Abstract
Functional polarization of leukocytes is a requisite to accomplish immune function. Immune synapse formation or chemotaxis requires asymmetric redistribution of membrane receptors, signaling molecules and the actin cytoskeleton. There is increasing evidence that compartmentalization of the plasma membrane into distinct lipid microdomains is pivotal in establishing and maintaining leukocyte polarity. Specific rafts assemble into large-scale domains to create plasma membrane asymmetries at specific cell locations, thus coordinating temporally and spatially cell signaling in these processes. In this review we discuss the roles of lipid rafts as organizers of T lymphocyte polarity during cell activation and migration.
Introduction
Migration and activation of T lymphocytes are critical processes of the adaptive immunity. Both T cell migration and activation require the compartmentalization of specific membrane receptors and signaling molecules in specific cell locations, a process termed polarization. At the plasma membrane, T cell polarization is accompanied by assemblies of specialized lipids with the characteristics of ‘lipid rafts’. Under resting conditions in the absence of an extracellular stimulus, lipid rafts are thought to be very small and highly unstable. Cross-linking of raft-preferring molecules (such as GPI-anchored receptors) may stabilize small/unstable rafts in larger structures that, by coalescence with other small/unstable rafts carrying signaling molecules, generate the lipid microdomains involved in signaling (Edidin [Citation2001], Subczynski & Kusumi [Citation2003]). Here we use the term ‘rafts’ to indicate the larger/stable domains, visible at the fluorescence microscope level, induced by either cross-linking with specific ligands or generated by selective stimuli, such as antigens on the surface of antigen presenting cells or chemotactic factors.
The use of fluorescent protein (FP) technology enables the direct examination of raft dynamics during T cell responses. This technique showed that the basic raft units, too small to be seen by conventional microscopy, become large platforms that transport membrane proteins and signaling partners to specific cell sites during lymphocyte migration and activation.
Lymphocyte migration
Cell migration is a crucial process in many biological events, including embryo implantation, developmental patterning, axon guidance and wound healing, among others. In the immune system, leukocyte migration is a prominent component in adaptive immune responses and tissue homeostasis; as a consequence, deregulated migration is involved in the development of many pathologies, including autoimmunity, chronic inflammation, immunodeficiency and cancer. To move, leukocytes – as well as all different cell types – must acquire and maintain morphological and functional asymmetry, a process termed polarization (Lauffenburger & Horwitz [Citation1996], Mañes et al. [Citation2000]). Polarity refers to the ability of a migrating cell to change its morphology in response to chemoattractants, and to maintain a stable asymmetric shape with two poles, the leading edge, which protrudes at the cell front, and the rear edge (termed uropod in leukocytes), which retracts (Sanchez-Madrid & del Pozo [Citation1999]). In mammalian cells, cell polarity is an absolute requirement for cell movement; concurrently, migration reinforces cell polarization (Mañes et al. [Citation2003]).
Several studies have shown that plasma membrane domains with specialized lipid composition are distributed asymmetrically in different moving cell types, including carcinoma cells, nerve growth cones, epithelial cells and leukocytes (Eaton & Simons [Citation1995], Ibañez [Citation2004], Ledesma et al., [Citation1998]; Mañes et al., [Citation1999]). Since a common feature of these domains is their cholesterol enrichment, we will designate them here as lipid rafts, although this is an oversimplification of the true complexity of plasma membrane domain segregation in migrating cells (Mañes & Martínez-A [Citation2004]).
Asymmetric distribution of lipid rafts during lymphocyte migration
An increasing number of reports have described asymmetric raft reorganization in several types of moving cells (Mañes et al. [Citation2003]), although where these domains localize is contradictory. Exclusive uropod GM1 accumulation was found in T lymphocytes (Millan et al. [Citation2002]) and neutrophils (Seveau et al. [Citation2001]). Studies using the lipid dye Laurdan, which displays a spectral shift depending on the degree of order in the lipid environment, suggest the presence of lipid rafts also at the front of polarized neutrophils (Kindzelskii et al. [Citation2004]). Unifying both types of observations, segregation of raft subtypes to each pole of polarized T cells and leukocytes has been also described (Gómez-Moutón et al. [Citation2001]), with GM3 at the leading edge (L-rafts) and GM1 at the uropod (U-rafts) (). Notably, two distinct raft types have been also implicated in pheromone-induced yeast polarization (Bagnat & Simons [Citation2002]).
