Abstract
In the past, research, policy and media have reported the use of anabolic androgenic steroids (AAS) primarily among younger males. However, recent studies have indicated the presence of an older cohort of men who use AAS in comparison to previous years. We carried out a scoping review of the extant literature to map and describe what is known about the use of AAS by older men (>40 years). A systematic search collected and analysed empirical research and grey literature relevant to the research question. Following application of inclusion and exclusion criteria, 44 studies were included which were subsequently charted and thematically analysed. The records included originated from the UK, USA, Canada, Australia, Slovenia, Norway, Spain, Turkey, Switzerland, Japan, and five global studies and were published between 1996 and 2021. Age ranged overall from 14 to 78 years old, however our review only discussed findings pertaining to those older than 40. Three main themes with subthemes were generated as follows: 1) Characteristics of AAS Use; Self-reported Adverse Effects from AAS Use; and Harms Diagnosed by Medical Professional. The review highlights the significant risks to hypothalamic-pituitary testicular function, cardiovascular health, and other organ systems as a result of the ageing man who is motivated to sustain masculine characteristics such as muscularity, youthfulness, sexual function, and perceived desirability and attractiveness. Future research is required to further understand the motivations of older men who use AAS. Furthermore, there is a need for age-specific research and recommendations to inform future policy and practice pertaining so that age-appropriate healthcare and policy decisions can be made in the future.
Background
The use of substances to modify physical appearance or athletic performance is not a new phenomenon. Image and performance enhancing drugs (IPEDs) are commonly used by individuals who want to improve physical strength and muscularity as well as having a healthy and attractive body (Brennan et al., Citation2017). IPEDs are pharmacologic agents with a variety of different functions on a person’s body. They include a range of substances whose function is primarily for the promotion of muscle growth, in particular anabolic androgenic steroids (AAS) which have received the most empirical attention for their non-medical use (Korkia & Stimson, Citation1993; McVeigh & Begley, Citation2017; Pope et al., Citation2014; Sagoe et al., Citation2014).
AAS are synthetic derivatives of testosterone, the primary male hormone which is accountable for masculinising (androgenic) and tissue building (anabolic) effects in males (Kanayama & Pope, Citation2018; Yesalis & Bahrke, Citation1995). The current study focuses on non-prescribed AAS use only. AAS are not typically consumed for intoxication or immediate psychoactive gratification (Bates et al., Citation2019). They are primarily taken to increase muscle size, definition and strength (Evans-Brown et al., Citation2012; McVeigh et al., Citation2012; van de Ven et al., Citation2019; Zahnow et al., Citation2018), however, they can produce rewarding secondary effects such as euphoria, increased libido, and increased self-confidence (Kanayama et al., Citation2009). More recently, research has reported the use of AAS for what is considered self-medicated testosterone replacement therapy (TRT) (Underwood et al., Citation2021). Those men were self-medicating with AAS as they either had not met the threshold for low testosterone diagnosis, had met the criteria but refused treatment by a physician, did not trust physicians so would not attend with low testosterone symptoms, or felt that black market testosterone is less expensive and easily accessible.
In the past global lifetime AAS prevalence rates were estimated at 3.3% with use higher amongst males (6.4%) than females (1.6%) (Sagoe et al., Citation2014). In the UK data from needle and syringe programmes (NSPs) in the UK observed a threefold increase over a ten year period in the number of clients who use AAS accessing services which accounts for 54.9% of clients (McVeigh & Begley, Citation2017). Prevalence estimations in the UK have been largely based on a household population-based survey; the Crime Survey for England and Wales (CSEW), has indicated that 31,000 people between aged 16–59 years are reportedly using AAS (Office for National Statistics, Citation2020). However, new and emerging research has reported that this is an under-estimation and it is likely ten-times higher than this (Hope et al., Citation2022) which is similar to estimates in the US (Pope et al., Citation2014) and wider regions globally (Sagoe et al., Citation2014).
Age of initiation to AAS use is reportedly also increasing compared to previous years (Bates & McVeigh, Citation2016; Begley et al., Citation2017). A systematic review of the trajectory of AAS use in 2014 reported most men who use AAS initiating use before 30 years of age (Sagoe et al., Citation2014). However, NSP data in the UK has observed an increased median age of AAS using clients from age 25 years in 1995 compared to 30 years in 2015 (Begley et al., Citation2017; McVeigh & Begley, Citation2017).
AAS use has been investigated in different settings such as gyms (Begley et al., Citation2017; Monaghan, Citation2001; Salinas et al., Citation2019), NSPs/drug and healthcare services (Bates & McVeigh, Citation2016), online communities/fora (Bonnecaze et al., Citation2020; Chandler & McVeigh, Citation2014; Perry et al., Citation2005; Underwood, Citation2017; Underwood et al., Citation2021); Underwood & Olson, Citation2019and among different user groups. Identified groups are generally non-competitive weightlifters, bodybuilders, recreational gym users (Begley et al., Citation2017), those who use for occupational benefits such as police (Hoberman, Citation2017) and security personnel/doormen (Monaghan, Citation2003), and those who use for TRT purposes (Underwood et al., Citation2021).
Research documents a number of motivating and influencing factors for AAS use such as to increase muscle mass and strength (Christiansen et al., Citation2016; Zahnow et al., Citation2018), to improve self-esteem and athletic performance (Petersson et al., Citation2010), to decrease muscle dysmorphia symptoms and body dissatisfaction (Greenway & Price, Citation2018), and for occupational reasons (Monaghan, Citation2003). However, motivations for AAS use are often complex and multi-faceted, and dependent on a variety of factors related to the individual themselves, their wider social network, and community, institutional, and societal influences which often change over time (Bates et al., Citation2019). Socio-cultural pressures drive an image and body conscious society defined by consumerism (Ricciardelli & Williams, Citation2012), particularly media saturation of idealised bodies which over time becomes normalised in society (Boothroyd et al., Citation2016; Thornborrow et al., Citation2020). For example, a v-shaped muscular body with particularly enhanced upper body, a flat stomach and low body fat percentage (Leit et al., Citation2001) known as a ‘mesomorph’ body (Grogan, Citation1999). Having a muscular body displayed as well-developed muscle mass is significant for masculine identity as it denotes strength and sexuality (Griffiths et al., Citation2015). A man’s failure to reach these media ideals of masculinity and muscularity can cause anxiety or stress, regarding gender role for the man, who will in then try to reach this goal by any means possible (Walker & Joubert, Citation2011). Brennan et al. (Citation2013) found that whilst risky drug taking practices such as injecting untested AAS products and with minimal medical support in place, the goal of body, image enhancement and perceived perfection justifies the behaviours and risks taken by the users.
The physical and psychological harms associated with the use of AAS are well documented (Pope et al., Citation2014) particularly those related to major organs and systems (Hartgens & Kuipers, Citation2004). Furthermore, the harms resulting from injecting AAS sourced from illicit unregulated markets which are potentially adulterated are a concern (Brennan et al., Citation2018; Coomber et al., Citation2014; Hope et al., Citation2015; Kimergard et al., Citation2014; van de Ven, Citation2016) such as localised injection site infections (Hope et al., Citation2015) and the transmission of blood borne viruses (BBVs) (Hope et al., Citation2013, Citation2021). The risk of BBV transmission however is lower among people who inject AAS and other IPEDs than those who inject psychoactive drugs (Hope et al., Citation2013). This may be due to the difference in injecting practices such as being on an off-cycle period (ACMD, Citation2010), injecting less frequently during an on-cycle period (Hope et al., Citation2015), and lower rates of needle-sharing among this cohort (Day et al., Citation2008; Hanley Santos & Coomber, Citation2017; Rowe et al., Citation2017).
Of concern is the long-term morbidity and mortality resulting from damage to cardiovascular health (Baggish et al., Citation2017; Darke et al., Citation2014; Frati et al., Citation2015; McCullough et al., Citation2021), the liver (Creagh et al., Citation1988; Schumacher et al., Citation1999), and cognitive effects from long-term high-dose AAS use (Bjørnebekk et al., Citation2019; Kanayama et al., Citation2013). Long-term high doses of AAS can be the result in changing patterns of use over time, from cycling i.e. an on-cycle period followed by an off-cycle period; to continuous cycles that include a high-dose period and a lower dose period known as ‘blast and cruise’ (Chandler & McVeigh, Citation2014; McVeigh & Begley, Citation2017). This results in the individual continuously using AAS, which is a concern for their health and the possibility of recovery from AAS related health issues. People who use AAS are often aware of risks to health however they continue to use them as they believe the benefits outweigh the harms (Maycock & Howat, Citation2005). This can be for a number of reasons such as fear of loss of muscle mass and social status, or withdrawal-like symptoms such as low mood, low libido, and depression (Griffiths et al., Citation2017; Kanayama et al., Citation2015; Pope & Kanayama, Citation2022).
Prolonged use of AAS is a concern as it can result in anabolic steroid induced hypogonadism (ASIH), also known as secondary hypogonadism. This occurs as hypothalamic-pituitary testicular (HPT) function is suppressed (Kanayama et al., Citation2008, Citation2015) and natural testosterone function cannot recover, which may result in ASIH even long after cessation of AAS use (Kanayama et al., Citation2015; Tan & Scally, Citation2009). Symptoms of ASIH have been reported to include erectile dysfunction, infertility, decreased sex drive, and depression (Kanayama et al., Citation2015; Pope & Kanayama, Citation2022). This is a concern for older males who experience lowered testosterone levels and decreased HPT function as part of the natural ageing process (Bhasin et al., Citation2006) and may be an indicator as to why some initiate, continue, or restart the use of AAS, particularly when the body is ageing.
We do know that many people who use AAS in the general population who began using in their youth in the 1980s and have a lifetime history of AAS use, are now in their 50 s or older and may present to health services with AAS related health issues such as cardiomyopathy and atherosclerotic disease (Kanayama et al., Citation2008; Kanayama & Pope, Citation2018). In 2017, media reports emerged, of men using testosterone purchased on the illicit market for self-prescribed TRT to increase libido, mood and the desire to look younger (Kemp, Citation2017; Marsh, Citation2017; Moody, Citation2017). Whilst media reports are anecdotal, this may be supported by the increased age of initiation to AAS use and ageing cohort effect. Considering the reported rise in age of initiation to AAS use, and the harms of long-term AAS use, specifically the effect on HPT function, we conducted a scoping review of extant literature to describe and map what is known about the use of AAS among older men (>40).
Methodology
Scoping reviews
Scoping reviews are increasingly used to address broader research questions than systematic reviews can answer (Arksey & O'Malley, Citation2005; Khalil et al., Citation2016; Levac et al., Citation2010; Peters et al., Citation2015). Tricco et al. (Citation2018, p. 1) define a scoping review approach as ‘a type of knowledge synthesis, following a systematic approach to map evidence on a topic and identifying main concepts, theories, sources, and knowledge gaps’. This approach is generally adopted to identify knowledge gaps, examine the nature/characteristics, size, and the range/variety) of a specific subject (in the case of this research, men who use AAS), summarise the findings of a large diverse body of knowledge, and propose health agendas for future policy, interventions and research (Arksey & O'Malley, Citation2005; Brandt et al., Citation2014; Daudt et al., Citation2013; Levac et al., Citation2010; Tricco et al., Citation2016).
