Abstract
Membrane rafts may act as platforms for membrane protein signalling. Rafts have also been implicated in the sorting of membrane components during membrane budding. We have studied by fluorescence microscopy cross-linking of ganglioside GM1 in the human erythrocyte membrane, and how membrane proteins CD47 and CD59 distribute in GM1 patched discoid cells and calcium-induced echinocytic cells. Patching of gangliosideM1 (GM1) by cholera toxin subunit B (CTB) plus anti-CTB resulted in the formation of usually 40–60 GM1 patches distributed over the membrane in discoid erythrocytes. Pre-treatment of erythrocytes with methyl-β-cyclodextrin abolished GM1 patching. GM1 patching was insensitive to pre-fixation (paraformaldehyde) of cells. Patching of GM1 did not affect the discoid shape of erythrocytes. Membrane proteins CD47 and CD59 did not accumulate into GM1 patches. No capping of patches occurred. GM1 accumulated in calcium-induced echinocytic spiculae. Also CD59, but not CD47, accumulated in spiculae. However, CD59 showed a low degree of co-localization with GM1 and frequently accumulated in different spiculae than GM1. In conclusion, our study describes a novel method for examining properties and composition of rafts. The study characterizes raft patching in the human erythrocyte membrane and emphasizes the mobility and ‘echinophilicity’ of GM1. Glycosyl phosphatidylinositol-anchored CD59 was identified as a mobile ‘echinophilic’ but ‘raftophobicGM1’ protein. Largely immobile CD47 showed no segregation.
Introduction
Induction of membrane curvature is a way for cells to simultaneously segregate membrane components and initiate vesiculation, where sorted matter and information are transported in endocytic and exocytic routes. Mobile membrane microdomains have roles in such sorting, budding and vesiculation processes Citation[1–4]. Membrane microdomains may also be important for the signalling of membrane proteins. The presence of CD47 and CD59 in membrane rafts has been reported Citation[5], Citation[6].
We recently observed that gangliosideM1 (GM1) and some integral and cytosolic proteins may distribute laterally according to erythrocyte membrane curvature Citation[7]. Accumulation of cholera toxin subunit B (CTB)-stained GM1 and proteins stomatin, synexin and sorcin to calcium-induced outward-curved spiculae in echinocytic cells was observed. Analysis of the composition of calcium-induced vesicles shed from the spiculae supported the microscopic observations Citation[7], and indicated the presence of these proteins in membrane rafts Citation[8]. It was suggested that the lateral distribution of membrane components in curved membrane is due to the molecular shape of skeleton-detached mobile membrane components and to the intermolecular interactions between the components.
To further investigate the possible preferential lateral distribution of membrane proteins to GM1-cholesterol-based microdomains and/or outward curved membrane, we have studied by fluorescence microscopy, first the characteristics of GM1 raft patching in the human erythrocyte, and subsequently the distribution of membrane proteins CD47 and CD59 in GM1 patched discoid cells and calcium-induced echinocytic (spiculated) cells. In the novel method patching of GM1 was induced by CTB plus anti-CTB. Our idea was that laterally mobile proteins showing a preferential association with GM1, i.e., lipid rafts, would be detectable in GM1-patched domains. Similarly, mobile proteins preferring membrane-skeleton free out-ward curved membrane would accumulate and be detectable in echinocytic spiculae independently from GM1 patching. The two proteins chosen for this study, CD47 and CD59 are, like GM1, stainable on the outer surface of erythrocytes. They represent different types of bilayer lipid binding and membrane anchoring, and have been reported in nucleated cells to associate with rafts Citation[5], Citation[6], Citation[9]. CD47 is a mainly membrane-skeleton associated transmembrane glycoprotein that functions as a marker of self in erythrocytes Citation[10], Citation[11]. CD59 is a mobile major erythrocyte glycosyl phosphatidylinositol (GPI)-anchored protein that inhibits complement-mediated lysis Citation[12], Citation[13]. A preliminary note on GM1 patching in human erythrocytes has been published Citation[14].
