Abstract
Background and objective: Renal ischaemia reperfusion injury (IRI), characterized by excessive cell apoptosis and inflammation, remains a clinical challenge. Mitochondrial membrane potential is related to apoptosis and inflammation of IRI. Previous studies have indicated that uncoupling protein 2 (UCP2) and its receptors play an important role in inflammation, apoptosis and injuries, especially in oxidative stress injury. However, the underlying mechanisms of UCP2 in IRI are still not fully understood.
Methods and results: In the present study, male C57 mice were randomly divided into three groups:sham, IR, and UCP2-/-+IR. The IRI model was established by removing the right kidney and clamping the left kidney for 45 min followed by reperfusion. Blood urea nitrogen (BUN) and creatinine were higher in UCP2-/-+IR mouse serum than in IR mouse serum. In addition, relative to the IR group, UCP2-/-+IR mouse renal cells had increased reactive oxygen species (ROS) production, aggravating tissue damage. We examined changes in the NFκB pathway and found that after UCP2 knockdown, IκB and IKK phosphorylation increased, and nuclear NFκB increased, which stimulated inflammation. Moreover, there was an increase in apoptosis in the UCP2-/-+IR group.
Conclusion: UCP2 can prevent IRI in C57 mice. Mechanistically, UCP2 may decrease ROS expression, NFκB activation and caspase-3 cleavage, rendering UCP2 a potential therapeutic target against IRI.
Introduction
Renal ischaemia reperfusion injury (IRI) is a common clinical complication of renal impairment, which often occurs in renal transplantation, shock and heart failure (Day et al., Citation2006; Lee et al., Citation2004b). Although many studies have focused on the development of IRI, the molecular mechanism of IRI is still unclear. Generally, IRI occurs due to a general or partial lack of oxygen and nutrient transport, which leads to the accumulation of nitrogenous waste, including blood urea nitrogen (BUN) and creatinine (Wang et al., Citation2018). In addition, renal tubular epithelial cells are damaged, and apoptosis and necrosis occur in severe cases. There is growing evidence that reactive oxygen species (ROS) in renal tubular epithelial cells are the main cause of IRI; oxidative stress theory demonstrates that ROS are produced in the renal tissue due to the ischaemia and reperfusion state, and ROS are thought to be minuscule molecules such as the superoxide anion (O−2), hydroxyl radicals (-OH) and hydroperoxides (H2O2), which are mainly produced in mitochondria; this leads to lipid peroxidation, inflammatory reactions and apoptosis (Moniruzzaman et al., Citation2018; Simon et al. Citation2000; Solati et al., Citation2018).
Mitochondrial membrane potential has been linked to apoptosis and inflammation of IRI (Ji et al., Citation2017). Previous studies have shown that the mitochondrial electron transport chain is the primary source of ROS production in cells (Ruiz-Ramírez et al. Citation2011; Zorov et al., Citation2000). Uncoupling protein 2 (UCP2) has received much attention in recent years due to its role in ROS. (Kawanishi et al., Citation2018). The mitochondrial inner membrane protein UCP2 is involved in electron transport (Pecqueur et al., Citation2008). As a stress protein, UCP2 production is limited by negative feedback regulation when intracellular ROS is upregulated (Ruiz-Ramírez et al., Citation2011). It has been shown that overexpression of UCP2 can inhibit cell inflammation and apoptosis (Deng et al., Citation2012; Derdak et al., Citation2007). However, it is still unclear whether UCP2 can alleviate IRI by downregulating ROS.
Therefore, in this study, we decided to use the IRI mouse model to investigate the regulation of UCP2 on renal IRI and its mechanism.
Materials and methods
Animals
All experimental procedures were approved by the Institutional Animal Care and Use Committee and the Ethics Committee of General Hospital of the Western War Zone of the Chinese People's Liberation Army (Chengdu, Sichuan, China). Twelve male C57 mice and 6 male UCP2−/− C57 mice, weighing 16–24 g, were obtained from Chengdu Dashuo Biotechnology Co., Ltd., and the experimental animal license number was: SCXK 2008–24. Mice were randomly divided into the sham group (n = 6), IR group (n = 6) and UCP2−/−+IR group (n = 6). Humane research was conducted according to the 3 R principle used in animal experiments.