Search strategy
The review approach was underpinned by the research question: ‘What is known about the use of anabolic steroids by older men?’ We defined an older man as older than 40 years of age for two reasons. First, there is only one quantitative study in the USA, with specific focus on the older male who uses AAS (Ip et al., Citation2015). This study considered those aged >40 as ‘older’ and based this choice on emerging evidence of a subset of individuals older than 40 using AAS (Cohen et al., Citation2007; Hakansson et al., Citation2012; Ip et al., Citation2011; Perry et al., Citation2005). Whilst some UK studies (see (Begley et al., Citation2017; Chandler & McVeigh, Citation2014; Korkia, Citation1994; Lenehan et al., Citation1996; McVeigh et al., Citation2015), report older males (>40) in their demographic findings, none have specifically focused on older men who use AAS (OMAAS). Secondly, the UK national IPED survey in 2017 reported a similar proportion of men under 25 (17%) using AAS and those over 40 years of age (20%) (Begley et al., Citation2017) indicating a possible emerging cohort of men older than 40 using AAS.
Arksey and O’Malley’s (2005) five-stage iterative process scoping review methodology was closely adhered to. The five stages are: (1) identifying the essential research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collecting, summarising, and reporting the results. A search was undertaken in April 2022, in Liverpool John Moores University Library catalogues using the following databases: Scopus, MEDLINE, CINAHL, PsychINFO, SPORTDiscus, Cochrane, PubMED, Web of Science and Wiley. There was no restriction on publication dates when carrying out searches. Search terms used were broad to ensure records pertaining to AAS use were included (see ). Inclusion criteria focused on the use of AAS among men older than 40 years of age (see ). We reviewed relevant and available published empirical and grey literature in the English language.
Table 1. Search Strategy/Terms.
Table 2. Inclusion and exclusion criteria.
Study selection
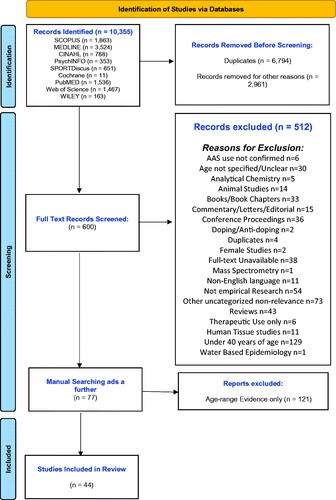
The initial search identified 10,355 records based on the search terms outlined in , which were then imported into Endnote® citation manager where they were managed and categorised. Following initial examination by author one (EH), 9,755 records were removed (e.g. duplicates, animal studies, female only studies) (see flow chart for detailed breakdown). This was followed by title and abstract screening of the remaining 600 records. Studies included were empirical studies in peer-reviewed journals, clinical case reports, and grey literature such as national policy reports and documents and needs assessments. At this stage, 600 records were identified for charting and full text screening whereby a further 512 were removed leaving a final number of 88. Manual hand-searching of the reference lists of these eighty-nine records was carried out as an additional step to capture any further empirical studies and in particular grey literature, resulting in a final number of 165 records for charting and analysis. A final search for grey literature was carried out on google and on websites of relevant public health and community organisations. This phase was supported by a key expert consultation exercise with a multi-stake holder expert group representing key identified academics working in the field, professionals and former people who use AAS, in order to identify any gaps in what was retrieved. From this analysis, a further 121 records were removed from the final dataset as, whilst their demographics had age-ranges higher than 40, the findings did not distinguish the age-ranges and so were not included in the review (see Supplementary Table). At this stage author two reviewed and agreed on the included records, which was carried out to avoid possible bias and subjective interpretation of author one. The final dataset consisted of 44 records for inclusion in the review.
Charting
Using Microsoft Excel, a spreadsheet was created to chart relevant data with the headings: author, year of publication, country of publication, study type, sample type, sample size, age range, age of first AAS use, mean/median age, and key findings pertaining to men older than 40 years. The extracted data were then thematically analysed to identify commonalities, emergent issues, themes, and gaps in the literature using Microsoft Excel spreadsheet. The spreadsheet data extraction table was broad, to ensure all records included were thoroughly analysed. Keywords and themes emerging were charted in parallel to the data extraction. Author one re-read the textual dataset numerous times so as to familiarise with the data and coded emergent themes. Themes were then reviewed and cross checked by all members of the research team who have specialist expert knowledge in scoping review methods and IPED use, which guided the final reporting of the results. Disagreements were addressed through discussion among all authors ().
Table 3. Charting of records.
Results
Profile of studies included
The final 44 records comprised of qualitative studies (n = 3), surveys (n = 8), mixed method studies (n = 2), retrospective data analysis (n = 2), clinical case reports (n = 23), forensic case reports (n = 2), observational studies (n = 1), prospective studies (n = 1), biomedical analysis (n = 1) and reviews (n = 1). The records originated from a variety of different countries including the UK (Bates & McVeigh, Citation2016; Begley et al., Citation2017; Boregowda et al., Citation2011; Graham et al., Citation2006; Hanley Santos & Coomber, Citation2017; Kimergard, Citation2015; Kimergard & McVeigh, Citation2014; Lenehan et al., Citation1996; McVeigh & Begley, Citation2017; Patil et al., Citation2007; Ravindran et al., Citation2020; Zahnow et al., Citation2018), the USA (Ahlgrim & Guglin, Citation2009; Ahmed et al., Citation2019; Baggish et al., Citation2017; Cabb et al., Citation2016; Cohen et al., Citation2007; Colburn et al., Citation2017; Farzam, Citation2021; Flo et al., Citation2018; Gangadharamurthy et al., Citation2018; Ip et al., Citation2015; Kovac et al., Citation2015; Long et al., Citation2019; Pope et al., Citation2021; Rashid, Citation2000; Rothman et al., Citation2011; Shiber, Citation2013; Shinya et al., Citation2013), Slovenia (Alibegović, Citation2018), Canada (Rosenfeld et al., Citation2011; Weinreb et al., Citation2010), Australia (Ding et al., Citation2013; Lovelock et al., Citation2021), Norway (Havnes et al., Citation2019), Spain (Llamas-Velasco et al., Citation2021), Japan Tashiro et al. (Citation2021), Switzerland (Fisler et al., Citation2018), Turkey (Ilhan et al., Citation2010) and five global studies (Bonnecaze et al., Citation2020; Chandler & McVeigh, Citation2014; Christiansen et al., Citation2016; Harvey et al., Citation2021; Zahnow et al., Citation2017). Studies included ranged from 1996 to 2021. Participants in the included studies were described as gym users, bodybuilders, athletes, IPED users, prisoners, needle and syringe program (NSP)/Harm reduction service clients, men who have sex with men (MSM), online bodybuilding, fitness and health forum members, and patients from clinical settings. It must be noted that these terms are interchangeable and not mutually exclusive within studies. Oldest age reported in studies included was 78 years. Following final charting and analysis of the data as outlined above, three themes with subthemes emerged: 1) Characteristics of AAS Use (age of initiation, motivations for use, sustained harmful patterns of use); 2) Self-Reported Adverse Effects from AAS Use (effects on sexual function and fertility, other self-reported adverse effects); and 3) Health Harms Diagnosed by a Medical Professional (cardiovascular harms, hepatotoxicity, other diagnosed health issues, experiences of healthcare) (see for themes).
Table 4. Themes and categories emerging during thematic analysis.
Theme 1: characteristics of AAS use
Age of initiation
First use of AAS at aged 40 years or older was reported in eight studies. These were in the UK (Bates & McVeigh, Citation2016; Begley et al., Citation2017; Lenehan et al., Citation1996), USA (Ip et al., Citation2015; Rothman et al., Citation2011), Norway (Havnes et al., Citation2019) and two global studies (Bonnecaze et al., Citation2020; Harvey et al., Citation2021). The global study by Bonnecaze et al. (Citation2020) included respondents from the USA, Canada, Europe, Australia, New Zealand, Asia, and Africa, with 7% (175/2385) of the total participants having initiated AAS use when older than 40 (Bonnecaze et al., Citation2020). Harvey et al. (Citation2021) reported oldest age of initiation to use at 57 years in their global study which included fifteen different countries. The oldest reported age of initiation among all studies was 69 years (Ip et al., Citation2015). Another US study reported older age of initiation by a male who began at 68 years old (Cohen et al., Citation2007). Recruitment for both US studies was primarily using online methods aimed at those using AAS and engaging in regular strength training.
In the UK, national annual IPED surveys reported age of initiation for both oral and injectable AAS (Bates & McVeigh, Citation2016; Begley et al., Citation2017; Chandler & McVeigh, Citation2014). In 2013 the age of initiation to oral AAS was mostly among the 18- to 24-year-old age-group. In the 40 to 54 year age-group 6% (5/80) and 8% (6/74) reported first use of oral and injectable AAS respectively (Chandler & McVeigh, Citation2014). The 2015 survey reported 3% (18/572) of participants’ older than 41 initiating use of injectable IPEDs, and 1.6% (8/505) initiating oral IPED use older than 41 (Bates & McVeigh, Citation2016). This was slightly higher in 2017 with initiation to use over 40 years for injectable IPEDs 5% (25/537) and for oral, 3% (20/590) (Begley et al., Citation2017).
Motivations for AAS use
Motivations by for initiating, continuing, and restarting AAS use by men older than 40 years of age were documented in twenty-three records (Ahlgrim & Guglin, Citation2009; Ahmed et al., Citation2019; Bonnecaze et al., Citation2020; Boregowda et al., Citation2011; Christiansen et al., Citation2016; Cohen et al., Citation2007; Fisler et al., Citation2018; Graham et al., Citation2006; Hanley Santos & Coomber, Citation2017; Harvey et al., Citation2021; Ilhan et al., Citation2010; Ip et al., Citation2015; Kimergard, Citation2015; Llamas-Velasco et al., Citation2021; Long et al., Citation2019; Pope et al., Citation2021; Rashid, Citation2000; Ravindran et al., Citation2020; Rothman et al., Citation2011; Shiber, Citation2013; Shinya et al., Citation2013; Tashiro et al., Citation2021; Zahnow et al., Citation2018).
Motivations to use AAS were reported as for aesthetics which included increased muscle mass, improved physique and appearance (Ahmed et al., Citation2019; Bonnecaze et al., Citation2020; Christiansen et al., Citation2016; Hanley Santos & Coomber, Citation2017; Harvey et al., Citation2021; Ip et al., Citation2015; Kimergard, Citation2015; Zahnow et al., Citation2018), improved physical strength for bodybuilding, sport and athletic performance, and other unspecified ‘performance enhancement’ (Bonnecaze et al., Citation2020; Harvey et al., Citation2021; Ilhan et al., Citation2010; Ip et al., Citation2015; Llamas-Velasco et al., Citation2021; Pope et al., Citation2021; Rothman et al., Citation2011; Tashiro et al., Citation2021), increased libido and endurance or stamina (Harvey et al., Citation2021), improved confidence and self-esteem (Bonnecaze et al., Citation2020; Harvey et al., Citation2021; Pope et al., Citation2021; Rashid, Citation2000), perceived low testosterone (Rashid, Citation2000), fat/weight loss (Christiansen et al., Citation2016; Cohen et al., Citation2007; Harvey et al., Citation2021), combating the ageing process and achieving feelings of youthfulness (Christiansen et al., Citation2016; Hanley Santos & Coomber, Citation2017; Ip et al., Citation2015; Kimergard, Citation2015), increasing musculature for bodybuilding (competitive and non-competitive) (Ahlgrim & Guglin, Citation2009; Boregowda et al., Citation2011; Fisler et al., Citation2018; Graham et al., Citation2006; Long et al., Citation2019; Ravindran et al., Citation2020); and for occupational reasons (Pope et al., Citation2021).