Materials and methods
Chemicals
Vybrant lipid raft labelling kit (Cholera Toxin B Alexa 555 and anti-Cholera toxin, V34404) and Alexa Fluor® 488 goat anti-mouse IgG (A11029) were from Invitrogen. Mouse anti-human IgG CD47 (556044) was from BD Pharmingen, mouse anti-human IgG CD59-FITC (MCA1054F) from Serotec, anti-CD95 (CH11) from MBL, Watertown, MA, USA and FITC conjugated mouse anti-human CD95 (clone ICO-160) a gift from Thomas Söderström, Department of Biology, Åbo Akademi University. Fish skin gelatine (Fsg, G-7041) and methyl-β-cyclodextrin (C-4555) were from Sigma.
Isolation of erythrocytes
Blood was drawn from the authors and other donors by venipuncture into heparinized tubes. Blood was washed three times with buffer (145 mM NaCl, 5 mM KCl, 4 mM Na2HPO4, 1 mM NaH2PO4, 1 mM MgSO4, 1 mM CaCl2, 10 mM glucose, pH 7.4). Erythrocytes were suspended in the buffer, stored at +4°C and used usually within 5 h.
Staining
The concentration of CTB, anti-CTB and antibodies, as well as the incubation time and temperature, giving good GM1 patching and staining, respectively, were initially determined. In a typical experiment, erythrocytes (1.65×108 cells/ml, ∼1.5% haematocrit) were blocked with Fsg (1%, 30 min, room temperature (RT)). 50 µl cell suspension was incubated with 10 µl CTB 555 (1/125, 20 min, RT). Following two washes the cell suspension was incubated with 10 µl anti-CTB (1/25, 20 min, RT) and washed twice. For double staining, 10 µl cell suspension was incubated (30 min, RT) with 10 ul anti-CD47 or 20 ul anti-CD59-FITC or anti-CD95 (1/200) and washed twice. 10 µl secondary antibody (Alexa Fluor® 488 (1/150) was incubated with the pellets (20 min, RT). Discoid cells were fixed with paraformaldehyde (PFA) and/or glutaraldehyde (GA). Following washing, cells were settled on polylysine-treated (0.1 mg/ml, 10 min) cover glasses and mounted on 80% glycerol. The cover slips were sealed with nail polish. To obtain echinocytic cells erythrocytes were treated with A23187 (2µM, 20 min, 37°C) plus calcium as previously described Citation[15]. The morphology of treated erythrocytes was followed by transmission light microscopy. Echinocytic cells were fixed in 5% PFA and 0.01% GA (60 min, RT). Treatment of erythrocytes with methyl-β-cyclodextrin (5 mM, 30 min, 37°C) was performed as previously indicated Citation[16]. Methyl-β-cyclodextrin treatment (5 mM, 20 min, 37°C) removes ∼33% of erythrocyte membrane cholesterol and result in abolishment of raft composition Citation[12].
Microscopy
Samples from several separate experiments were studied using a Leica DM RXA microscope (100×/1.4 aperture immersion oil objective, 10× ocular). Fluorescence data was not analysed in a strict quantitative way. In double staining experiments of GM1 patched cells, stained cells were compared to cells treated with the secondary antibody only. Images (single section) were acquired with a Leica DC300F CCD-camera. Where not otherwise indicated, multiple inset figures represent one and the same treatment.
Results
Discoid cells
CTB plus anti-CTB treatment formed distinct GM1 patches in the cell membrane of discoid human erythrocytes (A and B). The GM1 patches were dispersed lateral units with a spot-like appearance. The number of GM1 patches was usually 40–60 per cell in samples fixed with PFA (A, 5% PFA), assuming that all patches are detected in the haemoglobin-depleted erythrocytes (ghosts). Erythrocytes fixed with GA alone (B, 1% GA), or in addition to PFA, preserved the cell shape better than those fixed with PFA only. GA fixation hinders haemoglobin depletion. The fluorescence emitted by GA, and by haemoglobin, aided in orientation by giving a three-dimensional view of the cell. The use of GA fixative was therefore particularly advantageous when studying the distribution of membrane components in the spiculae of echinocytic cells (see below). GA preserved better than PFA the spiculae of echinocytic cells. Furthermore, GA may hinder vesiculation and arrest GM1 (rafts) and other membrane components in the tips of spiculae. A pre-fixation protocol involving GA may seem favourable because the many washing steps involved in the double-staining protocol are destructive for the morphology of echinocytic spiculae. However, GA may immobilize membrane proteins and diminish subsequent staining of membrane components by blocking binding sites. It was observed that GA in a concentration-dependent way affected the formation of GM1 patches. We found that post-fixation (60 min, RT) with 5% PFA plus 0.01% GA, gave an acceptable result. For a review on PFA and GA fixation in raft research see Citation[17].