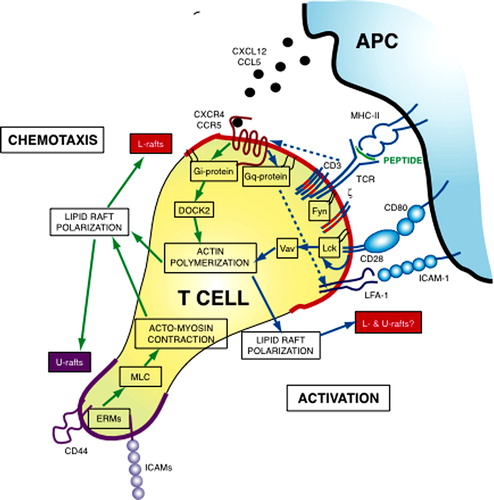
Figure 1. A molecular model for lipid rafts in T cell polarization. The scheme shows the main signaling events leading to T cell polarization during chemotaxis (green arrows) and activation (blue arrows). Chemotactic signaling is initiated when chemokines activate chemokine receptors in lipid rafts. In the absence of other signaling pathways, activated chemokine receptors couple to Gi proteins initiating actin rearrangements through a DOCK2/Rac-dependent pathway. Actin rearrangements probably lead to lipid rafts polarization, which culminate in segregation of two lipid raft types: L-rafts (red lines) at the leading edge and U-rafts (violet line) at the uropod. L-rafts polarization enhanced directional sensing by increasing chemokine receptors and integrins (such as Lymphocyte Function-Associated Antigen-1, LFA-1) at the leading edge; at this cell site, L-rafts may also favor protrusive activity by concentrating cholesterol-rich domains that help actin-induced membrane deformation. U-rafts concentrate adhesion receptors at the rear, triggering localized activation of ERM proteins. Activated ERM then stimulate cell contraction by regulating Myosin Light Chain (MLC) phosphorylation, likely through a RhoA/ROCK pathway. Actomyosin contraction reinforces lipid raft polarization, enabling the persistence in polarization. When the T lymphocyte reaches the proper APC, TCR/CD28 signaling induces cell polarization toward the antigenic stimulus and organizes the mature immune synapse. Lipid rafts are recruited at the T cell/APC contact region, even if it is not known whether L- and U-rafts segregate to opposite cell poles during IS formation or if both L- and U-rafts concentrate at the IS. TCR activation also leads to a major change in chemokine receptor signaling that, in this condition, couple preferentially to Gq/11 instead of Gi. Signaling through Gq/11 induce T cell adhesion rather than migration either by enhancing LFA-1 affinity or by impeding Gi-mediated chemotactic signaling. Lipid rafts recruitment into the IS requires CD28 signaling through its cytoplasmic proline motif and Vav-1 activity, suggesting that raft dynamics are controlled by actin remodeling. By reorganizing lipid rafts, CD28 recruits signaling molecules, such as Lck, into the IS, and generates an environment where signal transduction is protected and amplified. Moreover, by controlling stiffness of the plasma membrane, lipid rafts enrichment at the IS may regulate fitting between T cell and APC.This Figure is reproduced in colour in Molecular Membrane Biology online.
More recently, the use of the FP technology has permitted to visualize lipid raft dynamics in leukocytes engaged in chemotaxis. Real-time confocal videomicroscopy studies have shown that a lipid raft probe (glycosylphosphatidyl-tagged GFP; GFP-GPI), redistribute to, and persist at, the leading edge and uropod in directionally-stimulated lymphocytes, promyelocytic and neutrophil-like cells (Gómez-Moutón et al. [Citation2004]). A similar redistribution was observed with a probe for the inner plasma membrane leaflet of lipid rafts (Gri et al. [Citation2004]), suggesting that both inner and outer leaflet rafts are coupled during the polarization process. By contrast, a transmembrane, non-raft GFP probe (GFP-GT46) showed a non-polarized distribution during chemotaxis in these cells. Raft segregation takes place shortly after chemoattractant stimulation, and depends on chemoattractant receptor signaling and actin cytoskeleton integrity. These results thus confirm that lipid rafts segregate to each cell pole during migration of immune cells.
All together, the results suggest that lipid rafts comprise one of the mechanisms implicated in cell polarity during migration. The differences reported in their location in polarized cells may reflect the differences between the different cell types or the modes of migration analyzed. Indeed, ezrin-radixin-moesin (ERM) family proteins, which link filamentous actin (F-actin) to the cell membrane, associate to GM1-enriched rafts and redistribute to the leading edge in fibroblast but to the uropod in lymphocytes (Michaely et al. [Citation1999], Tomas et al. [Citation2002]). On the other hand, the lipid raft component caveolin-1 polarizes to the cell front in transmigrating endothelial cells, but to the rear when these cells migrate in a two-dimensional system (Parat et al. [Citation2003]).
Lipid rafts as platforms for spatial location of receptors in migrating cells
A consequence of cell polarity is the asymmetric localization of membrane receptors and signaling molecules between the leading edge and the uropod (Sanchez-Madrid & del Pozo [Citation1999]). Although there are differences among cell types, the leading edge usually contains the machinery that induces localized actin polymerization and that senses the environment; in immune cells, the uropod contains receptors and signaling molecules involved in cell adhesion, such as ERM, CD43, CD44 or ICAM1-3 (reviewed in Mañes et al. [Citation2005]). Redistribution of lipid raft markers, such as GFP-GPI, suggests that proteins with no functional significance in cell polarization or migration distribute in migrating lymphocytes as a function of their raft association. Evidence also indicates that membrane receptor association with distinct raft domains dictates their redistribution to the appropriate location during leukocyte chemotaxis. Chemosensory receptors of the chemokine family, such as CXCR4, CCR5, CCR2 and CXCR1, N-formyl peptide receptors, the receptor for Epidermal Growth Factor, CD44 or ICAMs, among other membrane receptors, are reported to partition in rafts and to be redistributed in migrating cells (Ge & Pachter [Citation2004], Jiao et al. [Citation2005], Mañes et al. [Citation2003]). Moreover, different reports indicates that raft partitioning influences activation and signaling of some of these receptors (Jiao et al. [Citation2005], Nguyen & Taub [Citation2002], Wysoczynski et al. [Citation2005], Xue et al. [Citation2004])
An important question is whether modification of proteins to impede association with lipid rafts inhibits their asymmetrical redistribution in moving cells. This has been demonstrated for the raft-associated proteins EphrinB1 (Mañes et al. [Citation1999]) and influenza virus hemagglutinin (HA) (Gómez-Moutón et al. [Citation2001]) ectopically expressed in breast cancer and Jurkat cells, respectively. It was found that either Ephrin B1 and HA redistribute asymmetrically in chemoattractant stimulated cells, whereas expression of non-raft mutant versions of these proteins results in homogeneous protein distribution on the cell membrane (Scheiffele et al. [Citation1997], Bruckner et al. [Citation1999]). These results emphasize the role of lipid rafts in asymmetric redistribution of membrane proteins during cell polarization.