Mouse model of kidney ischaemia reperfusion injury
The model was performed as Y.J. Day (Day et al., Citation2006) described, Briefly, the bilateral renal pedicle vessels were exposed and bluntly separated from the back incision. In the wild-type IR group and UCP2−/−+IR group, the right kidney was excised and the left kidney was clamped for 45 min to restore blood supply. Sham-operated animals underwent identical surgery for renal pedicle isolation without pedicle occlusion.
Changes in serum creatinine and urea nitrogen levels
Blood samples were collected from the abdominal aorta 24 h after reperfusion and clotted at room temperature 2 h, The blood was centrifuged at 4000 rpm for 20 min, and the serum was separated. The renal function was determined using levels of serum creatinine and blood urea nitrogen (BUN) (JL18267, JL20491, Jonln, China.)
Morphological changes of renal tissue
The injured left kidney tissues from C57 mice were cleared of blood with ice-cold PBS and kept in 4% paraformaldehyde for 24 h at 4 °C, sliced into 4-µm thick sections with a cryostat. Six paraffin sections (4 µm) were randomly selected for haematoxylin and eosin (HE) staining. Pathological changes of the kidneys were observed under a light microscope (Leica, DM3000, Germany).
Apoptosis detection
Next, 6 paraffin sections (4 µm) for each mouse were randomly selected, routinely dewaxed and rehydrated, and then renal cell apoptosis was measured by using the fluorescent terminal deoxynucleotidyl transferase (dUTP) nick-end labelling (TUNEL) assay kit (Dr. Dekker, MK1020, China). Microscopy (DM3000, Leica) was used to observe the distribution of apoptotic cells in the kidney and Image-Pro Plus was used to measure the proportion of apoptotic cells.
Immunofluorescence analysis
Three groups of mice were selected, and the left kidneys were treated 24 h after surgery. Normal saline was used to rapidly flush intravascular blood. Six frozen sections (8 µm) were randomly selected for each mouse and washed twice in PBS for 5 min each time. Then, 2% Triton-100 was added for 10 min at room temperature, and the sections were washed twice in PBS for 5 min each time. The final concentration of DHE (Biyuntian, S0063, China) was adjusted to 10 µM and incubated with the sections at 37 °C for 60 min with 5% BSA 37 °C for 30 min. The cells were washed 3 times with PBS for 5 min each time. Fluorescence microscopy (Olympus, IX81, Japan) was performed at a wavelength of 488 nm, and Image-Pro Plus was used to plot and calculate the positive area expression ratio.
Western blot analysis
Three groups of mice were selected and the left kidneys were treated 24 h after surgery. The blood was rapidly washed in physiological saline and quickly stored in liquid nitrogen. The total proteins were extracted using the KGP protein extraction kit (KGP2100, China), and protein quantification was performed using the BCA method (Bi Yuntian, P0006, China). The proteins were separated by SDS-PAGE and blotted onto a pre-wet PVDF membrane (Millipore HVLP14250, USA). The membrane was blocked with 5% skim milk powder at room temperature for 60 min; the primary antibody rabbit anti-mouse cleaved caspase-3 (Abcam, ab-2302, UK), rabbit anti-mouse UCP2 (CST, 89326, USA), rabbit anti-mouse IKK (Abcam, ab-178870, UK), rabbit anti-mouse p-IKK (Abcam, ab-55341, UK), rabbit anti-mouse IκB (Abcam, ab-32518, UK), rabbit anti-mouse p-IκB (Abcam, ab-133462, UK), rabbit anti-mouse NFκB (Abcam, ab-16502, UK), rabbit anti-mouse GAPDH (Abcam, ab-9485, UK), or rabbit anti-mouse H3 (Abcam, ab-1791, UK) was incubated at room temperature overnight at 4 °C for 30 min; secondary antibody (Proteintech, SA00001-2, USA) goat anti-rabbit 1:4000 was incubated at room temperature for 1 h; the blot was exposed in a dark room, images were collected and quantified with a gel imaging system (UVP, EC3300, USA) with the greyscale values for each group.
