulf of Bothnia (northern Baltic, 5 psu) and the Irish Sea (35 psu), cultivated for 5 weeks at different salinities with high concentrations of nutrients and DIC (details as ). F v : F m after 1 h recovery at 30 µmol m−2 s−1 and 10 min dark adaptation, following exposure to high PAR for 1 h, is expressed as a percentage of initial F v/F m. Histograms show means of 10 replicates and error bars are 95% confidence limits (calculated after angular transformation of percentage data).
Discussion
P:E-curves
Fucus vesiculosus from the Baltic possessed a lower ETRmax and photosynthesis became light saturated at a lower irradiance than in F. vesiculosus from the Irish Sea. Photoacclimation and/or photoadaptation to low light environments frequently results in reduced P max (when expressed per unit chlorophyll) and reduced saturating photon irradiance (E k; see Dring, Citation1992, .5), and is often accompanied by an increase in the initial slope of P : E curves (α) when photosynthesis is expressed per unit area or weight (e.g. Figueroa et al., Citation2003) and an increased Chl a content (Ramus et al., Citation1977; Ruokalahti & Rönnberg, 1988). In the present study, no differences were found in α between the two populations but this would not be expected because the calculation of ETR involves division by the absorption factor, so that ETR is more closely related to photosynthesis per unit chlorophyll than to photosynthesis per unit area. The feature of these plants that does not accord with the norm for high and low light populations is that the pigment content of F. vesiculosus from the Baltic has been shown previously to be lower than for F. vesiculosus from the Atlantic (Nygård & Ekelund, Citation2006). It seems, therefore, that, even though F. vesiculosus in the Baltic and the Irish Sea experience different light regimes because of the absence of tides in the Baltic, the observed differences in photosynthesis cannot be completely explained by light acclimation, but are probably affected by other abiotic differences between the Baltic and the Atlantic waters, such as will be discussed below.
Optimal temperature
The results indicate that F. vesiculosus from the Baltic is better adapted to low water temperatures than F. vesiculosus from the Irish Sea. The plants in the northern Baltic are usually exposed to lower winter temperatures than those in the Irish Sea, and this may have generated a different temperature optimum for the two populations. Temperature is a major factor controlling the rate of photosynthesis in all plants (Davison, Citation1991). It has been found to affect the availability of inorganic carbon (Surif & Raven, Citation1989) and the rate of carbon fixation by RuBisCO (Sukenik et al., Citation1987). For some algal species, the rate of nutrient uptake has been found to be correlated with temperature, but this has not been found so far in Fucus (Topinka, Citation1978). The upper thermal tolerance limit of photosynthesis appears to be set by the thermal stability of PSII, which in turn is controlled by the degree of saturation of fatty acids in the thylakoid membrane (Lynch & Thompson, Citation1984). Photorespiration has also been found to increase at high temperatures (Berry & Raison, Citation1981). The general trend is that algae from low-temperature environments show higher photosynthetic rates at low temperatures and have a lower optimal temperature for photosynthesis than algae from warmer environments (Kübler et al., Citation1991). This is supported by the present study, since F. vesiculosus from the Gulf of Bothnia had a lower temperature optimum than F. vesiculosus from the Irish Sea. The relatively broad range of optimal temperatures for photosynthesis observed here is typical of other fucoid species (Madsen & Maberly, Citation1990).
Salinity
Both the photosynthesis and growth of F. vesiculosus from the Irish Sea decreased sharply between salinities of 20 and 10 psu (, ). This result is in line with the observations of Tropin et al. (Citation2003) on F. vesiculosus in the Barents Sea. A salinity of 20 psu (with an exposure time of 14 days) had no effect on cell structure, whereas a lower salinity resulted in severe damage to cell structure. Earlier work on F. vesiculosus from the Irish Sea, however, has shown that growth rate was not affected by a 50% reduction in salinity (approximately 16 psu; Gordillo et al., Citation2002), so that the tolerance limit for F. vesiculosus from the Irish Sea seems to occur in the range of 10–16 psu.
