Abstract
Reabsorption of amino acids is an important function of the renal proximal tubule. pH-dependent amino acid transport has been measured previously using rabbit renal brush-border membrane vesicles (BBMV). The purpose of this investigation was to determine whether this pH-dependent uptake represents H+/amino acid cotransport via a PAT1-like transport system. The rabbit PAT1 cDNA was isolated (2296bp including both 5′ and 3′ untranslated regions and poly(A) tail) and the open reading frame codes for a protein of 475 amino acids (92% identity to human PAT1). Rabbit PAT1 mRNA was found in all tissues investigated including kidney. When expressed heterologously in a mammalian cell line, rabbit PAT1 mediates pH-dependent, Na+-independent uptake of proline, glycine, l-alanine and α-(methylamino)isobutyric acid. Proline uptake was maximal at pH 5.0 (Km 2.2±0.7 mM). A transport system with identical characteristics (ion dependency, substrate specificity) was detected in rabbit renal BBMV where an overshoot was observed in the absence of Na+ but in the presence of an inwardly directed H+ gradient. In the presence of Na+ and under conditions in which PAT1 transport function was suppressed, a second proline uptake system was detected that exhibited functional characteristics similar to those of the IMINO system. The functional characteristics of rabbit PAT1 in either mammalian cells or renal BBMV suggest that PAT1 is the low-affinity transporter of proline, glycine and hydroxyproline believed to be defective in patients with iminoglycinuria.
| Acronyms | ||
| α-aminoisobutyric acid | = | AIB |
| brush-border membrane vesicles | = | BBMV |
| γ-aminobutyric acid | = | GABA |
| human retinal pigment epithelial cell | = | HRPE |
| α-(methylamino)isobutyric acid | = | MeAIB |
| glyceraldehyde-3-phosphate dehydrogenase | = | G3PDH |
| lysosomal amino acid transporter 1 | = | LYAAT1 |
| Na+/H+ exchanger 3 | = | NHE3 |
| Online Mendelian Inheritance in Man | = | OMIM |
| H+-coupled amino acid transporter 1 | = | PAT1 |
| reverse-transcriptase polymerase chain reaction | = | RT-PCR |
| solute carrier family 36 | = | SLC36 |
| solute carrier family 36 member 1 | = | SLC36A1 |
Introduction
Renal tubular reabsorption of amino acids from the glomerular filtrate is a function of a number of amino acid transport systems which have distinct substrate specificities and which are localized at the brush-border surface of the proximal tubule. The relative efficiency of renal tubular reabsorption depends upon the transport capacity of the amino acid carrier systems and the plasma concentration of each amino acid. The fundamental importance of any individual transport system, and in some cases single membrane proteins, is apparent when a genetic defect leads to loss of tubular reabsorptive function and thus hyperaminoaciduria. This is evident in cystinuria (Online Mendelian Inheritance in Man (OMIM) database, OMIM 220100, at www.ncbi.nlm.nih.gov), where there is a defect in system b0,+ (Palacin et al. [Citation1998]), and Hartnup disease (OMIM 234500), where there is a defect in system B0 (Bröer et al. [Citation2004], Bröer et al. [Citation2005]). Similarly an increase in plasma amino acid levels, as observed in hyperprolinemia (Scriver et al. [Citation1964]), can unmask a previously undetected defect in a reabsorptive transport process. Indeed studies in patients exhibiting specific hyperaminoacidurias led to the suggestion that certain amino acids might share reabsorptive mechanisms (Scriver et al. [Citation1961], [Citation1964]). For example, in some patients with familial hyperprolinemia (characterized by a hyperaminoaciduria of glycine and the imino acids proline and hydroxyproline), prolinuria was observed only when the plasma proline concentration was greater than 0.8 mM (as opposed to the normal levels which are less than 0.3 mM) (Scriver et al. [Citation1964]). However, when plasma proline was >0.8 mM there was also an increase in excretion of hydroxyproline and glycine (despite plasma levels remaining normal) suggesting that there exists a low affinity transport system shared by proline, glycine and hydroxyproline (Scriver et al. [Citation1964]). At lower plasma concentrations, the imino acids and glycine are reabsorbed by separate high-affinity transport systems (Scriver et al. [Citation1964]). A genetic defect in the low affinity, shared transport system seems likely to be responsible for the autosomal recessive disorder iminoglycinuria (OMIM 242600) (Scriver et al. [Citation1961]; Scriver [Citation1968]; Lasley & Scriver [Citation1979]).
Mohyuddin & Scriver ([Citation1970]) characterized imino acid and glycine transport in rat kidney and identified a series of transport systems with characteristics that could account for the reabsorptive processes identified in man (Scriver et al. [Citation1964]; Scriver [Citation1968]). These include separate high affinity [Michaelis constant (Km), 0.1 mM] transport systems for glycine and proline, and a low affinity transport system (glycine Km, 2.7 mM; proline Km, 5 mM) which is shared by proline, hydroxyproline, glycine, alanine and α-aminoisobutyric acid (AIB) (Mohyuddin & Scriver, [Citation1970]). Unlike many mammalian membrane transport systems, this low affinity transport system did not show absolute dependence on extracellular Na+ as proline accumulation in rat kidney cortex slices was reduced by only 31% at a proline concentration of 3 mM when extracellular Na+ was removed from the incubation buffers (Mohyuddin & Scriver, [Citation1970]). This low affinity proline-hydroxyproline-glycine system is not only found in human and rat kidney. A series of studies using brush-border membrane vesicles (BBMV) prepared from the pars convoluta of rabbit kidney characterized a low affinity transport system which had several unusual characteristics in that transport of neutral dipolar amino acids was rheogenic (even in the absence of Na+), was increased by lowering extravesicular pH and was pH gradient-dependent (reduced in the presence of the H+ ionophore FCCP). These data suggested that the observed transport occurred via a H+/amino acid symport mechanism (Røigaard-Petersen & Sheikh [Citation1984]; Rajendran et al. [Citation1987]; Røigaard-Petersen et al. [Citation1987], [Citation1989], [Citation1990]; Jessen et al. [Citation1988a], [Citation1988b], [Citation1989], [Citation1991]; Jessen & Sheikh [Citation1991]; Wunz & Wright [Citation1993]). A Na+-independent, pH-dependent, rheogenic uptake of proline, hydroxyproline, glycine, alanine, β-alanine, AIB, taurine and betaine was indeed demonstrable in these studies (Røigaard-Petersen & Sheikh [Citation1984]; Rajendran et al. [Citation1987]; Røigaard-Petersen et al. [Citation1987], [Citation1989], [Citation1990]; Jessen et al. [Citation1988a], [Citation1988b], [Citation1989], [Citation1991]; Jessen & Sheikh [Citation1991]; Wunz & Wright [Citation1993]) and the relative low affinity (Km, 2.8–9.7 mM) was similar to that determined either using rat kidney slices in vitro or in vivo in humans (Scriver et al. [Citation1964]; Mohyuddin & Scriver [Citation1970]). An additional unusual feature of this low affinity transport system is the inability to discriminate between l- and d-enantiomers of some amino acids including alanine, proline and hydroxyproline (Jessen et al. [Citation1988a], [Citation1988b]; Røigaard-Petersen et al. [Citation1989]).