Figure 1. Fluorescence micrographs showing (discoid) human erythrocytes treated with CTB plus anti-CTB. Erythrocytes post-fixed (60 min, RT) with either PFA (5%) or GA (1%) are shown in (A) and (B), respectively. (A inset); erythrocytes incubated with CTB only. Erythrocytes post- or pre-treated with methyl-β-cyclodextrin relative to CTB plus anti-CTB treatment are shown in (C) and (C inset), respectively. Cells were fixed with PFA (5%) and GA (0.01%). Erythrocytes fixed with PFA (5%) or PFA (5%) and GA (0.01%) prior to CTB plus anti-CTB treatment are shown in (D) and (D insets), respectively.
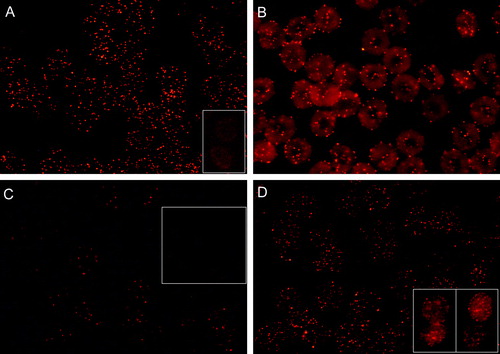
Erythrocytes treated with CTB (which may bind GM1 pentavalently) only, showed that GM1, prior to anti-CTB treatment, is uniformly (at the detection level of the fluorescence microscope) distributed over the erythrocyte membrane (A inset). Post-treatment of GM1 patched erythrocytes with methyl-β-cyclodextrin did not markedly affect the outlook of GM1 patching (C). On the other hand, little GM1 patching occurred in methyl-β-cyclodextrin pre-treated erythrocytes (C inset). This indicates that cholesterol is an important structural component of the GM1 patches. GM1 patching was rather insensitive to pre-fixation (PFA 5%) of cells (D). However, in PFA (5%) plus GA (0.01%) pre-fixed cells, besides patches also some less well defined staining occurred (D inset). CTB plus anti-CTB treatment (formation of GM1 patches) did not affect the discoid shape of erythrocytes (B).
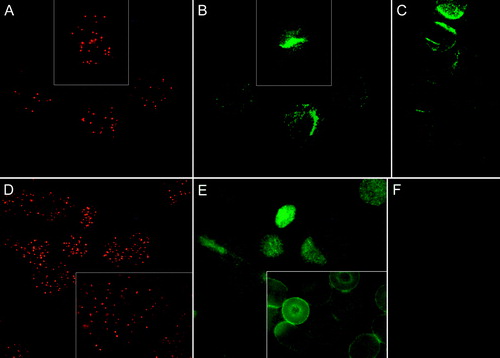
We could detect no accumulation of CD47 (A and B) or CD59 (D and E) to GM1 patches. CD47 (B and C) and CD59 (E and F) showed a homogenous patchy distribution both in the presence and absence of CTB plus anti-CTB treatment (GM1 patches). A partial co-localisation of CD47 and CD59 with GM1 cannot be excluded.
Figure 2. Fluorescence micrographs showing (discoid) human erythrocytes treated with CTB plus anti-CTB and stained for either CD47 or CD59. The CTB and CD47 signals from the same fields are indicated in (A) and (B), respectively. (C); cells stained for CD47 only. Similarly, CTB and CD59 signals from the same fields are indicated in (D) and (E), respectively. (F); cells stained for CD59 only. Cells were fixed with PFA (5%), except (D inset) and (E inset) which were fixed with PFA (5%) and GA (0.01%).
Spiculated echinocytic erythrocytes
Erythrocytes were treated with ionophore A23187 plus calcium to get echinocytic (spiculated) cells. Following staining, cells were fixed with PFA (5%) plus GA (0.01%).