Successful growth provides a good indication of the plant's ability to tolerate a changed salinity because it requires maintenance of cell turgor (Hellebust, Citation1976). It is also important to measure growth because it reflects the balance between photosynthesis and respiration (Kirst, Citation1989). The strong correlation between RGR and ETR in the present study () indicates that growth was largely controlled by photosynthesis, and that nutrient supplies were limiting only in so far as they limited photosynthetic capacity (possibly by restricting the concentration of RuBisCO). The constant ratio of photosynthesis and growth indicates that the same amount of energy relative to photosynthesis is used for growth in plants from the two localities and at the different salinities. The smaller size of Baltic plants is probably caused, therefore, by lower photosynthesis. The ratio of photosynthesis to growth also shows that F. vesiculosus from the Baltic and the Irish Sea respond in a similar way to salinity, nutrients and DIC, but within a different range. The plot of RGR against ETR also indicates that a certain amount of energy from photosynthesis is required before the plant starts to grow. The limit seems to be an ETR of approximately 20 µmol electrons m−2 s−1, below which no growth occurs (). Since the ETRmax of plants from the Gulf of Bothnia in their natural seawater was about 21–22 µmol electrons m−2 s−1 (, , ), they were close to the minimum demand of photosynthesis for growth, and this may explain their small size. If their photosynthesis decreased any further, it is likely that the plants would stop growing.
Alternative explanations for the small size of Baltic plants may be that they do not live as long as on the Atlantic shores, or that they are more heavily grazed or abraded in the Baltic. The evidence, however, suggests the opposite. The average life span of F. vesiculosus on the North Sea coast of the British Isles is 3 years, with a maximum of 4–5 years in sheltered areas (Knight & Parke, Citation1950), whereas F. vesiculosus does not become reproductive in the Baltic Sea until 4–5 years and single fronds in sheltered areas may be up to 20 years old (Malm & Kautsky, Citation2004). In addition, marine limpets and littorinid grazers are absent from the less saline areas of the Baltic, and the only grazing gastropods that occur in substantial quantities on rocky shores are the freshwater snails Theoduxus fluviatilis and Lymnaea peregra (Malm et al., Citation1999). Grazing by these two species was not found to affect the frond growth of F. vesiculosus in the Baltic Sea (Malm et al., Citation1999). One of the commonest herbivores in the northen Baltic is the isopod Idotea baltica, but this isopod has a preference for filamentous algae and mainly uses F. vesiculosus as shelter (Orav-Kotta & Kotta, Citation2001). Abrasion by wave action is also likely to be lower in the Baltic than in the Atlantic because storms are less severe and the plants are continually submerged.
The optimum salinities for ETR and RGR, as measured here, were 10–20 psu for F. vesiculosus from the Baltic and 20–35 psu for F. vesiculosus from the Irish Sea (, ). Thus, F. vesiculosus from the Baltic has the ability to grow in lower salinities than F. vesiculosus from the Irish Sea. The optimum salinity for photosynthesis was found in previous studies to be 6 psu for F. vesiculosus from the Baltic and 11–35 psu for F. vesiculosus from the Atlantic (Russell, Citation1988; Bäck et al., Citation1992a), but the acclimation times in these studies were considerably shorter (24–48 h) than in the present work. Russell (Citation1988) found that F. vesiculosus from the Baltic was harmed by salinities above 6 psu. This result was not supported by the present work, since ETR and RGR both increased significantly from 5 to 10 psu. Again, a possible explanation is the longer cultivation times in the present study (5 weeks). Other work in which a longer cultivation time was used (7 weeks) did not include photosynthetic measurements but showed a maximum growth rate (measured as increase in length) of F. vesiculosus from the Baltic at 6–12 psu and from the Atlantic at 12 psu (Bäck et al., Citation1992b). Although no salinities were tested between 12 and 35 psu, these data may indicate that the salinity tolerance limit for F. vesiculosus from the Irish Sea can be narrowed to 12–16 psu. Future work on the salinity tolerance limits for F. vesiculosus should use exposures of several weeks and salinities within this range. Fucus vesiculosus from the Baltic maintained ETR and RGR over a wider range of salinities, and appeared to be more tolerant of salinity variations, than plants from the Irish Sea, although they were less resistant to hyper-saline conditions (45 psu). These differences are similar to those observed between estuarine and fully marine strains of the filamentous brown alga Pilayella littoralis (Reed & Barron, Citation1983).