A clinical case study of a 48 year old male reported fear of losing muscle mass as a motivating factor for continued use (Ahmed et al., Citation2019). However, in 6 of the 23 studies documenting motivations, the main reported motivation for continued use by older men centred on ‘wellbeing’ and overall quality of life (QoL) especially during the ageing process (Bonnecaze et al., Citation2020; Christiansen et al., Citation2016; Harvey et al., Citation2021; Ip et al., Citation2015; Kimergard, Citation2015; Rashid, Citation2000). Wellbeing was not explicitly defined but represented a subjective measure in each of these studies. Feelings of mental, physical and social wellbeing were associated with training outcomes such as an attractive more youthful body (Christiansen et al., Citation2016) and enhanced overall QoL (Bonnecaze et al., Citation2020). Maintaining physical appearance and slowing the ageing process were also associated with feelings of wellbeing and youthfulness among older males (Harvey et al., Citation2021; Ip et al., Citation2015; Kimergard, Citation2015).
Image and increased size to promote authority in an occupational setting was reported as a motivation for restarting AAS use following a period of cessation in one case study (Pope et al., Citation2021). Secondary reinforcement motivating continued use was identified as arising from the positive effects of AAS use such as increased muscle tone, self-confidence, and social recognition (Pope et al., Citation2021). Motivations to recommence AAS use in one case was reported by Pope et al. (Citation2021) to increase confidence to impress a female partner. Another case study reported recommencing AAS use for self-directed TRT as use in the past improved quality of life (QoL) through confidence, happiness, image, and outgoingness (Rashid, Citation2000). Harvey et al. (Citation2021) noted that motivation to use AAS of an older male (43yrs) had changed over time, and the primary reason to continue or restart was linked to emotional wellbeing associated with increased libido. Literature reports that as the use of AAS causes suppression of the hypothalamic-pituitary-testicular axis, resulting in natural testosterone production shutting down (Tan & Scally, Citation2009) as individuals who cease use may quickly begin again to negate the dysphoric feelings associated with this such as decreased libido and low mood (Kanayama & Pope, Citation2018). Furthermore as older men experience lowered testosterone levels as part of the natural ageing process (Bhasin et al., Citation2006) they may be motivated to begin use later in life.
Sustained harmful patterns of use
AAS dependence (Havnes et al., Citation2019; Ip et al., Citation2015) and attempts at cessation (Bonnecaze et al., Citation2020; Fisler et al., Citation2018; Rashid, Citation2000) by older men were reported in five records. A study of 67 men who use AAS, specifically explored AAS dependence utilising the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; (American Psychiatric Association, Citation2000) as a measure (Ip et al., Citation2015). It found similarities with traditional illicit drug seeking behaviours, such as requiring higher dosages over longer periods of time, to reach desired effects. Moreover, 27.4% (17/67) of their sample met the criteria for DSM-IV-TR substance use disorder, however it did not report on whether those who met the criteria for dependence had presented for medical treatment or advice pertaining to this, or if they wished to seek advice but did not do so. Havnes et al. (Citation2019) reported 26% (62/232) of their participants said they experience dependence on AAS. However, this was based on self-disclosure and not on any dependence measures. Difficulty in attempts at cessation and high-levels of recommencement of AAS use by participants (663/2,385) were reported in the global study by Bonnecaze et al. (Citation2020). Rashid (Citation2000) noted that their case study participant reported feeling low, had decreased energy and decreased libido two months following cessation. However, in conjunction with his psychiatrist, he remained abstinent from AAS. Both points here underscore the importance of specific treatment interventions for those wishing to cease AAS use (Bates et al., Citation2019).
Theme 2: self-reported adverse effects from AAS use
Adverse effects described by older men resulting from the use of AAS were reported in ten records (Ahlgrim & Guglin, Citation2009; Ahmed et al., Citation2019; Bonnecaze et al., Citation2020; Boregowda et al., Citation2011; Ip et al., Citation2015; Kovac et al., Citation2015; Llamas-Velasco et al., Citation2021; Shiber, Citation2013; Shinya et al., Citation2013; Weinreb et al., Citation2010).
Effects on sexual function and fertility
The most self-reported adverse effects of AAS use centred on erectile dysfunction (Bonnecaze et al., Citation2020; Boregowda et al., Citation2011; Kovac et al., Citation2015), testicular atrophy (Bonnecaze et al., Citation2020; Boregowda et al., Citation2011), low libido (Boregowda et al., Citation2011), hypersexuality (Bonnecaze et al., Citation2020). Psychological effects such as low mood and depressive symptoms were directly associated with symptoms of low testosterone specifically low libido and erectile dysfunction (Harvey et al., Citation2021; Kovac et al., Citation2015; Rashid, Citation2000) and decreased energy (Rashid, Citation2000). Of interest is one of the participants of Harvey et al. (Citation2021) study was receiving therapeutic TRT from his physician but continued to use non-prescribed AAS if he planned to compete in a sporting event. A forensic case report of a deceased male who used AAS was diagnosed with secondary hypogonadism following autopsy (Alibegović, Citation2018). However, it is unknown if the deceased suffered hypogonadal symptoms prior to death. AAS suppress HPT function in males resulting in hypogonadal symptoms such as erectile dysfunction, loss of libido (Pope et al., Citation2014) and infertility (McBride & Coward, Citation2016). In the current review, Ip et al. (Citation2015) reported participants consuming clomiphene citrate and human chorionic gonadotropin in a bid to reduce these negative effects on sexual function and fertility. Infertility was reported by one male who had undergone investigations for this with his partner, and following cessation of AAS use (30 months after) primary gonadal failure was diagnosed (Boregowda et al., Citation2011). Infertility is the failure to successfully achieve pregnancy following one year or more of unprotected intercourse (de Souza & Hallak, Citation2011). Infertility resulting from AAS use is sometimes reversible after AAS discontinuation but the length of time to recovery cannot be ascertained, and should be considered by clinicians who are treating patients for infertility (McBride & Coward, Citation2016). Endogenous testosterone production and fertility are more likely to be restored in males who have used AAS in non-excessive doses and for a shorter length of time (Bonnecaze et al., Citation2021).
Other Self-Reported adverse effects
Other self-reported harms from AAS use included early-stage gynaecomastia in a 41-year old male (Llamas-Velasco et al., Citation2021), hormonal imbalances (acne, gynaecomastia, hair loss) (Bonnecaze et al., Citation2020), and fatigue, breathlessness and dyspnoea affecting one’s ability to carry out resistance training (Ahlgrim & Guglin, Citation2009). Injecting harms were reported in three studies (Bonnecaze et al., Citation2020; Shiber, Citation2013; Weinreb et al., Citation2010). Shiber (Citation2013) reported their case of a 45-year-old bodybuilder who presented with pain and swelling three days following injecting AAS. Here the patient had surgical debridement and following recovery was discharged after five days. Weinreb at al. (2010) reported a patient with swelling at the injecting site of a 49-year-old man however in this case no treatment was necessary. Bonnecaze et al. (Citation2020) note that OMAAS were more likely than younger males to report injecting harms.
Theme 3: health harms diagnosed by medical professional
Health harms diagnosed by a medical professional were reported in twenty-two records (Ahlgrim & Guglin, Citation2009; Ahmed et al., Citation2019; Alibegović, Citation2018; Baggish et al., Citation2017; Boregowda et al., Citation2011; Cabb et al., Citation2016; Colburn et al., Citation2017; Ding et al., Citation2013; Farzam, Citation2021; Fisler et al., Citation2018; Flo et al., Citation2018; Gangadharamurthy et al., Citation2018; Ilhan et al., Citation2010; Long et al., Citation2019; Lovelock et al., Citation2021; Patil et al., Citation2007; Ravindran et al., Citation2020; Rosenfeld et al., Citation2011; Rothman et al., Citation2011; Shinya et al., Citation2013; Tashiro et al., Citation2021).
Cardiovascular harms
The most commonly reported diagnosed adverse health effects resulting from AAS use were cardiovascular harms, reported in fourteen studies. These included eleven clinical case reports (Ahlgrim & Guglin, Citation2009; Ahmed et al., Citation2019; Farzam, Citation2021; Flo et al., Citation2018; Graham et al., Citation2006; Ilhan et al., Citation2010; Lovelock et al., Citation2021; Ravindran et al., Citation2020; Rothman et al., Citation2011; Shinya et al., Citation2013; Tashiro et al., Citation2021), an observational study (Baggish et al., Citation2017), and two cross-sectional surveys (Bonnecaze et al., Citation2020; Ip et al., Citation2015). Cardiovascular health problems attributed to the use of AAS included (see for definitions of cardiovascular harms terminology) cardiomyopathy (Ahlgrim & Guglin, Citation2009; Ahmed et al., Citation2019; Flo et al., Citation2018), left ventricular systolic dysfunction (Ahlgrim & Guglin, Citation2009; Flo et al., Citation2018; Ravindran et al., Citation2020), left ventricular hypertrophy (Farzam, Citation2021), right coronary artery infarct (Flo et al., Citation2018), decreased right ventricular function (Farzam, Citation2021) dyslipidaemia (Bonnecaze et al., Citation2020), myocardial infarction (Ilhan et al., Citation2010; Ravindran et al., Citation2020; Tashiro et al., Citation2021), Class IV heart failure (Ahlgrim & Guglin, Citation2009), myocardial dysfunction (Baggish et al., Citation2017), coronary atherosclerosis (Baggish et al., Citation2017), hypertension (Bonnecaze et al., Citation2020; Farzam, Citation2021), elevated hs-CRP (Farzam, Citation2021), hyperlipidaemia (Farzam, Citation2021), hyperhomocysteinaemia (Graham et al., Citation2006), increased haematocrit levels (Graham et al., Citation2006), infective endocarditis (Lovelock et al., Citation2021), refractory supraventricular tachycardia (Shinya et al., Citation2013). A U.S. cross-sectional cohort study found that long-term AAS use may have led to premature coronary artery disease in their eighty-six older male participants (34–54 years old) (Baggish et al., Citation2017). Of interest in this study is that systolic dysfunction was reversible on cessation of AAS use, however, diastolic dysfunction was less reversible. The study also found that atherosclerotic disease was significantly linked to lifetime use of AAS. In a clinical case report, a 41-year-old male was reported as having cardiac failure associated with the use of supraphysiological doses of AAS and other IPEDs and continuing to carry out heavy resistance training after the diagnosis. This resulted in the patient being evaluated for a heart transplant (Ahlgrim & Guglin, Citation2009). The patient unfortunately died as a result before his follow-up could be carried out.
Table 5. Cardiovascular harms terminology.
Hepatotoxicity
Diagnosed liver injury from AAS use was reported in five clinical case reports (Ahmed et al., Citation2019; Cabb et al., Citation2016; Ding et al., Citation2013; Fisler et al., Citation2018; Patil et al., Citation2007). Liver injury in an older male who used AAS was reported in one clinical case report, whereby a 46-year-old man experienced nausea, anorexia, and pruritus. Even though he had not been using AAS for at least five months, liver injury was associated with his past history of AAS use (Ding et al., Citation2013). The patient had been ingesting the oral AAS stanozolol (40 mg) and methandrostenolone (40 mg) daily for two months prior to cessation. Cabb et al. (Citation2016) reported their case, a 45-year-old male who had been consuming Anavar (50 mg orally daily) and injecting testosterone (once a week) for three months and was diagnosed with AAS-induced liver injury. He discontinued AAS after the onset of his symptoms. It is documented that AAS induced hepatotoxicity among older men is related to oral AAS use (Carrasco et al., Citation1985; Neri et al., Citation2011). As oral AAS resist immediate degradation in the hepatic system, it takes longer for the liver to clear them, increasing their hepatotoxic potential. Hepatotoxicity of the liver is evident in the form of acute cholestatic syndrome, elevated liver transaminases, hepatic tumours, vascular injury, and fatty liver disease. Most of these can be reversed on cessation of AAS use (Niedfeldt, Citation2018). People who use AAS orally are an under researched group of older male users who are at risk of non-injecting related harms, particularly liver hepatotoxicity (van de Ven et al., Citation2020). Attributing hepatotoxicity to AAS use is not always possible due those using AAS not divulging their use to them. Non-disclosure to healthcare professionals may make treatment decisions difficult (Pope et al., Citation2004; Zahnow et al., Citation2017).