Many proximal tubular transport systems are also localized at the brush-border membrane of the small intestine where they absorb amino acids from diet. We have characterized a H+-coupled, pH gradient-dependent, rheogenic, low affinity transport system (system PAT) at the brush-border membrane of the human intestinal epithelial cell line Caco-2 (Thwaites et al. [Citation1993a], [Citation1993b], [Citation1995b], [Citation1999]; Thwaites & Stevens [Citation1999]) that has remarkable similarities with the low affinity transport system shared by glycine, proline and hydroxyproline in human, rat and rabbit kidney. We have recently isolated a cDNA from a Caco-2 cDNA library which codes for this transport protein (Chen et al. [Citation2003]; Anderson et al. [Citation2004]). We named this transporter hPAT1 (for human Proton-coupled Amino acid Transporter 1). PAT1 (or SLC36A1) is the first member of solute carrier family 36 (SLC36) which includes three other closely related sequences which have been designated as PAT2-4 or SLC36A2-4 (Boll et al. [Citation2003a]). hPAT1 mRNA is expressed ubiquitously (including kidney) and PAT1 protein has been immunolocalized to the brush-border of Caco-2 cell monolayers, and human and rat small intestine (Chen et al. [Citation2003]; Anderson et al. [Citation2004]). Our hypothesis is that PAT1 represents the renal low affinity transport system of glycine, proline and hydroxyproline which has been characterized in rabbit renal BBMV and which is thought to be defective in iminoglycinuria. Functional evidence exists for this H+-coupled amino acid transport system only from studies using the human Caco-2 cell line and rabbit renal BBMV. Therefore, the purpose of this investigation was to isolate the PAT1 cDNA from rabbit and to compare the characteristics of this transport system in isolation (following heterologous expression in the human retinal pigment epithelial [HRPE] cell line using the vaccinia virus expression system [Blakely et al. [Citation1991]; Chen et al. [Citation2003]]) with those in the endogenous tissue (using rabbit renal BBMV).
Material and methods
Materials
[3H]-α-(Methylamino)isobutyric acid (MeAIB) (specific radioactivity, 85 Ci/mmol) was purchased from American Radiolabeled Chemicals (St Louis, MO, USA). [3H]Glycine (specific radioactivity, 65.4 Ci/mmol), [3H]-l-alanine (specific radioactivity, 40 Ci/mmol) and [3H]-l-proline (specific radioactivity, 9.9 Ci/mmol) were purchased from Moravek Biochemicals (Brea, CA, USA).
Isolation of rabbit PAT1 cDNA from a rabbit jejunal cDNA library
The rabbit PAT1 cDNA was isolated from a jejunal cDNA library using the human PAT1 cDNA as a probe. The cDNA was labeled by random priming using the Ready-to-go Oligo labeling beads (Amersham Biosciences). The cDNA library was screened under medium stringency conditions (Kekuda et al. [Citation1997]) and positive clones were purified by secondary and tertiary screening.
DNA sequencing
The sequence of both strands of rabbit PAT1 cDNA was determined using an automated PerkinElmer Applied Biosystems 377 Prism DNA sequencer (Foster City, CA, USA) and the Taq DyeDeoxy terminator cycle sequencing protocol.
Rabbit PAT1 mRNA tissue expression profile
A male New Zealand White rabbit was humanely killed with an overdose of sodium pentobarbitone injected via an ear vein, following Schedule 1 methods (UK Home Office guidelines). Whole rabbit tissue samples and intestinal mucosal scrapings (from duodenum, jejunum, ileum and colon) were collected. Total RNA was isolated using TRI Reagent (Sigma) and reverse-transcribed using Omniscript (Qiagen, Crawley, UK). Control reactions were prepared without the addition of reverse transcriptase (no bands were amplified demonstrating that there was no genomic DNA contamination within the samples, not shown). PCR primers specific for the rabbit PAT1 sequence were designed to amplify a 739bp product (forward, 5′-GCATCGTGGCCGTGCACTGCATGG-3′; reverse, 5′-GACTGGTACAACCAGCAGTTG-3′). Following alignment of the mouse and human IMINO (SLC6A20) nucleotide sequences (GenBank accession nos. NM_011731, BC013484, NM_139142, NM_022405 and NM_020208) PCR primers (forward, 5′-CAGCGCATGCGGCAGGGCAGC-3′; reverse, 5′-GTGGCTGCATTGATCCAGGCC-3′) were designed to amplify a predicted 539bp product of the rabbit IMINO sequence. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) transcript abundance, amplified with primers (forward, 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′; reverse, 5′-CATGTGGGCCATGAGGTCCACCAC-3′) to produce a 983bp product, was used to standardize the amount of cDNA in each reaction. PCR amplification was performed using HotStarTaq DNA Polymerase (Qiagen). Each reaction mixture contained 0.5 µM of each primer, 1 µg of cDNA and dNTPs (200 µM) in a final volume of 25 µl. Thermal cycling parameters (35 cycles) were: 95oC for 1min, 55oC for 1 min and 72oC for 1 min. PCR products and 100 bp ladder were separated by electrophoresis on a 1% agarose gel and visualized by ethidium bromide staining.
Functional expression of rabbit PAT1 cDNA in mammalian cells
Uptake measurements were performed in human retinal pigment epithelial (HRPE) cells transfected either with cDNA or vector using the vaccinia virus expression system, as described previously (Blakely et al. [Citation1991]; Kekuda et al. [Citation1997]; Chen et al. [Citation2003]). Subconfluent HRPE cells grown on 24-well plates were first infected with a recombinant (VTF7-3) vaccinia virus encoding T7 RNA polymerase and then transfected with the plasmid carrying the full-length rabbit PAT1 cDNA. 12–15 h post-transfection, uptake measurements were made at 37oC with radiolabelled amino acids. The uptake medium contained 140 mM N-methyl-d-glucamine (NMDG) chloride (Na+-free buffers) or NaCl (Na+-containing buffers), and 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose. The pH 5.0 buffer contained 25 mM MES/Tris whereas the pH 7.5 buffer contained 25 mM HEPES/Tris (pH 7.5). In experiments in which the influence of pH on the transport process was investigated, transport buffers of different pH values were prepared by varying the concentrations of Tris, HEPES, and MES (total concentration 25 mM). The time of incubation in most experiments was 5 min. Endogenous transport was always determined in parallel using cells transfected with pSPORT1 vector alone. The transport activity in cDNA-transfected cells was adjusted for the endogenous activity to calculate the cDNA-specific transport activity.