Effects of DIC concentration
Earlier studies have suggested that Fucaceae in marine waters are saturated with DIC at ambient levels (Surif & Raven, Citation1989; Mercado et al., Citation1998). The present study shows, however, that the DIC concentration in the Baltic is not sufficient for maximal photosynthesis and growth of F. vesiculosus. The results obtained emphasize the importance of maintaining the DIC level when diluting seawater to obtain lower salinities. Dilution also reduces DIC, which will inevitably reduce photosynthesis. The multivariate regression analysis showed that the concentration of DIC had a significant influence on the photosynthesis of F. vesiculosus, even when the dominant factor was salinity. Consequently, increasing DIC does not compensate for decreases in salinity, but it is a contributing factor.
Effects of nutrients
A high nutrient content in the water not only increased the photosynthesis and growth rates of F. vesiculosus from both localities, but also provided increased tolerance to low salinities (except for F. vesiculosus from the Irish Sea at 5 psu, which lost tissue probably because of osmotically damaged cells). The growth of F. vesiculosus has been found to be stimulated by high nutrient loads from sewage outlets in areas with low salinity (Waern, Citation1952; Pekkari, Citation1973). These authors found plants of F. vesiculosus just outside untreated sewage outlets in areas with salinity below the usual tolerance limit. If this applies to species other than F. vesiculosus, then high eutrophication in the Baltic Sea could make it possible for new marine species to enter the Baltic ecosystem. An increased nutrient concentration might also enable marine species already present in the northern Baltic to extend further north.
As in higher plants, N is important for all enzymatic reactions in algae and N-limitation has been found to decrease both growth and fluorescence yield in brown algae (Kuppers & Weidner, Citation1980). It is clear that N-limitation affects the photosynthetic rate, but the mechanisms are not completely known, although carbon fixation and nitrogen metabolism have been found to be coupled (Turpin, Citation1991). The content of RuBisCO (and hence photosynthetic rate) has been shown to be dependent on nitrogen availability and sensitive to nitrogen starvation (Wheeler & Weidner, Citation1983). Therefore, the low ETRmax obtained at low nutrient concentration is probably caused by a limitation of RuBisCO. Few studies have been performed of the effects of phosphorus on the photosynthesis of macroalgae, but work with microalgae has shown that phosphorus affects the active uptake of NO3 (Beardall et al., Citation2005).
Tolerance to high PAR
A lowering of optimal fluorescence yield (F v/F m) that persists for 40 min after exposure to high light has been used as a measure of photoinhibition of photosynthesis in flowering plants (Schreiber, Citation1997). If that definition is applied in the present study, where a recovery time of 1 h was used, the Baltic F. vesiculosus show photoinhibition at all salinities used, suggesting a low tolerance to high PAR. The photoinhibition in these plants was least pronounced in salinities 10–20 psu, indicating that the low salinity in the Baltic generates a low tolerance to high PAR. The plants from the Irish Sea showed almost full recovery of F v/F m in salinities of 20–35 psu, but enhanced photoinhibition in low salinity (5–10 psu), where also a deterioration of tissue was observed. Low salinities appear, therefore, to make the photosynthetic apparatus more sensitive to photoinhibition. Even though the statistical analysis showed a strong correlation between tolerance to high PAR and salinity, the multivariate regression showed that DIC and nutrients also contributed to photoinhibition. Another possible factor affecting the tolerance to high PAR for the Baltic plants could be the different light regimes in the Baltic and the Irish Seas. In the Baltic the plants grow constantly submerged and are not exposed to the high levels of PAR that plants in the Irish Sea experience at low tides.
Conclusions
Fucus vesiculosus from the Irish Sea had a higher ETRmax and RGR than F. vesiculosus from the Baltic. The main explanation for these differences was found to be salinity, followed by DIC and nutrient concentrations. Although the addition of DIC (to the levels found in the Irish Sea) increased photosynthesis and growth of F. vesiculosus from the Baltic, the values of ETRmax and RGR never equalled those of F. vesiculosus from the Irish Sea. The differences in tolerance to high PAR could also be partly explained by the salinity, followed by DIC and nutrient concentration, but the degree of acclimation to high PAR at the natural growth sites seems to be a significant contributor.