More recently, the function of lipid rafts as platforms to deliver proteins to specific cell locations has been further reinforced by analyzing the dynamics of CXCR4 and CCR5 receptors during extravasation or crawling of lymphocytes (Gómez-Moutón et al. [Citation2004], van Buul et al. [Citation2003]). These receptors associate to GM3-enriched lipid rafts and consequently are redistributed to the leading edge of moving cells (Gómez-Moutón et al. [Citation2001]). In these studies, receptor polarization did not parallel accumulation of an inert, non-raft membrane probe, indicating that receptor asymmetry is likely the consequence of an active mechanism that deliver the receptor to the lymphocyte leading edge. Disturbance of lipid raft organization prevents redistribution of these receptors and impairs chemotaxis, suggesting that these microdomains are closely linked to the polarization of the proteins. Taken together, these data suggest a model in which raft domain redistribution causes the polarization of membrane receptors and lipid-linked ordered domain partitioning proteins.
Lipid rafts as organizational centers for signaling in migrating cells
Current evidence suggests that rafts are platforms in which interactions take place between activated receptors and signal transduction partners in an efficient manner. In migrating cells, lipid rafts might increase signaling efficiency but also restrict and/or organize signaling to specific cell areas. Leukocyte movement requires the interplay of many different signaling pathways initiated by chemoattractant receptors and integrins, which regulate gradient sensing and cytoskeletal rearrangements (for a review see Mañes et al. [Citation2005]). Heterotrimeric G proteins initiate most of these signaling pathways; ligand binding to G-protein-coupled receptors dissociates Gαi and Gβγ subunits of G-proteins, leading to calcium flux and activation of the phosphatidylinositol 3-kinase (PI3K) and the small Rho GTPases signaling pathways, among others. Double-acylated Gαi subunits concentrate in lipid rafts (Moffett et al. [Citation2000], Oh & Schnitzer [Citation2001]) in such a manner that active chemoattractant receptors and G-proteins concentrate in a common lipid environment, thus enabling signaling. Since chemoattractant receptors show a preferential affinity for lipid rafts distributed to the leading edge, redistribution of microdomains may permit the spatial restriction of G-protein activation at the cell front ().
Activation of G-proteins leads to dissociation of Gα and Gβγ subunits, which induce F-actin polymerization via two possible pathways: (i) one involves Gα signaling to Rac or Cdc42 activation through a DOCK2-dependent mechanism (Nombela-Arrieta et al. [Citation2004]), and (ii) the other involves free Gβγ activating PI3Kγ, which in turn activates Rac or Cdc42 (Hill et al. [Citation2005]). The first is the main signaling pathway to F-actin formation in lymphocytes, whereas the second pathway is likely more prominent in neutrophils than in lymphocytes. At present there is evidence that lipid rafts spatially control PI3K pathway during chemotaxis. It is well established that PI3K products phosphatidylinositol 3,4,5 triphosphate (PtdIns(3,4,5)P3) and PtdIns(3,4)P2 are generated and accumulate at the leading edge of moving cells (Mañes et al. [Citation2005]). Accumulation of these lipid enables localized recruitment of proteins with specific pleckstrin homology (PH) domains, thought to be important in cells sensing of the chemoattractant source and directed F-actin formation. It has been observed that different PI3K isoforms, including PI3Kγ, are recruited to lipid rafts at the leading edge shortly after chemoattractant stimulation (Gómez-Moutón et al. [Citation2004]). Inhibition of raft redistribution impedes asymmetric PI3Kγ activation but not homogenous production of PtdIns(3,4,5)P3, suggesting that lipid rafts are involved in directional sensing.
An important question is how PtdIns(3,4,5)P3 is confined to the leading edge. In Dictyostelium it has been shown that GFP-tagged Phosphatase and Tensin homolog on chromosome 10 (PTEN) is recruited at the sides and back of moving cell (reviewed in Mañes et al. [Citation2005]); however, PTEN is evenly distributed in migrating neutrophils and T cells (Lacalle et al. [Citation2004]). Since chemoattractant receptors require lipid rafts to function (see above), it is tempting to speculate that accumulation of rafts at the leading edge deliver ‘active’ receptors at thus permitting restricted PI3K activation at this cell site.