Preparation of brush-border membrane vesicles (BBMV) and uptake measurements
Brush border membrane vesicles were prepared from rabbit kidney cortex by the Mg2 + -aggregation method, as described previously (Tiruppathi et al. [Citation1990]). The membrane vesicles were preloaded with 20 mM Tris/Hepes buffer (pH 8.4) or 20 mM Hepes/Tris buffer (pH 7.5), each containing 300 mM mannitol. The protein concentration of the membrane preparation was adjusted to 8 mg/ml. Uptake of [3H]proline (10 µM) in these membrane vesicles was measured by the rapid filtration method (Tiruppathi et al. [Citation1990]). Uptake was initiated by mixing 40 µl of membrane suspension with 160 µl of uptake buffer containing radiolabeled proline. The uptake buffer was either 20 mM Mes/Tris (pH 6) containing 300 mM mannitol or 20 mM Hepes/Tris (pH 7.5) containing 150 mM NaCl and 10 mM alanine. Thus, the transport function of PAT1 was monitored as the uptake of proline in the presence of an inwardly directed H+ gradient (intravesicular pH 8.4; extravesicular pH 6) and in the absence of Na+. The transport function of the IMINO system was monitored as the uptake of proline in the presence of an inwardly directed Na+ gradient and 10 mM alanine and in the absence of a H+ gradient (intravesicular pH 7.5; extravesicular pH 7.5). Substrate specificity studies were performed by competition experiments in which the ability of various amino acids (5 mM) to inhibit the uptake of [3H]proline (10 µM) was assessed. In these studies, a 15s incubation was used to determine uptake rates. Uptake measurements were made in duplicate or triplicate and the experiments were repeated with two different membrane preparations.
Statistics
Uptake measurements in HRPE cells were made in triplicate and experiments were repeated at least three times with separate transfections. Uptake measurements in BBMV were made in duplicate or triplicate and each experiment was repeated in two different membrane preparations. The results are expressed as mean±SEM of multiple measurements. The Michaelis constant (Km) was determined by the Eadie–Hofstee plot. Statistical comparisons of mean values were made using paired 2-tailed Student t-test or, for multiple comparisons, one-way analysis of variance (ANOVA) (using the Bonferroni multiple comparisons post-test). Curve-fitting was performed using GraphPad Prism version 3.00 (GraphPad, San Diego, CA, USA).
Results
Structural features of the rabbit PAT1 cDNA
The rabbit PAT1 cDNA is 2,296bp long (GenBank accession no. AY989816), with a 1,428 bp-long open reading frame (including the stop codon) coding for a protein of 475 amino acids (). The open reading frame is flanked by a 112 bp-long 5′ untranslated region and a 756 bp-long 3′ untranslated region. The cDNA possesses a poly(A) tail. Comparison of the rabbit PAT1 sequence demonstrates that PAT1 is highly conserved between mammalian species and is 92% identical to hPAT1 (Chen et al. [Citation2003]) at the protein level () and 85% identical to both rat PAT1 (Sagné et al. [Citation2001]) and mouse PAT1 (Boll et al. [Citation2002]). A comparison of rabbit PAT1 with other predicted amino acid sequences (based upon available genomic sequence, http://www.ensembl.org/) demonstrates 92% and 93% identity with the chimp (Pan troglodytes) and dog (Canis familiaris) PAT1 sequences, respectively.
Tissue expression pattern of PAT1 and IMINO mRNA
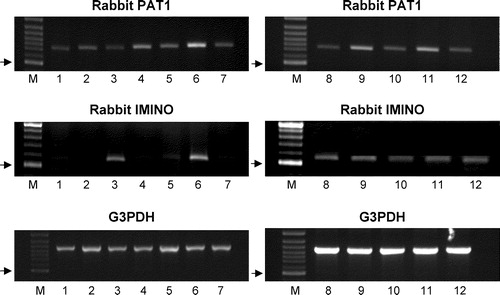
PCR analysis of rabbit cDNA prepared from multiple tissues using an oligonucleotide pair specific for the rabbit PAT1 sequence produced a single product of the predicted size (739 bp) in all tissues examined (). Thus it seems likely that rabbit PAT1 has a ubiquitous distribution at the mRNA level being expressed in heart, liver, kidney, duodenum, jejunum, ileum, colon and brain (cerebellum, hippocampus, striatum, frontal cortex and parietal cortex) (). In contrast, another proline transporter, the IMINO system (SLC6A20) (see Results below), was only identified at the mRNA level in rabbit small intestine (a strong band was observed in the ileum and a very weak band in the jejunum), kidney and brain (all regions investigated) (). G3PDH was used as a positive control.
Figure 2. Expression of PAT1 and IMINO mRNA in rabbit tissues. Products of PCR using primers specific for PAT1 (739bp), IMINO (539bp) and G3PDH (983bp) and cDNA from multiple rabbit tissues. Lanes are: M, 100 bp marker; 1, heart; 2, liver; 3, kidney; 4, duodenum; 5, jejunum; 6, ileum; 7, colon; 8–12, brain (8, cerebellum; 9, hippocampus; 10, striatum; 11, frontal cortex; 12, parietal cortex). PCR products were separated on a 1% agarose gel and were visualized by ethidium bromide staining. The arrows indicate the position of the 500 bp marker in lane M on each gel.
Functional characteristics of rabbit PAT1 in HRPE cells and renal BBMV
The functional features of the cloned rabbit PAT1 were investigated in a mammalian cell heterologous expression system in which rabbit PAT1 cDNA was expressed functionally in HRPE cells using the vaccinia virus system. Experiments in rabbit renal BBMV have identified that proline (Røigaard-Petersen & Sheikh [Citation1984]; Røigaard-Petersen et al. [Citation1987]), glycine (Rajendran et al. [Citation1987]; Røigaard-Petersen et al. [Citation1990]) and alanine (Jessen et al. [Citation1988b]) are all good substrates for the low affinity, pH-dependent, Na+-independent transport system in pars convoluta. Uptake of these three substrates, which are also substrates for hPAT1 (Chen et al. [Citation2003]), was compared in this study with another hPAT1 substrate MeAIB (20 µM) (Chen et al. [Citation2003]) in control HRPE cells transfected with vector alone and in cells transfected with rabbit PAT1 cDNA (). The cDNA-induced uptake of all four imino/amino acids could not be detected when measured at extracellular pH 7.5 in the presence of Na+ ((a)) (p>0.05 versus vector alone). However, when the experiments were performed at extracellular pH 5.0 in the absence of Na+, a significant increase (p<0.001 versus vector alone) in uptake of all four substrates was observed in rabbit PAT1 cDNA-transfected cells compared to vector-transfected cells (MeAIB, 6.6-fold; proline, 6.2-fold; glycine, 5.3-fold; alanine, 2.5-fold) ((b) & (c)). Subsequent uptake experiments were performed using proline as the substrate.