Acknowledgements
The authors thank Dan Isaksson and Maurice O’Connor for field assistance during algal collection, Nils G.A. Ekelund for valuable support and discussion, Nigel Tooke for comments and corrections and Tomas Palo for help with statistics. Much of the work was conducted while C.N. held a Marie Curie Fellowship from the European Commission (HPMT-CT-2001-00268).
References
- Andersson , LBH , Lundqvist , JE , Omstedt , A , Rahm , LA , Sjöberg , B and Svensson , J . 1992 . “ Water in the marine and water in the brackish ” . In Sea and Coast Swedish National Atlas , Edited by: Sjöberg , B . 56 – 72 . Stockholm, , Sweden : Almqvist & Wiksell .
- Bäck , S and Ruuskanen , A . 2000 . Distribution and maximum growth depth of Fucus vesiculosus along the Gulf of Finland . Mar. Biol. , 136 : 303 – 307 .
- Bäck , S , Collins , JC and Russell , G . 1992a . Comparative ecophysiology of Baltic and Atlantic Fucus vesiculosus . Mar. Ecol. Prog. Ser. , 84 : 71 – 82 .
- Bäck , S , Collins , JC and Russell , G . 1992b . Effects of salinity on growth of Baltic and Atlantic Fucus vesiculosus . Br. Phycol. J. , 27 : 39 – 47 .
- Beardall , J , Roberts , S and Raven , JA . 2005 . Regulation of inorganic carbon acquisition by phosphorus limitation in the green alga Chlorella emersonii . Can. J. Bot. , 83 : 859 – 864 .
- Beer , S and Axelsson , L . 2004 . Limitations in the use of PAM fluorometry for measuring photosynthetic rates of macroalgae at high irradiances . Eur. J. Phycol. , 39 : 1 – 7 .
- Beer , S , Larsson , C , Poryan , O and Axelsson , L . 2000 . Photosynthetic rates of Ulva (Chlorophyta) measured by pulse amplitude modulated (PAM) fluorometry . Eur. J. Phycol. , 35 : 69 – 74 .
- Bergström , L , Tatarenkov , A , Johannesson , K , Jönsson , RB and Kautsky , L . 2005 . Genetic and morphological identification of Fucus radicans sp. nov. (Fucales, Phaeophyceae) in the brackish Baltic Sea . J. Phycol. , 41 : 1025 – 1038 .
- Berry , JA and Raison , JK . 1981 . “ Responses of macrophytes to temperature ” . In Physiological Plant Ecology. I. Responses to the Physical Environment. Encyclopedia of Plant Physiology New series , Edited by: Lange , OL , Nobel , PS , Osmond , CB and Ziegler , H . Vol. 12A , 277 – 338 . Berlin, , Germany : Springer-Verlag .
- Davison , IR . 1991 . Environmental effects on algal photosynthesis: temperature . J. Phycol. , 27 : 2 – 8 .
- DEFRA . 2005 . MEMG Contribution to Charting Progress – an Integrated Assessment of the Strategy of UK Seas , Department for Environment, Food and Rural Affairs (DEFRA) .
- Dring , MJ . 1992 . The Biology of Marine Plants , Cambridge, , UK : Cambridge University Press .
- Dring , MJ and Brown , FA . 1982 . Photosynthesis of intertidal brown algae during and after periods of emersion: a renewed search for physiological causes of zonation . Mar. Ecol. Prog. Ser. , 8 : 301 – 308 .
- Elmgren , R . 1989 . Man's impact on the ecosystem of the Baltic Sea: energy flows today and at the turn of the century . Ambio , 18 : 326 – 332 .
- Eriksson , BK , Johansson , G and Snoeijs , P . 1998 . Long-term changes in sublittoral zonation of brown algae in the southern Bothnian Sea . Eur. J. Phycol. , 33 : 241 – 249 .
- Figueroa , FL , Nygård , C , Ekelund , N and Gomez , I . 2003 . Photobiological characteristics and photosynthetic UV responses in two Ulva species (Chlorophyta) from southern Spain . J. Photochem. Photobiol. , 72 : 35 – 44 .