Other diagnosed health issues
Other health problems diagnosed by medical professionals included acute kidney injury (Colburn et al., Citation2017; Fisler et al., Citation2018; Rosenfeld et al., Citation2011; Shinya et al., Citation2013), renal infarction (Ilhan et al., Citation2010) acute respiratory distress syndrome (ARDS) (Shinya et al., Citation2013), type 2 Diabetes Mellitus diagnosis (Farzam, Citation2021), cholesterol imbalance (Bonnecaze et al., Citation2020), acute pancreatitis (Rosenfeld et al., Citation2011), ischaemic stroke (Long et al., Citation2019), hypercalcaemia, inflammatory myositis, rhabdomyolysis (Ravindran et al., Citation2020) and erythrocytosis (Gangadharamurthy et al., Citation2018).
Experiences of healthcare
Only a small number of included records (n = 3) reported on experiences of healthcare by OMAAS (Harvey et al., Citation2021; Havnes et al., Citation2019; Lenehan et al., Citation1996). There are reports in these studies that those using AAS were reluctant to engage with medical professionals, but those who did seek medical advice were in the ‘older’ cohort, although specific age was not reported (Zahnow et al., Citation2017). A Norwegian study reported that older people who use AAS with a longer lifetime of use than their younger counterparts wished to seek treatment for cessation of use Havnes et al. (Citation2019). The reluctance of people who use AAS in general to seek medical professional advice and mistrust in GPs is documented in the literature (Hope et al., Citation2015; NICE, Citation2017; Pope et al., Citation2004). However, Zahnow et al. (Citation2017) did report that concerns regarding sexual function increased the likelihood of those individual’s engaging with healthcare. A US participant in the study by Harvey et al. (Citation2021) described feeling dissatisfaction at the lack of empathy from medical professionals toward him, resulting in accessing support from a private online clinic for AAS-related healthcare.
Discussion
This is the first known mapping exercise to scope the extant literature on the use of AAS by older men (>40). Whilst we closely adhered to the scoping review approach (Arksey & O'Malley, Citation2005), we wish to note the limitations of the study. First, as studies were restricted to those in the English language, our findings are only generalizable to those in English. Thus, our restriction to the English language may have limited the breadth of the review. Second, of the 165 articles that were initially found indicating the presence of OMAAS, 121 were removed as whilst they had respondents surpassing 40 years of age, they did not distinguish the age-ranges in the text and were therefore not included in our review. Participants in the older age-range within studies included have likely used AAS long-term and who may have begun using AAS in the 1980s, when the widespread use of illicit AAS and IPEDs emerged (McVeigh & Begley, Citation2017; Pope et al., Citation2014). Considering the long-term effects of AAS use on liver, cardiovascular, neuroendocrine, and cognitive health (Baggish et al., Citation2017; Bjørnebekk et al., Citation2019; Creagh et al., Citation1988; Kanayama et al., Citation2013, Citation2015; Schumacher et al., Citation1999; Tan & Scally, Citation2009), future researchers should acknowledge, distinguish, and separately analyse older males who use AAS as they may have specific long-term health effects from use compared to their younger counterparts. The studies included indicating men older than 40 using AAS were from the UK, USA, Canada, Australia, Slovenia, Norway, Spain, Switzerland, Japan, and five global studies. Historically, people who use AAS have been reported as young adults within the 20–29-year age group (Kanayama & Pope, Citation2018; Lenehan et al., Citation1996) with research, practice, and policy generally focused on this age-group (ACMD, Citation2010; NICE, Citation2017). However recent research has identified different populations of people who use AAS that are not consistent with the stereotypical young male who uses AAS (McVeigh et al., Citation2021). The global presence of OMAAS within studies in this scoping review highlight the need for age-specific research and recommendations to inform future policy and practice pertaining to this older cohort.
A comprehensive review of the global aetiology and trajectory of AAS use in 2014, reported that most men initiate use before 30 years of age. The authors found in their study age of initiation ranged from 14 to 54 years (Sagoe et al., Citation2014). However, the current scoping review found increasing numbers of men initiating AAS use later in life ranging as far as 69 years old (Bates & McVeigh, Citation2016; Begley et al., Citation2017; Bonnecaze et al., Citation2020; Cohen et al., Citation2007; Havnes et al., Citation2019; Ip et al., Citation2015; Lenehan et al., Citation1996).
Of significance in the UK national IPED studies is the increased age of initiation to use over the last decade (Bates & McVeigh, Citation2016; Begley et al., Citation2017; Chandler & McVeigh, Citation2014). It is important to note the different recruitment strategies in these studies which developed over time from those attending gymnasiums (Lenehan et al., Citation1996) to online forums and NSP clients (Chandler & McVeigh, Citation2014) and those in community drug/healthcare settings, NSPs, and harm reduction outreach (Bates & McVeigh, Citation2016; Begley et al., Citation2017), Thus, there is a possibility that under- or over-recruiting from these groups may have occurred. Whilst we know that there has been a significant increase in the numbers of people using AAS in the UK over the last quarter of a century (McVeigh & Begley, Citation2017), our scoping review has also indicated an increased age of initiation to use found within the included studies. Whilst the findings are not indicative of a definite trend of increased age of initiation, there are suggestions of a change over the decades.
There is limited research examining what motivates older men to initiate, continue, or restart AAS use following a period of cessation. People who use AAS and IPEDs are a heterogeneous population in terms of their lifestyle and motivations for the use of enhancement substances (Christiansen et al., Citation2016; Zahnow et al., Citation2018). However, a commonality among them exists in that improving body image, appearance, and/or performance are often the main drivers for initiating use (Brennan et al., Citation2017;; Cohen et al., Citation2007; Evans-Brown et al., Citation2012). Research illustrates primary motivations for younger males to use AAS to increase muscle size and weight (Antonopoulos & Hall, Citation2016; Cohen et al., Citation2007; Hanley Santos & Coomber, Citation2017; Underwood, Citation2017; Zahnow et al., Citation2017) which results in their perceived masculine identity (Cranswick et al., Citation2020) and feeling as though they have achieved ‘male status’ (Calogero & Thompson, Citation2010). Whilst motivations by older males to initiate AAS use in the current study do not differ greatly from young males, the motives for continued or restarting use are significant. The concept of AAS use for wellbeing was considered a primary motivating factor for continued use and restarting use following a period of cessation by OMAAS in 4 of the 23 studies documenting motivations (Bonnecaze et al., Citation2020; Harvey et al., Citation2021; Ip et al., Citation2015; Kimergard, Citation2015).
Wellbeing is associated with possessing and exercising specific human capabilities pertaining to human functioning and without these, an individual may feel they are not experiencing dignified and fulfilling lives (Nussbaum, Citation2011; Seligman, Citation2011; Sen, Citation1993). Enhanced wellbeing was associated with emotional happiness resulting from sexual function (Harvey et al., Citation2021) and feelings of youthfulness (Christiansen et al., Citation2016; Harvey et al., Citation2021; Ip et al., Citation2015; Kimergard, Citation2015; Rashid, Citation2000). In the case of the OMAAS here, continued use of AAS was likely a method to uphold their male status through their enhanced muscular physique (Calogero & Thompson, Citation2010) and ability to perform sexually later in life (Potts, Citation2000).
In contrast, participants of studies included in this review associated poor QoL with dysphoric feelings resulting from low testosterone such as low mood, decreased libido, and sexual dysfunction on cessation of AAS use (Harvey et al., Citation2021; Ip et al., Citation2015; Rashid, Citation2000). These were also the most self-reported adverse effect from AAS use (Boregowda et al., Citation2011; Harvey et al., Citation2021; Kovac et al., Citation2015). Western discourse on male sexuality indicates that the healthy functioning man must be capable of producing erections thus providing sexual satisfaction to his partner (Potts et al., Citation2004) and loss of erectile function is often correlated with loss of masculinity or manhood (Potts, Citation2000). This is in part a response to the increase in biomedical enhancements and pharmaceuticals for erectile dysfunction over the last decade aimed at older or ageing men (Potts et al., Citation2006). Furthermore, inability to perform sexually, particularly as older men experience lowered testosterone levels is part of the natural ageing process (Bhasin et al., Citation2006) and may result in emotional, psychological, and social distress for a man (Potts et al., Citation2006), thus affecting his sense of wellbeing. Literature has underscored the likelihood that individuals who value traditional male gender roles and masculine values are likely to use AAS (Kanayama et al., Citation2006; Keane, Citation2005) allowing them to uphold a socially acceptable masculine status (Connell, Citation1995; Courtenay, Citation2000; Kimmel et al., Citation2005). Of interest is that the older male participants’ perceived wellbeing could be considered as situated within traditional hegemonic masculine values concerned with desirability, libido and sexual function (Connell, Citation1995; Connell & Messerschmidt, Citation2005). Literature documents that men feel an internal need and intense pressure to have sex and to perform the stereotypical male sex-role (Kimmel et al., Citation2005). Not having the ability to perform this role can result in poor mental wellbeing and QoL (Courtenay, Citation2000). Our review indicates that motivations for initiating AAS use for aesthetics and performance enhancement differ in the older man who is using later in life for sexual function, desirability, and wellbeing (Christiansen et al., Citation2016; Harvey et al., Citation2021; Ip et al., Citation2015). This suggests that older men who continue to use AAS for this reason may be doing so to sustain their previously achieved ‘male status,’ embodied by their ability to continue to perform sexually. Our review has underscored the issue of older males who rarely seek medical or professional advice pertaining to their use of AAS. This is due to mistrust and lack of confidence in the knowledge of the primary care physician, feeling stigmatised because of their choice to use AAS (Bonnecaze et al., Citation2020; Zahnow et al., Citation2017) and their capability to self-manage their use (Harvey et al., Citation2021). Moreover, it is reported that men who hold or conform to strong masculine values are often reluctant to engage with healthcare (Courtenay, Citation2000). This has significance for healthcare practitioners who are urged to consider the wider spectrum of wellbeing to include PERMA (positive emotion, engagement, relationships, meaningful activities and accomplishment) (Seligman, Citation2011) in all areas of their lives, and how this impacts on male self-image, and the stereotypical masculine identity (Connell, Citation1995; Courtenay, Citation2000; Kimmel et al., Citation2005).