Figure 3. Influence of Na+ and H+ gradients on rabbit PAT1 cDNA-mediated amino acid uptake. Uptake of [3H]proline (5 µM), [3H]glycine (5 µM), [3H]alanine (5 µM) and [3H]MeAIB (20 µM) was measured for 5 min in HRPE cells transfected either with the empty pSPORT1 vector (open bars) or rabbit PAT1 cDNA (filled bars). (a) The influence of extracellular Na+. Uptake was measured at extracellular pH 7.5 in the presence of a Na+-containing buffer (containing 140 mM NaCl). (b) & (c) The influence of a pH or H+ gradient. Uptake was measured at extracellular pH 5.0 in a Na+-free buffer (140 mM NMDGCl replacing 140 mM NaCl). Results are mean±SEM (n=6). **p < 0.01 rabbit PAT1 versus pSPORT.
![Figure 3. Influence of Na+ and H+ gradients on rabbit PAT1 cDNA-mediated amino acid uptake. Uptake of [3H]proline (5 µM), [3H]glycine (5 µM), [3H]alanine (5 µM) and [3H]MeAIB (20 µM) was measured for 5 min in HRPE cells transfected either with the empty pSPORT1 vector (open bars) or rabbit PAT1 cDNA (filled bars). (a) The influence of extracellular Na+. Uptake was measured at extracellular pH 7.5 in the presence of a Na+-containing buffer (containing 140 mM NaCl). (b) & (c) The influence of a pH or H+ gradient. Uptake was measured at extracellular pH 5.0 in a Na+-free buffer (140 mM NMDGCl replacing 140 mM NaCl). Results are mean±SEM (n=6). **p < 0.01 rabbit PAT1 versus pSPORT.](/cms/asset/a57c5e3c-d4d9-4420-babb-c3fc27dd7368/imbc_a_142160_f0003_b.jpg)
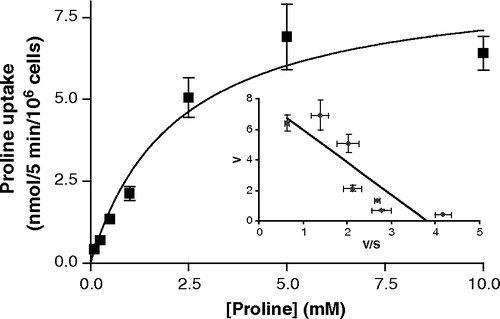
The pH-dependent nature of rabbit PAT1 transport is emphasized by the results in . In the absence of extracellular Na+, there was only a small increase in proline uptake in rabbit PAT1 cDNA-transfected cells at pH 7.0–7.5 (p>0.05 versus vector), but the rabbit PAT1-mediated proline uptake increased as pH decreased until there was an 8.1-fold stimulation above vector-transfected cells at pH 5.0 (p<0.001) (). These data show that rabbit PAT1-mediated proline uptake is H+-dependent and that Na+ has no direct role in the uptake process. Subsequent uptake measurements were made in the absence of Na+ and at an extracellular pH of 5.0 and under these conditions proline uptake via rabbit PAT1 is saturable with a Km of 2.2±0.7 mM ().
Figure 4. pH-dependent proline uptake via rabbit PAT1. [3H]Proline uptake (10 µM) into vector-transfected (open squares) or rabbit PAT1 cDNA-transfected (filled squares) HRPE cells was measured for 5 min in the absence of Na+ over the extracellular pH range 5.0–7.5. The pH of the uptake buffer was varied by adjusting the concentrations of MES, HEPES and Tris base. Results are mean±SEM (n=6).
![Figure 4. pH-dependent proline uptake via rabbit PAT1. [3H]Proline uptake (10 µM) into vector-transfected (open squares) or rabbit PAT1 cDNA-transfected (filled squares) HRPE cells was measured for 5 min in the absence of Na+ over the extracellular pH range 5.0–7.5. The pH of the uptake buffer was varied by adjusting the concentrations of MES, HEPES and Tris base. Results are mean±SEM (n=6).](/cms/asset/46a3eb5b-a25d-4fec-9fab-3ccaefc8bc25/imbc_a_142160_f0004_b.jpg)
Figure 5. Saturation kinetics of rabbit PAT1-mediated proline uptake. Proline uptake (0.1–10 mM) was measured at pH 5 in the absence of extracellular Na+ in cells transfected either with the empty pSPORT1 vector or rabbit PAT1 cDNA. Uptake represents the rabbit PAT1-specific uptake (calculated by subtraction of uptake in the vector-only transfected cells from uptake in the rabbit PAT1 cDNA-transfected cells). Inset, Eadie–Hofstee plot where V (uptake rate) is plotted against V/S (uptake rate/proline concentration). Results are mean±SEM (n=6).
The substrate specificity of rabbit PAT1 was determined by assessing the potency of various amino acids (all 5 mM) to inhibit rabbit PAT1-mediated [3H]proline (10 µM) uptake (). These experiments were done in parallel in vector-transfected cells and in rabbit PAT1 cDNA-transfected cells and the data for cDNA-specific uptake were analysed to determine substrate specificity of rabbit PAT1. The results demonstrate that glycine, alanine, β-alanine, γ-aminobutyric acid (GABA), α-aminoisobutyric acid (AIB), proline, pipecolate, hydroxyproline, betaine, and MeAIB significantly (p<0.001 versus control) reduce rabbit PAT1-mediated [3H]proline uptake, suggesting that they may all be substrates for this transporter. In contrast, phenylalanine, leucine and glutamine did not cause a significant reduction (p>0.05 versus control) in [3H]proline uptake, suggesting that these amino acids are not substrates for rabbit PAT1. This profile of selective inhibition is identical to results using the hPAT1, mouse PAT1 and rat PAT1 clones (Sagné et al. [Citation2001]; Boll et al. [Citation2002]; Chen et al. [Citation2003]; Boll et al. [Citation2003b]; Anderson et al. [Citation2004]). The apparent substrate selectivity is also similar to that observed for the low affinity, pH-dependent, Na+-independent transport system characterized using rabbit renal BBMV (Røigaard-Petersen & Sheikh [Citation1984]; Rajendran et al. [Citation1987]; Røigaard-Petersen et al. [Citation1987], [Citation1989], [Citation1990]; Jessen et al. [Citation1988a], [Citation1988b], [Citation1989], [Citation1991]; Jessen & Sheikh [Citation1991]; Wunz & Wright [Citation1993]). The data in demonstrate that rabbit PAT1 is expressed in the kidney at the mRNA level but this does not allow us to determine whether the PAT1 protein is expressed nor in which cell or membrane this protein may function. Currently we do not have a suitable antibody to allow investigation of the level of expression of rabbit PAT1 protein but functional measurements can be made. Although there have been a number of studies using rabbit renal BBMV, cross-competition experiments have usually been limited to one or two substrates. Therefore, to confirm that rabbit PAT1 is expressed functionally at the brush-border of the renal tubule and is responsible for the low affinity, pH-dependent, Na+-independent transport system characterised previously, uptake and competition experiments have been performed in this investigation using rabbit renal BBMV (see below).
