ABSTRACT
The diversity and phylogenetic relationships of specimens in the brown algal genus Cystoseira sensu lato from the eastern Atlantic and Mediterranean were examined using DNA sequences for four genetic markers (psbA, mt23S, cox1 and nad1). Our results provide evidence to resolve most of the species documented in the eastern Atlantic and Mediterranean as Cystoseira in three clades that are interpreted here as representing three genera. Morphological studies confirmed the distinctness of the genera and identified characters that distinguish species. One clade corresponds to Cystoseira sensu stricto, including the type species C. foeniculacea, together with C. compressa, C. humilis and the resurrected species C. aurantia. Species in Cystoseira are characterized by a caespitose habit and receptacles with grouped conceptacles lacking spiny appendages. The second clade corresponds to Treptacantha gen. emend., with T. abies-marina as the type species. A further 11 Atlantic and/or Mediterranean species were transferred to this genus. The concept of Treptacantha was better redefined, using the following morphological characters: smooth primary branches at least in the basal region, cortical cells with thickened walls and square meristoderm cells in cross section. The third clade corresponds to Carpodesmia gen. emend., with C. zosteroides as the type species. Another six Mediterranean species and the Atlantic/Mediterranean C. tamariscifolia were transferred to this genus. The concept of Carpodesmia was better defined to include new morphological features: all branches having spines, cortical cells with thin walls and rectangular meristoderm cells in cross section.
Introduction
Large brown algae of the genus Cystoseira C. Agardh nom. cons. (Fucales, Phaeophyceae) are among the dominant and ecologically key macrophytes in the warm temperate region of the eastern Atlantic, including the Mediterranean, the Adriatic Sea and the Macaronesian archipelagos (Sangil et al., Citation2011; Rozic et al., Citation2012; Blanfune et al., Citation2015). Cystoseira forms extensive beds on rocky substrata from exposed to sheltered conditions and from intertidal pools to more than 50 m depth (Hereu et al., Citation2008; Rodríguez-Prieto et al., Citation2013; Capdevila et al., Citation2016; Tsiamis et al., Citation2016). They play a pivotal role, providing habitats for a great biodiversity of epiphytic algae, invertebrates and fishes (Gómez-Garreta et al., Citation2001; Draisma et al., Citation2010; Sales & Ballesteros, Citation2010; Martínez et al., Citation2015). These macroalgal canopies moderate extreme temperature and salinity oscillations and protect recruits and initial stages of development of other organisms from stressors (Chapman, Citation1995; Dijkstra et al., Citation2011; Wahl et al., Citation2011; Jueterbock et al., Citation2013; Hernández et al., Citation2015). Unfortunately, the productive marine forests dominated by Cystoseira and other fucalean species are seriously threatened by habitat destruction, pollution and environmental changes linked to climate change. As a consequence, many populations have declined along the Atlantic and Mediterranean coasts over the last few decades, with some species disappearing from many locations (Jueterbock et al., Citation2013; Sansón et al., 2014, Citation2017; Mineur et al., Citation2015; Riera et al., Citation2015; Valdazo et al., Citation2017).
With 46 currently recognized species, Cystoseira sensu lato is the second most diverse genus among fucalean macrophytes, after Sargassum which includes 360 recognized species (Guiry & Guiry, Citation2018). Although it is a conspicuous component of the warm temperate eastern Atlantic flora, the high morphological plasticity of Cystoseira has hindered species identification based on morphological characters (Rozic et al., Citation2012). Roberts (Citation1967) described in detail the morphology, development of the thallus and reproductive structures of Cystoseira and discussed the validity of these characters for species separation. The structure of the thallus in Cystoseira consists of an inner medulla, a cortex and an outer meristoderm that have been described as uniform among species, and have consequently been little used in species identification (Gómez-Garreta et al., Citation2001; Rodríguez-Prieto et al., Citation2013). Previous studies suggested that morphology alone is insufficient to differentiate species, indicating the need for molecular tools to set the limits between them and study their phylogenetic relationships. Draisma et al. (Citation2010) demonstrated the polyphyly of the genus Cystoseira and other genera of Sargassaceae, based on the most abundant taxa in the Mediterranean. They used the plastid psbA gene to study the family level phylogeny, and the mitochondrial mt23S gene and mt23S-tRNA Val spacer for genus and species delineation, respectively. Following this study, Rozic et al. (Citation2012) tested these mitochondrial markers in two Cystoseira species (C. montagnei J. Agardh, as C. spinosa Sauvageau; and C. squarrosa De Notaris) from the Adriatic, showing that the intergenic spacer has a lower potential than mt23S to discriminate them.
Previous investigations based on floristic and morphological data have recorded 21 species of Cystoseira sensu lato in the warm temperate region of the eastern Atlantic, with 15 reported from Macaronesian Islands (Azores, Madeira, Salvage Is., Canary Is. and Cape Verde Is.) (Gallardo et al., Citation2016; Guiry & Guiry, Citation2018). Most are known from several of the archipelagos, except that C. mauritanica Sauvageau ex Hariot and C. wildpretii Nizamuddin are only documented in the Canary Islands, and C. sonderi (Kützing) Piccone only in the Cape Verde Islands (John et al., Citation2004). Draisma et al. (Citation2010) studied most of the European species of Cystoseira, suggesting that they should be divided into three genera, but their analyses did not include some of the species from these islands. The aim of the present study is to resolve the diversity and phylogenetic relationships of Cystoseira sensu lato from the eastern Atlantic and Mediterranean. To this end, we obtained 31 new DNA sequences for four molecular markers (psbA, mt23S, cox1 and nad1) of Canarian specimens and examined morphological characters of 18 species of Cystoseira sensu lato from Atlantic and Mediterranean localities to determine which diagnostic vegetative and reproductive characters can assist in identifying taxa.
Materials and methods
Study area and sampling
Specimens for molecular and morphological analyses were collected from the intertidal and subtidal zones in four localities of the Canary Islands (Supplementary table S1). Field samples were kept in separate bags and transported to the laboratory. Clean fragments of each specimen were dried in silica gel for DNA extraction. Samples for morphological studies were preserved as voucher specimens, and they were deposited in the herbarium of the University of La Laguna (TFC, Tenerife Ciencias, Spain). Morphological studies of non-Canarian species were made on material deposited in HGI-A (Herbarium of Universitat de Girona, Spain) (Thiers, Citation2018).
DNA extraction and amplification
Taxa used in the phylogenetic analyses are listed in Supplementary table S1. Total DNA was extracted from silica-dried branches. Lysis matrices type A (MP, Biomedicals, USA) were used for material fragmentation, with the aid of FastPrep-24®56 (MP, Biomedicals, USA). Subsequently, DNA was extracted using the E.Z.N.A. SP Plant kit (Omega Bio-tek, USA) following the manufacturer’s instructions. Total DNA was quantified using a Nanodrop ND-1000 (Thermo Science, USA) and samples taken to a final concentration of 1 ng µl–1. Four partial genes were analysed, three mitochondrial (mt23S, nad1 and cox1) and one plastid (psbA). Sequence primers, PCR conditions and components are shown in Supplementary table S2. All amplifications were carried out in a Veriti 96-well thermocycler (Applied Biosystems, USA). Aliquots of amplified products were electrophoresed on 1.7% agarose gel to verify DNA amplification. Subsequently, PCR products were enzymatically purified with ExoSAP-IT kit (GE Healthcare, Illustra, UK) according to the manufacturer’s instructions. Finally, all samples were sequenced with the same primers used for amplification at the Genomics Service (SEGAI) of the University of La Laguna, Spain.
Morphological observations
Macromorphological characters were observed under a Leica EZ4 stereomicroscope (Leica Geosystems, Germany). Macroscopic analysis for all specimens included examination of morphology and size of holdfast or basal system, main axis, branches and receptacles. Thallus length, diameter of axes and receptacles were measured. Images were captured using a Fujifilm X10 digital camera. Fragments of primary branches were selected for cross-sections, made by hand with stainless-steel razor blades. When necessary, material was stained with 1% aqueous aniline blue solution, acidified with 1N HCl and mounted in 40% Karo® solution (Tsuda & Abbott, Citation1985). Microscopic characters such as the shape of medullary, cortical and meristoderm cells were observed and the diameter of 10 cells per tissue per thallus was measured with a Leica DM500 (Leica Geosystems, Germany) microscope. Anatomical images were captured using a Fujifilm X10 digital camera attached to the microscope.
Data analyses
Sequences were edited using MEGA version 7 (Kumar et al., Citation2016) and aligned with CLUSTAL W (Thompson et al., Citation1994) as implemented in MEGA 7 and then visually assessed. In addition, p-distances were calculated using MEGA 7. Indels present in the mt23S dataset were coded as binary characters using the simple coding method by Simmons & Ochoterena (Citation2000) as implemented in FastGap software (Borchsenius, Citation2009). For all genes, the alignments were trimmed to fit the sequence lengths to those from GenBank. Accession numbers of DNA sequences used in this study are shown in Supplementary table S1. JModelTest 2 software (Darriba et al., Citation2012) was used to select the best nucleotide substitution model for each dataset according to the Bayesian Information Criterion (BIC) (Schwarz, Citation1978). These models were TPM2uf+G+I for mt23S, HKY+G for cox1 and nad1, and TrN+I for psbA. Since there is a very limited number of species sequenced for all four markers, it was necessary to carry out two analyses: the first one with the concatenated sequences of mt23S and psbA (1146 base pairs, bp), to establish the position of the Canarian species in relation to species from other regions; and a second analysis with those species with information for the four markers (mt23S + nad1 + cox1 + psbA) aimed to resolve species boundaries within the species of the Canary Islands (2449 bp).
Phylogenetic trees were inferred from the concatenated sequences by Maximum likelihood (ML) and Bayesian inference (BI), using the previously selected models of nucleotide substitution.
ML analysis was conducted using the RAxML software (Ott et al., Citation2007) with the GTR+CAT approximation to accommodate rate heterogeneity among partitions and 1000 bootstrap replicates. The MrBayes software (Huelsenbeck & Ronquist, Citation2001) was used for BI. Two independent runs were performed with default prior values, running 10 million generations and sampling every 100 generations. All parameters were unlinked across partitions. Convergence of runs was assessed by comparing the average standard deviation of split frequencies (< 0.01). The first 25% of trees were discarded as burn-in and the remaining trees were summarized in a majority consensus tree.