In addition, lipid rafts can organize directly the activation and/or recruitment of the small GTPases implicated in F-actin remodeling. Although these GTPases are recruited to the membrane through isoprenyl-based lipid anchors, which are supposed not fit well in raft membranes, both Cdc42 (Golub & Caroni [Citation2005]) and Rac (del Pozo et al. [Citation2004]) partition in lipid rafts; Rac partitioning in lipid rafts is nonetheless contended (Pierini et al. [Citation2003]). Independent of direct Rac association to lipid rafts, these reports implicate lipid rafts as key elements in regulating sustained Rac activation (del Pozo et al. [Citation2004], Pierini et al. [Citation2003]). Further supporting this view, crosslinking of lipid rafts components, or raft targeting of activated signaling proteins trigger Rho GTPase-dependent actin cytoskeleton rearrangements (Lacalle et al. [Citation2002], Nguyen et al. [Citation2005]).
T cell activation
T lymphocyte activation is the consequence of the interaction between T cell receptors (TCRs) and specific antigenic complexes formed by antigen-derived peptides bound to integral membrane proteins encoded by the class I or class II genes of the major histocompatibility complex (MHC). Whereas the interaction between an individual TCR and its ligand occurs over a time frame of a few seconds, the interaction between a single T cell and the antigen-presenting cell (APC) can be sustained for several hours. During the prolonged interaction with APCs, T lymphocytes scan the surface of their partners, integrate signals continually delivered by TCRs and costimulatory receptors, and organize ‘supramolecular activation clusters’ (SMACs) at the immunological synapse (IS) (Monks et al. [Citation1998], Dustin & Shaw [Citation1999]). Indeed, productive T cell activation requires both recognition of MHC/peptide complexes by the TCR and accessory signals generated by other cell membrane molecules. T-cell priming is strongly influenced by signals delivered by the costimulatory molecule CD28, which lower the T cell activation threshold and allow T cell priming by few antigenic complexes (Viola & Lanzavecchia [Citation1996]). CD28 ligands, namely B7.1 and B7.2, are expressed at high levels by professional APC, such as mature dendritic cells, activated B cells and macrophages. The presence of activation thresholds in T cell responses allows a precise discrimination of different antigens, as well as contexts in which they are presented.
Asymmetric distribution of lipid rafts during lymphocyte activation
Several lines of evidence indicate that receptor patterning is accompanied by lipid membrane remodeling leading to selective recruitment of raft domains into the IS.
In resting T lymphocytes, stimulation of T cells with anti-CD3 plus anti-CD28 antibody-coated beads induces recruitment of the ganglioside GM1 to the TCR triggering site (Viola et al. [Citation1999]). Several studies confirmed and expanded this initial observation (Dupre et al. [Citation2002], Paccani et al. [Citation2005], Round et al. [Citation2005]) and suggested that rafts recruitment to the IS occurs only in a subset of T cells characterized by high activation stringency (Ebert et al. [Citation2000], Kovacs et al. [Citation2002], Balamuth et al. [Citation2001]) and requires CD28 signaling (Viola et al. [Citation1999], Tavano et al. [Citation2004]).
Recently, using immortalized Jurkat T cells, it was suggested that lipid rafts are randomly distributed during cell stimulation by antibodies-coated beads and that the apparent enrichment in GM1 observed at the TCR contact site is the sole consequence of unspecific membrane ruffling (Glebov & Nichols [Citation2004]). Moreover, using the same experimental model, i.e., Jurkat T cells instead of peripheral blood CD4+ T cells, it was demonstrated that membrane microdomains generated during cell activation are created by protein-protein networks, suggesting that lipid rafts play no role in the IS formation (Douglass & Vale [Citation2005]). Unfortunately, in both the studies technically sophisticated approaches were applied to the wrong cells. Indeed, most of the published studies used resting T cells to observe lipid rafts mobilization in response to antibody-coated beads. Moreover, Douglass and Vale stimulated Jurkat cells using anti-CD3 antibodies, an experimental condition that does not induce raft mobilization in T cells (Viola et al. [Citation1999]).
Antibody-induced T cell activation is a highly useful experimental tool, but bears little resemblance to physiological situations. To understand whether and how lipid-based compartmentalization of plasma membrane occurs during T cell stimulation by APC, lipid rafts were visualized using a cyan fluorescent protein (CFP) carrying consensus sequences for myristoylation plus palmitoylation (MyrPalm-mCFP) (Zacharias et al. [Citation2002]). MyrPalm-mCFP allow to visualize rafts without the use of cross-linking reagents such as cholera toxin or antibodies and to follow the dynamics of lipid microdomains present at the inner plasma membrane leaflet, where the signaling molecules are recruited upon T cell activation. It has been shown that MyrPalm-mCFP accumulate at the IS of T cells stimulated by APCs, indicating that rafts are mobilized in response to physiological stimulation and participate in T cell synapse organization (Tavano et al. [Citation2004]). This rafts recruitment is highly specific, since fluorescent markers of non-raft membranes do not accumulate at the IS. These data, together with many previous results, demonstrate that lipid-based membrane asymmetries are generated during physiological processes in lymphocytes, suggesting a pivotal role for lipid membrane microdomains in controlling the IS formation ().
Lipid rafts as platforms for spatial location of receptors at the IS
The fact that T cells are activated through an interaction with another cell has three intriguing consequences: (i) several receptors are simultaneously triggered; (ii) signaling is compartmentalized; and (iii) effector functions are localized.