Figure 6. Effects of other amino acids on rabbit PAT1-mediated [3H]proline uptake. [3H]Proline uptake (10 µM, pH 5, Na+-free conditions) into vector-transfected or rabbit PAT1 cDNA-transfected HRPE cells in the presence or absence of unlabeled amino acids (all 5 mM). Results are mean±SEM (n=6). ***p<0.001 rabbit PAT1 versus pSPORT.
![Figure 6. Effects of other amino acids on rabbit PAT1-mediated [3H]proline uptake. [3H]Proline uptake (10 µM, pH 5, Na+-free conditions) into vector-transfected or rabbit PAT1 cDNA-transfected HRPE cells in the presence or absence of unlabeled amino acids (all 5 mM). Results are mean±SEM (n=6). ***p<0.001 rabbit PAT1 versus pSPORT.](/cms/asset/4857d90f-e84b-4398-825a-9a56970843d3/imbc_a_142160_f0006_b.jpg)
A complication in determining transport in renal BBMV is that many transport systems with overlapping specificity are expressed at the renal brush-border membrane. The high-affinity glycine-insensitive transport system for proline (Km, 0.1 mM) and hydroxyproline, identified in rat and human kidney in the studies by Scriver and colleagues (Scriver et al. [Citation1964]; Scriver [Citation1968]; Mohyuddin & Scriver [Citation1970]), is almost certainly the Na+-dependent IMINO system described in a series of studies using rabbit small intestinal BBMV (Stevens & Wright [Citation1985], [Citation1987]). Wright and colleagues (Mircheff et al. [Citation1982]) had earlier identified 6 different amino acid transport systems in rabbit renal BBMV and the IMINO system most likely represents System 6 (as suggested by Wunz & Wright [Citation1993]). The IMINO system (SLC6A20) has recently been identified at the molecular level (in rat, human and mouse), is expressed in both small intestine and kidney () and has a Km of 0.1–0.2 mM for proline (Takanaga et al. [Citation2005]; Kowalczuk et al. [Citation2005]). Thus in the experiments using rabbit renal BBMV (see below) it is important to be able to distinguish between IMINO- and PAT1-mediated proline transport processes. demonstrates that more than 80% of rabbit PAT1-mediated proline uptake (in HRPE cells) is inhibited by 10 mM alanine. Alanine is a very poor substrate for the IMINO carrier and the Na+-dependent, alanine-insensitive proline uptake is defined as the IMINO system (Stevens & Wright [Citation1985], [Citation1987]; Takanaga et al. [Citation2005]; Kowalczuk et al. [Citation2005]). Therefore, PAT1-specific transport activity in rabbit BBMV was monitored as the uptake of proline in the absence of Na+ but in the presence of an inwardly directed H+ gradient whereas IMINO-specific transport activity was monitored as the uptake of proline in the presence of an inwardly directed Na+ gradient. In the case of the latter, 10 mM alanine was present in the uptake medium to suppress PAT1 activity.
Figure 7. Effect of alanine on rabbit PAT1-mediated proline uptake. Rabbit PAT1-specific [3H]proline uptake (5 µM, pH 5, Na+-free conditions) in HRPE cells was measured in the presence and absence of alanine (0.01–10 mM). Results are mean±SEM (n=3).
![Figure 7. Effect of alanine on rabbit PAT1-mediated proline uptake. Rabbit PAT1-specific [3H]proline uptake (5 µM, pH 5, Na+-free conditions) in HRPE cells was measured in the presence and absence of alanine (0.01–10 mM). Results are mean±SEM (n=3).](/cms/asset/420063a8-17a4-4f93-a5e0-4e1ae66b839f/imbc_a_142160_f0007_b.jpg)
(a) demonstrates that there is a 9.1-fold overshoot (above equilibrium, 60 min) in proline uptake into rabbit renal BBMV in the presence of an inwardly-directed H+ gradient (extravesicular pH 6.0, intravesicular pH 8.4) in the absence of Na+. The initial H+-dependent proline uptake (at 15 s) is 8.9-fold greater than that in the absence of a H+ gradient (extravesicular pH 8.4, intravesicular pH 8.4) ((a)). Under conditions in which PAT1-mediated proline uptake will be minimal (the presence of 10 mM alanine and the absence of a pH gradient), an inwardly directed Na+ gradient caused a 3.7-fold accumulation in proline uptake above equilibrium (60 min) ((b)). The initial Na+-dependent proline uptake (at 15 s) is 14.7-fold greater than that in the absence of a Na+ gradient ((b)). The ability of glycine, GABA, proline, hydroxyproline, pipecolate, AIB, MeAIB, β-alanine and betaine (all 5 mM) to significantly reduce (p<0.001 versus control) the Na+-independent, H+-gradient driven uptake of [3H]proline into renal BBMV demonstrates the brush-border localization of rabbit PAT1 ((a)). In the presence of 10 mM alanine and in the absence of a H+ gradient (to inhibit PAT1-mediated uptake), the Na+-gradient driven [3H]proline uptake is significantly inhibited (p<0.001 versus control) by proline, hydroxyproline, pipecolate, MeAIB and betaine (all 5 mM) whereas glycine, GABA, AIB and β-alanine were without effect (p>0.05 versus control) which demonstrates that the IMINO system is also functionally expressed in the rabbit renal brush-border membrane ((b)).
Figure 8. Influence of H+ (a) and Na+ (b) gradients on proline uptake into rabbit renal brush-border membrane vesicles (BBMV). Time-dependent [3H]proline (10 µM) uptake into rabbit renal BBMV: (a) in the presence (extravesicular pH 6.0, intravesicular pH 8.4, Na+-free conditions) or absence (Control, extravesicular pH 8.4, intravesicular pH 8.4, Na+-free conditions) of an inwardly directed H+ gradient; or (b) in the presence (extravesicular 150 mM NaCl, intravesicular 300 mM mannitol) or absence (Control, extravesicular 300 mM mannitol, intravesicular 300 mM mannitol) of a Na+ gradient (both extra- and intravesicular pH 7.5). The Na+ gradient experiments (b) were performed in the presence of 10 mM alanine in the extravesicular uptake solution to inhibit any PAT1-mediated uptake. Results are mean±SEM (n=4).