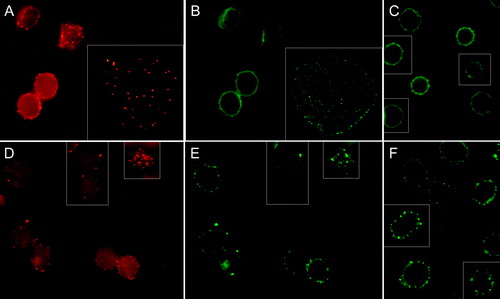
GM1 accumulated in calcium-induced echinocytic spiculae (). CD47 did not accumulate in spiculae, neither without (C) nor following (B) CTB plus anti-CTB treatment. Absence of accumulation of CD47 to GM1 patches was also indicated in studies of spread flake-like membranes occasionally formed from echinocytic cells (A inset and 4B inset). In contrast, CD59 accumulated in spiculae both in erythrocytes treated (E) and untreated (F) with CTB plus anti-CTB. However, CD59 (E) showed a low degree of co-localization with GM1 (D). CD59 often accumulated in different spiculae (membrane areas) than GM1. Pre- or post-treatment with CTB plus anti-CTB did not affect the shape-alterations induced by echinocytic and stomatocytic amphiphiles (results not shown).
Figure 3. Fluorescence micrograph (mid-plane cross-section) with insets showing echinocytic human erythrocytes treated with CTB plus anti-CTB. Cells were incubated with A23187 (2 µM, 20 min, 37°C) plus calcium to induce echinocytosis. Cells were fixed with PFA (5%) and GA (0.01%).
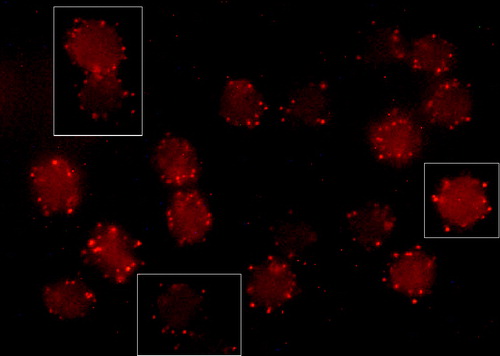
Figure 4. Fluorescence micrographs (mid-plane cross-sections) showing echinocytic human erythrocytes treated with CTB plus anti-CTB prior to staining for either CD47 or CD59. The CTB and CD47 signals from the same fields are indicated in (A) and (B), respectively. In insets spread flake-like membrane. (C); cells stained for CD47 only. Similarly, CTB and CD59 signals from the same fields are indicated in (D) and (E), respectively. (F); cells stained for CD59 only. Cells were incubated with A23187 (2 µM, 20 min, 37°C) plus calcium to induce echinocytosis. Cells were fixed with PFA (5%) and GA (0.01%).
Discussion
In the present study we have examined GM1 raft patching induced by CTB plus anti-CTB in the human erythrocyte membrane, how membrane curvature affect the distribution of GM1, and how membrane proteins CD47 and CD59 distribute in GM1 patched and curved membrane. Ionophore A23187 plus calcium was used to induce echinocytic cells carrying outward curved membrane spiculae.
GM1
The present study indicates that GM1 and cholesterol are major components of at least one type of erythrocyte membrane microdomains (lipid rafts) Citation[18–20]. The study shows that in discoid human erythrocytes highly dispersed CTB-labelled GM1 forms, following cross-linking with anti-CTB, form many distinct patches (). Interestingly, indirect immunofluorescence microscopy has revealed that in the sheep erythrocyte membrane adenylate cyclase toxin molecules form clusters Citation[21], that are distributed like the GM1 patches seen in our study. It was suggested that the cholesterol-dependent clustering of the toxin molecules occurs into membrane microdomains (rafts). An increase of GM1 raft domain size upon oligomerization of GM1 has previously been reported in nucleated cells Citation[5], Citation[22]. Notably, GM1 patches remained dispersed over the erythrocyte membrane and did not form one big patch. Why? The lateral movements of GM1 may be regionally restricted by direct or indirect interactions with the membrane skeleton [see Citation[23]]. Alternatively, the formation of many separate patches may be due to the Rabbit anti-CTB antisera used. This anti-CTB serum, which has an unknown antibody composition, might cluster CTB to a stable complex.