Trees were visualized and edited with FigTree v1.4.3 (Rambaut, Citation2016).
Results
Alignment properties
A total of 31 sequences for mt23S representing eight haplotypes were obtained (Supplementary table S1). Sequence lengths ranged between 370 bp (C. mauritanica) and 390 bp (C. tamariscifolia), making the introduction of alignment gaps necessary. Unexpectedly, differences in length occurred within C. tamariscifolia and C. abies-marina, with some specimens featuring an additional 11 bp and 6 bp, respectively.
Similarly, 31 sequences of 925 bp representing seven haplotypes were obtained for the gene psbA (Supplementary table S1). However, in order to compare with sequences deposited in GenBank, the alignments for mt23S and psbA had to be reduced to 363 bp and 783 bp, respectively. Cox1 (553 bp) and nad1 (562 bp) sequences were obtained from 31 specimens representing a total of seven and 10 haplotypes, respectively.
Phylogenetic analyses
For concatenated psbA + mt23S analysis, ML and BI tree topologies were identical to those obtained independently for each gene (data not shown), although with higher support. Therefore, only the BI tree for the concatenation of both genes is shown (). Likewise, ML and BI trees of the concatenated sequences including also cox1 and nad1 () had higher support than trees constructed independently for each gene. Again, topology of trees for each fragment gene were identical to the ones for the concatenated sequences, and therefore, only the BI tree for the concatenation of the four genes is shown ().
Fig. 1. Phylogenetic tree of concatenated psbA and mt23S partial genes obtained by Bayesian inference (BI). Maximum likelihood (ML) gave the same topology. ML bootstrap values (≥ 70%) and Bayesian posterior probabilities (≥ 0.95) respectively, are indicated adjacent to the branches. Sequences for taxa in bold were generated in this study. Sequences from GenBank correspond to those included in Supplementary table S1. Scale = 0.01.
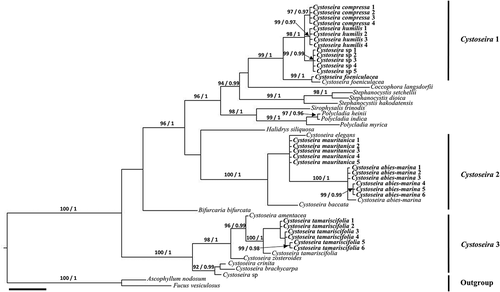
Fig. 2. Phylogenetic tree of concatenated psbA, mt23S, cox1 and nad1 partial genes obtained by Bayesian inference (BI) for the Canary Islands specimens of Cystoseira sensu lato. Maximum likelihood (ML) gave the same topology. ML bootstrap values (≥ 70%) and Bayesian posterior probabilities (≥ 0.95) respectively, are indicated adjacent to the branches. All sequences were generated in this study. Scale = 0.01.
shows Cystoseira sensu lato to be a polyphyletic genus. One of the clades (Cystoseira 3) includes Cystoseira sp. (Croatia), C. brachycarpa (Sicily, Italy), C. crinita (Sicily, Italy), C. zosteroides (Sicily, Italy), C. amentacea (Sicily, Italy), and a group of samples of C. tamariscifolia from the Canary Islands (C. tamariscifolia 1–6) and a specimen from the Atlantic Iberian Peninsula (Galicia, Spain) (BS = 100%; PP = 1.0) that exhibit some genetic differentiation among them (). In addition, two specimens (C. tamariscifolia 5–6) are clustered together in both trees (, ). Sequences of these two specimens have an insertion/deletion of 11 bp and several nucleotide substitutions in the mt23S gene. In addition, these two specimens have several nucleotide substitutions in the nad1 gene.
A second clade (Cystoseira 2) includes specimens of C. baccata (Galicia, Spain), C. elegans (Sicily, Italy), C. mauritanica and C. abies-marina (Tenerife, Canary Islands, Spain) (). The clade formed by all specimens of C. abies-marina shows a polytomy (C. abies-marina 1–3) and a subclade with significant support (BS = 99%; PP = 0.99) composed by C. abies-marina 4–6 (). It is worth noting the differences observed among specimens of C. abies-marina from the Canary Islands (). Although these differences are not as clear in the tree in , both trees show significant support ( BS = 99%; PP = 0.99 and BS = 97%; PP = 0.98). These specimens have an insertion/deletion of 6 bp and several nucleotide substitutions in the sequences of mt23S.
Finally, a third, well-supported clade ( BS = 96%; PP = 1.0) encompasses five clades representing as many distinct genera: Polycladia Montagne, Sirophysalis Kützing, Coccophora Greville, Stephanocystis Trevisan and Cystoseira sensu stricto. The Polycladia/Sirophysalis clade is the earliest diverged. In both trees (, ) the clade Cystoseira sensu stricto (Cystoseira 1) includes four species present in the Canary Islands: the generitype C. foeniculacea, as well as C. compressa, C. humilis and an initially unidentified Cystoseira sp., with the last three species displaying closer relationships ( BS = 98%; PP = 1.0 and BS = 92%; PP = 1.0).
Morphology of Cystoseira sensu lato species
A morphological survey of 18 species, some included in the phylogenetic analysis, shows that they exhibit unique combinations of attributes. Species in Cystoseira 1 are characterized by a caespitose habit, with several axes from a discoid base, and warty non-spinose receptacles with grouped conceptacles. Cystoseira 2 includes species showing smooth primary branches, at least in the basal region, occasionally with small and widely spaced spiny appendages upwards, and spiny receptacles with 1–3 (few) conceptacles located at the base of each fertile spine. Finally, species in Cystoseira 3 have branches all bearing spinose appendages, receptacles with spiny or filiform appendages and grouped conceptacles ().
Table 1. Comparison of morphological attributes exhibited by species of Cystoseira sensu lato studied. Groups: Cystoseira 1 (= Cystoseira sensu stricto), Cystoseira 2 (here proposed as Treptacantha) and Cystoseira 3 (here proposed as Carpodesmia)
The caespitose species in Cystoseira 1 are arborescent and epilithic. Primary branches are cylindrical or flattened. Among the cylindrical ones, those of Cystoseira sp. are smooth (, ); they are very thorny in C. foeniculacea () or show protuberances or scars in C. humilis (). In contrast, primary branches are flattened in C. compressa (, ) and the basal branches are spinose in C. foeniculacea, which shows a dimorphism in branches. Secondary and higher-order branches are thread-like and radially ramified, resulting in a spruce-shaped outline in C. foeniculacea and C. humilis (, ). In Cystoseira sp. and the spring-summer morphotype of C. compressa, secondary branches are distichous, while higher-order branches are radially ramified (, , , ). In the rosette-flattened winter morphotype of C. compressa (), all branches are distichous. Internal aerocysts are large (up to 7 mm in length) and swollen in Cystoseira sp. (), mainly arranged along last-order branches; they are small (up to 4 mm in length) and rarely evident in C. compressa, and inconspicuous in C. foeniculacea and C. humilis. Cryptostomata are scattered over the entire thallus, with deciduous phaeophycean hairs mainly protruding towards branch apices. Thalli of Cystoseira 1 have a compact parenchymatous structure, consisting of three tissues (medulla, cortex and meristoderm). In cross section, small polygonal cells (0.6 to 14.3 µm) forming a central unpigmented mass constitute the medulla (–). The cortex exhibits irregularly globose cortical cells, with thickened and shiny walls, progressively decreasing in size towards both the medulla and the meristoderm (–). The phaeoplastic meristoderm is unilayered, and in cross section cells are square and arranged in a palisade (–). Cell size in these tissues contributes to species differentiation (). Meristoderm cells of Cystoseira sp. are the narrowest, while those of C. foeniculacea are the widest. C. compressa shows the greatest diameter in cortical and medullary cells. Evidence of sexual reproduction was observed in all species of the group Cystoseira 1. Receptacles are formed at the ends of last-order terminal branches; they are cylindrical, fusiform or siliquiform, simple or sometimes branched, have grouped protruding conceptacles, and always lack spines. Outer appearance of receptacles is similar in all species of the group Cystoseira 1 (–), however the diameter varies slightly according to the species (). In all species, conceptacles are usually bisexual, with several rounded dark brown oogonia emerging from the bottom, and small yellowish antheridia formed at the apices of branched filaments. Diameter of conceptacles reaches 500 μm in all species ().
Table 2. Diameter (µm) of cells in cross section of primary branches, and diameter (mm) of reproductive structures of species of Cystoseira sensu lato
Figs 3–8. Habit of species of Cystoseira 1 (= Cystoseira sensu stricto) from the Canary Islands. Figs 3, 4. Cystoseira sp. (= C. aurantia) (TFC Phyc 15276). Fig. 3. Habit. Fig. 4. Detail of last order branches, with conspicuous aerocysts (arrows). Fig. 5. Cystoseira foeniculacea (TFC Phyc 15266). Fig. 6. Cystoseira humilis (TFC Phyc 15267). Fig. 7. Cystoseira compressa (TFC Phyc 15263). Spring-summer morphotype. Fig. 8. Cystoseira compressa (TFC Phyc 15291). Rosette morphotype. Note the caespitose habit of all species. All scale bars: 1 cm.

Species in the group Cystoseira 2 are non-caespitose, arborescent, radially ramified and epilithic. C. abies-marina (, ) and C. usneoides () have prostrate axes with haptera () that cut off several primary branches, and sometimes develop basal slightly compressed branches. By contrast, C. algeriensis, C. baccata, C. elegans, C. mauritanica, C. montagnei and C. nodicaulis have a rounded basal disc from which arises a cylindrical simple or branched axis (–). C. abies-marina and C. mauritanica are sometimes iridescent. All species of this group present smooth primary branches at least in the basal region, occasionally with small and widely spaced spiny appendages upwards. Branches of higher-order show lateral spines, except in C. baccata, C. barbata and C. susanensis. Inner aerocysts are inconspicuous (except in C. usneoides) and cryptostomata are scattered over the entire thallus. Cystoseira 2 is also characterized by a parenchymatous structure consisting of medulla, cortex and meristoderm. In cross section, small polygonal medullary cells form a central mass (–, –). The cortex exhibits globose cortical cells, sometimes deformed by pressure, with thickened walls (–, –). The phaeoplastic meristoderm is unilayered, with square cells in cross section, arranged in a palisade (–, –). Cell size in these tissues contributes to species differentiation (). Meristoderm cells of C. abies-marina are considerably wider than in other species. On the contrary, C. elegans and C. mauritanica have the smallest cells. The largest cortical cells are those of C. abies-marina and the shortest those of C. elegans. Medullary cells are more similar between species, with the largest in C. baccata (). Evidence of sexual reproduction was observed in all species. Receptacles are transformed ends of last-order branches and show spiny laterals in all species, except C. usneoides (–, –). Few (1–3) conceptacles are located at the base of each fertile spine (except in C. nodicaulis and C. usneoides, which show numerous grouped conceptacles). Diameters of receptacles varied according to the species but are always greater than those of Cystoseira 1 (). Conceptacles are usually bisexual, with several rounded dark brown oogonia emerging from the bottom, and small yellowish antheridia formed at the apices of branched filaments. C. mauritanica presents the largest diameter of conceptacles ().