Research has identified an AAS dependence syndrome whereby they are used for prolonged periods of time despite negative effects on physical and psychological well-being (Kanayama et al., Citation2008, Citation2009; Pope et al., Citation2014). It is difficult to determine though if they are being used to restore an individual to a normal baseline gonadal state, or, to surpass the normal baseline and achieve specific outcomes (Anawalt, Citation2019). More recently, researchers have demonstrated structural brain differences between AAS dependent and non-dependent people, specifically a thinner prefrontal cortex in those who are dependent. The prefrontal cortex is associated with inhibitory control and emotional regulation, indicating why some people who use AAS become dependent (Hauger, Westlye, Fjell, Walhovd, & Bjørnebekk et al., Citation2019). The literature documents that AAS are used in supraphysiologic doses to enhance muscularity (Anawalt, Citation2019; Kaufman et al., Citation2019) as without doing this, muscle mass at the level they desire cannot be gained (Bhasin et al., Citation1996). It is approximated that up to 30% of those who use AAS in supraphysiological doses for extended periods of time may become dependent (Kanayama et al., Citation2009; Pope et al., Citation2014). A complication of this pattern of use often means that natural hormone function cannot recover and may result in anabolic steroid induced hypogonadism (ASIH) on cessation of use (Tan & Scally, Citation2009). In some cases, there is no possibility for recovery of HPT function and fertility in older males who employ this method (Pope et al., Citation2014) resulting in continued AAS use. Self-reported dependence was noted (Havnes et al., Citation2019; Ip et al., Citation2015) and unsuccessful attempts at cessation were documented in some studies in the current review (Bonnecaze et al., Citation2020; Fisler et al., Citation2018; Rashid, Citation2000). Continued use or unsuccessful attempts at cessation may be an indication of the ‘neuroendocrine pathway’ for AAS dependence (Kanayama et al., Citation2020). Whilst AAS are not used for psychoactive effects like traditional illicit substances (Bates et al., Citation2019), their continued use to negate the symptoms of low testosterone certainly mimic traditional illicit drug seeking behaviours. These negative feelings are symptoms of AAS withdrawal hypogonadism which potentiates the need to self-treat with AAS, thus resulting in dependence (Kanayama et al., Citation2020). Furthermore, the OMAAS’ change in patterns of use from cycling to blast and cruise/prolonged use to improve overall wellbeing and QoL is indicative of psychological dependency, compounded by the fact than men are often unwilling to seek treatment for mental health issues such as depression (Courtenay, Citation2000). Other records which reported that participants simply did not perceive themselves as dependent, and that their AAS use is part of a healthy regime and a valued lifestyle (Cohen et al., Citation2007; Kimergard, Citation2015). The non-disclosure of AAS use to primary care physicians (Harvey et al., Citation2021; Pope et al., Citation2004; Zahnow et al., Citation2017), compounds this issue further and may result in inappropriate or lack of medical interventions and treatment. This results in the older man continuing to use AAS whilst also having undiagnosed and untreated AAS dependence syndrome.
Adverse health effects related to the use of AAS are well established in the literature, particularly the comprehensive review published in 2014 by the Endocrine Society (Pope et al., Citation2014). Physical harms outlined in their report include cardiovascular and liver harms, as well as psychological issues and dependence which have been identified in many of the studies included in our review. Cardiovascular harms diagnosed by medical professionals were more frequently reported in the current review than harms to other organ systems (Ahlgrim & Guglin, Citation2009; Ahmed et al., Citation2019; Baggish et al., Citation2017; Bonnecaze et al., Citation2020; Farzam, Citation2021; Flo et al., Citation2018; Graham et al., Citation2006; Ilhan et al., Citation2010; Ip et al., Citation2015; Lovelock et al., Citation2021; Ravindran et al., Citation2020; Rothman et al., Citation2011; Shinya et al., Citation2013; Tashiro et al., Citation2021). Supraphysiological doses of AAS can adversely affect other organ systems such as endocrine, and neurological systems, requiring medical intervention (Kanayama et al., Citation2008). It is important to also consider that OMAAS may be entering the period of natural age-related increased risk for cardiovascular health problems, an aging myocardium, combined with a history or current AAS use may be at even higher risk for cardiovascular health issues (Kanayama et al., Citation2008; Thiblin et al., Citation2015). Of interest, is that cardiovascular harms reported such as left ventricular hypertrophy (Farzam, Citation2021), dyslipidaemia (Bonnecaze et al., Citation2020), coronary atherosclerosis (Baggish et al., Citation2017) and hypertension (Bonnecaze et al., Citation2020; Farzam, Citation2021) are mostly diagnosed in sedentary or obese populations (McCullough et al., Citation2021) and not among people who use AAS that consider their AAS use part of a health regime and a valued lifestyle (Cohen et al., Citation2007; Kimergard, Citation2015). As the long-term effects of AAS use cause damage not only to cardiovascular health (Baggish et al., Citation2017), but also to the liver (Creagh et al., Citation1988; Schumacher et al., Citation1999), neuroendocrine health (Kanayama et al., Citation2015; Tan & Scally, Citation2009) and cognitive decline (Bjørnebekk et al., Citation2019; Kanayama et al., Citation2013) as highlighted in our review, the intervention and treatment needs of an older man who is using AAS will require a multi-disciplinary approach including physicians, endocrinologists, psychologists and psychiatrists, and addiction specialist practitioners (Casavant & Griffith, Citation2017).
Conclusion
This unique scoping review has mapped and described for the first time what is known about the use of AAS among older men. Increased age of initiation and an ageing cohort of people who user AAS and who are motivated by feelings of wellbeing and youthfulness was observed. This is embodied by their ability to perform sexually and to have a good quality of life which they achieve by using AAS, despite the harms of use experienced. The use of AAS by older males coupled with the natural ageing decline in testosterone and the possibility for cardiovascular ill-health later in life, is a concern for healthcare and medical professionals. Future research, qualitatively exploring the motivations of older men to initiate, continue, and recommence AAS use following a period of cessation, will aid in the development of effective interventions, treatment, and harm reduction specific to their needs and can also inform AAS specific training for medical and healthcare professionals. Moreover, it is recommended that researchers acknowledge, distinguish, and separately analyse older males in future studies so as to age-appropriately inform healthcare, policy, and practice. By integrating gender perspectives into healthcare, may result in increased numbers of men seeking treatment for sexual dysfunction resulting from AAS use as well as other healthcare specific to their needs thus improving their wellbeing and QoL. Policymakers are urged to consider the findings of this review and consider the different needs this age-group have so that informed healthcare and policy decisions can be made in the future.
Supplemental Material
Download MS Word (37.2 KB)Additional information
Funding
References
- ACMD. (2010). Consideration of the Anabolic Steroids. Retrieved from London (UK): https://www.gov.uk/government/publications/advisory-council-on-the-misuse-of-drugs-consideration-of-the-anabolic-steroids
- Ahlgrim, C., & Guglin, M. (2009). Anabolics and cardiomyopathy in a bodybuilder: case report and literature review. Journal of Cardiac Failure, 15(6), 496–500. https://doi.org/10.1016/j.cardfail.2008.12.014
- Ahmed, A., Vavrenyuk, A., Fallahi, A., Lin, E., & Hsi, D. (2019). Bodybuilding gone wrong: Anabolic steroid induced cardiomyopathy. Journal of the American College of Cardiology, 73(9), 2199. https://doi.org/10.1016/S0735-1097(19)32805-0
- Alibegović, A. (2018). Testicular morphology in hypogonadotropic hypogonadism after the abuse of anabolic steroids. Forensic Science, Medicine, and Pathology, 14(4), 564–567. https://doi.org/10.1007/s12024-018-0015-6
- American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision (DSM-IVTR). American Psychiatric Association.
- Anawalt, B. D. (2019). Diagnosis and management of anabolic androgenic steroid use. Journal of Clinical Endocrinology and Metabolism, 104(7), 2490–2500. https://doi.org/10.1210/jc.2018-01882
- Antonopoulos, G. A., & Hall, A. (2016). Gain with no pain’: Anabolic-androgenic steroids trafficking in the UK. European Journal of Criminology, 13(6), 696–713. https://doi.org/10.1177/1477370816633261
- Arksey, H., & O'Malley, L. (2005). Scoping studies: towards a methodological framework. International Journal of Social Research Methodology, 8(1), 19–32. https://doi.org/10.1080/1364557032000119616
- Baggish, A. L., Weiner, R. B., Kanayama, G., Hudson, J. I., Lu, M. T., Hoffmann, U., … Pope, H. G. Jr (2017). Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation, 135(21), 1991–2002. https://doi.org/10.1161/CIRCULATIONAHA.116.026945
- Bates, G., & McVeigh, J. (2016). Image and performance enhancing drugs 2015 survey results. Centre for public health.
- Bates, G., Tod, D., Leavey, C., & McVeigh, J. (2019). An evidence-based socioecological framework to understand men’s use of anabolic androgenic steroids and inform interventions in this area. Drugs: Education, Prevention and Policy, 26(6), 484–492. https://doi.org/10.1080/09687637.2018.1488947
- Bates, G., Van Hout, M. C., Tay Wee Teck, J., & McVeigh, J. (2019). Treatments for people who use anabolic androgenic steroids: a scoping review. Harm Reduction Journal, 16(1), 75. https://doi.org/10.1186/s12954-019-0343-1
- Begley, E., McVeigh, J., Hope, V. D., Bates, G., Glass, R., Campbell, J., … Smith, J. (2017). Image and performance enhancing drugs: 2016 national survey results. Liverpool.
- Bhasin, S., Cunningham, G. R., Hayes, F. J., Matsumoto, A. M., Snyder, P. J., Swerdloff, R. S., & Montori, V. M. (2006). Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism, 91(6), 1995–2010. https://doi.org/10.1210/jc.2005-2847
- Bhasin, S., Storer, T. W., Berman, N., Callegari, C., Clevenger, B., Phillips, J., Bunnell, T. J., Tricker, R., Shirazi, A., & Casaburi, R. (1996). The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. The New England Journal of Medicine, 335(1), 1–7. https://doi.org/10.1056/Nejm199607043350101
- Bjørnebekk, A., Westlye, L. T., Walhovd, K. B., Jorstad, M. L., Sundseth, O. O., & Fjell, A. M. (2019). Cognitive performance and structural brain correlates in long-term anabolic-androgenic steroid exposed and nonexposed weightlifters. Neuropsychology, 33(4), 547–559. https://doi.org/10.1037/neu0000537
- Bonnecaze, A. K., O'Connor, T., & Aloi, J. A. (2020). Characteristics and attitudes of men using Anabolic Androgenic Steroids (AAS): a survey of 2385 men. American Journal of Men’s Health, 14(6), 1557988320966536. https://doi.org/10.1177/1557988320966536
- Bonnecaze, A. K., O'Connor, T., & Burns, C. A. (2021). Harm reduction in male patients actively using Anabolic Androgenic Steroids (AAS) and Performance-Enhancing Drugs (PEDs): a review. Journal of General Internal Medicine, 36(7), 2055–2064. https://doi.org/10.1007/s11606-021-06751-3
- Boothroyd, L. G., Jucker, J.-L., Thornborrow, T., Jamieson, M. A., Burt, D. M., Barton, R. A., Evans, E. H., & Tovee, M. J. (2016). Television exposure predicts body size ideals in rural Nicaragua. British Journal of Psychology (London, England : 1953), 107(4), 752–767. https://doi.org/10.1111/bjop.12184
- Boregowda, K., Joels, L., Stephens, J. W., & Price, D. E. (2011). Persistent primary hypogonadism associated with anabolic steroid abuse. Fertility and Sterility, 96(1), E7–E8. https://doi.org/10.1016/j.fertnstert.2011.04.029
- Brandt, S. D., King, L. A., & Evans-Brown, M. (2014). The new drug phenomenon. Drug Testing and Analysis, 6(7-8), 587–597. https://doi.org/10.1002/dta.1686
- Brennan, R., Van Hout, M. C., & Wells, J. (2013). Heuristics of human enhancement risk: a little chemical help? International Journal of Health Promotion and Education, 51(4), 212–227. https://doi.org/10.1080/14635240.2013.818295
- Brennan, R., Wells, J. S., & Van Hout, M. C. (2017). The injecting use of image and performance-enhancing drugs (IPED) in the general population: a systematic review. Health & Social Care in the Community, 25(5), 1459–1531. https://doi.org/10.1111/hsc.12326
- Brennan, R., Wells, J. S., & Van Hout, M. C. (2018). “Raw juicing” – an online study of the home manufacture of anabolic androgenic steroids (AAS) for injection in contemporary performance and image enhancement (PIED) culture. Performance Enhancement & Health, 6(1), 21–27. https://doi.org/10.1016/j.peh.2017.11.001
- Cabb, E., Baltar, S., Powers, D. W., Mohan, K., Martinez, A., & Pitts, E. (2016). The diagnosis and manifestations of liver injury secondary to off-label androgenic anabolic steroid use. Case Reports in Gastroenterology, 10(2), 499–505. https://doi.org/10.1159/000448883
- Calogero, R. M., & Thompson, J. K. (2010). Gender and body image. In J. C. Chrisler & D. R. McCreary (Eds.), Handbook of Gender Research in Psychology: Volume 2: Gender Research in Social and Applied Psychology. (pp. 153–184). Springer New York.