A variety of receptors accumulate and segregate at the IS. Lipid rafts have been implicated in protein sorting in several cell types (reviewed in Schuck & Simons [Citation2004]). However, the precise role for lipid rafts in organizing receptor assembly at the IS is not yet clear. As already discussed above, recruitment into the IS of lipid raft markers, such as GFP-GPI or MyrPalm-mCFP, suggests that proteins with no functional significance in cell activation distribute as a function of their raft association. Even if it is yet to be fully appreciated the precise relationship between detergent resistant membranes (DRM) and lipid rafts (Lichtenberg et al. [Citation2005]), the correlation between the capacity of a molecule to be recruited into the IS and its preference for a ‘raft environment’ is quite strong.
While evidence for the presence of a fraction of CD4 and CD8 coreceptors in DRM have been provided (Parolini et al. [Citation1996], Arcaro et al. [Citation2001]), it is not clear yet whether the TCR constitutively resides in raft domains. Although pre-TCR seems to be constitutively present in DRM and its presence into rafts might be sufficient for eliciting signals needed to proceed in T cell development (Saint-Ruf et al. [Citation2000]), most of the data indicate that in mature resting cells the TCR is excluded from DRM and that antigen binding is required to induce a significant number of TCRs to associate with rafts (reviewed in Pizzo & Viola [Citation2003]). CD28 behaves similarly to the TCR: it is excluded from DRM in resting conditions, but partition in these membrane domains after triggering (Sadra et al. [Citation2004]).
Chemokine receptors have been recently described as new T cell costimulatory molecules (Molon et al. [Citation2005]). During T cell activation, CCR5 and CXCR4 are recruited to and accumulate at the IS by a mechanism requiring chemokine secretion by APCs. Recruitment of chemokine receptors to the IS results in stronger T cell-APC attraction, reduction of T cell responsiveness to chemotactic gradients, and in higher levels of T cell proliferation and cytokine production (Molon et al. [Citation2005]). As already discussed, both CCR5 and CXCR4 partition in DRM, and it is temping to speculate that their association with lipid rafts might be pivotal for recruitment into the IS ().
It has been originally proposed that dynamic raft reorganization at the IS favor T cell activation by generating an environment where signal transduction is protected and amplified (Rodgers & Rose [Citation1996], Viola et al. [Citation1999], Janes et al. [Citation1999]). Recently, this hypothesis was confirmed by a study on the transmembrane tyrosine phosphatase CD45 (Zhang et al. [Citation2005]). While it is well accepted that CD45 is responsible for switching on Src-family kinases (key initiators of lymphocyte receptor signaling), repeated findings of CD45 acting to inhibit lymphocyte activation indicate that CD45 may act as both a positive and negative regulator (Huntington & Tarlinton [Citation2004]). Interestingly, CD45 seems to be dynamically repositioned within lipid rafts and the IS during T cell activation. After TCR stimulation, the low endogenous CD45 associated with lipid rafts become actively excluded from the microdomains (Edmonds & Ostergaard [Citation2002], Zhang et al. [Citation2005]). Similarly, CD45 appear included in the immature IS for a short period in which the synapse is relatively devoid of phosphorylated proteins (Freiberg et al. [Citation2002]). Therefore, during T cell activation three events occur in parallel: exclusion of CD45 from lipid rafts, exclusion of CD45 from the central part of the IS, and accumulation of phosphoproteins within the IS. By using chimeric proteins to target CD45 activity to lipid rafts or to conventional membranes it has been demonstrated that CD45 partitioning into rafts dictate whether the phosphatase functions as a negative or positive regulator of T cell activation (Zhang et al. [Citation2005]). Thus, recruitment and clustering of rafts within the IS segregate negative and positive players of T cell activation and protect TCR signaling.
Lipid rafts as organizational centers for signaling at the IS
During lymphocyte activation lipid rafts may function as platforms for the formation of multi-component transduction complexes. Indeed, these microdomains are constitutively enriched in proteins involved in the early phases of TCR signaling, such as the Src-family kinases Lck and Fyn, the adapter protein LAT, phosphoprotein associated with glycosphingolipid-enriched domains (PAG) or Csk-activating protein (Cbp) and Lck-interacting molecule (LIME) (reviewed in Pizzo & Viola [Citation2005]). Whether targeting of these proteins to raft domains is crucial for their functions is not yet clear. Thus, while it was reported that a non-palmitoylated LAT mutant that do not localize to plasma membrane rafts is unable to signal properly (Zhang et al. [Citation1998]), new data demonstrate that LAT localization to lipid rafts is not essential in T cell activation as well as in development (Zhu et al. [Citation2005]). The composition of raft-associated proteins changes after T cell stimulation, suggesting that rafts are dynamic platforms for T cell signaling. Thus, upon TCR stimulation, many signaling proteins became concentrated in rafts, including the zeta-associated protein 70 (ZAP-70), the phospholipase Cγ (PLCγ), the exchange factor Vav, the protein kinase Cθ?(PKCθ) and the protein kinase B (PKB) (reviewed in Pizzo & Viola [Citation2005]). Based on these data and on the use of cholesterol-depleting reagents, a direct role for lipid rafts in controlling TCR triggering was initially proposed (Montixi et al. [Citation1998], Xavier et al. [Citation1998]). However, the recent literature indicates that TCR triggering, at least in its initial phases, is independent of cholesterol-based microdomains (Pizzo et al. [Citation2002], Pizzo et al. [Citation2004], Rouquette-Jazdanian et al. [Citation2005]). On the other hand, during physiological T cell stimulation, rafts might be required to organize a complete IS or for signal transduction associated to T cell costimulatory molecules.