Figs 9–17. Habit of species of Cystoseira 2 (here proposed as Treptacantha). Fig. 9. Cystoseira abies-marina (TFC Phyc 15256). Well-developed specimen. Fig. 10. Cystoseira abies-marina (TFC Phyc 15259). Specimen from upper sublittoral. Fig. 11. Cystoseira usneoides (TFC Phyc 1402). Fig. 12. Cystoseira algeriensis (HGI – A 1289). Fig. 13. Cystoseira mauritanica (TFC Phyc 15271). Fig. 14. Cystoseira baccata (TFC Phyc 1766). Fig. 15. Cystoseira nodicaulis (HGI – A 3813). Fig. 16. Cystoseira elegans (HGI – A 14577). Fig. 17. Cystoseira montagnei (TFC Phyc 599). All scale bars: 1 cm.

Figs 18–24. Habit of species of Cystoseira 3 (here proposed as Carpodesmia). Fig. 18. Cystoseira amentacea (HGI – A 14601). Fig. 19. Cystoseira brachycarpa (HGI – A 14552). Fig. 20. Cystoseira crinita (HGI – A 14570). Fig. 21. Cystoseira mediterranea (HGI – A 9427). Fig. 22. Cystoseira zosteroides (HGI – A 3054). Fig. 23. Cystoseira tamariscifolia (TFC Phyc 15285). Morphotype from sublittoral at La Graciosa islet. Fig. 24. Cystoseira tamariscifolia (TFC Phyc 15281). Morphotype from lower eulittoral at Gran Canaria island. Note: spiny appendages on primary branches are indicated with arrows. All scale bars: 1 cm.
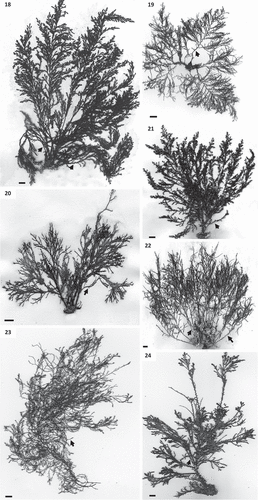
Figs 25–38. Cross sections of thalli and details of receptacles of Cystoseira 1 (= Cystoseira sensu stricto) from the Canary Islands. Figs 25–29. Primary branches. Fig. 25. Cystoseira sp. (= C. aurantia) (TFC Phyc 15276). Fig. 26. Cystoseira foeniculacea (TFC Phyc 15266). Fig. 27. Cystoseira humilis (TFC Phyc 15267). Fig. 28. Cystoseira compressa (TFC Phyc 15263). Spring-summer morphotype. Fig. 29. Cystoseira compressa (TFC Phyc 15291). Rosette morphotype. Figs 30–34. Details of the three vegetative tissues. Fig. 30. Cystoseira sp. (= C. aurantia) (TFC Phyc 15276). Fig. 31. Cystoseira foeniculacea (TFC Phyc 15266). Fig. 32. Cystoseira humilis (TFC Phyc 15267). Fig. 33. Cystoseira compressa (TFC Phyc 15263). Fig. 34. Cystoseira compressa (TFC Phyc 15291). Figs 35–38. Warty fusiform receptacles (arrows). Fig. 35. Cystoseira sp. (= C. aurantia) (TFC Phyc 15276). Fig. 36. Cystoseira foeniculacea (TFC Phyc 15266). Fig. 37. Cystoseira humilis (TFC Phyc 15267). Fig. 38. Cystoseira compressa (TFC Phyc 15263). Meristoderm (mt), cortex (c) and medulla (m). Scale bars: Figs 25–34 = 100 μm; Figs 35–38 = 1 mm.
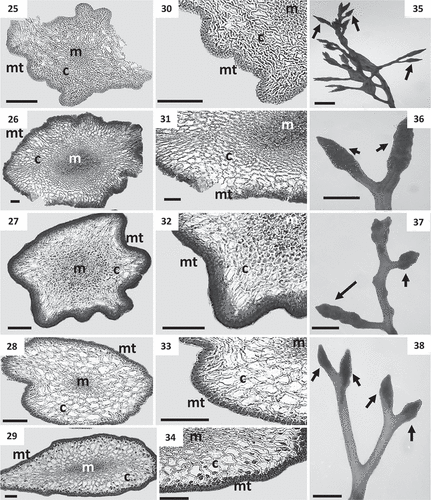
Figs 39–52. Cross sections of thalli and details of receptacles of Cystoseira 2 (here proposed as Treptacantha). Figs 39–48. Primary branches. Fig. 39. Cystoseira abies-marina (TFC Phyc 15259). Upper sublittoral morphotype. Fig. 40. Cystoseira abies-marina (TFC Phyc 15256). Sublittoral morphotype. Fig. 41. Cystoseira usneoides (TFC Phyc 1402). Fig. 42. Cystoseira algeriensis (HGI – A 1289). Fig. 43. Cystoseira mauritanica (TFC Phyc 15271). Figs 44–48. Details of the three vegetative tissues. Fig. 44. Cystoseira abies-marina (TFC Phyc 15259). Upper sublittoral morphotype. Fig. 45. Cystoseira abies-marina (TFC Phyc 15256). Sublittoral morphotype. Fig. 46. Cystoseira usneoides (TFC Phyc 1402). Fig. 47. Cystoseira algeriensis (HGI – A 1289). Fig. 48. Cystoseira mauritanica (TFC Phyc 15271). Figs 49–52. Receptacles (arrows). Fig. 49. Cystoseira abies-marina (TFC Phyc 15256). Fig. 50. Cystoseira usneoides (TFC Phyc 1402). Fig. 51. Cystoseira algeriensis (HGI – A 1289). Fig. 52. Cystoseira mauritanica (TFC Phyc 15271). Meristoderm (mt), cortex (c) and medulla (m). Scale bars: Figs 39–48 = 100 μm; Figs 49–52 = 1 mm.
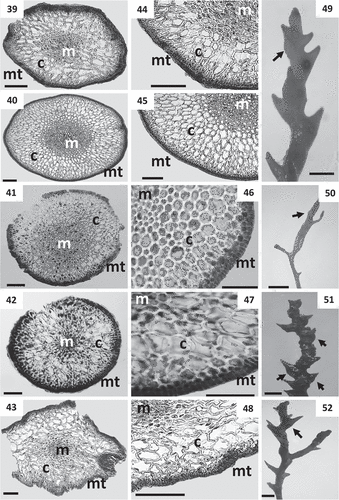
Figs 53–64. Cross sections of thalli and details of receptacles of Cystoseira 2 (continued). Figs 53–60. Primary branches. Fig. 53. Cystoseira baccata (TFC Phyc 1766). Fig. 54. Cystoseira nodicaulis (HGI – A 3813). Fig. 55. Cystoseira elegans (HGI – A 14577). Fig. 56. Cystoseira montagnei (TFC Phyc 599). Figs 57–60. Details of the three vegetative tissues. Fig. 57. Cystoseira baccata (TFC Phyc 1766). Fig. 58. Cystoseira nodicaulis (HGI – A 3813). Fig. 59. Cystoseira elegans (HGI – A 14577). Fig. 60. Cystoseira montagnei (TFC Phyc 599). Figs 61–64. Receptacles (arrows). Fig. 61. Cystoseira baccata (TFC Phyc 1766). Fig. 62. Cystoseira nodicaulis (HGI – A 3813). Fig. 63. Cystoseira elegans (HGI – A 14577). Fig. 64. Cystoseira montagnei (TFC Phyc 599). Meristoderm (mt), cortex (c) and medulla (m). Scale bars: Figs 53–60 = 100 μm; Figs 61–64 = 1 mm.
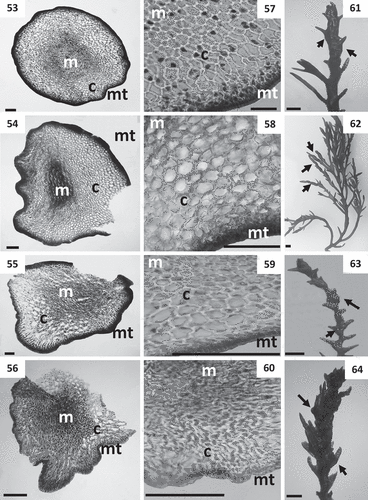
The arborescent species in Cystoseira 3 grow epilithically, attached to the substrata by haptera or disc, with a caespitose or non-caespitose habit (, –). Species in this group exhibit branches all with spinose laterals, except C. crinita, which lacks spines in secondary and higher-order branches. Specimens of C. tamariscifolia from the Canary Islands show two different morphotypes. One of the morphotypes () shows prostrate axes with haptera and several erect primary branches. Primary branches are cylindrical and profusely radially branched, with secondary branches decreasing in length to the apices. All orders of branches form numerous lateral spinose appendages. The second morphotype () show a discoid base, a dark brown cylindrical axis, with a non-prominent apex covered by small spines. In all species of Cystoseira 3, inner aerocysts are inconspicuous and cryptostomata are scattered over the entire thallus. Thalli develop a parenchymatous structure consisting of medulla, cortex and meristoderm. In cross section, the medulla is more developed than in the other two groups, exhibiting small polygonal medullary cells as a central mass. The cortex has globose cortical cells, sometimes deformed by pressure, with thin walls (–, , ). Exceptionally, C. mediterranea has small thickenings at the corners of the cells (). The phaeoplastic meristoderm is unilayered; cells are ellipsoidal to rounded, rectangular in cross section, twice as long as wide, arranged in palisade (–, , ). Cell size varied according to species, with C. crinita showing the smallest meristoderm cells, almost half of those of C. mediterranea. Cortical and medullary cells of C. zosteroides are the smallest, while C. amentacea has the largest (). Evidence of sexual reproduction was observed in all species. Receptacles are transformed ends of last-order branches. Fertile ends of branches show numerous grouped conceptacles and spiny appendages (–, , ). Conceptacles are usually bisexual, with several rounded dark brown oogonia emerging from the bottom, and small yellowish antheridia formed at the apices of branched filaments. Diameter of conceptacles is included in .