RT-qPCR analysis
Mice renal RNA was extracted with Trizol(Life Technologies Invitrogen, Shanghai, China), reverse transcribed (Takara, Dalian, China) into cDNA and used as a template for the amplification of UCP2. The following primer sequences were used: UCP2 forward primer, 5′-ATG GTT GGT TTC AAG GCC ACA-3′ and reverse primer, 5′-TTG GCG GTA TCC AGA GGG AA-3′; GAPDH forward primer, 5′-AGG TCG GTG TGA ACG GAT TTG-3′ and reverse primer, 5′-GGG GTC GTT GAT GGC AAC A-3′. The amplification was performed by quantitative real-time PCR by using SYBR Green Master Mix according to the manufacturer’s instructions (SYBR Real-Time PCR Kit, Takara, Dalian, China). Relative expressions of target genes were standardized to GAPDH, evaluated by the 2−ΔΔCT method and given as a ratio to normalize the experiments.
Statistical analysis
The results were analysed using the SPSS (Inc., Chicago, IL, USA) software. Data were expressed as the mean ± standard deviation (±S). One-way ANOVA was used to compare three groups, and the independent sample t test was used to compare two groups. p < 0.05 was considered statistically significant.
Results
UCP2 expression after ischaemia reperfusion injury
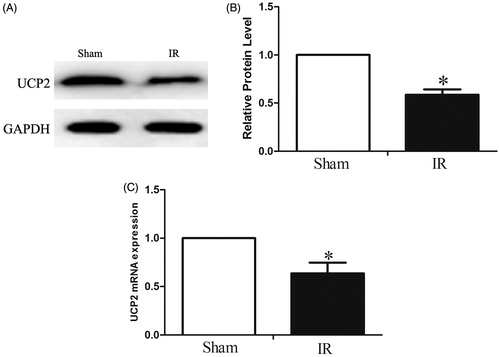
To investigate the role of UCP2 in IRI, we examined changes in the expression of UCP2 after renal tissue IRI. We first used RT-PCR and Western blotting to detect the expression of UCP2 after IRI. The results showed that UCP2 gene expression was significantly downregulated after IR compared with the sham-operated group (), decreased by 0.64 ± 0.11-fold (p < 0.05). On the other hand, UCP2 protein expression was significantly decreased after IR (), decreased by 0.59 ± 0.56-fold (p < 0.05). indicating that the impairment of UCP2 expression by IRI occurred at both the post-translational and transcriptional levels.
Figure 1. Detection of UCP2 changes in renal tissue by Western Blotting. AB: Downregulation of UCP2 protein expression after IR. C: Downregulation of UCP2 gene expression after IR. (The internal reference is GAPDH. The greyscale value of each group is compared with the internal reference, and the obtained result is compared with the sham group. In each group of six mice, the data are expressed as the mean ± standard deviation (±S). *p < 0.01, compared with sham.).