Our study emphasizes the ability of GM1 to accumulate in calcium-induced tubule-like echinocytic spiculae () and the un-anchored character of GM1 in these cells. Previous studies have similarly indicated enrichment of GM1 (CTB-labelled) in echinocytic spiculae Citation[7] and its presence in calcium-induced exovesicles Citation[8]. Enrichment of sphingolipid-cholesterol microdomains in curved membrane has been connected to their possible functional role in cellular and intracellular vesiculation and sorting of membrane components Citation[2–4].
CD47
Transmembrane glycoprotein CD47 functions as a marker of self in erythrocytes. Red blood cells that lack CD47 are rapidly cleared from the bloodstream by splenic red pulp macrophages. CD47 prevents this elimination by binding to the inhibitory receptor signal regulatory protein SIRPalpha Citation[10], Citation[11] However, it has also been reported that ligation of CD47 may induce phosphatidylserine exposure and loss of viability in human erythrocytes, thereby being part of a possible pathway for the induction of erythrocyte death Citation[24].
In erythrocytes CD47 is present both as a pool that is associated with the membrane skeleton Citation[25–27] and as a pool (∼30%) that is more mobile Citation[28]. It was suggested that the freely mobile pool of CD47 is of importance to accumulate CD47 in specific membrane areas in order to serve as a marker of self in contact with SIRPa-expressing phagocytic cells Citation[28]. It can be speculated that also the skeleton-associated pool of CD47 contribute to self-recognition by keeping some CD47 distributed all around the cell membrane.
Our results revealed that CD47 has a homogenous patchy distribution (B and C). Similarly, CD47 was previously shown to form small discrete spots in the erythrocyte membrane, spots that were suggested to reflect small clusters of CD47 Citation[28]. Our results indicated that CD47 does not accumulate into GM1 patches (A and B, 4A and B). In line with our results, CD47 was previously shown not to be associated with erythrocyte detergent-resistant membrane fraction (DRM) Citation[29]. In other cell types CD47 has been shown to exist both inside and outside rafts [see Citation[30]].
Our study further showed that CD47 does not accumulate in echinocytic spiculae (B and C). In accordance with our results, it was previously demonstrated that CD47 does not accumulate towards the tip of membrane skeleton-free tubular projections created in the human erythrocyte membrane by micropipette aspiration Citation[31]. CD47 mainly distributed at the base of the projection, as did the membrane skeleton, but also showed a small fraction that distributed homogenously over the membrane.
To summarize, while the major part of CD47 is immobile due to its association with the membrane-skeleton Citation[28], the mobile pool of CD47 does not have molecular properties that favour its diffusion and accumulation into membrane areas with high outward curvature or into patched GM1-containing membrane microdomains.
CD59
CD59 is a major GPI-anchored erythrocyte protein that inhibits complement-mediated lysis and has signalling properties Citation[12], Citation[32–34].
Our results showed that CD59 has a uniform patchy membrane distribution (E and F, see also Citation[35]. It has previously been reported that a part of the GPI-anchored protein pool can make small clusters in the membrane with the rest existing as ‘floating’ monomers Citation[36], Citation[37].
Our results indicated that CD59 does not accumulate into GM1 patches in discoid cells (D and E). GPI anchored proteins have previously been shown to reside outside GM1 rafts, e.g., cross-linking of GPI-linked protein Thy-1 and GM1 showed no co-localization with the other in the native membrane of mast cells Citation[38], Citation[39]. However, in Jurkat T cells CD59 was substantially concentrated in cross-linked CTB patches Citation[5]. The reason to these different results remains unclear. Eventually, they may be due to cell specific differences Citation[40].
In the study mentioned above, indicating the absence of co-localization of GPI-linked protein Thy-1 and GM1, both components were enriched in DRM Citation[38]. This shows that DRM associated GPI-linked proteins may occur outside rafts in the native membrane Citation[2], Citation[41]. Notably, human erythrocyte CD59 is also reported to accumulate in DRMs Citation[42]. Apparently, the lateral organization of GM1- and cholesterol-based membrane microdomains (rafts) is more complex and dynamic than what is predicted by biochemical analysis of DRM Citation[17], Citation[43], Citation[44]. DRM is made up of a collection of separate membrane domains with a common ability to resist detergent solubilisation Citation[35], Citation[38].