Figs 65–79. Cross sections of thalli and details of receptacles of Cystoseira 3 (here proposed as Carpodesmia). Figs 65–69. Primary branches. Fig. 65. Cystoseira amentacea (HGI – A 14601). Fig. 66. Cystoseira brachycarpa (HGI – A 14552). Fig. 67. Cystoseira crinita (HGI – A 14570). Fig. 68. Cystoseira mediterranea (HGI – A 9427). Fig. 69. Cystoseira zosteroides (HGI – A 3054). Figs 70–74. Details of the three vegetative tissues. Fig. 70. Cystoseira amentacea (HGI – A 14601). Fig. 71. Cystoseira brachycarpa (HGI – A 14552). Fig. 72. Cystoseira crinita (HGI – A 14570). Fig. 73. Cystoseira mediterranea (HGI – A 9427). Fig. 74. Cystoseira zosteroides (HGI – A 3054). Figs 75–79. Receptacles (arrows). Fig. 75. Cystoseira amentacea (HGI – A 14601). Fig. 76. Cystoseira brachycarpa (HGI – A 14552). Fig. 77. Cystoseira crinita (HGI – A 14570). Fig. 78. Cystoseira mediterranea (HGI – A 9427). Fig. 79. Cystoseira zosteroides (HGI – A 3054). Meristoderm (mt), cortex (c) and medulla (m). Scale bars: Figs 65–74 = 100 μm; Figs 75–79 = 1 mm.
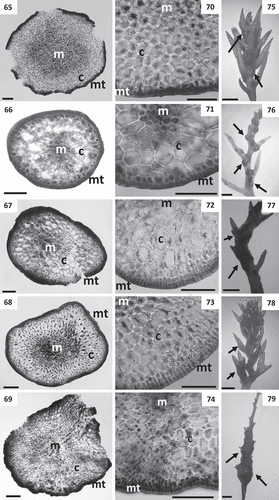
Figs 80–85. Cross sections of thalli and details of receptacles of Cystoseira 3 (continued). Figs 80–83. Primary branches of Cystoseira tamariscifolia. Fig. 80. Cystoseira tamariscifolia (TFC Phyc 15285). Sublittoral morphotype. Fig. 81. Cystoseira tamariscifolia (TFC Phyc 15281). Low eulittoral morphotype. Figs 82, 83. Details of the three vegetative tissues. Fig. 82. Cystoseira tamariscifolia (TFC Phyc 15285). Sublittoral morphotype. Fig. 83. Cystoseira tamariscifolia (TFC Phyc 15281). Low eulittoral morphotype. Figs 84, 85. Receptacles (arrows). Fig. 84. Cystoseira tamariscifolia (TFC Phyc 15285). Sublittoral morphotype. Fig. 85. Cystoseira tamariscifolia (TFC Phyc 15281). Low eulittoral morphotype. Meristoderm (mt), cortex (c) and medulla (m). Scale bars: Figs 80–83 = 100 μm; Figs 84–85 = 1 mm.
In accordance with our molecular and morphological results we propose resurrecting and amending the genera Treptacantha Kützing for the clade Cystoseira 2, with T. abies-marina (S.G. Gmelin) Kützing as the type species, and Carpodesmia Greville for the clade Cystoseira 3, with Carpodesmia zosteroides (C. Agardh) Greville as the type species. Among the new combinations of species proposed below, those marked with an asterisk could not be studied morphologically in this work and these proposals are based on phylogenies published by Draisma et al. (Citation2010).
Taxonomic proposals
Treptacantha Kützing, gen. emend
Diagnosis: Thallus attached by prostrate axes with haptera or a small basal disc, non-caespitose. Axes cylindrical to slightly compressed, simple or branched. Primary branches erect, cylindrical, radially ramified, smooth at least in the basal region, occasionally with small and widely spaced spiny appendages upwards. Higher order branches with spines (except in T. baccata, T. barbata and T. susanensis). Tophules present or absent. In cross section, cortical cells with thickened walls and square meristoderm cells. Spiny receptacles, with few (1–3) conceptacles located at the base of each fertile spine (except in T. nodicaulis and T. usneoides, with grouped conceptacles).
Lectotype species: Treptacantha abies-marina (S.G. Gmelin) Kützing. Type designated in De Toni (Citation1891).
observations: Treptacantha is the only remaining available name for this group of species that is resurrected herein.
Treptacantha abies-marina (S.G. Gmelin)
Kützing, Citation1843: 353
Basionym: Fucus abies-marinus S.G. Gmelin, 1768: 83. Historia fucorum. Ex typographia Academiae scientiarum, St. Petersburg. 239 pp.
Homotypic synonym: Cystoseira abies-marina (S.G. Gmelin) C. Agardh, Citation1821: 54.
Heterotypic synonyms: Treptacantha gracillima Kützing, Citation1843: 354; Phyllacantha moniliformis Kützing, Citation1843: 356; Treptacantha montagnei Kützing, Citation1849: 594.
Syntype locality: ‘Mare mediterraneum et oceanus, Angliam’. Type: original illustration (Tab. II.A fig. 1) (Gmelin, 1768: 83).
Representative DNA sequences: GenBank accession no. MH493055/MH493056 (mt23S partial gene), MH513830 (cox1 partial gene), MH513837 (nad1 partial gene) and MH513845 (psbA partial gene). Loc. Punta del Hidalgo (N Tenerife, Canary Islands); Dat. 5 November 2015; Legit. Sharay Orellana & Marta Sansón.
Treptacantha algeriensis (Feldmann) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira algeriensis Feldmann, 1945: 7. Une nouvelle espèce de Cystoseira (Fucales, Sargassacées) des côtes d’Algérie. Bulletin de la Société d’Histoire Naturelle de l’Afrique du Nord 35: 7–10.
Type locality: ‘Prope Saldas (Bougie) et Caesaream Mauretaniae (Cherchell)’. Lectotype: PC (Furnari et al., Citation1999: 20).
Treptacantha baccata (S.G. Gmelin) S. Orellana
& M. Sansón, comb. nov
Basionym: Fucus baccatus S.G. Gmelin, 1768: 90. Historia fucorum. Ex typographia Academiae scientiarum, St. Petersburg. 239 pp.
Homotypic synonym: Cystoseira baccata (S.G. Gmelin) P.C. Silva, Citation1952: 280.
Heterotypic synonyms: Fucus abrotanoides S.G. Gmelin, 1768: 89; Fucus fibrosus Hudson, Citation1778: 575; Cystoseira fibrosa (Hudson) C. Agardh, Citation1821: 65; Cystoseira thesiophylla Duby, Citation1830: 937; Phyllacantha fibrosa (S.G. Gmelin) Kützing, Citation1843: 356; Phyllacantha thesiophylla (Duby) Kützing, Citation1860: 37.
Type locality: ‘Scheuelingensi prope Hagam‘ (Scheveningen, The Hague, the Netherlands) (Gmelin, 1768: 90). Type: original illustration (Tab. III fig. 2).
Representative DNA sequences: GenBank accession no. FM958291 (psbA partial gene), FM958368 (mt23S partial gene). Loc. A Coruña, Galicia (Spain); Dat. 24 August 2005; Legit. Ignacio Bárbara.
*Treptacantha barbata (Stackhouse) S. Orellana
& M. Sansón, comb. nov
Basionym: Abrotanifolia barbata Stackhouse, 1809: 81. Tentamen marino-cryptogamicum, ordinem novum; in genera et species distributum, in Classe XXIVta Linnaei sistens. Memoires de la Société Impériale des Naturalistes de Moscou, 2: 50–97.
Homotypic synonym: Cystoseira barbata (Stackhouse) C. Agardh, Citation1821: 57.
Heterotypic synonyms: Fucus barbatus Goodenough & Woodward, Citation1797: 103, 128, nom. illeg.; Cystoseira hoppei C. Agardh, Citation1821: 59; Cystoseira barbata var. hoppei (C. Agardh) J. Agardh, Citation1842: 51; Cystoseira barbata f. hoppei (C. Agardh) Woronichin, 1908: 117.
Type locality: Devon, England (Silva et al., Citation1996: 647).
Representative DNA sequence: GenBank accession no. FM958378 (mt23S partial gene). Loc. Moll d’es Miami, Bay of Fornells, Menorca (Spain); Dat. 25 June 2006; Legit. Stefano G.A. Draisma.
Treptacantha elegans (Sauvageau) S. Orellana &
M. Sansón, comb. nov
Basionym: Cystoseira elegans Sauvageau, Citation1912: 292. A propos des Cystoseira de Banyuls et Guéthary. Bulletin de la Station Biologique d’Arcachon, 14: 133–556.
Type locality: Banyuls-sur-mer, France (Sauvageau, Citation1912). Lectotype: PC (Furnari et al., Citation1999: 21).
Representative DNA sequences: GenBank accession no. FM958292 (psbA partial gene), FM958375 (mt23S partial gene). Loc. Capo Passero, Sicily (Italy); Dat. 13 June 2005; Legit. Stefano G.A. Draisma.
Treptacantha mauritanica (Sauvageau) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira mauritanica Sauvageau in Hariot, 1911: 440. Algues de Mauritanie recueillies par M. Chudeau. Bulletin de la Société Botanique de France, 58: 438–445.
Heterotypic synonyms: Cystoseira selaginoides var. gibraltarica Sauvageau, Citation1920: 28; Cystoseira sauvageauana var. gibraltarica (Sauvageau) Hamel, Citation1939: 400; Cystoseira gibraltarica (Sauvageau) P.J.L. Dangeard, Citation1949: 128, 133.
Type locality: Port Étienne and Baie de Cansado (Hariot, 1911).
Representative DNA sequences: GenBank accession no. MH493071 (mt23S partial gene), MH513835 (cox1 partial gene), MH513842 (nad1 partial gene) and MH513850 (psbA partial gene). Loc. Punta del Hidalgo, Tenerife (Spain); Dat. 05 November 2015; Legit. Sharay Orellana & Marta Sansón.