![Figure 8. Influence of H+ (a) and Na+ (b) gradients on proline uptake into rabbit renal brush-border membrane vesicles (BBMV). Time-dependent [3H]proline (10 µM) uptake into rabbit renal BBMV: (a) in the presence (extravesicular pH 6.0, intravesicular pH 8.4, Na+-free conditions) or absence (Control, extravesicular pH 8.4, intravesicular pH 8.4, Na+-free conditions) of an inwardly directed H+ gradient; or (b) in the presence (extravesicular 150 mM NaCl, intravesicular 300 mM mannitol) or absence (Control, extravesicular 300 mM mannitol, intravesicular 300 mM mannitol) of a Na+ gradient (both extra- and intravesicular pH 7.5). The Na+ gradient experiments (b) were performed in the presence of 10 mM alanine in the extravesicular uptake solution to inhibit any PAT1-mediated uptake. Results are mean±SEM (n=4).](/cms/asset/d0f5ab6b-3b1f-41f7-ad58-f2879aa31262/imbc_a_142160_f0008_b.jpg)
Figure 9. Effects of other amino acids on PAT1- (a) and IMINO-mediated (b) proline uptake in rabbit renal BBMV. [3H]Proline (10 µM) uptake (15 s) into rabbit renal BBMV was measured in the presence or absence of unlabeled amino acids (all 5 mM) in the presence of either an inwardly directed H+ gradient (extravesicular pH 6.0, intravesicular pH 8.4, Na+-free conditions) (a) or an inwardly directed Na+ gradient (both extra- and intravesicular pH 7.5) (b). The Na+ gradient experiments (b) were performed in the presence of 10 mM alanine in the extracellular uptake solution to inhibit any PAT1-mediated uptake. Results are mean±SEM (n=6). ***p<0.001 rabbit PAT1 versus control.
![Figure 9. Effects of other amino acids on PAT1- (a) and IMINO-mediated (b) proline uptake in rabbit renal BBMV. [3H]Proline (10 µM) uptake (15 s) into rabbit renal BBMV was measured in the presence or absence of unlabeled amino acids (all 5 mM) in the presence of either an inwardly directed H+ gradient (extravesicular pH 6.0, intravesicular pH 8.4, Na+-free conditions) (a) or an inwardly directed Na+ gradient (both extra- and intravesicular pH 7.5) (b). The Na+ gradient experiments (b) were performed in the presence of 10 mM alanine in the extracellular uptake solution to inhibit any PAT1-mediated uptake. Results are mean±SEM (n=6). ***p<0.001 rabbit PAT1 versus control.](/cms/asset/a0686a66-ea4a-40d9-8eb8-5994099bb0b5/imbc_a_142160_f0009_b.jpg)
Discussion
The only functional evidence for the role of the H+-electrochemical gradient as a driving force in amino acid transport across mammalian epithelial cell membranes comes from studies using either brush-border membrane vesicles prepared from rabbit kidney (Røigaard-Petersen & Sheikh [Citation1984]; Rajendran et al. [Citation1987]; Røigaard-Petersen et al. [Citation1987], [Citation1989], [Citation1990]; Jessen et al. [Citation1988a], [Citation1988b], [Citation1989], [Citation1991]; Jessen & Sheikh [Citation1991]; Wunz & Wright [Citation1993]) or confluent monolayers of the human intestinal epithelial cell line Caco-2 (Thwaites et al. [Citation1993a], [Citation1993b], [Citation1995b], [Citation1999]; Thwaites & Stevens [Citation1999]). This investigation reveals the molecular identity of the rabbit low affinity, H+-dependent, Na+-independent amino acid transport system characterized in the studies using renal BBMV. This transporter has been named rabbit PAT1 due to its high degree of homology to human PAT1 isolated previously from a human Caco-2 cDNA library (Chen et al. [Citation2003]). PAT1 (SLC36A1) is the first member of solute carrier family SLC36. The rat orthologue of PAT1 was the first to be identified, being isolated from rat brain and named LYAAT1 because of the predominant lysosomal localization in the neural tissues investigated (Sagné et al. [Citation2001]). Rat PAT1 (LYAAT1) is also functionally-expressed at the plasma-membrane of neurones (Wreden et al. [Citation2003]). Mouse PAT1 was isolated from small intestine (Boll et al. [Citation2002]). All orthologues identified thus far function as H+-coupled, pH gradient-dependent, Na+-independent amino acid transporters. The transport characteristics of rabbit PAT1 reported here are similar to those reported for rat, mouse and human PAT1. The high degree of sequence homology between PAT1 transporters isolated from different mammalian species and sequences in the genomes of species as diverse as Pan troglodytes, Drosophila melanogaster and Caenorhabditis elegans suggest that this transporter has an important function(s) conserved through evolution (Sagné et al. [Citation2001]).
The functional characteristics of rabbit PAT1 reported here can account for the previous functional measurements in rabbit renal brush-border membrane vesicles (Røigaard-Petersen & Sheikh [Citation1984]; Rajendran et al. [Citation1987]; Røigaard-Petersen et al. [Citation1987], [Citation1989], [Citation1990]; Jessen et al. [Citation1988a], [Citation1988b], [Citation1989], [Citation1991]; Jessen & Sheikh [Citation1991]; Wunz & Wright [Citation1993]). However, it is clear that PAT1 is not the only transporter of imino acids (proline and hydroxyproline) in the proximal tubule. In this study we have demonstrated functional activity of both PAT1 and the IMINO system in rabbit renal BBMV ( and ). IMINO transport activity in renal BBMV is demonstrated under conditions in which PAT1 transport will be minimal (absence of a pH gradient, presence of 10 mM alanine). A third proline transporter, system B0, is also present in the proximal tubule (Bröer et al. [Citation2004]). System B0 is a Na+-dependent transporter that is inhibited at low pH (Bröer et al. [Citation2004]) suggesting that it is not responsible for the H+gradient-driven proline uptake in renal BBMV ((a) & (a)). Under the conditions used, system B0 is also not responsible for the Na+-dependent proline uptake in renal BBMV ((b) & (b)) as system B0 will be saturated by 10 mM alanine and is not inhibited by MeAIB (Bröer et al. [Citation2004]). The relative contributions of these various carriers to renal tubular reabsorption of amino and imino acids will depend upon the local transmembrane ionic gradients within the renal tubule and substrate concentration in the filtrate. The earliest evidence for the presence of multiple amino and imino acid transport systems in the kidney comes from studies of hyperaminoacidurias in humans (Scriver et al. [Citation1961]; Scriver et al. [Citation1964]; Scriver [Citation1968]; Lasley & Scriver [Citation1979]). Although iminoglycinuria is characterized by urinary excretion of proline, hydroxyproline and glycine, even within families there are notable phenotypical differences in the patterns of amino/imino acid excretion. This suggests that multiple genotypes may be responsible for the disorder which could include defects in multiple transport proteins or multiple defects in individual transport systems.
Studies of the PAT1 clones and the endogenous carrier in Caco-2 cells have identified that PAT1 can transport a broad spectrum of substrates including imino and amino acids, the conditionally essential amino acid taurine, the osmolyte betaine, drugs used to treat schizophrenia and epilepsy, and some short-chain fatty acids (Thwaites et al. [Citation1995a], [Citation1995b], [Citation2000]; Boll et al. [Citation2002], [Citation2003b]; Chen et al. [Citation2003]; Anderson et al. [Citation2004]; Metzner et al. [Citation2004]; Foltz et al. [Citation2004]). It is not known whether any of these pharmacologically relevant compounds have unusual pharmacokinetics in iminoglycinuric patients, but this does emphasize that care should be taken in drug regimes used in patients with specific hyperaminoacidurias.