Our results further showed that CD59 accumulates into echinocytic spiculae, both in cells treated and untreated with CTB plus anti-CTB ( E and F). In line with our results, CD59 was shown to accumulate towards the tip of membrane skeleton-free projections created in the human erythrocyte membrane by micropipette aspiration Citation[31], Citation[45]. While this accumulation may be mainly due to a sterical exclusion of CD59 from the membrane skeleton occupied area Citation[28], beneficial molecular shape of CD59 may also favour its accumulation towards the tip Citation[7]. Interestingly, CD59 but not CD47 was shown to internalize into erythrocytic malarian vacuoles Citation[12], Citation[29]. The presence of CD59 in inward bend vacuoles may be due to the fact that vacuoles are large and mainly offer regions of low inward bend curvature that differs little from that of the normal membrane. Furthermore, malaria-induced interactions between CD59 and other membrane components may affect its curvature-dependence and thereby distribution.
Molecular shape-dependent accumulation of membrane proteins Citation[7], Citation[46], like CD59, into echinocytic spiculae may be one reason to they being lost (as vesicles) during RBC aging and storing Citation[47], a phenomenon that has bearing for the quality of transfused blood Citation[48].
Interestingly, and in congruence with our results showing that CD59 does not accumulate into GM1 patches in discoid cells (D and E), CD59 showed a low degree of co-localization with GM1 in echinocytic cells (D and E). CD59 appeared to mainly accumulate in different spiculae and/or in different membrane areas, than GM1.
To summarize, CD59 does not prefer partition into patched GM1-containing microdomains, although CD59 should be freely diffusible and not laterally restricted by associations with the membrane skeleton Citation[28], Citation[31]. However, CD59, like GM1, prefers partition into echinocytic spiculae, which may offer regions of skeleton-free membrane area and outward membrane curvature.
CD95
Presence of transmembrane protein CD95 (Fas/Apo-1) in human erythrocytes was recently reported Citation[49]. Aged and oxidatively stressed erythrocytes showed co-localization of CD95 with DRM. The possible functional role of CD95 in human erythrocytes remains unclear. In nucleated cells CD95 can trigger rapid apoptosis, but has also non-apoptotic functions [see Citation[50]]. Ligand binding of CD95 can induce its capping Citation[30]. Co-capping of CD95 and membrane rafts (GM1) has been observed Citation[50].
Using two antibodies previously successfully utilised to detect CD95 Citation[50], we could detect no accumulation of CD95 into GM1 patches or into calcium-induced echinocytic spiculae (results not shown). Neither was capping of CD95 (or GM1) observed. It was concluded from staining experiments with discoid control erythrocytes (positive control) that the level of CD95 in the human erythrocyte membrane may be too low for the detection protocol and technique used. However, we anticipate that a substantial accumulation of CD95 in GM1 patches or spiculae would have been detected.
Conclusion
Our study introduces the human erythrocyte as a convenient model for studying at the outer membrane surface of intact cells properties and composition of microdomain patches. The erythrocyte model can be used for distinguishing between mobile membrane components that accumulate in (raftophilicGM1) or are excluded from (raftophobicGM1) GM1 patches, and those that do (echinophilic) or do not (echinophobic) accumulate towards the tip of outward curved membrane-skeleton depleted membrane spiculae. In the present study CD59 was identified as a raftophobicGM1 but echinophilic protein. While CD47 is largely immobile, no signs of raftophilicityGM1 or echinophilicity that could be related to the mobile pool of CD47 were revealed. Notably, echinophilic GM1 and CD59 did not show a high degree of co-localization in membrane spiculae. This observation shows that carefulness is needed not to describe adjacent membrane components as co-localizing. Similarly, accumulation of membrane components and GM1 (rafts) in membrane vesicles does not indicate their co-localization. Finally, it should be noted that activation of a membrane receptor, e.g., by ligand binding, may alter its interactions with other membrane components and microdomains.