Treptacantha montagnei (J. Agardh) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira montagnei J. Agardh, Citation1842: 47. Algae maris Mediterranei et Adriatici, observationes in diagnosin specierum et dispositionem generum. Apud Fortin, Masson et Cie, Paris. 164 pp.
Heterotypic synonyms: Fucus erica-marina S.G. Gmelin, 1768: 128, nom. illeg.; Cystoseira erica-marina (S.G. Gmelin) Naccari, Citation1828: 96; Cystoseira spinosa Sauvageau, Citation1912: 69, 387; Cystoseira adriatica Sauvageau, Citation1912: 249, 518; Cystoseira jabukae Ercegovic, Citation1952: 109.
Type locality: “Hab. e profundiori mari rejectam ad Cette mense aprilis frequentem, ad Massiliam rariorem legi; ex Adriatico dedit Biasoletto!” (J. Agardh, Citation1842). Lectotype: LD 00528 (Sellam et al., Citation2017).
Representative DNA sequence: GenBank accession no. FM958374 (mt23S partial gene). Loc. Cala Mica, Menorca (Spain); Dat. 23 June 2006; Legit. Stefano G.A. Draisma.
Treptacantha nodicaulis (Withering) S. Orellana
& M. Sansón, comb. nov
Basionym: Fucus nodicaulis Withering, 1796: 111. An arrangement of British plants; according to the latest improvements of the Linnaean system. To which is prefixed, an easy introduction to the study of botany. Illustrated by copper plates. The third edition, in four volumes. Vol. IV. Birmingham & London: Printed for the Author, by M. Swinney. 418 pp.
Homotypic synonym: Cystoseira nodicaulis (Withering) M. Roberts, Citation1967: 349.
Heterotypic synonym: Fucus mucronatus Turner (Greville, Citation1830).
Syntype localities: On the coast of Cornwall; at Penzance and Acton Castle (Withering, 1796).
Representative DNA sequence: GenBank accession no. FM958369 (mt23S partial gene). Loc. Santec, Brittany (Atl. France); Dat. June 1995; Legit. Florence Rousseau.
*Treptacantha sauvageauana (Hamel) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira sauvageauana Hamel, Citation1939: 399 (as ‘sauveageauiana’). Phéophycées de France. Fasc. V. Published by author, Paris. pp. 337–431.
Heterotypic synonyms: Cystoseira selaginoides var. polyoedematis Sauvageau, Citation1912: 288–292, 517; Cystoseira sauvageauana var. polyoedematis (Sauvageau) Hamel, Citation1939: 400; Cystoseira sicula Schiffner ex Gerloff & Nizamuddin, Citation1976: 169.
Type locality: Mediterranean France, Algeria (Silva, Citation1996).
Representative DNA sequence: GenBank accession no. FM993038 (mt23S partial gene, IGS, tRNA-Lys gene, IGS and tRNA-Val partial gene). Loc. Salina I., Aeolian Is., Sicily (Italy); Dat. 26 May 2006; Legit. Marcello Catra.
*Treptacantha squarrosa (De Notaris) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira squarrosa De Notaris, 1841: 200. La descrizione di quattro nuove specie di alghe indigene del mare Ligustico. In Atti della seconda riunione degli scienziati italiani tenuta in Torino nell’settembre del 1840 (Anon., editor), Tipografia Cassone e Marzopati, Turin. pp. 199–202.
Heterotypic synonyms: Cystoseira spinosa var. squarrosa (De Notaris) Giaccone, Citation1978; Cystoseira erica-marina var. squarrosa (De Notaris) De Toni & Levi, Citation1887.
Type locality: Nice, France (De Notaris, 1841).
Representative DNA sequence: GenBank accession no. HQ438491 (mt23S partial gene). Loc. Dubrovnik city area (Croatia). Dat. 2010. Legit. J. Puizina, S. Rozic, I. Samanic & A Zuljevic.
*Treptacantha susanensis (Hamel) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira susanensis Nizamuddin, 1985: 119. A new species of Cystoseira C. Ag. (Phaeophyta) from the Eastern part of Libya. Nova Hedwigia 42: 119–122.
Type locality: West side off the lighthouse, Susa, Libya. Holotype Mus. Bot. Berol. 32143 (Nizamuddin, 1985).
Representative DNA sequence: GenBank accession no. FM958379 (mt23S partial gene). Loc. Marzameni, Sicily (Italy); Dat. 26 May 2006; Legit. Marcello Catra.
Treptacantha usneoides (Linnaeus) S. Orellana
& M. Sansón, comb. nov
Basionym: Fucus usneoides Linnaeus, 1759: 1345. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Editio decima, reformata. Editio decima revisa. Vol. 2. Holmiae, Impensis Direct, Laurentii Salvii, Stockholm. pp. 825–1384.
Homotypic synonym: Cystoseira usneoides (Linnaeus) M. Roberts, Citation1968: 259.
Heterotypic synonyms: Fucus granulatus Linnaeus, Citation1763: 1629; Fucus aculeatus Esper, Citation1798: 33, nom. illeg.; Cystoseira granulata C. Agardh, Citation1821: 55.
Type locality: ‘Oceano Indico‘ (Indian Ocean) (Silva et al., Citation1996). Type: LINN sheet 1274:11 (Roberts, Citation1968).
Representative DNA sequence: GenBank accession no. FM958367 (mt23S partial gene). Loc. A Coruña, Galicia (Spain); Dat. 14 June 2005; Legit. Ignacio Bárbara.
Carpodesmia Greville, gen. emend
Diagnosis: Thallus attached by prostrate axes with haptera or a small basal disc, caespitose or non-caespitose. Axes cylindrical, simple or branched. Primary branches erect, cylindrical, radially ramified. All orders of branches with spiny appendages. Tophules present or absent. In cross section, cortical cells with thin walls and ellipsoidal to rounded rectangular meristoderm cells twice as long as wide. Receptacles with grouped conceptacles and spiny appendages.
Type species: Carpodesmia zosteroides (C. Agardh) Greville.
observations: The combination Fucus zosteroides Turner is illegitimate and Cystoseira zosteroides C. Agardh (Citation1821) is a nomen novum to be credited only to C. Agardh. Greville (Citation1830) described the new monotypic genus Carpodesmia with the only species Carpodesmia zosteroides (C. Agardh) Greville (Furnari pers. com.). Thus, Carpodesmia Greville is an available name for the clade Cystoseira 3 and has been resurrected herein.
Carpodesmia zosteroides (C. Agardh) Greville,
Citation1830: 34
Basionym: Cystoseira zosteroides C. Agardh, Citation1821: 71. Species algarum rite cognitae, cum synonymis, differentiis specificis et descriptionibus succinctis. Volumen primum. Pars prima. ex officina Berlingiana, Lundae [Lund]. 168 pp.
Heterotypic synonyms: Cystoseira opuntioides Bory ex Montagne, Citation1846: 14; Phyllacantha opuntioides (Bory ex Montagne) Kützing, Citation1849: 598; Carpodesmia opuntioides (Bory ex Montagne) Kützing, Citation1860: 13.
Type locality: “Locus natalis ignotus” (unknown) (C. Agardh, Citation1821).
Representative DNA sequences: GenBank accession no. FM958290 (psbA partial gene), FM958366 (mt23S partial gene). Loc. Santa Maria la Scala, Sicily (Italy); Dat. 14 June 2005; Legit. Stefano G.A. Draisma.
Carpodesmia amentacea (C. Agardh) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira ericoides var. amentacea C. Agardh, Citation1821: 53. Species algarum rite cognitae, cum synonymis, differentiis specificis et descriptionibus succinctis. Volumen primum. Pars prima. Ex officina Berlingiana, Lund. 168 pp.
Homotypic synonyms: Cystoseira amentacea (C. Agardh) Bory, Citation1832: 319; Halerica amentacea (C. Agardh) Kützing, Citation1843: 354.
Heterotypic synonyms: Cystoseira stricta (Montagne) Sauvageau, Citation1911: 468; Cystoseira spicata subsp. elegans Ercegovic, Citation1952: 111.
Type locality: ‘In mari Mediterraneo‘ (C. Agardh, Citation1821).
Representative DNA sequence: GenBank accession no. FM958359 (mt23S partial gene). Loc. Capo Passero, Sicily (Italy); Dat. 13 June 2005; Legit. Stefano G.A. Draisma.
*Carpodesmia barbatula (Kützing) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira barbatula Kützing, Citation1860: 17. Tabulae phycologicae; oder, Abbildungen der Tange. Vol. X. Gedruckt auf kosten des Verfassers, Nordhausen. 39 pp.
Heterotypic synonym: Cystoseira graeca Schiffner ex Gerloff & Nizamuddin, Citation1975: 565.
Type locality: Golfo di Napoli (Silva, Citation1996). Holotype: Herb. Sonder; MEL folio 694721 (Cormaci et al., Citation1992).
Representative DNA sequence: GenBank accession no. FM958365 (mt23S partial gene). Loc. Marzameni, Sicily (Italy); Dat. 13 June 2005; Legit. G. Furnari et al.
Carpodesmia brachycarpa (J. Agardh) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira brachycarpa J. Agardh, Citation1896: 38. Analecta algologica, Continuatio III. Lunds Universitets Års-Skrift, Andra Afdelningen, Kongl. Fysiografiska Sällskapets i Lund Handlingar, 32: 1–140.
Heterotypic synonyms: Cystoseira balearica Sauvageau, Citation1912: 390; Cystoseira brachycarpa var. balearica (Sauvageau) Giaccone in Ribera et al., Citation1992: 124.
Type locality: Salerno (J. Agardh, Citation1896).
Representative DNA sequence: GenBank accession no. FM958361 (mt23S partial gene). Loc. Cala Viola de Llevant, Menorca (Spain); Dat. 25 June 2006; Legit. Stefano G.A. Draisma.
Carpodesmia crinita (Bory) S. Orellana & M. Sans
ón, comb. nov
Basionym: Cystoseira crinita Duby, Citation1830: 936. Aug. Pyrami de Candolle Botanicon gallicum sen synopsis plantarum in flora gallica descriptarum. Editio secunda. Ex herbariis et schedis Candollianis propriisque digestum a J. É. Duby V.D.M. Pars secunda plantas cellulares continens. Ve Desray, Paris. 1068 pp.