- Gordillo , FJL , Dring , MJ and Savidge , G . 2002 . Nitrate and phosphate uptake characteristics of three species of brown algae cultured at low salinity . Mar. Ecol. Prog. Ser. , 234 : 111 – 118 .
- Hellebust , JA . 1976 . Osmoregulation . Ann. Rev. Plant Physiol. , 27 : 485 – 505 .
- Ignatius , H , Axberg , S , Niemistö , L and Winterhalter , B . 1981 . “ Quaternary geology of the Baltic Sea ” . In The Baltic Sea , Edited by: Voipio , A . 54 – 104 . Amsterdam, , The Netherlands : Elsevier Scientific .
- Kangas , P and Hällfors , G . 1985 . “ On the decline of Fucus vesiculosus at the south coast of Finland ” . In Academy of Sciences of the Estonian SSR , Institute of Zoology and Botany, Hydrobiological researches XV .
- Kautsky , H . 1992 . The impact of pulp-mill effluents on phytobenthic communities in the Baltic Sea . Ambio , 21 : 308 – 313 .
- Kirst , GO . 1989 . Salinity tolerance of eukaryotic marine algae . Annu. Rev. Plant Physiol. Plant Mol. Biol. , 40 : 21 – 53 .
- Knight , M and Parke , M . 1950 . A biological study of Fucus vesiculosus and Fucus serratus . J. Mar. Biol. Ass. UK , 29 : 439 – 501 .
- Kübler , JE , Davidson , IR and Yarish , C. 1991 . Photosynthetic adaptation to temperature in the red algae Lomentaria baileyana and Lomentaria arcadensis . Br. Phycol. J. , 26 : 9 – 19 .
- Kuppers , U and Weidner , M . 1980 . Seasonal variations of enzyme activities in Laminaria hyperborea . Planta , 148 : 222 – 230 .
- Lynch , DV and Thompson , GA . 1984 . Chloroplast phospholipid molecular species alterations during low temperature acclimation in Dunaliella . Plant Physiol. , 74 : 198 – 203 .
- Madsen , TV and Maberly , SC . 1990 . A comparison of air and water as environments for photosynthesis by the intertidal alga Fucus spiralis . J. Phycol. , 26 : 24 – 30 .
- Malm , T and Kautsky , L . 2004 . Are bladderwrack (Fucus vesiculosus L.) holdfasts that support several fronds composed of one or several genetic individuals? . Aquatic. Bot. , 80 : 221 – 226 .
- Malm , T , Engkvist , R and Kautsky , L . 1999 . Grazing effects by two freshwater snails on juvenile Fucus vesiculosus L. in the Baltic Sea . Mar. Ecol. Progr. Ser. , 188 : 63 – 71 .
- McGovern , E , Monaghan , E. , Bloxham , M , Rowe , A , Duffy , C. , Quinn , A , McHugh , B , McMahon , T , Smyth , M. , Naughton , M. , McManus , T and Nixon , E. 2002 . Winter nutrient monitoring of the Western Irish sea – 1990 to 2000 . Mar. Environ. Health Ser. , No. 4 : 73 pp
- Mercado , JM , Gordillo , FJL , Figueroa , FL and Niell , FX . 1998 . External carbonic anhydrase and affinity for inorganic carbon in intertidal macroalgae . J. Exp. Mar. Biol. Ecol. , 221 : 209 – 220 .
- Nygård , CA and Ekelund , NGA . 2006 . Photosynthesis and UV-B tolerance of the marine alga Fucus vesiculosus at different sea water salinities . J. Appl. Phycol. , 18 : 461 – 467 .
- Omstedt , A and Axell , LB . 2003 . Modeling the variations of salinity and temperature in the large Gulfs of the Baltic Sea . Continental Shelf Res. , 23 : 265 – 294 .
- Orav-Kotta , H and Kotta , J . 2001 . Food and habitat choice of the isopod Idotea baltica in the northeastern Baltic Sea . Hydrobiologia. , 514 : 79 – 85 .
- Pekkari , S . 1973 . Effects of sewage water on benthic vegetation . Oikos Suppl. , 15 : 185 – 188 .