UCP2 protects renal function in IRI mice
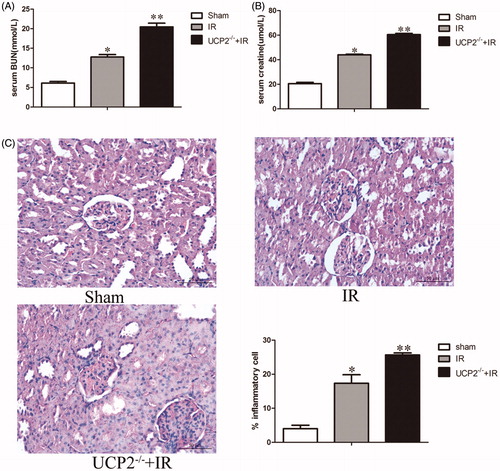
The expression levels of creatinine and BUN in serum are important indicators of renal function. Normally, impaired renal function leads to a sharp increase in serum creatinine and BUN (Wang et al., Citation2019). The results showed that the creatinine in the IR group was 43.93 ± 0.62 umol/L, which was 2.14 ± 0.13-fold (p < 0.05) in the sham operation group (. In the UCP2−/−+IR group, creatinine increased further to 60.41 ± 1.00 umol/L, which was 1.38 ± 0.04-fold (p < 0.05) in the IR group (. On the other hand, the BUN in the IR group was 12.75 ± 0.63 mmol/L, which was 2.09 ± 0.05-fold that of the sham-operated group (p < 0.05). In the UCP2−/−+IR group, serum BUN content was 20.78 ± 0.92 mmol/L, which was 1.61 ± 0.10-fold (p < 0.05) in the IR group (. The results showed that the IRI model was successfully established, and knockout the UCP2 gene significantly aggravated IR damage, suggesting that UCP2 can protect renal function in IRI mice.
Figure 2. UCP2−/− induced renal ischaemia reperfusion injury (IRI) aggravation. A, B: ELISA determination of serum creatinine and BUN in 3 groups. (For the six mice in each group, data are expressed as the mean ± standard deviation (±S). The upper panel * is p < 0.01, compared with sham; ** is p < 0.01, compared with IR group.) C: Pathological changes in the kidneys of mice in each group after ischaemia-reperfusion.
UCP2 alleviates renal tissue injury
Renal tissue pathology can directly reflect the degree of renal tissue damage and the location of lesions (Solati et al., Citation2018). HE staining showed renal tubular epithelial cell degeneration, water-like lesions, infiltration of inflammatory cells around the renal tubules, interstitial cells, and partial apoptosis in the IR group; interstitial oedema in the UCP2−/−+IR group was greater than that in the IR group. Inflammatory cell infiltration and apoptosis also increased (. After UCP2 knockout, inflammatory cell infiltration was 1.50 ± 0.19-fold (p < 0.05) in the IR group, suggesting that UCP2 can protect renal tissue by reducing inflammation.
UCP2 downregulates ROS expression in renal tissue
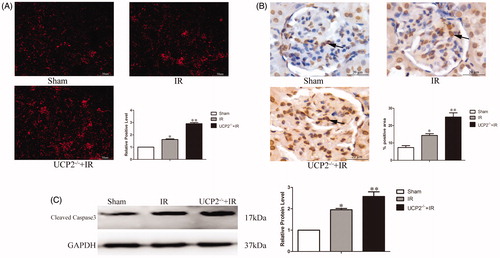
ROS is a key indicator of oxidative stress. It can directly reflect the content and localization of ROS tissue and is used to judge the severity of oxidative stress damage in tissues (Hee & Seung-Hyo, Citation2018). Immunofluorescence DHE staining indicated ROS (the red area is a positive expression). The results showed that ROS levels in the IR group were significantly higher than those in the sham group, up to 1.16 ± 0.05-fold (p < 0.01). Compared with the IR group, the UCP2−/−+IR group showed significantly increased ROS expression, increased by 1.79 ± 0.06-fold (p < 0.01) (. It is suggested that UCP2 may alleviate renal IRI by decreasing ROS expression.
Figure 3. (A) Immunofluorescence determination of ROS expression in the kidneys of 3 groups. (Six mice in each group, data are expressed as the mean ± standard deviation (±S). The upper panel * is p < 0.01, compared with sham; ** is p < 0.01, compared with the IR group, × 200) (B) Immunohistochemical detection of apoptotic cell content in the three groups. (The black arrows in the figure indicate areas of apoptosis-positive cells; in each group six mice, the data are expressed as the mean ± standard deviation (±S). The upper panel* is p < 0.01, compared with sham; ** is p < 0.01, compared with UCP2-/-+IR group. ×200) (C) Detection of cleaved caspase-3 expression in the kidneys by Western blotting. (The internal reference is GAPDH. The greyscale value of each group is compared with the internal reference, and the obtained result is compared with the sham group. In each group of six mice, the data are expressed as the mean ± standard deviation (±S). *p < 0.01, compared with sham; **p < 0.01 compared to IR group.).