- Carrasco, D., Prieto, M., Pallardo, L., Moll, J. L., Cruz, J. M., Munoz, C., & Berenguer, J. (1985). Multiple hepatic adenomas after long-term therapy with testosterone enanthate. Review of the literature. Journal of Hepatology, 1(6), 573–578. https://doi.org/10.1016/S0168-8278(85)80001-5
- Casavant, M. J., & Griffith, J. R. K. (2017). Anabolic steroid use disorder. New Jersey.
- Chandler, M., & McVeigh, J. (2014). Steroids and image enhancing drugs; 2013 survey results. Center for Public Health.
- Christiansen, A. V., Vinther, A. S., & Liokaftos, D. (2016). Outline of a typology of men’s use of anabolic androgenic steroids in fitness and strength training environments. Drugs: Education, Prevention and Policy, 24(3), 295–305.
- Cohen, J., Collins, R., Darkes, J., & Gwartney, D. (2007). A league of their own: demographics, motivations and patterns of use of 1,955 male adult non-medical anabolic steroid users in the United States. Journal of the International Society of Sports Nutrition, 4(12), 12. https://doi.org/10.1186/1550-2783-4-12
- Colburn, S., Childers, W. K., Chacon, A., Swailes, A., Ahmed, F. M., & Sahi, R. (2017). The cost of seeking an edge: recurrent renal infarction in setting of recreational use of anabolic steroids. Annals of Medicine and Surgery (2012), 14, 25–28. https://doi.org/10.1016/j.amsu.2017.01.015
- Connell, R. W. (1995). Masculinities. Polity.
- Connell, R. W., & Messerschmidt, J. W. (2005). Hegemonic masculinity: rethinking the concept. Gender & Society, 19(6), 829–859. https://doi.org/10.1177/0891243205278639
- Coomber, R., Pavlidis, A., Hanley Santos, G., Wilde, M., Schmidt, W., & Redshaw, C. (2014). The supply of steroids and other performance and image enhancing drugs (PIEDs) in one English city: Fakes, counterfeits, supplier trust, common beliefs and access. Performance Enhancement & Health, 3(3-4), 135–144. https://doi.org/10.1016/j.peh.2015.10.004
- Courtenay, W. H. (2000). Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Social Science & Medicine (1982), 50(10), 1385–1401. https://doi.org/10.1016/S0277-9536(99)00390-1
- Cranswick, I., Richardson, D., Littlewood, M., & Tod, D. (2020). “Oh take some man-up pills”: A life-history study of muscles, masculinity, and the threat of injury. Performance Enhancement & Health, 8(2-3), 100176. https://doi.org/10.1016/j.peh.2020.100176
- Creagh, T. M., Rubin, A., & Evans, D. J. (1988). Hepatic tumours induced by anabolic steroids in an athlete. Journal of Clinical Pathology, 41(4), 441–443. https://doi.org/10.1136/jcp.41.4.441
- Darke, S., Torok, M., & Duflou, J. (2014). Sudden or unnatural deaths involving anabolic-androgenic steroids. Journal of Forensic Sciences, 59(4), 1025–1028. https://doi.org/10.1111/1556-4029.12424
- Daudt, H. M. L., van Mossel, C., & Scott, S. J. (2013). Enhancing the scoping study methodology: a large, inter-professional team’s experience with Arksey and O’Malley’s framework. BMC Medical Research Methodology, 13(1), 48. https://doi.org/10.1186/1471-2288-13-48
- Day, C. A., Topp, L., Iversen, J., Maher, L. N., Collaboration of Australian NSPs (2008). Blood-borne virus prevalence and risk among steroid injectors: results from the Australian Needle and Syringe Program Survey. Drug and Alcohol Review, 27(5), 559–561. https://doi.org/10.1080/09595230801956132
- de Souza, G. L., & Hallak, J. (2011). Anabolic steroids and male infertility: a comprehensive review. BJU International, 108(11), 1860–1865. https://doi.org/10.1111/j.1464-410X.2011.10131.x
- Ding, N. S., De Cruz, P., Lim, L., Thompson, A., & Desmond, P. (2013). Androgenic-anabolic steroid drug-induced liver injury. Internal Medicine Journal, 43(2), 215–216. https://doi.org/10.1111/imj.12054
- Evans-Brown, M., McVeigh, J., Perkins, C., & Bellis, M. A. (2012). Human enhancement drugs: the emerging challenges to public health. Retrieved from Liverpool. : North West Public Health Observatory.
- Farzam, K. (2021). Anabolic-androgenic steroids and cardiometabolic derangements. Cureus, 13(1), e12492. https://doi.org/10.7759/cureus.12492
- Fisler, A., Breidthardt, T., Schmidlin, N., Hopfer, H., Dickenmann, M., König, K., & Hirt-Minkowski, P. (2018). Bile cast nephropathy: the unknown dangers of online shopping. Case Reports in Nephrology and Dialysis, 8(2), 98–102. https://doi.org/10.1159/000489771
- Flo, F. J., Kanu, O., Teleb, M., Chen, Y., & Siddiqui, T. (2018). Anabolic androgenic steroid-induced acute myocardial infarction with multiorgan failure. Proceedings, 31(3), 334–336. https://doi.org/10.1080/08998280.2018.1460130
- Frati, P. P., Busardo, F., Cipolloni, L., De Dominicis, E., & Fineschi, V. (2015). Anabolic androgenic steroid (AAS) related deaths: autoptic, histopathological and toxicological findings. Current Neuropharmacology, 13(1), 146–159. https://doi.org/10.2174/1570159X13666141210225414
- Gangadharamurthy, D., Pandian, N., Malhotra, S., Tahir, S., & Mukherjee, J. (2018). Anabolic androgenic steroid abuse and reversible cardiomyopathy: an emerging and under-recognized cardiovascular public health problem among fitness enthusiasts. Journal of the American College of Cardiology, 71(11), A2426–2426. https://doi.org/10.1016/S0735-1097(18)32967-X
- Graham, M. R., Grace, F. M., Boobier, W., Hullin, D., Kicman, A., Cowan, D., Davies, B., & Baker, J. S. (2006). Homocysteine induced cardiovascular events: a consequence of long term anabolic-androgenic steroid (AAS) abuse. British Journal of Sports Medicine, 40(7), 644–648. https://doi.org/10.1136/bjsm.2005.025668
- Greenway, C. W., & Price, C. (2018). A qualitative study of the motivations for anabolic-androgenic steroid use: The role of muscle dysmorphia and self-esteem in long-term users. Performance Enhancement & Health, 6(1), 12–20. https://doi.org/10.1016/j.peh.2018.02.002
- Griffiths, S., Henshaw, R., McKay, F. H., & Dunn, M. (2017). Post-cycle therapy for performance and image enhancing drug users: A qualitative investigation. Performance Enhancement & Health, 5(3), 103–107. https://doi.org/10.1016/j.peh.2016.11.002
- Griffiths, S., Murray, S. B., & Touyz, S. (2015). Extending the masculinity hypothesis: an investigation of gender role conformity, body dissatisfaction, and disordered eating in young heterosexual men. Psychology of Men & Masculinity, 16(1), 108–114. https://doi.org/10.1037/a0035958
- Grogan, S. (1999). Body image: understanding body dissatisfaction in men, women and children. Routledge.
- Hakansson, A., Mickelsson, K., Wallin, C., & Berglund, M. (2012). Anabolic androgenic steroids in the general population: user characteristics and associations with substance use. European Addiction Research, 18(2), 83–90. https://doi.org/10.1159/000333037
- Hanley Santos, G., & Coomber, R. (2017). The risk environment of anabolic-androgenic steroid users in the UK: Examining motivations, practices and accounts of use. The International Journal on Drug Policy, 40, 35–43. https://doi.org/10.1016/j.drugpo.2016.11.005
- Hartgens, F., & Kuipers, H. (2004). Effects of androgenic-anabolic steroids in athletes. Sports Medicine, 34(8), 513–554. https://doi.org/10.2165/00007256-200434080-00003
- Harvey, O., Parrish, M., van Teijlingen, E., & Trenoweth, S. (2021). Libido as a motivator for starting and restarting non-prescribed anabolic androgenic steroid use among men: a mixed-methods study. Drugs: Education, Prevention & Policy, 29(3), 1–13. https://doi.org/10.1080/09687637.2021.1882940
- Hauger, L. E., Westlye, L. T., Fjell, A. M., Walhovd, K. B., & Bjørnebekk, A. (2019). Structural brain characteristics of anabolic-androgenic steroid dependence in men. Addiction (Abingdon, England), 114(8), 1405–1415. https://doi.org/10.1111/add.14629
- Havnes, I. A., Jorstad, M. L., & Wisloff, C. (2019). Anabolic-androgenic steroid users receiving health-related information; health problems, motivations to quit and treatment desires. Substance Abuse Treatment, Prevention, and Policy, 14(1), 20. https://doi.org/10.1186/s13011-019-0206-5
- Hoberman, J. (2017). Dopers in Uniform The hidden world of police on steroids. Texas.