In the presence of CD28 costimulation, T cell responses are achieved at a lower threshold of triggered TCRs, suggesting that CD28 can increase the potency of the signal transduced by each TCR engaged. Indeed, several reports have demonstrated that CD28 can enhance diverse T cell signaling pathways (reviewed in Viola [Citation2001]), suggesting that the molecule acts as a general amplifier of early TCR signaling. It was proposed that CD28-induced amplification of the TCR signaling cascade is based on lipid rafts (Viola [Citation2001]). By reorganizing lipid rafts, CD28 recruits signaling molecules, such as Lck, into the IS, thus explaining the mechanism by which CD28 triggering leads to higher and more stable tyrosine phosphorylation of several substrates (Viola et al. [Citation1999], Tavano et al. [Citation2004]).
In addition to their role in recruiting signaling mediators, rafts may represent key elements in determining which molecules accumulate at the IS and thus they can protect signaling from the action of negative regulators of TCR activation pathways (Rodgers & Rose [Citation1996], Viola et al. [Citation1999], Janes et al. [Citation1999], Zhang et al. [Citation2005]).
The classical view of chemokine receptor signaling and function proposes that, when engaged by specific chemokines, chemokine receptors induce cell migration via a Gi-mediated signaling. Thus, it has been proposed that go signals mediated by chemokine receptors compete with stop signals delivered by TCRs engaged by specific peptide-MHC complexes on APCs (Dustin [Citation2004]). However, CCR5 and CXCR4 are recruited to the IS and, in this context, instead of delivering signals competing with those induced by TCR and adhesion molecules, they are responsible of a signaling pathway resulting in T cell costimulation (Molon et al. [Citation2005]). Compatible with this, during T cell activation by APCs, CCR5 does not associates with Gi but with Gq/11, explaining the higher stability of the T cell-APC pair when CCR5 is triggered (Molon et al. [Citation2005]). Indeed, it has been reported that this change in G-protein resulted in preferential chemokine-induced cell adhesion rather than chemotaxis (Mellado et al. [Citation2001]). In this scenario, chemokine receptors prolong the duration of T cell-APC interaction, and facilitate T cell activation by reinforcing T cell-APC pair attraction and by avoiding pre-mature splitting due to chemoattractant sources. In addition, Gq-mediated signaling also triggers the translocation of nuclear factor of activated T cells (NFAT) to the nucleus (Boss et al. [Citation1996]), providing another mechanism by which chemokine receptor engagement at the IS may promote T cell activation.
An intriguing question is how and when the G-protein coupling to the chemokine receptors is specified. Different G-proteins may be differently targeted to distinct membrane microdomains by acylation of the α-subunit. For example, in endothelial and epithelial cells Gq interacts with caveolin, whereas Gi and Gs seem to be localized in other lipid domains (Oh & Schnitzer [Citation2001]). It was therefore proposed that G-protein coupled receptors (GPCR) can induce distinct signaling pathways depending on their association with membrane rafts (Chini & Parenti [Citation2004]). The human oxytocin receptor (OTR) is one example of a GPCR whose localization in lipid rafts modulates the specificity of Gα coupling (Rimoldi et al. [Citation2003]). OTR coupling to Gi occurs when the receptor is excluded from lipid rafts, while it signals through Gq when localized in caveolae. As already discussed, in moving T cells, there is segregation of two lipid raft subtypes between the two opposite cell poles, resulting in a preferential location of GM3-enriched rafts at the leading edge and GM1-based rafts at the uropod (Gómez-Mouton et al. [Citation2001]). CCR5 and CXCR4 associate specifically to GM3-enriched lipid rafts at the front of moving cells and likely they are segregated from Gq, which is active at the uropod. GM1 rafts are known to accumulate at the IS during T cell stimulation (Viola et al. [Citation1999]). It is possible that IS formation overrides the segregation between chemokine receptors and Gq/11-proteins, thus permitting Gq/11 coupling to these receptors ().
Linking lipid rafts to the actin cytoskeleton
T cell activation by APCs as well as chemoattractant-induced cell polarization and migration depend largely on F-actin rearrangements. Different reports have identified several molecules that participate in tethering lipid rafts to the actin cytoskeleton (Rodgers et al. [Citation2005]). These include actin binding proteins, such as ERM proteins, talin, and vinculin, among others, that have been found associated to DRMs, and the lipid PtdIns(4,5)P2, which is also enriched in lipid rafts. PtdIns(4,5)P2 is a major regulator the actin polymerization machinery by regulating directly the activity of Rac and Cdc42; these GTPases, in turn, regulate the Wiskott-Aldrich syndrome (WASp) and Scar/WAVE proteins and, ultimately, the Arp2/3 complex nucleation and branching activity (Mañes et al. [Citation2005]). Notably, PtdIns(4,5)P2 generation also depends on cholesterol levels at the plasma membrane.
Increasing evidence indicates the prevalence of feedback loops between lipid rafts and the actin cytoskeleton during migration and activation (). On one hand, lipid rafts contain part of the elements involved in the regulation of F-actin rearrangements. On the other hand, the actin cytoskeleton can participate in inducing and sustaining lipid raft polarization in triggered cells. One hypothesis is that small rafts in the plasma membrane of unstimulated cells migrate through the bilayer to form large raft clusters in discrete regions on the cell surface in an F-actin dependent manner. Direct visualization of lipid rafts in T cells and neutrophils engaged in chemotaxis supports this notion (Gómez-Moutón et al. [Citation2004]). Nonetheless, it is also possible that raft polarization is achieved by intracellular trafficking of lipid raft-enriched vesicles (Viola et al. [Citation1999], Martin et al. [Citation2001]). Indeed, it has been reported that there is a specific association between microtubule plus ends and lipid rafts (Golub & Caroni [Citation2005]).