Acknowledgements
This study was supported by grants from the Research Institute of Åbo Akademi University, the Borg Foundation ÅA and the Paulo Foundation. Thomas Söderström, Christer Lindqvist, Kimmo Michelsen, Gunilla Henriksson, Esa Nummelin and Thomas Bymark are gratefully acknowledged for advice and technical assistance. Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry-US 1988; 27: 6197–6202
- Ikonen E. Roles of lipid rafts in membrane transport. Curr Opin Cell Biol 2001; 13: 470–477
- Huttner WB, Zimmerberg J. Implications of lipid microdomains for membrane curvature, budding and fission. Curr Opin Cell Biol 2001; 13: 478–484
- Sprong H, van der Sluijs P, van Meer G. How proteins move lipids and lipid move proteins. Nat Cell Biol 2001; 2: 504–513
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol 1999; 147: 447–461
- McDonald JF, Zheleznyak A, Frazier WA. Cholesterol-independent interactions with CD47 enhance alphavbeta3 avidity. J Biol Chem. 2004; 279: 17301–17311
- Hägerstrand H, Mrówczyńska L, Salzer U, Prohaska R, Michelsen K, Kralj-Iglič V, Iglič A. Curvature dependent lateral distribution of raft markers in the human erythrocyte membrane. Mol Membr Biol 2006; 23: 277–288
- Salzer U, Hinterdorfer P, Hunger U, Borken C, Prohaska R. Ca(+ + )-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood 2002; 99: 2569–2577
- Green JM, Zhelesnyak A, Chung J, Lindberg FP, Sarfati M, Frazier WA, Brown EJ. Role of cholesterol in formation and function of a signaling complex involving alphavbeta3, integrin-associated protein (CD47), and heterotrimeric G proteins. J Cell Biol 1999; 146: 673–682
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science 2000; 288: 2051–2054
- Oldenborg PA. Role of CD47 in erythroid cells and in autoimmunity. Leukemia Lymphoma 2004; 45: 1319–1327
- Samuel BU, Mohandas N, Harrison T, McManus H, Rosse W, Reid M, Haldar K. The role of cholesterol and glycosylphosphatidylinositol-anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection. J Biol Chem 2001; 276: 29319–29329
- Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check – a new role for complement regulators?. Trends Immunol 2006; 27: 102–108
- Mrówczyńska L, Hägerstrand H. Patching of ganglioside GM1 in human erythrocytes. Chem Phys Lip 2007; 149S: 34
- Hägerstrand H, Danieluk M, Bobrowska-Hägerstrand M, Iglic A, Wrobel A, Isomaa B, Nikinmaa M. Influence of band 3 protein absence and skeletal structures on amphiphile- and Ca2 + -induced shape alterations in erythrocytes – a study with lamprey (Lampetra fluviatilis), trout (Oncorhynchus mykiss) and human erythrocytes. Biochim Biophys Acta 2000; 1466: 125–138
- Bobrowska-Hägerstrand M, Wróbel A, Mrówczyńska L, Söderström T, Hägerstrand H. Modulation of MRP-like efflux activity in human erythrocytes caused by membrane perturbing agents. Mol Membr Biol 2003; 20: 255–259
- Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta 2005; 1746: 234–251
- Gomez-Mouton C, Abad JL, Mira E, Lacalle RA, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Manes S, Martinez-A C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci 2001; 98: 9642–9647
- Janich P, Corbeil D. GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett 2007; 581: 1783–1787
- Fujita A, Cheng J, Hirakawa M, Furukawa K, Kusunoki S, Fujimoto T. Gangliosides GM1 and GM3 in the living cell membrane form clusters susceptible to cholesterol depletion and chilling. Mol Biol Cell 2007; 18: 2112–2122
- Vojtová J, Kofronová O, Sebo P, Benada O. Bordetella adenylate cyclase toxin induces a cascade of morphological changes of sheep erythrocytes and localizes into clusters in erythrocyte membranes. Microsc Res Tech 2006; 69: 119–129
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 1998; 141: 929–942
- Lillemeier BF, Pfeiffer JR, Surviladze Z, Wilson BS, Davis MM. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc Natl Acad Sci 2006; 103: 18992–18997
- Head DJ, Lee ZE, Swallah MM, Avent ND. Ligation of CD47 mediates phosphatidylserine expression on erythrocytes and a concomitant loss of viability in vitro. Br J Haematol 2005; 130: 788–790