Heterotypic synonyms: Fucus crinitus Desfontaines, Citation1799: 425, nom. illeg.; Cystoseira granulata Schousboe in Bornet, Citation1892: 256, nom. inval.
Type locality: Corsica and French Riviera (Nice) (Berov et al., Citation2015).
Representative DNA sequences: GenBank accession no. FM958287 (psbA partial gene), FM958360 (mt23S partial gene). Loc. Marzameni, Sicily (Italy); Dat. 13 June 2005; Legit. Stefano G.A. Draisma.
* Carpodesmia funkii (Schiffner ex Gerloff &
Nizamuddin) S. Orellana & M. Sansón, comb. nov
Basionym: Cystoseira funkii Schiffner ex Gerloff & Nizamuddin, Citation1976: 167. New species of the genus Cystoseira C. Ag. Nova Hedwigia, 27: 167–182.
Type locality: Scoglio Vervece, Naples, Italy. Holotype: Funk. Herbarium Museum Natural History Vinndabon (Gerloff & Nizamuddin, Citation1976).
Representative DNA sequence: GenBank accession no. FM958357 (mt23S partial gene). Loc. Salina Is., Aeolian Is., Sicily (Italy); Dat. 04 June 2005; Legit. Stefano G.A. Draisma.
Carpodesmia mediterranea (Sauvageau) S. Orellana
& M. Sansón, comb. nov
Basionym: Cystoseira mediterranea Sauvageau, Citation1912: 209. A propos des Cystoseira de Banyuls et Guéthary. Bulletin de la Station Biologique d’Arcachon, 14: 133–556.
Type locality: Banyuls-sur-Mer (Sauvageau, Citation1912). Lectotype: C. Sauvageau. PC SA4480 (Gómez-Garreta & Ribera, Citation2002).
Representative DNA sequence: GenBank accession no. FM958371 (mt23S partial gene). Loc. Le Troc, Banyuls-sur-Mer (France); Dat. 24 May 2005; Legit. Olivier de Clerck.
Carpodesmia tamariscifolia (Hudson) S. Orellana
& M. Sansón, comb. nov
Basionym: Fucus tamariscifolius Hudson, 1762: 469. Flora anglica; exhibens plantas per regnum angliae sponte crescentes, distributas secundum systema sexuale: cum differentiis specierum, synonymis auctorum, nominibus incolarum, solo locorum, tempore florendi, oficinalibus pharmacopoeorum. Strand et C. Moran, London. 506 pp.
Homotypic synonym: Cystoseira tamariscifolia (Hudson) Papenfuss, Citation1950: 185.
Type locality: Cornwall, England (Hudson, 1762).
Representative DNA sequences: GenBank accession no. FM958286 (psbA partial gene), FM958370 (partial mt23S gene). Loc. A Coruña, Galicia (Spain); Dat. 19 August 2005; Legit. Ignacio Bárbara.
Discussion
Our phylogenetic analyses, combining sequence data for two genetic markers, psbA and mt23s, confirm that Cystoseira sensu lato resolved in three clades, interpreted here as three genera. Accordingly, we transferred 20 species to the resurrected genera Carpodesmia and Treptacantha. Some species for which molecular data are available were not included in our phylogenetic analyses due to the lack of sequences for some markers used in this study. In addition to the species included in our phylogeny, we transferred other species to the resurrected genera, considering previous results by Draisma et al. (Citation2010).
The clade Cystoseira 1 corresponds to Cystoseira nom. cons. with the type species C. concatenata (Linnaeus) C. Agardh, currently regarded as a synonym of C. foeniculacea (Guiry & Guiry, Citation2018). This clade was recognized before by Draisma et al. (Citation2010) whose species C. foeniculacea and C. compressa display a wide distribution in the Mediterranean Sea and the eastern Atlantic Islands (Azores, Madeira, Salvage Islands, Canary Islands and Cape Verde Islands). Our results add to this clade C. humilis and the herein resurrected C. aurantia (as Cystoseira sp. in , ). C. aurantia was described for the first time by Kützing (Citation1843) based on morphological characters and later considered as a variety of C. barbata (as C. barbata var. aurantia (Kützing) Giaccone) or a form of C. concatenata (as C. concatenata f. repens A.D. Zinova & Kalugina) (Amico et al., Citation1985; Gómez-Garreta et al., Citation2001; Cormaci et al., Citation2012). C. barbata (now Treptacantha barbata (Stackhouse) S. Orellana & M. Sansón, comb. nov.) is phylogenetically distant from C. aurantia so our morphological and molecular data support the restoration of its original specific status in Cystoseira sensu stricto.
The clade Cystoseira 2, proposed herein as the resurrected Treptacantha, includes C. abies-marina and C. mauritanica from the Canary Islands. Two sequences of Canarian specimens of C. abies-marina were included before in the clade Cystoseira-6 in Draisma et al. (Citation2010) together with 11 other species: C. baccata, C. barbata, C. elegans, C. montagnei (as C. jabukae and C. spinosa), C. nodicaulis, C. sauvageauana, C. sonderi, C. squarrosa, C. susanensis and C. usneoides. These species are distributed in the Mediterranean Sea and/or the north-eastern Atlantic Ocean, and some of them (C. barbata, C. spinosa and C. usneoides) have been previously documented in the Canary Islands (Price et al., Citation1978; John et al., Citation2004; Guiry & Guiry, Citation2018). However, no specimen was found in TFC or collected in the field during our study and consequently, we consider the presence of these three species in the Canary Islands uncertain. Our phylogenetic results incorporate C. mauritanica in this group, which is closely related to C. elegans. Only four species of this clade were included in our trees due to the lack of available sequences for the markers we used in other species. The transfer to Treptacantha of C. barbata, C. sauvageauana, C. squarrosa and C. susanensis is based on information from previous published work (see ) and further studies are necessary to confirm some of the diagnostic characters of Treptacantha proposed here. C. sonderi, endemic to Cape Verde, was excluded from this study because its potential synonymy with C. abies-marina is unclear (Piccone, Citation1886, Citation1890; Sauvageau, Citation1912, Citation1920, Draisma et al., Citation2010).
Our third clade Cystoseira 3, proposed as the resurrected genus Carpodesmia, is represented in the Canary Islands by Cystoseira tamariscifolia, which is also known from the Atlantic coast of Spain (Galicia) and the Mediterranean Sea. Nevertheless, a certain genetic differentiation in the mt23S and nad1 genes was detected between samples. C. tamariscifolia in the Canary Islands exhibits a high morphological variability related to the habitat (intertidal vs. subtidal) with two morphotypes: the intertidal (C. tamariscifolia 1–4 in , ) has a basal disc and rigid erect primary branches, while the subtidal (as C. tamariscifolia 5 and 6 in , ) has haptera and long flaccid primary branches. The subtidal morphotype was described by Nizamuddin (Citation1995) as Cystoseira wildpretii Nizamuddin, based on specimens from Montaña Clara (TFC Phyc 5610, 5676) and La Graciosa (TFC Phyc 4581) in the Canary Islands. Our molecular and morphological studies included specimens of both morphotypes. The subtidal morphotype was collected in Alegranza, another small Canarian islet (identified as C. tamariscifolia 5 and 6; , ). Although we detected some sequence divergence between morphotypes (), it does not support the recognition of two species and we treated C. wildpretii as a synonym of C. tamariscifolia. According to Draisma et al. (Citation2010), C. tamariscifolia forms a clade with other seven species: C. amentacea, C. barbatula, C. brachycarpa, C. crinita, C. funkii, C. mediterranea and C. zosteroides. All these species are distributed in the Mediterranean Sea, except C. amentacea and C. zosteroides which are also documented as probably present in the South Atlantic Iberian Peninsula (Gallardo et al., Citation2016). Some of the species (C. barbatula, C. funkii and C. mediterranea) were not included in our trees due to the absence of available sequences for all the molecular markers we used in our analyses. Besides, specimens of C. barbatula and C. funkii were not available for morphological studies, so data included herein are based on information from previous references (see ). More studies on these species are still needed to confirm some of the diagnostic characters of Carpodesmia.
Table 3. Inferred pairwise-distances (%) for the four genetic markers studied among nearby species of Cystoseira sensu lato from the Canary Islands. Groups: Cystoseira 1 (= Cystoseira sensu stricto), Cystoseira 2 (here proposed as Treptacantha) and Cystoseira 3 (here proposed as Carpodesmia)
The four genetic markers chosen in this study were useful at different taxonomic levels. Cox1 and nad1 are helpful at the species level, mt23S partial gene is suitable for delineating species and genera and the more conserved psbA is adequate for analysing higher taxonomic levels. When analysing the p-distances, cox1 gene divergence is 0.9–1.1% among C. humilis, C. compressa and C. aurantia (as Cystoseira sp.), values higher than those obtained by Kucera & Saunders (Citation2008) for this marker between Fucus serratus and F. distichus (0.7%). For this same marker the highest values were those between C. abies-marina and C. mauritanica (4.7%). For nad1, nucleotide differentiation ranges from 0.7% (C. compressa – C. humilis) to 3.2% (Cystoseira sp. – C. foeniculacea). Finally, of the four genes analysed, psbA exhibits the least differentiation and the lowest discriminatory power (see ).