UCP2 promotes renal cell apoptosis
The cleavage of chromosomal DNA in apoptosis is a gradual, phased process in which chromosomal DNA is first degraded to a large fragment of 50–300 kb by the action of an endogenous nuclease (Lin et al., Citation2014). The cleavage DNA will be exposed to –OH, and the apoptosis can be accurately quantified and localized by the Terminal-deoxynucleotide transferase Mediated Nick End Labeling (TUNEL) method (Lee et al., Citation2004a). TUNEL staining marked apoptotic cells (positive expression in the brown area), mainly concentrated in the glomerular part. The results showed that compared with the sham group, the apoptotic cells in the IR group increased significantly (p < 0.05), up to 1.98 ± 0.43-fold. apoptosis increased significantly in the UCP2−/−+IR group compared with the IR group (p < 0.01), up to 1.74 ± 0.10-fold (. It is suggested that UCP2 may reduce IRI by down-regulating renal tissue apoptosis.
UCP2 decreases cleavage of apoptotic protein cleaved caspase-3 and activation of NFκB
Caspase3 is involved in the process of cell apoptosis. Cleaved caspase-3 specifically cleaves DNApoly (ADP-ribose) polymerase, an enzyme involved in DNA repair and gene integrity monitoring. The study found that cleaved caspase-3 is highly expressed in apoptotic cells. Therefore, by detecting the content of cleaved caspase-3 in tissues, it can reflect the severity of apoptosis in tissues (Seong et al., Citation2017). Western blot analysis detected changes in apoptotic cleaved caspase-3 protein. The results showed that compared with the IR group, the UCP2−/−+IR group significantly increased the expression of cleaved caspase-3 (p < 0.05), increased by 1.32 ± 0.14-fold (. It is suggested that UCP2 can reduce the cleaved caspase-3 to alleviate IRI.
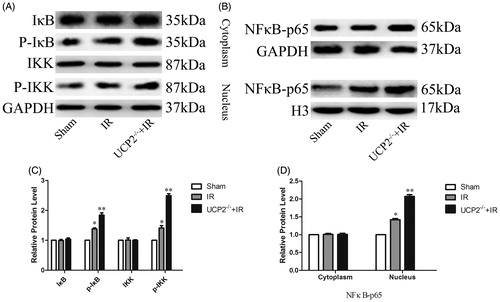
UCP2 protects renal function by inhibiting NFκB
IRI-induced ROS increase activates the inflammatory response, and studies have shown that ROS is closely related to the NFκB signaling pathway, but the mechanism is unknown. Typically, the NFκB signaling pathway is activated by stress, pro-inflammatory cytokines and antigen receptors (Kemmner and Bachmann Citation2019). During the whole conduction process, NFκB protein is bound and inhibited by IκB protein. After activation by stress reaction, IKK complex phosphorylates, and this complex can phosphorylate IκB protein. Phosphorylation of IκB releases NFκB, and NFκB released as a transcription factor enters the nucleus from the cytoplasm and binds to the target gene, regulating cell inflammation and expression of apoptotic proteins (Rostasy et al., Citation2000). By detecting key molecules of the NFκB signaling pathway, the distribution of various factors in the cytoplasm and nucleus can be clearly observed. Western blot analysis detected changes in the NFκB pathway. NFκB expression in the IR group was significantly higher than that in the sham group (p < 0.05). In addition, the results showed that compared with the IR group, the UCP2-/-+IR group significantly increased the expression of NFκB. These results suggest that UCP2 may protect renal function by inhibiting NFκB (p < 0.05) (). It is suggested that UCP2 can alleviate IR inflammatory injury by down-regulating NFκB signaling pathway.