- Bruce LJ, Beckmann R, Ribeiro ML, Peters LL, Chasis JA, Delaunay J, Mohandas N, Anstee DJ, Tanner MJ. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 2003; 101: 4180–4188
- Dahl KN, Parthasarathy R, Westhoff CM, Layton DM, Discher DE. Protein 4.2 is critical to CD47-membrane skeleton attachment in human red cells. Blood 2004; 103: 1131–1136
- Subramanian S, Tsai R, Sen S, Dahl KN, Discher DE. Membrane mobility and clustering of Integrin Associated Protein (IAP,CD47) – major differences between mouse and man and implications for signaling. Blood Cell Mol Dis 2006; 36: 364–372
- Dahl KN, Westhoff CM, Discher DE. Fractional attachment of CD47 (IAP) to the erythrocyte cytoskeleton and visual colocalization with Rh protein complexes. Blood 2003; 101: 1194–1199
- Murphy SC, Samuel BU, Harrison T, Speicher KD, Speicher DW, Reid ME, Prohaska R, Low PS, Tanner MJ, Mohandas N, Haldar K. Erythrocyte detergent-resistant membrane proteins: Their characterization and selective uptake during malarial infection. Blood 2004; 103: 1920–1928
- Manna PP, Dimitry J, Oldenborg PA, Frazier WA. CD47 augments Fas/CD95-mediated apoptosis. J Biol Chem 2005; 280: 29637–29644
- Discher DE, Mohandas N, Evans EA. Molecular maps of red cell deformation: hidden elasticity and in situ connectivity. Science 1994; 266: 1032–1035
- Meri S, Morgan BP, Davies A, Daniels RH, Olavesen MG, Waldmann H, Lachmann PJ. Human protectin (CD59), an 18,000–20,000 MW complement lysis restricting factor, inhibits C5b-8 catalysed insertion of C9 into lipid bilayers. Immunology 1990; 71: 1–9
- Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol 1990; 144: 3478–3483
- Kimberley FC, Sivasankar B, Paul Morgan B. Alternative roles for CD59. Mol Immunol 2007; 44: 73–81
- Chatterjee S, Mayor S. The GPI-anchor and protein sorting. Cell Mol Life Sci 2001; 58: 1969–1987
- Varma R, Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 1998; 394: 798–801
- Sharma P, Varma R, Sarasij RC, Ira, Gousset K,Krishnamoorthy G, Rao M, Mayor S. 2004. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116:577–589.
- Wilson BS, Steinberg SL, Liederman K, Pfeiffer JR, Surviladze Z, Zhang J, Samelson LE, Yang LH, Kotula PG, Oliver JM. Markers for detergent-resistant lipid rafts occupy distinct and dynamic domains in native membranes. Mol Biol Cell 2004; 15: 2580–2592
- Kenworthy AK, Petranova N, Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell 2000; 11: 1645–1655
- Shaikh SR, Edidin MA. Membranes are not just rafts. Chem Phys Lip 2006; 144: 1–3
- Zajchowski LD, Robbins SM. Lipid rafts and little caves: Compartmentalized signalling in membrane microdomains. Eur J Biochem 2002; 269: 737–752
- Lauer S, VanWye J, Harrison T, McManus H, Samuel BU, Hiller NL, Mohandas N, Haldar K. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J 2000; 19: 3556–3564
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci 2005; 30: 430–436
- Brown DA. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 2006; 21: 430–439
- Knowles DW, Tilley L, Mohandas N, Chasis JA. Erythrocyte membrane vesiculation: model for the molecular mechanism of protein sorting. Proc Natl Acad Sci 1997; 94: 12969–12974
- Kralj-Iglič V, Heinrich V, Svetina S, Žekš B. Free energy of closed membrane with anisotropic inclusions. Eur Phys J 1999; 10: 5–8
- Pascual M, Danielsson C, Steiger G, Schifferli JA. Proteolytic cleavage of CR1 on human erythrocytes in vivo: Evidence for enhanced cleavage in AIDS. Eur J Immunol 1994; 24: 702–708
- Högman CF, Meryman HT. Red blood cells intended for transfusion: Quality criteria revisited. Transfusion 2006; 46: 137–142
- Mandal D, Mazumder A, Das P, Kundu M, Basu J. Fas-, caspase 8-, and caspase 3-dependent signaling regulates the activity of the aminophospholipid translocase and phosphatidylserine externalization in human erythrocytes. J Biol Chem 2005; 280: 39460–39467
- Söderström TS, Nyberg SD, Eriksson JE. CD95 capping is ROCK-dependent and dispensable for apoptosis. J Cell Sci 2005; 118: 2211–2223