We have identified (Anderson et al. [Citation2004]) that the transport system induced by the PAT1 cDNA represents a transporter that was first characterized in rat small intestine in the 1960s and named variously as the sarcosine carrier (Newey & Smyth [Citation1964]), the imino acid carrier (Munck [Citation1966]; Munck et al. [Citation1994]) and the methionine-insensitive sarcosine-glycine-proline system (Thompson et al. [Citation1970]). This transport system in rat small intestine has the same substrate specificity to system PAT at the brush-border membrane of human intestinal Caco-2 cell monolayers (Thwaites et al. [Citation1993a], [Citation1995b]; Anderson et al. [Citation2004]). Despite the fact that rabbit PAT1 was cloned from jejunum and PAT1 mRNA is expressed in rabbit duodenum, jejunum and ileum (), we and others have failed to detect PAT1-like function in rabbit small intestine (Munck [Citation1985]; Anderson et al. [Citation2004]). This may suggest that in the rabbit small intestine: PAT1 protein has a predominantly intracellular localization – as found in rat brain (Sagné et al. [Citation2001]); there is region-specific surface PAT1 protein expression (transport experiments have been performed only using rabbit ileum and not rabbit jejunum [Munck [Citation1985]; Anderson et al. [Citation2004]]) as observed for other amino acid transporters in rabbit jejunum and ileum (Munck & Munck [Citation1992a], [Citation1992b]); there may be low levels of PAT1 protein expression at the intestinal surface with intestinal transport of imino acids in the rabbit being mediated principally via the IMINO system (Munck [Citation1985]; Stevens & Wright [Citation1985], [Citation1987]). In contrast, PAT1 function is observed in both human intestinal (Caco-2) cells and rat small intestine which corresponds to the surface expression of PAT1 protein as determined by immunocytochemistry (Anderson et al. [Citation2004]).
Previously we have identified that hPAT1 function in small intestinal epithelia is partially Na+-dependent due to a requirement for the coexpression and cooperative activity of the Na+/H+ exchanger NHE3 (Thwaites et al. [Citation1999]; Anderson et al. [Citation2004]; Anderson & Thwaites [Citation2005]). Whether there is a similar relationship between PAT1 and NHE3 function in the renal proximal tubule is not known but low-affinity proline influx into rat kidney cortex slices also shows partial Na+-dependence being reduced by 31% in the absence of Na+ (Mohyuddin & Scriver [Citation1970]). The expression of the high capacity H+-coupled PAT1 in the S1 segment of the proximal tubule (as detected functionally in the BBMV studies in the 1980s) along with NHE3 lends support to the idea that a similar functional coupling (of H+-coupled absorptive transport and NHE3) will take place at the brush-border of the renal proximal tubule.
In conclusion this study reports the isolation and functional characterization of rabbit PAT1 which is a low affinity, high capacity carrier of amino and imino acids in the renal proximal tubule. PAT1 is likely to play a key role in the tubular reabsorption of amino/imino acids and small amino acid-like pharmacologically relevant compounds. The potential role of a defective PAT1 transporter in the autosomal recessive disorder iminoglycinuria (characterized by increased urinary excretion of proline, hydroxyproline and glycine) requires identification of the genotype of iminoglycinuric patients.
This study was supported by the National Institutes of Health grants HL64196 and AI49849 to VG and an MRC Career Establishment Grant (G9801704) to DTT.
References
- Anderson CMH, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, Munck LK, Miyauchi S, Taylor PM, Campbell FC, Munck BG, Daniel H, Ganapathy V, Thwaites DT. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: An intestinal nutrient/drug transporter in human and rat. Gastroenterology 2004; 127: 1410–1422
- Anderson CMH, Thwaites DT. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1). J Cell Physiol 2005; 204: 604–613
- Blakely RD, Clark JA, Rudnick G, Amara SG. Vaccinia-T7 RNA polymerase expression system: Evaluation for the expression cloning of plasma membrane transporters. Anal Biochem 1991; 194: 302–308
- Boll M, Daniel H, Gasnier B. The SLC36 family: proton-coupled transporters for absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Arch 2003a; 447: 776–779
- Boll M, Foltz M, Anderson CMH, Oechsler C, Kottra G, Thwaites DT, Daniel H. Substrate recognition by the mammalian proton-dependent amino acid transporter PAT1. Mol Membr Biol 2003b; 20: 261–269
- Boll M, Foltz M, Rubio-Aliaga I, Kottra G, Daniel H. Functional characterization of two novel mammalian electrogenic proton-dependent amino acid cotransporters. J Biol Chem 2002; 277: 22966–22973
- Bröer S, Cavanaugh JA, Rasko JEJ. Neutral amino acid transport in epithelial cells and its malfunction in Hartnup disorder. Biochem Soc Trans 2005; 33: 233–236
- Bröer A, Klingel K, Kowalczuk S, Rasko JEJ, Cavanaugh J, Bröer S. Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to Hartnup disorder. J Biol Chem 2004; 279: 24467–24476
- Chen Z, Fei YJ, Anderson CMH, Wake KA, Miyauchi S, Huang W, Thwaites DT, Ganapathy V. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J Physiol 2003; 546: 349–361
- Foltz M, Boll M, Raschka L, Kottra G, Daniel H. A novel bifunctionality: PAT1 and PAT2 mediate electrogenic proton/amino acid and electroneutral proton/fatty acid symport. FASEB J 2004; 18: 1758–1760
- Jessen H, Jorgensen KE, Røigaard-Petersen H, Sheikh MI. Demonstration of H+- and Na+-coupled cotransport of β-alanine by luminal membrane vesicles of rabbit proximal tubule. J Physiol 1989; 411: 517–528
- Jessen H, Sheikh MI. Renal transport of taurine in luminal membrane vesicles from rabbit proximal tubule. Biochim Biophys Acta 1991; 1064: 189–198
- Jessen H, Vorum H, Jorgensen KE, Sheikh MI. Characteristics of d-alanine transport by luminal membrane vesicles from pars convoluta and pars recta of rabbit proximal tubule. Biochim Biophys Acta 1988a; 942: 262–270
- Jessen H, Vorum H, Jorgensen KE, Sheikh MI. Energetics of renal Na+ and H+/l-alanine co-transport systems. Biochem J 1988b; 256: 299–302