The morphological survey of all specimens included in our DNA analyses supports the recognition of three genera. We propose that they can be distinguished by combinations of the following characters: the morphology of axes/primary branches, the receptacle appearance and the characteristics of cortical and meristoderm cell (size and morphology). The two former characters were selected among those used in previous studies for species delineation (Sauvageau, Citation1912, Citation1920; Ercegovic, Citation1952; Roberts, Citation1967). Our results confirm that the presence/absence of appendages (spine-like or filiform branches of limited growth) on branches of different order in the thalli is useful to separate genera. Cystoseira sensu stricto lacks these appendages (except C. foeniculacea that shows spines on primary branches), Treptacantha presents smooth or occasionally widely spaced spinose primary branches, and Carpodesmia shows all branches with spine-like appendages. According to Roberts (Citation1967), the morphology of receptacles is difficult to apply as a specific diagnostic character. Nevertheless, we observed that it is constant at generic level in Cystoseira sensu stricto (warty and non-spinose) and Carpodesmia (always spinose), but not in Treptacantha (with or without spines). The number of conceptacles and their distribution along the receptacles are constant in each genus (grouped conceptacles in Cystoseira sensu stricto and Carpodesmia; 1–3 conceptacles at the base of each fertile spine in Treptacantha). Other characters used for species delineation, such as the axis morphology or presence/absence of tophules (swollen basal regions of branches) (Roberts, Citation1967), are not applicable for generic circumscription. By contrast, we propose that the thickness of cortical cell walls and the dimensions of meristoderm cell in cross section are useful for separation of genera (Cystoseira sensu stricto: thick-walled cortical cells and rectangular meristoderm cells; Treptacantha: thick-walled cortical cells and square meristoderm cells; Carpodesmia: thin-walled cortical cells and rectangular meristoderm cells). This character has been previously poorly used for species identification (Gómez-Garreta et al., Citation2001; Rodríguez-Prieto et al., Citation2013), and a compact parenchymatous tissue-like structure is described for all species in Cystoseira sensu lato (Roberts, Citation1967; Rodríguez-Prieto et al., Citation2013). Other morphological characteristics, such as apical structure, occurrence and distribution of cryptostomata, require more studies to test their validity for generic delineation. Morphological differentiation among species and genera in Cystoseira sensu lato is subtle. High similarity among species can be explained by the active recent speciation (Roberts, Citation1968; Amico et al., Citation1985: Draisma et al. Citation2010). Alternative explanations for these similarities among species and genera could be due to the existence of small or null morphological change (morphological stasis), adaptative convergence or genetic constraints.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://10.1080/09670262.2019.1590862
Supplementary table S1. Specimens of Cystoseira sensu lato and other fucalean species used in the present study. GenBank accession numbers for the new sequences (this study) are shown in bold.
Supplementary table S2. Sequence primers, PCR conditions and components used in this study.
Author contributions
S. Orellana: original concept, morphology, taxonomy, generation and analysis of molecular data, drafting and editing manuscript; M. Hernández-Ferrer: generation and analysis of molecular data, drafting manuscript; M. Sansón: original concept, morphology, taxonomy, analysis of molecular data, drafting and editing manuscript.
Acknowledgements
The authors would like to thank C. Rodríguez-Prieto (Universitat di Girona and HGI-A herbarium) and E. Ballesteros (CSIC Blanes) for the loan of material of Mediterranean species of Cystoseira. Thanks to I. Bárbara (Universidad de A Coruña) for the photograph of receptacles of Cystoseira nodicaulis. Thanks to G. Furnari for comments on nomenclatural issues. We thank two anonymous reviewers for all comments and suggestions and Adrián Báez Ortega for English improvement of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agardh, C.A. (1821). Species algarum rite cognitae, cum synonymis, differentiis specificis et descriptionibus succinctis. Volumen primum. Pars prima. Ex officina Berlingiana, Lund.
- Agardh, J.G. (1842). Algae maris Mediterranei et Adriatici, observationes in diagnosin specierum et dispositionem generum. Apud Fortin, Masson et Cie, Paris.
- Agardh, J.G. (1896). Analecta algologica, Continuatio III. Lunds Universitets Års-Skrift, Andra Afdelningen, Kongl. Fysiografiska Sällskapets i Lund Handlingar, 32: 1–140.
- Amico, V., Giaccone, G., Colonna, P., Mannino, A.M. & Randazzo, R. (1985). Un nuovo approccio allo studio della sistematica del genere Cystoseira C. Agardh (Phaeophyta, Fucales). Bollettino delle sedute della Accademia Gioenia di Scienze Naturali in Catania, 18: 887–985.
- Berov, D., Ballesteros, E., Sales, M. & Verlaque, M. (2015). Reinstatement of species rank for Cystoseira bosphorica Sauvageau (Sargassaceae, Phaeophyceae). Cryptogamie, Algologie, 36: 65–80.
- Blanfune, A., Boudouresque, C.F., Verlaque, M. & Thibaut, T. (2015). Cystoseira crinita, a long-lived habitat-forming species: the fate of the French Mediterranean Sea populations. European Journal of Phycology, 50: 213–214.
- Borchsenius, F. (2009). FastGap 1.2. Available: www.aubot.dk/FastGap_home.htm.
- Bornet, E. (1892). Les algues de P.-K.-A. Schousboe. Mémoires de la Société des Sciences Naturelles et Mathématiques de Cherbourg, 28: 165–376.
- Bory de Saint-Vincent, J.B.G.M. (1832). Hydrophytes. In Expédition scientifique de Morée. Section des sciences physiques. Tome III. 2e partie. Botanique (Bory de Saint-Vincent, J.B.G.M., editor), 316–337. Chez F. G. Levrault, imprimeur-libraire, Paris & Strasbourg.
- Capdevila, P., Hereu, B., Riera, J.L. & Linares, C. (2016). Unravelling the natural dynamics and resilience patterns of underwater Mediterranean forests: insights from the demography of the brown alga Cystoseira zosteroides. Journal of Ecology, 104: 1799–1808.
- Chapman, A.R.O. (1995). Functional ecology of fucoid algae: twenty-three years of progress (Phycologia Reviews 14). Phycologia, 34: 1–32.
- Cormaci, M., Furnari, G., Giaccone, G., Scammacca, B. & Serio, D. (1992). Observations taxonomiques et biogéographiques sur quelques espèces du genre Cystoseira C. Agardh. Bulletin de l’Institut Océanographique de Monaco, 9: 21–35.
- Cormaci, M., Furnari, G., Catra, M., Alongi, G. & Giaccone, G. (2012). Flora marina bentonica del Mediterraneo: Phaeophyceae. Bollettino Accademia Gioenia Scienze Naturali, 45: 1–508.
- Dangeard, P.J.L. (1949). Les algues marines de la côte occidentale du Maroc. Le Botaniste, 34: 89–189.
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9: 772.
- Desfontaines, R. (1799). Flora atlantica, sive historia plantarum, quae in Atlante, agro Tunetano et Algeriensi crescunt. Tomus secundus. L.G. Desgranges, Paris.
- De Toni, G.B. (1891). Systematische Übersicht der bisher bekannten Gattungen der echten Fucoideen. Flora, 74: 171–182.
- De Toni, G.B. & Levi, D. (1887). Algae novae. Notarisia, 2: 333–353.
- Dijkstra, J.A., Westerman, E.L. & Harris, L.G. (2011). The effects of climate change on species composition, succession and phenology: a case study. Global Change Biology, 17: 2360–2369.
- Draisma, S.G.A., Ballesteros, E., Rousseau, F. & Thibaut, T. (2010). DNA sequence data demonstrate the polyphyly of the genus Cystoseira and other Sargassaceae genera (Phaeophyceae). Journal of Phycology, 46: 1329–1345.
- Duby, J.É. (1830). Aug. Pyrami de Candolle Botanicon gallicum sen synopsis plantarum in flora gallica descriptarum. Editio secunda. Ex herbariis et schedis Candollianis propriisque digestum a J.É. Duby V.D.M. Pars secunda plantas cellulares continens. Ve Desray, Rue Hautefueille, Paris.
- Ercegovic, A. (1952). Fauna i Flora Jadrana. Jadranske cistozire. Njihova morfologija, ekologija i razvitak/Fauna et Flora Adriatica. Sur les cystoseira adriatiques. Leur morphologie, écologie et évolution. Vol. 2. Map. Institut za Oceanografiju i Ribarstvo Split/Institut d’Océanograpie et de Peche, Split.
- Esper, E.J.C. (1798). Icones fucorum cum characteribus systematicis, synonimis auctorum et descriptionibus novarum specierum. Abbildungen der Tange mit beygefügten systematischen Kennzeichen, Anführungen der Schriftsteller, und Beschribungen der neuen Gattungen. Vol. Erster Theil, Part 2. Raspe, Nürnberg.
- Furnari, G., Cormaci, M. & Alongi, C. (1999). Lectotypification of Cystoseira algeriensis J. Feldmann and Cystoseira elegans Sauvageau (Cystoseiraceae, Phaeophyta). Cryptogamie, Algologie, 20: 19–23.
- Gallardo, T., Bárbara, I., Afonso-Carrillo, J., Bermejo, R., Altamirano, M., Gómez Garreta, A., Barceló Martí, C., Rull Lluch, J., Ballesteros, E. & De la Rosa, J. (2016). Nueva lista crítica de las algas bentónicas marinas de España. Algas, Boletín de la Sociedad Española de Ficología, 51: 7–52.
- Gerloff, J. & Nizamuddin, M. (1975). Three new species of the genus Cystoseira C. Ag. Willdenowia, 7: 565–582.
- Gerloff, J. & Nizamuddin, M. (1976). New species of the genus Cystoseira C. Ag. Nova Hedwigia, 27: 167–182.
- Giaccone, G. (1978). Revisione della flora marina de Mare Adriatico. Annuario Parco Marino Miramare, 6: 1–118.
- Gómez-Garreta, A. & Ribera, M.A. (2002). Lectotypification of several species of Cystoseira (Cystoseiraceae, Fucales) described by Sauvageau. Cryptogamie, Algologie, 23: 291–300.
- Gómez-Garreta, A., Barceló-Martí, M.C., Gallardo-García, T., Pérez-Ruzafa, I.M., Ribera-Siguán, M.A. & Rull Lluch, J. (2001). Flora Phycologica Iberica. Vol.1. Fucales. Universidad de Murcia, Murcia.
- Goodenough, S. & Woodward, T.J. (1797). Observations on the British Fuci, with particular descriptions of each species. Transactions of the Linnean Society of London, 3: 84–235.
- Greville, R.K. (1830). Algae britannicae, or descriptions of the marine and other inarticulated plants of the British islands, belonging to the order Algae; with plates illustrative of the genera. McLachlan & Stewart, Baldwin & Cradock, Edinburgh & London.
- Guiry, M.D. & Guiry, G.M. (2018). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org.
- Hamel, G. (1939). Phéophycées de France. Fasc. V. Published by author, Paris.
- Hereu, B., Mangialajo, L., Ballesteros, E. & Thibaut, T. (2008). On the occurrence, structure and distribution of deep-water Cystoseira (Phaeophyceae) populations in the Port-Cros National Park (Northwestern Mediterranean). European Journal of Phycology, 43: 263–273.
- Hernández, C.A., Sangil, C., Clemente, S. & Hernández, J.C. (2015). High-resolution ocean pH dynamics in four subtropical Atlantic benthic habitats. Biogeosciences Discussions, 12: 19481–19498.