Figure 4. Detection of NFκB pathway changes in renal tissue by Western blotting. AC: UCP2 prevents IRI by inhibiting cytoplasmic IκB and IKK phosphorylation. BD: UCP2 inhibits NFκB. (The internal references are GAPDH (cytoplasm) and histone H3 (nucleus). The greyscale value of each group is compared with the internal reference, and the obtained result is compared with the sham group. In each group of six mice, the data are expressed as the mean ± standard deviation (±S). *p < 0.01, compared with sham; **p < 0.01 compared to IR group).
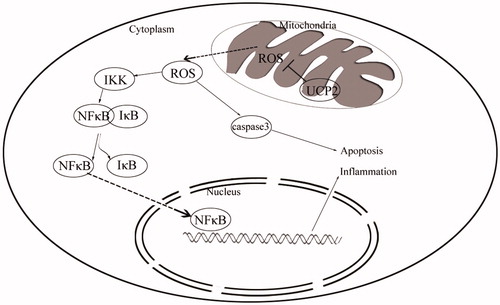
The molecular mechanism by which UCP2 alleviates IRI
We combined a simplified diagram of the protective mechanism of UCP2 on IRI. We observed a dramatic increase in ROS in kidney tissue after IR. Kidney tissue damage is severe due to oxidative damage of ROS to biological macromolecules. We knocked out the UCP2 gene and found that renal tissue damage and ROS were increased in the knockdown group compared to that in the IR group. The NFκB pathway was detected in the cells. IKK/IκB was activated by phosphorylation after IR, and NFκB bound to IκB was separated and localized the nucleus. At the same time, we detected an increase in nuclear NFκB after UCP2 gene knockout compared with that in the IR group, thereby activating the downstream inflammatory pathway and aggravating the damage, which is consistent with the BNU and creatinine levels detected in serum. In addition, we observed that the expression of cleaved caspase-3 in cells also showed an increase in apoptosis after UCP2 gene knockout ().
Discussion
We have identified the function of UCP2 in attenuating renal IRI through the reduction of ROS and subsequent inhibition of the NFκB pathway. Renal ischaemia reperfusion is a common cause of renal injury, with a high incidence and poor prognosis. Its pathogenesis involves oxidative stress, inflammation, and apoptosis (Donnahoo et al., Citation1999; Lee et al., Citation2004b). The oxidative stress theory accounts for an important part, and the main starting point is determining how to effectively eliminate the free radical and ROS generated after reperfusion. Previous reports have shown that a large amount of ROS is produced after IRI, leading to tissue damage and apoptosis (Indran et al., Citation2011; Kelly Citation2003). We detected apoptotic cells and apoptotic proteins, The results showed that IRI can effectively upregulate the production of the apoptotic protein caspase 3. Some studies have focused on the use of proprietary Chinese medicines for the antioxidation and anti-apoptosis relief of IRI, but the effects of these exogenous treatments are uneven and have a detrimental effect on other tissues (Bo et al., Citation2015; Hill et al., Citation2008; Lin et al., Citation2003). Thus, the activation of endogenous protection is still the focus of research. Although there are certain mitigating effects, the effects are still not obvious, and the surgery is not stable enough to be effectively used in clinical research.
A large number of studies have shown that UCP2 can inhibit cell inflammation and apoptosis by downregulating ROS (Derdak et al., Citation2009). UCP2 as a mitochondrial uncoupling protein can effectively downregulate ROS (Bhattacharya et al., Citation2018; Brand & Esteves, Citation2005). In vitro and in vivo experiments have shown that the reduction of reactive oxygen species caused by the uncoupling effect of UCP2 can reduce the damage of ROS to cells and have a protective effect on cells and tissues (Deng et al., Citation2012; Derdak et al., Citation2007; Pecqueur et al., Citation2008). However, the role of UCP2 in IRI remains unclear, and the discussion of the mechanism is insufficient. Another study showed that UCP2 overexpression inhibits ROS production and protects tissues from oxidative stress damage (Kukat et al., Citation2014). This study showed that UCP2 protects renal function by reducing BUN and creatinine. This suggests the potential application value of UCP2 in IRI. Many studies have applied UCP2 to oxidative stress and inflammation-related diseases, and its protective effect on tissue damage has been widely recognized (Xu et al., Citation2015). It has been reported that UCP2 regulates NFκB-dependent inflammatory responses and promotes NFκB nuclear regulation of the transcription of inflammatory mediators (Bai et al. Citation2005; Hee & Seung-Hyo, Citation2018; Kelly et al., Citation2006). Therefore, UCP2 plays an important role in the inflammatory response. The results of this experiment indicate that UCP2 can downregulate IκB and IKK phosphorylation and reduce the inflammatory response of tissues by regulating the NFκB pathway. However, these studies lack cell experiments. It is still unclear which specific cell types use UCP2, and in vivo studies are a big challenge in the next step.