A new emerging paradigm is the possible function of specific raft lipids in regulating membrane elasticity that, in turn, controls the acquisition of a motile cell phenotype and the fitting of the T cell-APC at the IS. Actin cytoskeleton-induced membrane deformation is lower in artificial membranes with low cholesterol than in membranes with physiological cholesterol content (Vasanji et al. [Citation2004]). Moreover, in endothelial cells cholesterol depletion results in a significant decrease in membrane deformability and a corresponding increase in the value of the elastic coefficient of the membrane, indicating that cholesterol-depleted cells are stiffer than control cells (Byfield et al. [Citation2004]). This is an apparent paradox, since it is well-established that cholesterol addition to phospholipid bilayers increases their rigidity and diminishes their elasticity (Brown et al. [Citation2002]). A recent study based on artificial membranes showed that vesiculation preferentially occurs from cholesterol-poor, liquid disordered-phase membranes (Roux et al. [Citation2005]). Notably, the increased membrane stiffness of cholesterol depleted cells was reversed by treatment with Latrunculin A, suggesting that cholesterol can regulate rigidity by altering the properties of the submembrane F-actin and/or its membrane association (Byfield et al. [Citation2004]). Accumulation of lipid rafts at the leading edge may thus control the F-actin-induced membrane protrusions and the stability of the F-actin network by increasing membrane cholesterol content at this cell site.
During the organization of the IS, CD28 signaling is responsible of actin rearrangement and lipid rafts recruitment. Vav-1 is a guanine nucleotide exchange factor (GEF) for the Rho/Rac family of protein G, and is involved in the activation of Rac-1 and Cdc42 (Khosravi-Far et al. [Citation1994]). Vav-1 is considered one of the main effector molecules of CD28. Although TCR is able to activate Vav-1, the most prominent role in Vav-1 activation is played by CD28 (Michel et al. [Citation2000]; Tuosto & Acuto [Citation1998]). Vav-1 is a key regulator of the actin cytoskeleton rearrangements that are necessary to the accumulation of signaling molecules and lipid rafts at the APC/T cell interface (Wulfing et al. [Citation2000], Villalba et al. [Citation2001], Tavano et al. [Citation2004]). To induce Vav-1 phosphorylation and Cdc42 activation, CD28 seems to use a signaling pathway distinct from the TCR and involving the Src kinases Lck and /or Fyn (Raab et al. [Citation2001], Salazar-Fontana et al. [Citation2003]). It has been proposed that C-terminal proline residues of CD28 are required for Lck binding as well as for CD28-mediated T cell costimulation (Holdorf et al. [Citation1999]). Notably, the same proline residues of CD28 were identified as the region responsible for CD28-mediated lipid rafts recruitment to the IS (Tavano et al. [Citation2004]). Thus, the interaction of CD28 with Lck seems to be relevant for CD28 signaling leading to actin remodeling, lipid rafts mobilization and T cell costimulation ().
This paper was first published online on prEview on 27 January 2006.
References
- Arcaro A, Gregoire C, Bakker TR, Baldi L, Jordan M, Goffin L, Boucheron N, Wurm F, van der Merwe PA, Malissen B, Luescher IF. CD8beta endows CD8 with efficient coreceptor function by coupling T cell receptor/CD3 to raft-associated CD8/p56(lck) complexes. J Exp Med 2001; 194: 1485–1495
- Bagnat M, Simons K. Cell surface polarization during yeast mating. Proc Natl Acad Sci USA 2002; 99: 14183–14188
- Balamuth F, Leitenberg D, Unternaehrer J, Mellman I, Bottomly K. Distinct patterns of membrane microdomain partitioning in Th1 and th2 cells. Immunity 2001; 15: 729–738
- Boss V, Talpade DJ, Murphy TJ. Induction of NFAT-mediated transcription by Gq-coupled receptors in lymphoid and non-lymphoid cells. J Biol Chem 1996; 271: 10429–10432
- Brown M, Thurmond R, Dodd S, Otten D, Beyer K. Elastic deformation of membrane bilayers probed by deuterium NMR relaxation. J Am Chem Soc 2002; 124: 8471–8484
- Bruckner K, Labrador J, Scheiffele P, Herb A, Seeburg P, Klein R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 1999; 22: 511–524
- Byfield F, Aranda-Espinoza H, Romanenko V, Rothblat G, Levitan I. Cholesterol depletion increases membrane stiffness of aortic endothelial cells. Biophys J 2004; 87: 3336–3343
- Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there?. J Mol Endocrinol 2004; 32: 325–338
- del Pozo M, Alderson N, Kiosses W, Chiang H, Anderson R, Schwartz M. Integrins regulate Rac targeting by internalization of membrane domains. Science 2004; 303: 839–842
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 2005; 121: 937–950