- Hope, V. D., McVeigh, J., Begley, E., Glass, R., Edmundson, C., Heinsbroek, E., Kean, J., Campbell, J., Whitfield, M., Morgan, G., Acreman, D., & Smith, J. (2021). Factors associated with hepatitis C and HIV testing uptake among men who inject image and performance enhancing drugs. Drug and Alcohol Review, 40(4), 586–596. https://doi.org/10.1111/dar.13198
- Hope, V. D., McVeigh, J., Marongiu, A., Evans-Brown, M., Smith, J., Kimergård, A., Croxford, S., Beynon, C. M., Parry, J. V., Bellis, M. A., & Ncube, F. (2013). Prevalence of, and risk factors for, HIV, hepatitis B and C infections among men who inject image and performance enhancing drugs: A cross-sectional study. BMJ Open, 3(9), e003207-e003207. https://doi.org/10.1136/bmjopen-2013-003207
- Hope, V. D., McVeigh, J., Marongiu, A., Evans-Brown, M., Smith, J., Kimergård, A., Parry, J. V., & Ncube, F. (2015). Injection site infections and injuries in men who inject image- and performance-enhancing drugs: prevalence, risks factors, and healthcare seeking. Epidemiology and Infection, 143(1), 132–140. https://doi.org/10.1017/S0950268814000727
- Hope, V. D., Walker Bond, V., Boardley, I., Smith, J., Campbell, J., Bates, G., Ralphs, R., Van Hout, M.-C., & McVeigh, J. (2022). Anabolic androgenic steroid use population size estimation: a first stage study utilising a Delphi exercise. Drugs: Education, Prevention and Policy, 1–13. https://doi.org/10.1080/09687637.2022.2070058
- Ilhan, E., Demirci, D., Güvenç, T. S., & Çalik, A. N. (2010). Acute myocardial infarction and renal infarction in a bodybuilder using anabolic steroids. Turk Kardiyoloji Dernegi Arsivi, 38(4), 275–278. https://www.scopus.com/inward/record.uri?eid=2-s2.0-79953282539&partnerID=40&md5=82294b6296631aac49e09e09940f8a45
- Ip, E. J., Barnett, M. J., Tenerowicz, M. J., & Perry, P. J. (2011). The anabolic 500 survey: characteristics of male users versus nonusers of anabolic-androgenic steroids for strength training. Pharmacotherapy, 31(8), 757–766. https://doi.org/10.1592/phco.31.8.757
- Ip, E. J., Trinh, K., Tenerowicz, M. J., Pal, J., Lindfelt, T. A., & Perry, P. J. (2015). Characteristics and behaviors of older male anabolic steroid users. Journal of Pharmacy Practice, 28(5), 450–456. https://doi.org/10.1177/0897190014527319
- Kanayama, G., & Pope, H. G. J. (2018). History and epidemiology of anabolic androgens in athletes and non-athletes. Molecular and Cellular Endocrinology, 464(C), 4–13. https://doi.org/10.1016/j.mce.2017.02.039
- Kanayama, G., Barry, S., Hudson, J. I., & Pope, H. G. J. (2006). Body image and attitudes toward male roles in anabolic-androgenic steroid users. The American Journal of Psychiatry, 163(4), 697–703. https://doi.org/10.1176/appi.ajp.163.4.697
- Kanayama, G., Brower, K. J., Wood, R. I., Hudson, J. I., & Pope, H. G. J. (2009). Anabolic-androgenic steroid dependence: an emerging disorder. Addiction (Abingdon, England), 104(12), 1966–1978. https://doi.org/10.1111/j.1360-0443.2009.02734.x
- Kanayama, G., Hudson, J. I., & Pope, H. G. J. (2008). Long-term psychiatric and medical consequences of anabolic-androgenic steroid use: A looming public health concern? Drug and Alcohol Dependence, 98(1-2), 1–12. https://doi.org/10.1016/j.drugalcdep.2008.05.004
- Kanayama, G., Hudson, J. I., Pope, & H. G. J. (2020). Anabolic-androgenic steroid use and body image in men: a growing concern for clinicians. Psychotherapy and Psychosomatics, 89(2), 65–73. https://doi.org/10.1159/000505978
- Kanayama, G., Hudson, J. I., DeLuca, J., Isaacs, S., Baggish, A., Weiner, R., Bhasin, S., & Pope, H. G. (2015). Prolonged hypogonadism in males following withdrawal from anabolic–androgenic steroids: An under‐recognized problem. Addiction, 110(5), 823–831. https://doi.org/10.1111/add.12850
- Kanayama, G., Kean, J., Hudson, J. I., & Pope, H. G. J. (2013). Cognitive deficits in long-term anabolic-androgenic steroid users. Drug and Alcohol Dependence, 130(1-3), 208–214. https://doi.org/10.1016/j.drugalcdep.2012.11.008
- Kaufman, M. J., Kanayama, G., Hudson, J. I., & Pope, H. G. J. (2019). Supraphysiologic-dose anabolic-androgenic steroid use: a risk factor for dementia? Neuroscience and Biobehavioral Reviews, 100, 180–207.
- Keane, H. (2005). Diagnosing the male steroid user: drug use, body image and disordered masculinity. Health, 9(2), 189–208. https://doi.org/10.1177/1363459305050585
- Kemp, R. (2017). Middle-aged men are taking steroids to look young – but do they know the alarming consequences? The Telegraph, 3rd April 2017.
- Khalil, H., Peters, M., Godfrey, C. M., McInerney, P., Soares, C. B., & Parker, D. (2016). An evidence-based approach to scoping reviews. Worldviews on Evidence-Based Nursing, 13(2), 118–123. https://doi.org/10.1111/wvn.12144
- Kimergard, A. (2015). A qualitative study of anabolic steroid use amongst gym users in the United Kingdom: motives, beliefs and experiences. Journal of Substance Use, 20(4), 288–294. https://doi.org/10.3109/14659891.2014.911977
- Kimergard, A., & McVeigh, J. (2014). Environments, risk and health harms: a qualitative investigation into the illicit use of anabolic steroids among people using harm reduction services in the UK. BMJ Open, 4(6), e005275–e005275. https://doi.org/10.1136/bmjopen-2014-005275
- Kimergard, A., Breindahl, T., Hindersson, P., & McVeigh, J. (2014). The composition of anabolic steroids from the illicit market is largely unknown: implications for clinical case reports. QJM : monthly Journal of the Association of Physicians, 107(7), 597–598. https://doi.org/10.1093/qjmed/hcu101
- Kimmel, M., Hearn, J., & Connell, R. W. (2005). Handbook of studies on men and masculinities. Sage Publications, Inc.
- Korkia, P. (1994). Anabolic steroid use in Great Britain: a study. Relay, 1(1), 10–11.
- Korkia, P., & Stimson, G. V. (1993). Anabolic Steroid Use in Great Britain: An Exploratory Investigation: Final Report to the Departments of Health for England, Scotland and Wales. Centre for Research on Drugs Health Behaviour.
- Kovac, J. R., Scovell, J., Ramasamy, R., Rajanahally, S., Coward, R. M., Smith, R. P., & Lipshultz, L. I. (2015). Men regret anabolic steroid use due to a lack of comprehension regarding the consequences on future fertility. Andrologia, 47(8), 872–878. https://doi.org/10.1111/and.12340
- Leit, R. A., Pope, H. G., & Gray, J. J. (2001). Cultural expectations of muscularity in men: THE evolution of Playgirl centerfolds. International Journal of Eating Disorders, 29(1), 90–93. https://doi.org/10.1002/1098-108X(200101)29:1<90::AID-EAT15>3.0.CO;2-F
- Lenehan, P., Bellis, M. A., & McVeigh, J. (1996). A study of anabolic steroid use in the North West of England. Journal of Performance Enhancing Drugs, 1(2), 57–70.
- Levac, D., Colquhoun, H., & O'Brien, K. K. (2010). Scoping studies: advancing the methodology. Implementation Science : IS, 5(1), 69. https://doi.org/10.1186/1748-5908-5-69
- Llamas-Velasco, M., Bianciardi Valassina, M. F., Ovejero-Merino, E., Massi, G., & Mentzel, T. (2021). Bilateral nipple enlargement as a secondary effect of anabolic drugs: a histopathological mimicker of smooth muscle hamartoma. Dermatopathology, 8(2), 103–106. https://doi.org/10.3390/dermatopathology8020016
- Long, N., Bassi, S., Pepito, D., & Akhondi, H. (2019). Gerstmann syndrome complicating polycythemia secondary to anabolic steroid use. BMJ Case Reports, 12(6), e229004. https://doi.org/10.1136/bcr-2018-229004
- Lovelock, T., Dean, A., Saran, A., & Vasudevan, T. (2021). A case of brachial artery infected aneurysm secondary to infective endocarditis from intramuscular steroid use. Indian Journal of Vascular and Endovascular Surgery, 8(3), 283–285. https://doi.org/10.4103/ijves.ijves_123_20
- Marsh, S. (2017). More middle-aged men taking steroids to look younger. The Guardian.
- Maycock, B., & Howat, P. (2005). The barriers to illegal anabolic steroid use. Drugs-Education Prevention and Policy, 12(4), 317–325. https://doi.org/10.1080/09687630500103622
- McBride, J. A., & Coward, R. M. (2016). Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian Journal of Andrology, 18(3), 373–380. https://doi.org/10.4103/1008-682X.173938
- McCullough, D., Webb, R., Enright, K. J., Lane, K. E., McVeigh, J., Stewart, C. E., & Davies, I. G. (2021). How the love of muscle can break a heart: Impact of anabolic androgenic steroids on skeletal muscle hypertrophy, metabolic and cardiovascular health. Reviews in Endocrine & Metabolic Disorders, 22(2), 389–405. https://doi.org/10.1007/s11154-020-09616-y
- McVeigh, J., & Begley, E. (2017). Anabolic steroids in the UK: an increasing issue for public health. Drugs: Education, Prevention and Policy, 24(3), 278–285. https://doi.org/10.1080/09687637.2016.1245713
- McVeigh, J., Bates, G., & Chandler, M. (2015). Steroids and image enhancing drugs. Centre for Public Health.
- McVeigh, J., Evans-Brown, M., & Bellis, M. A. (2012). Human enhancement drugs and the pursuit of perfection. Adicciones, 24(3), 185–190.
- McVeigh, J., Hearne, E., Boardley, I. D., Bates, G., Hope, V., Ralphs, R., & Van Hout, M. C. (2021). Generating evidence on the use of Image and performance enhancing drugs in the UK: Results from a scoping review and expert consultation by the Anabolic Steroid UK network. Harm Reduction Journal, 18, 107. https://doi.org/10.1186/s12954-021-00550-z
- Monaghan, L. F. (2001). Bodybuilding, drugs and risk. london. Routledge.
- Monaghan, L. F. (2003). Danger on the doors: Bodily risk in a demonised occupation. Health, Risk & Society, 5(1), 11–31. https://doi.org/10.1080/1369857021000069814
- Moody, O. (2017). Middle-aged men use steroids to boost sex drive. The Times.
- Neri, M., Bello, S., Bonsignore, A., Cantatore, S., Riezzo, I., Turillazzi, E., & Fineschi, V. (2011). Anabolic androgenic steroids abuse and liver toxicity. Mini Reviews in Medicinal Chemistry, 11(5), 430–437. https://doi.org/10.2174/138955711795445916
- NICE. (2017). Drug misuse prevention: targeted interventions. London.
- Niedfeldt, M. W. (2018). Anabolic steroid effect on the liver. Current Sports Medicine Reports, 17(3), 97–102. https://insights.ovid.com/pubmed?pmid=29521706
- Nussbaum, M. (2011). Creating Capabilities. Harvard University Press.