- Jessen H, Vorum H, Jorgensen KE, Sheikh MI. Na+- and H+-gradient-dependent transport of α-aminoisobutyrate by luminal membrane vesicles from rabbit proximal tubule. J Physiol 1991; 436: 149–167
- Kekuda R, Torres-Zamorano V, Fei YJ, Prasad P, Li HW, Mader LD, Leibach FH, Ganapathy V. Molecular and functional characterization of intestinal Na+-dependent neutral amino acid transporter B0. Am J Physiol 1997; 272: G1463–G1472
- Kowalczuk S, Bröer A, Munzinger M, Tietze N, Klingel K, Bröer S. Molecular cloning of the mouse IMINO system, a Na+ and Cl−-dependent proline transporter. Biochem J 2005; 386: 417–422
- Lasley L, Scriver CR. Ontogeny of amino acid reabsorption in human kidney. Evidence from the homozygous infant with familial renal iminoglycinuria for multiple proline and glycine systems. Pediatr Res 1979; 13: 65–70
- Metzner L, Kalbitz J, Brandsch M. Transport of pharmacologically active proline derivatives by the human proton-coupled amino acid transporter hPAT1. J Pharmacol Exp Ther 2004; 309: 28–35
- Mircheff AK, Kippen I, Hirayama B, Wright EM. Delineation of sodium-stimulated amino acid transport pathways in rabbit kidney brush border vesicles. J Membr Biol 1982; 64: 113–122
- Mohyuddin F, Scriver CR. Amino acid transport in mammalian kidney: Multiple systems for imino acids and glycine in rat kidney. Am J Physiol 1970; 219: 1–8
- Munck BG. Amino acid transport by the small intestine of the rat. Biochim Biophys Acta 1966; 120: 97–103
- Munck BG. Transport of imino acids and non-α-amino acid across the brush-border membrane of the rabbit ileum. J Membr Biol 1985; 83: 15–24
- Munck BG, Munck LK, Rasmussen SN, Polache A. Specificity of the imino acid carrier in rat small intestine. Am J Physiol 1994; 266: R1154–R1161
- Munck LK, Munck BG. Distinction between chloride-dependent transport systems for taurine and β-alanine in rabbit ileum. Am J Physiol 1992a; 262: G609–G615
- Munck LK, Munck BG. Variation in amino acid transport along the rabbit small intestine. Mutual jejunal carriers of leucine and lysine. Biochim Biophys Acta 1992b; 1116: 83–90
- Newey H, Smyth DH. The transfer system for neutral amino acids in the rat small intestine. J Physiol 1964; 170: 328–343
- Palacin M, Estevez R, Zorzano A. Cystinuria calls for heteromultimeric amino acid transporters. Curr Opin Cell Biol 1998; 10: 455–461
- Rajendran VM, Barry JA, Kleinman JG, Ramaswamy K. Proton-gradient dependent transport of glycine in rabbit renal brush border membrane vesicles. J Biol Chem 1987; 262: 14974–14977
- Røigaard-Petersen H, Jacobsen C, Jessen H, Mollerup S, Sheikh MI. Electrogenic uptake of d-imino acids by luminal membrane vesicles from rabbit kidney proximal tubule. Biochim Biophys Acta 1989; 984: 231–237
- Røigaard-Petersen H, Jacobsen C, Sheikh MI. H+-l-proline cotransport by vesicles from pars convoluta of rabbit proximal tubule. Am J Physiol 1987; 253: F15–F20
- Røigaard-Petersen H, Jessen H, Mollerup S, Jorgensen KE, Jacobsen C, Sheikh MI. Proton-gradient dependent renal transport of glycine: evidence from vesicle studies. Am J Physiol 1990; 258: F388–F396
- Røigaard-Petersen H, Sheikh MI. Renal transport of neutral amino acids. Biochem J 1984; 220: 25–33
- Sagné C, Agulhon C, Ravassard P, Darmon M, Hamon M, El Mestikawy S, Gasnier B, Giros B. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci USA 2001; 98: 7206–7211
- Scriver CR. Renal tubular transport of proline, hydroxyproline, and glycine. III. Genetic basis for more than one mode of transport in human kidney. J Clin Invest 1968; 47: 823–835
- Scriver CR, Efron ML, Schafer IA. Renal tubular transport of proline, hydroxyproline, and glycine in health and in familial hyperprolinemia. J Clin Invest 1964; 43: 374–385
- Scriver CR, Schafer IA, Efron ML. New renal tubular amino-acid transport system and a new hereditary disorder of amino-acid metabolism. Nature 1961; 192: 672–673
- Stevens BR, Wright EM. Substrate specificity of the intestinal brush-border proline/sodium (IMINO) transporter. J Membr Biol 1985; 87: 27–34
- Stevens BR, Wright EM. Kinetics of the intestinal brush border proline (Imino) carrier. J Biol Chem 1987; 262: 6546–6551
- Takanaga H, Mackenzie B, Suzuki Y, Hediger MA. Identification of a mammalian proline transporter (SIT1, SLC6A20) with characteristics of classical system IMINO. J Biol Chem 2005; 280: 8974–8984
- Thompson E, Levin RJ, Jackson MJ. The stimulating effect of low pH on the amino acid transferring systems of the small intestine. Biochim Biophys Acta 1970; 196: 120–122
- Thwaites DT, Armstrong G, Hirst BH, Simmons NL. d-Cycloserine transport in human intestinal epithelial (Caco-2) cells: mediation by a H+-coupled amino acid transporter. Br J Pharmacol 1995a; 115: 761–766
- Thwaites DT, Basterfield L, McCleave PMJ, Carter SM, Simmons NL. Gamma-aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br J Pharmacol 2000; 129: 457–464
- Thwaites DT, Ford D, Glanville M, Simmons NL. H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J Clin Invest 1999; 104: 629–635
- Thwaites DT, McEwan GTA, Brown CDA, Hirst BH, Simmons NL. Na+-independent, H+-coupled transepithelial β-alanine absorption by human intestinal Caco-2 cell monolayers. J Biol Chem 1993a; 268: 18438–18441
- Thwaites DT, McEwan GTA, Cook MJ, Hirst BH, Simmons NL. H+-coupled (Na+-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett 1993b; 333: 78–82
- Thwaites DT, McEwan GTA, Simmons NL. The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J Membr Biol 1995b; 145: 245–256
- Thwaites DT, Stevens BC. H+/zwitterionic amino acid symport at the brush-border membrane of human intestinal epithelial (Caco-2) cells. Exp Physiol 1999; 84: 275–284
- Tiruppathi C, Ganapathy V, Leibach FH. Kinetic evidence for a common transporter for glycylsarcosine and phenylalanylalanine in renal brush-border membrane vesicles. J Biol Chem 1990; 265: 14870–14874
- Wreden CC, Johnson J, Tran C, Seal RP, Copenhagen DR, Reimer RJ, Edwards RH. The H+-coupled electrogenic lysosomal amino acid transporter LYAAT1 localizes to the axon and plasma membrane of hippocampal neurons. J Neurosci 2003; 23: 1265–1275
- Wunz TM, Wright SH. Betaine transport in rabbit renal brush-border membrane vesicles. Am J Physiol 1993; 264: F948–F955