- Powell , HT . 1963 . “ Speciation in the genus Fucus L., and related genera ” . In Speciation in the Sea , Edited by: Harding , JP and Tebble , N. 63 – 77 . London : Systematics Association .
- Ramus , J , Lemons , F and Zimmerman , C . 1977 . Adaption of light-harvesting pigments to downwelling light and the consequent photosynthetic performance of the eulittoral rockweeds Ascophyllum nodosum and Fucus vesiculosus . Mar. Biol. , 42 : 293 – 303 .
- Raven , JA and Samuelsson , G . 1988 . Ecophysiology of Fucus vesiculosus L. close to its northern limit in the Gulf of Bothnia . Bot. Mar. , 31 : 399 – 410 .
- Reed , RH and Barron , JA . 1983 . Physiological adaptation to salinity change in Pilayella littoralis from marine and eustarine sites . Bot. Mar. , 26 : 409 – 416 .
- Rönnberg , C and Bonsdorff , E . 2004 . Baltic Sea eutrophication: area-specific ecological consequences . Hydrobiologia , 514 : 227 – 241 .
- Ruokolahti , C. and Rönnberg , O . 1988 . Seasonal variation in chlorophyll a content of Fucus vesiculosus in a northern Baltic archipelago . Am. Bot. Fennici , 25 : 385 – 388 .
- Russell , G . 1988 . The seaweed flora of a young semi-enclosed sea: The Baltic. Salinity as a possible agent of flora divergence . Helgoländer Meeresunters. , 42 : 243 – 250 .
- Schreiber , U . 1997 . Chlorophyll Fluorescence and Photosynthetic Energy Conversion , Effeltrich, , Germany : Walz .
- Snoeijs , P . 1999 . Marine and brackish waters . Acta Phytogeogr. Suec. , 84 : 187 – 212 .
- Stumm , W and Morgan , JJ . 1996 . Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters , New York, , USA : John Wiley & Sons Inc. .
- Sukenik , A , Bennett , J and Falkowski , P . 1987 . Light-saturated photosynthesis-limitation by electron transport or carbon fixation? . Biochim. Biophys. Acta , 891 : 205 – 215 .
- Surif , MB and Raven , JA . 1989 . Exogenous inorganic carbon sources for photosynthesis in seawater by members of the Fucales and the Laminariales (Phaeophyta): ecological and taxonomic implications . Oecologia , 78 : 97 – 105 .
- Surif , MB and Raven , JA . 1990 . Photosynthetic gas exchange under emersed conditions in eulittoral and normally submersed members of the Fucales and the Laminariales: interpretation in relation to C isotope ratio and N and water use efficiency . Oecologia , 82 : 68 – 80 .
- Swedish Environmental Protection Agency . 2001 . Environmental Quality in Swedish Coast and Sea. Official Statistics of Sweden
- Swedish Meteorological And Hydrological Institute, SMHI . 2005 . Data extracted from the Swedish Ocean Archive (SHARK), and originating from SMHI and Stockholm Marine Research Centre within the Swedish co-ordinated environmental monitoring programme
- Topinka , JA . 1978 . Nitrogen uptake by Fucus spiralis (Phaeophyceae) . J. Phycol. , 14 : 241 – 247 .
- Tropin , IV , Radzinskaya , NV and Voskoboinikov , GM . 2003 . The influence of salinity on the rate of dark respiration and structure of the cells of brown algae thalli from the Barents Sea littoral . Biol. Bull. , 30 : 40 – 47 .
- Turpin , DH . 1991 . Effects of inorganic N availability on algal photosynthesis and carbon metabolism . J. Phycol. , 27 : 14 – 20 .
- Van Den Hoek , C , Mann , DG and Jahns , HM . 1995 . Algae – An Introduction to Phycology , UK : Cambridge University Press .
- Waern , M . 1952 . Rocky shore algae in the Öregrund archipelago . Acta phytogeogr. Suec. , 30 : 1 – 298 .
- Wheeler , WN and Weidner , M . 1983 . Effects of external inorganic nitrogen concentration on metabolism, growth and activities of key carbon and nitrogen assimilating enzymes of Laminaria saccharina (Phaeophyceae) in culture . J. Phycol. , 19 : 92 – 106 .