- Office for National Statistics (2020). Drug misuse in England and Wales: year ending March 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/drugmisuseinenglandandwales/yearendingmarch2020
- Patil, J. J., O'Donohoe, B., Loyden, C. F., Shanahan, D., Patil, J. J., O'Donohoe, B., … Shanahan, D. (2007). Near-fatal spontaneous hepatic rupture associated with anabolic androgenic steroid use: a case report. British Journal of Sports Medicine, 41(7), 462–463. https://doi.org/10.1136/bjsm.2006.031617
- Perry, P. J., Lund, B. C., Deninger, M. J., Kutscher, E. C., & Schneider, J. (2005). Anabolic steroid use in weightlifters and bodybuilders - An internet survey of drug utilization. Clinical Journal of Sport Medicine : official Journal of the Canadian Academy of Sport Medicine, 15(5), 326–330. https://doi.org/10.1097/01.jsm.0000180872.22426.bb
- Peters, M. D. J., Godfrey, C. M., Khalil, H., McInerney, P., Parker, D., & Soares, C. B. (2015). Guidance for conducting systematic scoping reviews. International Journal of Evidence-Based Healthcare, 13(3), 141–146. https://doi.org/10.1097/xeb.0000000000000050
- Petersson, A., Bengtsson, J., Voltaire-Carlsson, A., & Thiblin, I. (2010). Substance abusers’ motives for using anabolic androgenic steroids. Drug and Alcohol Dependence, 111(1-2), 170–172. https://doi.org/10.1016/j.drugalcdep.2010.04.008
- Pope, H. G. J., & Kanayama, G. (2022). Body image disorders and anabolic steroid withdrawal hypogonadism in men. Endocrinology and Metabolism Clinics of North America, 51(1), 205–216. https://doi.org/10.1016/j.ecl.2021.11.007
- Pope, H. G. J., Kanayama, G., Athey, A., Ryan, E., Hudson, J. I., & Baggish, A. (2014). The lifetime prevalence of anabolic-androgenic steroid use and dependence in Americans: current best estimates. The American Journal on Addictions, 23(4), 371–377. https://doi.org/10.1111/j.1521-0391.2013.12118.x
- Pope, H. G. J., Kanayama, G., Hudson, J. I., & Kaufman, M. J. (2021). Anabolic-androgenic steroids, violence, and crime: two cases and literature review. The American Journal on Addictions, 30(5), 423–432. https://doi.org/10.1111/ajad.13157
- Pope, H. G. J., Kanayama, G., Ionescu-Pioggia, M., & Hudson, J. I. (2004). Anabolic steroid users’ attitudes towards physicians. Addiction, 99(9), 1189–1194. https://doi.org/10.1111/j.1360-0443.2004.00781.x
- Pope, H. G. J., Wood, R. I., Rogol, A., Nyberg, F., Bowers, L., & Bhasin, S. (2014). Adverse health consequences of performance-enhancing drugs: an endocrine society scientific statement. Endocrine Reviews, 35(3), 341–375. https://doi.org/10.1210/er.2013-1058
- Potts, A. (2000). “The Essence of the Hard On”: hegemonic masculinity and the cultural construction of “Erectile Dysfunction”. Men and Masculinities, 3(1), 85–103. https://doi.org/10.1177/1097184X00003001004
- Potts, A., Grace, V. M., Gavey, N., & Vares, T. (2004). “Viagra stories": challenging 'erectile dysfunction. Social Science & Medicine, 59(3), 489–499. https://doi.org/10.1016/j.socscimed.2003.06.001
- Potts, A., Grace, V. M., Vares, T., & Gavey, N. (2006). Sex for life’? Men’s counter-stories on 'erectile dysfunction’, male sexuality and ageing. Sociology of Health & Illness, 28(3), 306–329. https://doi.org/10.1111/j.1467-9566.2006.00494.x
- Rashid, W. (2000). Testosterone abuse and affective disorders. Journal of Substance Abuse Treatment, 18(2), 179–184. https://doi.org/10.1016/S0740-5472(99)00023-9
- Ravindran, R., Witczak, J., Bahl, S., Premawardhana, L., & Adlan, M. (2020). Myositis, rhabdomyolysis and severe hypercalcaemia in a body builder. Endocrinology Diabetes and Metabolism Case Reports, 2020, 20–0032. https://doi.org/10.1530/edm-20-0032
- Ricciardelli, L. A., & Williams, R. J. (2012). Beauty over the Centuries – Male. Elsevier Inc.
- Rosenfeld, G. A., Chang, A., Poulin, M., Kwan, P., & Yoshida, E. (2011). Cholestatic jaundice, acute kidney injury and acute pancreatitis secondary to the recreational use of methandrostenolone: A case report. Journal of Medical Case Reports, 5(1), 138. https://doi.org/10.1186/1752-1947-5-138
- Rothman, R. D., Weiner, R. B., Pope, H., Kanayama, G., Hutter, A. M., Fifer, M. A., Dec, G. W., & Baggish, A. L. (2011). Anabolic androgenic steroid induced myocardial toxicity: an evolving problem in an ageing population. Case Reports, 2011(Aug 17 1), bcr0520114280–bcr0520114280. https://doi.org/10.1136/bcr.05.2011.4280
- Rowe, R., Berger, I., Yaseen, B., & Copeland, J. (2017). Risk and blood-borne virus testing among men who inject image and performance enhancing drugs, Sydney, Australia. Drug and Alcohol Review, 36(5), 658–666. https://doi.org/10.1111/dar.12467
- Sagoe, D., Andreassen, C. S., & Pallesen, S. (2014). The aetiology and trajectory of anabolic-androgenic steroid use initiation: a systematic review and synthesis of qualitative research. Substance Abuse Treatment, Prevention, and Policy, 9, 27.
- Sagoe, D., Molde, H., Andreassen, C. S., Torsheim, T., & Pallesen, S. (2014). The global epidemiology of anabolic-androgenic steroid use: a meta-analysis and meta-regression analysis. Annals of Epidemiology, 24(5), 383–398. https://doi.org/10.1016/j.annepidem.2014.01.009
- Salinas, M., Floodgate, W., & Ralphs, R. (2019). Polydrug use and polydrug markets amongst image and performance enhancing drug users: Implications for harm reduction interventions and drug policy. The International Journal on Drug Policy, 67, 43–51. https://doi.org/10.1016/j.drugpo.2019.01.019
- Schumacher, J., Muller, G., & Klotz, K. F. (1999). Large hepatic hematoma and intraabdominal hemorrhage associated with abuse of anabolic steroids. The New England Journal of Medicine, 340(14), 1123–1124. https://doi.org/10.1056/Nejm199904083401420
- Seligman, M. E. P. (2011). Flourish: A visionary new understanding of happiness and well-being. Free Press.
- Sen, A. (1993). Capability and well-being. In M. Nussbaum & A. Sen (Eds.), The Quality of Life. (pp. 30–54). Oxford University Press.
- Shiber, J. R. (2013). Pyomyositis due to anabolic steroid injection. The Journal of Emergency Medicine, 44(1), E69–E70. https://doi.org/10.1016/j.jemermed.2011.06.008
- Shinya, U., Miessau, J., Karbowski, P., Baram, M., Cavarocchi, N. C., & Hitoshi, H. (2013). Caution for Anabolic Androgenic Steroid use: A case report of multiple organ dysfunction syndrome. Respiratory Care, 58(12), e159-163–e163. https://doi.org/10.4187/respcare.02338
- Tan, R. S., & Scally, M. C. (2009). Anabolic steroid-induced hypogonadism–towards a unified hypothesis of anabolic steroid action. Medical Hypotheses, 72(6), 723–728. https://doi.org/10.1016/j.mehy.2008.12.042
- Tashiro, K., Iso, Y., Tsujiuchi, M., Sone, H., Sasai, M., Mori, H., Wakatsuki, D., Shoji, M., Sato, T., & Suzuki, H. (2021). Subacute stent thrombosis after primary percutaneous coronary intervention in a middle-aged anabolic steroid–abusing bodybuilder. JACC. Case Reports, 3(4), 537–541. https://doi.org/10.1016/j.jaccas.2020.09.038
- Thiblin, I., Garmo, H., Garle, M., Holmberg, L., Byberg, L., Michaelsson, K., & Gedeborg, R. (2015). Anabolic steroids and cardiovascular risk: A national population-based cohort study. Drug and Alcohol Dependence, 152, 87–92. https://doi.org/10.1016/j.drugalcdep.2015.04.013
- Thornborrow, T., Onwuegbusi, T., Mohamed, S., Boothroyd, L. G., & Tovée, M. J. (2020). Muscles and the media: a natural experiment across cultures in men’s body image. Frontiers in Psychology, 11, 495. https://doi.org/10.3389/fpsyg.2020.00495
- Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K., Colquhoun, H., Kastner, M., Levac, D., Ng, C., Sharpe, J. P., Wilson, K., Kenny, M., Warren, R., Wilson, C., Stelfox, H. T., & Straus, S. E. (2016). A scoping review on the conduct and reporting of scoping reviews. BMC Medical Research Methodology, 16, 15–15. https://doi.org/10.1186/s12874-016-0116-4
- Tricco, A. C., Lillie, E., Zarin, W., O'Brien, K. K., Colquhoun, H., Levac, D., Moher, D., Peters, M. D. J., Horsley, T., Weeks, L., Hempel, S., Akl, E. A., Chang, C., McGowan, J., Stewart, L., Hartling, L., Aldcroft, A., Wilson, M. G., Garritty, C., … Straus, S. E. (2018). PRISMA extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Annals of Internal Medicine, 169(7), 467–473. https://doi.org/10.7326/M18-0850
- Underwood, M. (2017). Exploring the social lives of image and performance enhancing drugs: An online ethnography of the Zyzz fandom of recreational bodybuilders. The International Journal on Drug Policy, 39, 78–85. https://doi.org/10.1016/j.drugpo.2016.08.012
- Underwood, M., & Olson, R. (2019). ‘Manly tears exploded from my eyes, lets feel together brahs’: Emotion and masculinity within an online body building community. Journal of Sociology, 55(1), 90–107. https://doi.org/10.1177/1440783318766610
- Underwood, M., van de Ven, K., & Dunn, M. (2021). Testing the boundaries: Self-medicated testosterone replacement and why it is practised. The International Journal on Drug Policy, 95, 103087. https://doi.org/10.1016/j.drugpo.2020.103087
- van de Ven, K. (2016). ‘ Blurred lines’: Anti-doping, national policies, and the performanceand image enhancing drug (PIED) market in Belgium and TheNetherlands. Performance Enhancement & Health, 4(3-4), 94–102. https://doi.org/10.1016/j.peh.2016.03.003
- van de Ven, K., Mulrooney, K. J., & McVeigh, J. (2019). Human enhancement drugs. (1 ed.) Routledge.
- van de Ven, K., Zahnow, R., McVeigh, J., & Winstock, A. (2020). The modes of administration of anabolic-androgenic steroid users (AAS): are non-injecting people who use steroids overlooked? Drugs: Education, Prevention and Policy, 27(2), 131–135. https://doi.org/10.1080/09687637.2019.1608910
- Walker, D. M., & Joubert, H. E. (2011). Attitudes of injecting male anabolic androgenic steroid users to media influence, health messages and gender constructs. Drugs and Alcohol Today, 11(2), 56–70. https://doi.org/10.1108/17459261111174019
- Weinreb, I., Goldblum, J. R., & Rubin, B. P. (2010). Factitial soft tissue pseudotumor due to injection of anabolic steroids: a report of 3 cases in 2 patients. Human Pathology, 41(3), 452–455. https://doi.org/10.1016/j.humpath.2009.10.001
- Yesalis, C. E., & Bahrke, M. S. (1995). Anabolic-Androgenic Steroids. Sports Medicine (Auckland, N.Z.), 19(5), 326–340. https://doi.org/10.2165/00007256-199519050-00003
- Zahnow, R., McVeigh, J., Bates, G., Hope, V. D., Kean, J., Campbell, J., & Smith, J. (2018). Identifying a typology of men who use Anabolic Androgenic Steroids (AAS). The International Journal of Drug Policy, 55, 105–112. https://ac.els-cdn.com/S0955395918300616/1-s2.0-S0955395918300616-main.pdf?_tid=59ce28a0-d1f9-4505-b3ec-dd09b60d52a7&acdnat=1550157796_2b9651fce38b637e3f9ed0d6744983fd
- Zahnow, R., McVeigh, J., Ferris, J., & Winstock, A. (2017). Adverse effects, health service engagement, and service satisfaction Among Anabolic Androgenic Steroid users. Contemporary Drug Problems, 44(1), 69–83. https://doi.org/10.1177/0091450917694268