- Hudson, G. (1778). Flora anglica; exhibens plantas per regnum Britanniæ sponte crescentes, distributas secundum systema sexuale: cum differentiis specierum, synonymis auctorum, nominibus incolarum, solo locorum, tempore florendi, officinalibus pharmacopæorum. Tomus II. Editio altera, emendata et aucta. Impressed for J. Nourse by author, Strand, London.
- Huelsenbeck, J.P. & Ronquist, F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics, 17: 754–755.
- John, D.M., Prud’homme van Reine, W.F., Lawson, G.W., Kostermans, T.B. & Price, J.H. (2004). A taxonomic and geographical catalogue of the seaweeds of the western coast of Africa and adjacent islands. Beihefte zur Nova Hedwigia, 127: 1–339.
- Jueterbock, A., Tyberghein, L., Verbruggen, H., Coyer, J.A., Olsen, J.L. & Hoarau, G. (2013). Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecology and Evolution, 3: 1356–1373.
- Kucera, H. & Saunders, G.W. (2008). Assigning morphological variants of Fucus (Fucales, Phaeophyceae) in Canadian waters to recognized species using DNA barcoding. Botany, 86: 1065–1079.
- Kumar, S., Stecher, G. & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution, 33: 1870–1874.
- Kützing, F.T. (1843). Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange. Mit 80 farbig gedruckten Tafeln, gezeichnet und gravirt vom Verfasser. F.A. Brockhaus, Leipzig.
- Kützing, F.T. (1849). Species algarum. F.A. Brockhaus, Leipzig.
- Kützing, F.T. (1860). Tabulae phycologicae; oder, Abbildungen der Tange. Vol. X. Gedruckt auf kosten des Verfassers, Nordhausen.
- Linnaeus, C. (1763). Species plantarum. Vol. 2. Stockholm.
- Martínez, B., Afonso-Carrillo, J., Anadón, R., Araujo, R., Arenas, F., Arrontes, J., Bárbara, I., Borja, A., Díez, I., Duarte, L., Fernández, C., García Tasende, M., Gorostiaga, J.M., Juanes, J.A., Peteiro, C., Puente, A., Rico, J.M., Sangil, C., Sansón, M., Tuya, F. & Viejo, R.M. (2015). Regresión de las algas marinas en la costa atlántica de la Península Ibérica y en las Islas Canarias por efecto del cambio climático. Algas, Boletín de la Sociedad Española de Ficología, 49: 5–12.
- Mineur, F., Arenas, F., Assis, J., Davies, A.J., Engelen, A.H., Fernandes, F., Malta, E., Thibaut, T., Van Nguyen, T., Vaz-Pinto, F., Vranken, S., Serrão, E. & De Clerck, O. (2015). European seaweeds under pressure: consequences for communities and ecosystem functioning. Journal of Sea Research, 98: 91–108.
- Montagne, C. (1846). Flore d’Algérie. Ordo I. Phyceae Fries. In Exploration scientifique de l’Algérie pendant les années 1840, 1841, 1842…Sciences physiques. Botanique. Cryptogamie (Durieu De Maisonneuve, M.C., editor), 1–197. Imprimerie Royale, Paris.
- Naccari, F.L. (1828). Flora veneta o descrizione delle piante che nascono nella provincia de Venezia disposta secondo il sistema Linneano e colla indicazione al metodo di Jueeieu modification dal De-Candolle arriccmita di osservationi medico-economicae di Fortunato Luigi Naccari. Vol VI. L. Bonvecchiato Ed., Merceria A.S. Bartolomeo, Venice.
- Nizamuddin, M. (1995). Cystoseira wildpretii Nizamuddin sp. nov. from Canary Islands. Pakistan Journal of Botany, 27: 263–266.
- Ott, M., Zola, J., Stamatakis, A. & Aluru, S. (2007). Large-scale maximum likelihood-based phylogenetic analysis on the IBM BlueGene/L. Proceedings of the 2007 ACM/IEEE Conference on Supercomputing: 1–11.
- Papenfuss, G.F. (1950). Review of the genera of algae described by Stackhouse. Hydrobiologia, 2: 181–208.
- Piccone, A. (1886). Alghe del Viaggio di Circumnavigazione della Vettor Pisani. Genova.
- Piccone, A. (1890). Nuove alghe del viaggio di circumnavigazione della ‘Vettor Pisani’. Atti della Reale Accademia nazionale dei Lincei Memorie ser. 4: 10–63.
- Price, J.H., John, D.M. & Lawson, G.W. (1978). Seaweeds of the western coast of tropical Africa and adjacent islands: a critical assessment. II. Phaeophyta. Bulletin of the British Museum (Natural History) Botany 6: 87–182.
- Rambaut, A. (2016). Figtree v 1.4.3. http://tree.bio.ed.ac.uk/software/figtree/.
- Ribera, M.A., Gómez-Garreta, A., Gallardo, T., Cormaci, M., Furnari, G. & Giaccone, G. (1992). Check-list of Mediterranean Seaweeds. I. Fucophyceae (Warming 1884). Botanica Marina, 35: 109–130.
- Riera, R., Sangil, C. & Sansón, M. (2015). Long-term herbarium data reveal the decline of a temperate-water alga at its southern range. Estuarine, Coastal and Shelf Science, 165: 159–165.
- Roberts, M. (1967). Studies on marine algae of the British Isles. 3. The genus Cystoseira. British Phycological Bulletin, 3: 345–366.
- Roberts, M. (1968). Taxonomic and nomenclatural notes on the genus Cystoseira C. Ag. Journal of the Linnean Society of London, Botany, 60: 251–264.
- Rodríguez-Prieto, C., Ballesteros, E., Boisset, F. & Afonso-Carrillo, J. (2013). Guía de las macroalgas y fanerógamas marinas del Mediterráneo occidental. Ediciones Omega S.A., Barcelona.
- Rozic, S., Puizina, J., Samanic, I., Zuljevic, A. & Antolic, B. (2012). Molecular identification of the brown algae, Cystoseira spp. (Phaeophycae, Fucales) from the Adriatic Sea – preliminary results. Acta Adriatica, 53: 447–456.
- Sales, M. & Ballesteros, E. (2010). Long-term comparison of algal assemblages dominated by Cystoseira crinita (Fucales, Heterokontophyta) from Cap Corse (Corsica, North Western Mediterranean). European Journal of Phycology, 45: 404–412.
- Sangil, C., Sansón, M. & Afonso-Carrillo, J. (2011). Spatial variation patterns of subtidal seaweed assemblages along a subtropical oceanic archipelago: thermal gradient vs herbivore pressure. Estuarine, Coastal and Shelf Science, 94: 322–333.
- Sansón, M., Sangil, C., Orellana, S. & Afonso-Carrillo, J. (2014). Do the size shifts of marine macroalgae match the warming trends in the Canary Islands? Algas, Boletín de la Sociedad Española de Ficología, 48: 12–13.
- Sansón, M., Martín, L., Rancel, N., Sangil, C., Reyes, J., Brito, A., Afonso, J. & Barquín, J. (2017). Análisis de distribución histórica y distribución actual de las especies Cystoseira abies-marina, Cystoseira tamariscifolia y Cystoseira mauritanica en la provincia occidental canaria para la toma de decisiones en la elaboración de sus planes de recuperación. Universidad de La Laguna-Gobierno de Canarias.
- Sauvageau, C. (1911). Sur Cystoseira. Compte Rendu Hebdomadaire des Séances de l’Académie des Sciences, Paris, 17: 467–686.
- Sauvageau, C. (1912). A propos des Cystoseira de Banyuls et Guéthary. Bulletin de la Station Biologique d’Arcachon, 14: 133–556.
- Sauvageau, C. (1920). A propos des Cystoseira de Banyuls et Guéthary. Bulletin de la Station Biologique d’Arcachon, 17: 1–51.
- Schwarz, G. (1978). Estimating the dimension of a model. Annals of Statistics, 6: 461–464.
- Sellam, L.N., Blanfuné, A., Boudouresque, C.F., Thibaut, T., Rebzani Zahaf, C. & Verlaque, M. (2017). Cystoseira montagnei J. Agardh and C. spinosa Sauvageau (Phaeophyceae, Sargassaceae): a taxonomic reappraisal of misused names, with the proposal of Cystoseira michaelae Verlaque et al. nom. et stat. nov. Cryptogamie, Algologie, 38: 133–157.
- Silva, P.C. (1952). A review of nomenclatural conservation in the algae from the point of view of the type method. University of California Publications in Botany, 25: 241–323.
- Silva, P.C. (1996). Index Nominum Algarum, University Herbarium, University of California, Berkeley. http://ucjeps.berkeley.edu/INA.html. [ continuously updated].
- Silva, P.C., Basson, P.W. & Moe, R.L. (1996). Catalogue of the benthic marine algae of the Indian Ocean. University of California Publications in Botany, 79: 1–1259.
- Simmons, M. P. & Ochoterena, H. (2000). Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology, 49: 369–381.
- Thiers, B. (2018). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/[ continuously updated].
- Thompson, J.D., Higgins, D.G. & Gibson, T.J. (1994). CLUSTAL W: improving sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties, and weight matrix choice. Nucleic Acids Research, 22: 4673–4680.
- Tsiamis, K., Salomidi, M., Kytinou, E., Issaris, Y. & Gerakaris, V. (2016). On two new records of rare Cystoseira taxa (Fucales, Phaeophyceae) from Greece (Eastern Mediterranean). Botanica Marina, 59: 73–77.
- Tsuda, R.T. & Abbott, I.A. (1985). Collecting, handling, preservation and logistics. In Handbook of Phycological Methods. Ecological Field Methods: Macroalgae (Littler, M.M. & Littler, D.S., editors), 67–86. Cambridge University Press, Cambridge.
- Valdazo, J., Viera-Rodríguez, M.A., Espino, F., Haroun, R. & Tuya, F. (2017). Massive decline of Cystoseira abies-marina forests in Gran Canaria Island (Canary Islands, eastern Atlantic). Scientia Marina, 81: 499–507.
- Wahl, M., Jormalainen, V., Eriksson, B.K., Coyer, J.A., Molis, M., Schubert, H., Dethier, M., Karez, R., Kruse, I., Lenz, M., Pearson, G., Rohde, S., Wikström, S.A. & Olsen, J.L. (2011). Stress ecology in Fucus: abiotic, biotic and genetic interactions. Advances in Marine Biology, 59: 37–105.