In summary, UCP2 can be used as a new potential target for alleviating renal injury after IRI. The mechanism may be to downregulate ROS expression and thereby inhibit apoptosis and the NFκB signalling pathway, which could be a pharmacological target for the treatment of renal ischaemia reperfusion injury.
Acknowledgements
We thank Jiong Yang, Xiangyun Wu, for assistance during establishing the ischaemia injury model.
Disclosure statement
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Additional information
Funding
References
- Bai Y, Onuma H, Xu B, Medvedev AV, Misukonis M, Weinberg JB, et al. 2005. Persistent NF-κB activation in Ucp2-/-mice leads to enhanced nitric oxide and inflammatory cytokine production. J Biol Chem 280:19062–19069.
- Bhattacharya R, Singh P, John JJ, Gujar NL. 2018. Oxidative damage mediated iNOS and UCP-2 upregulation in rat brain after sub-acute cyanide exposure: dose and time-dependent effects. Drug Chem Toxicol 1–8.
- Bo J, Xie S, Guo Y, Zhang C, Guan Y, Li C, et al. 2015. Methylglyoxal impairs insulin secretion of pancreatic Î2-Cells through increased production of ROS and mitochondrial dysfunction mediated by upregulation of UCP2 and MAPKs. J Diabetes Res 2016:2029854.
- Brand MD, Esteves TC. 2005. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab 2:85–93.
- Day Y-J, Huang L, Ye H, Li L, Linden J, Okusa MD. 2006. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. J Immunol 176:3108.
- Deng S, Yang Y, Han Y, Li X, Wang X, Li X, et al. 2012. UCP2 inhibits ROS-mediated apoptosis in A549 under hypoxic conditions. PLoS One 7:e30714.
- Derdak Z, Garcia TA, Baffy G. 2009. Detection of Uncoupling Protein-2 (UCP2) as a mitochondrial modulator of apoptosis. Methods Mol Biol 559:205–217.
- Derdak Z, Mark N, Wands J, Baffy G. 2007. Mitochondrial uncoupling protein-2 inhibits oxidative stress and p53-mediated apoptosis in HCT116 cells. Cancer Res 67:3617–3617.
- Donnahoo KK, Shames BD, Harken AH, Meldrum DR. 1999. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol 162:196–203.
- Hee KY, Seung-Hyo L. 2018. TGF-β/SMAD4 mediated UCP2 downregulation contributes toAspergillusprotease-induced inflammation in primary bronchial epithelial cells. Redox Biol 18:104–113.
- Hill P, Shukla D, Tran MGB, Aragones J, Cook HT, Carmeliet P, Maxwell PH. 2008. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 19:39–46.
- Indran IR, Hande MP, Pervaiz S. 2011. hTERT overexpression alleviates intracellular ROS production, improves mitochondrial function, and inhibits ROS-mediated apoptosis in cancer cells. Cancer Res 71:266–276.
- Ji F, Shen T, Zou W, Jiao J. 2017. UCP2 Regulates Embryonic Neurogenesis via ROS‐mediated Yap Alternation in the Developing Neocortex. Stem Cells 35:1479–1492.
- Kawanishi M, Fukuda T, Shimomura M, Inoue Y, Wada T, Tasaka R, et al. 2018. Expression of UCP2 is associated with sensitivity to platinum-based chemotherapy for ovarian serous carcinoma. Oncol Lett 15:9923.
- Kelly KJ. 2003. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14:1549–1558.
- Kelly M, Ruderman NB, Tomas E. 2006. AMP-activated protein kinase and its regulation by adiponectin and interleukin-6. Food Nutr Res 50:85–91.
- Kemmner S, Bachmann Q. 2019. Normothermic machine perfusion may prevent regulated cell death following renal ischemia-reperfusion injury. Am J Transplant. 19(4):1245.
- Kukat A, Dogan SA, Edgar D, Mourier A, Jacoby C, Maiti P, et al. 2014. Loss of UCP2 attenuates mitochondrial dysfunction without altering ROS production and uncoupling activity. PLoS Genetics 10:e1004385.
- Lee HT, Gallos G, Nasr SH, Emala CW. 2004a. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal Ischemia-Reperfusion injury in mice. J Am Soc Nephrol 15:102–111.
- Lee HT, Ota-Setlik A, Fu Y, Nasr SH, Emala CW. 2004b. Differential protective effects of volatile anesthetics against renal ischemia-reperfusion injury in vivo. Anesthesiology 101:1313–1324.
- Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, et al. 2003. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal Ischemia-Reperfusion injury in mice. J Am Soc Nephrol 14:1188–1199.
- Lin M, Li L, Li L, Pokhrel G, Zhu T. 2014. The protective effect of baicalin against renal ischemia-reperfusion injury through inhibition of inflammation and apoptosis. BMC Complem Altern Med 14:19.
- Moniruzzaman M, Ghosal I, Das D, Chakraborty SB. 2018. Melatonin ameliorates H 2 O 2 -induced oxidative stress through modulation of Erk/Akt/NFkB pathway. Biol Res 51:17.
- Pecqueur C, Bui T, Gelly C, Hauchard J, Barbot C, Bouillaud F, et al. 2008. Uncoupling protein-2 controls proliferation by promoting fatty acid oxidation and limiting glycolysis-derived pyruvate utilization. Faseb J 22:9–18.
- Rostasy K, Monti L, Yiannoutsos C, Wu J, Bell J, Hedreen J, et al. 2000. NFKB activation, TNF-α expression, and apoptosis in the AIDS-Dementia-Complex. J Neurovirol 6:537–543.
- Ruiz-Ramírez A, Chávez-Salgado M, Peñeda-Flores JA, Zapata E, El-Hafidi M. 2011. High-sucrose diet increases ROS generation, FFA accumulation, UCP2 level, and proton leak in liver mitochondria. Am J Physiol Endocrin Metab 301:E1198–1207.
- Seong H, Ryu J, Yoo W-S, Kim SJ, Han Y‑S, Park JM, et al. 2017. Resveratrol ameliorates retinal ischemia/reperfusion injury in C57BL/6J mice via downregulation of caspase-3. Curr Eye Res 42:1650–1659.
- Simon HU, Haj-Yehia A, Levi-Schaffer F. 2000. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 5:415–418.
- Solati Z, Edel AL, Shang Y, O K, Ravandi AZ. 2018. Oxidized phosphatidylcholines are produced in renal ischemia reperfusion injury. PLoS One 13:e0195172.
- Wang L, Lin R, Guo L, Hong M. 2018. Rosuvastatin relieves myocardial ischemia/reperfusion injury by upregulating PPAR-γ and UCP2. Mol Med Rep 18:789–798.
- Wang Q, Xu J, Li X, Liu Z, Li X. 2019. Sirt3 modulate renal ischemia‐reperfusion injury through enhancing mitochondrial fusion and activating the ERK‐OPA1 signaling pathway. J Cell Physiol 234:23495–23506.
- Xu H, Hertzel AV, Steen KA, Wang Q, Bernlohr DA. 2015. Uncoupling lipid metabolism from inflammation through FABP-dependent Expression of UCP2. Mol Cell Biol 35:1055–1065.
- Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. 2000. Reactive oxygen species (Ros-Induced) ros release a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192:1001.