- Dupre LA, Aiuti A, Trifari S, Martino S, Saracco P, Bordignon C, Roncarolo MG. Wiskott-Aldrich syndrome protein regulates lipid raft dynamics during immunological synapse formation. Immunity 2002; 17: 157–166
- Dustin ML. Stop and go traffic to tune T cell responses. Immunity 2004; 21: 305–314
- Dustin ML, Shaw AS. Costimulation: building an immunological synapse. Science 1999; 283: 649–650
- Eaton S, Simons K. Apical, basal, and lateral cues for epithelial polarization. Cell 1995; 82: 5–8
- Ebert PJ, Baker JF, Punt JA. Immature CD4 + CD8+ thymocytes do not polarize lipid rafts in response to TCR-mediated signals. J Immunol 2000; 165: 5435–5442
- Edidin M. Shrinking patches and slippery rafts: scales of domains in the plasma membrane. Trends Cell Biol 2001; 11: 492–496
- Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol 2002; 169: 5036–5042
- Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol 2002; 3: 911–917
- Ge S, Pachter J. Caveolin-1 knockdown by small interfering RNA suppresses responses to the chemokine monocyte chemoattractant protein-1 by human astrocytes. J Biol Chem 2004; 279: 6688–6695
- Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol 2004; 6: 238–243
- Golub T, Caroni P. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J Cell Biol 2005; 169: 151–165
- Gómez-Moutón C, Abad J, Mira E, Lacalle R, Gallardo E, Jiménez-Baranda S, Illa I, Bernad A, Mañes S, Martínez-A C. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci USA 2001; 98: 9642–9647
- Gómez-Moutón C, Lacalle R, Mira E, Jiménez-Baranda S, Barber D, Carrera A, Martínez-A C, Mañes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol 2004; 164: 759–768
- Gri G, Molon B, Mañes S, Pozzan T, Viola A. The inner side of T cell lipid rafts. Immunol Lett 2004; 94: 247–252
- Hill K, Krugmann S, Andrews S, Coadwell W, Finan P, Welch H, Hawkins P, Stephens L. Regulation of P-Rex1 by phosphatidylinositol (3,4,5)-trisphosphate and Gbetagamma subunits. J Biol Chem 2005; 280: 4166–4173
- Holdorf AD, Green JM, Levin SD, Denny MF, Straus DB, Link V, Changelian PS, Allen PM, Shaw AS. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J Exp Med 1999; 190: 375–384
- Huntington ND, Tarlinton DM. CD45: direct and indirect government of immune regulation. Immunol Lett 2004; 94: 167–174
- Ibañez C. Lipid rafts as organizing platforms for cell chemotaxis and axon guidance. Neuron 2004; 42: 3–5
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol 1999; 147: 447–461
- Jiao X, Zhang N, Xu X, Oppenheim J, Jin T. Ligand-induced partitioning of human CXCR1 chemokine receptors with lipid raft microenvironments facilitates G-protein-dependent signaling. Mol Cell Biol 2005; 25: 5752–5762
- Kindzelskii A, Sitrin R, Petty H. Cutting edge: Optical microspectrophotometry supports the existence of gel phase lipid rafts at the lamellipodium of neutrophils: Apparent role calcium signaling. J Immunol 2004; 172: 4681–4685
- Khosravi-Far R, Chrzanowska-Wodnicka M, Solski PA, Eva A, Burridge K, Der CJ. Dbl and Vav mediate transformation via mitogen-activated protein kinase pathways that are distinct from those activated by oncogenic Ras. Mol Cell Biol 1994; 14: 6848–6857
- Kovacs B, Maus MV, Riley JL, Derimanov GS, Koretzky GA, June CH, Finkel TH. Human CD8+ T cells do not require the polarization of lipid rafts for activation and proliferation. Proc Natl Acad Sci USA 2002; 99: 15006–15011
- Lacalle R, Gómez-Moutón C, Barber D, Jiménez-Baranda S, Mira E, Martínez-A C, Carrera A, Mañes S. PTEN regulates motility but not directionality during leukocyte chemotaxis. J Cell Sci 2004; 117: 6207–6215
- Lacalle R, Mira E, Gómez-Moutón C, Jiménez-Baranda S, Martínez-A C, Mañes S. Specific SHP-2 partitioning in raft domains triggers integrin-mediated signaling via Rho activation. J Cell Biol 2002; 157: 277–289
- Lauffenburger D, Horwitz A. Cell migration: a physically integrated molecular process. Cell 1996; 84: 359–369
- Ledesma M, Simons K, Dotti C. Neuronal polarity: Essential role of protein-lipid complexes in axonal sorting. Proc Natl Acad Sci USA 1998; 95: 3966–3971
- Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci 2005; 30: 430–436
- Mañes S, del Real G, Lacalle R, Lucas P, Gómez-Moutón C, Sánchez-Palomino S, Delgado R, Alcamí J, Mira E, Martínez-A C. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Reports 2000; 1: 190–196
- Mañes S, Gómez-Moutón C, Lacalle R, Jiménez-Baranda S, Mira E, Martínez-A C. Mastering time and space: immune cell polarization and chemotaxis. Semin Immunol 2005; 17: 77–86
- Mañes S, Lacalle R, Gómez-Moutón C, Martínez-A C. From rafts to crafts: membrane asymmetry in moving cells. Trends Immunol 2003; 24: 320–326
- Mañes S, Martínez-A C. Cholesterol domains regulate the actin cytoskeleton at the leading edge of moving cells. Trends Cell Biol 2004; 14: 275–278
- Mañes S, Mira E, Gómez-Moutón C, Lacalle R, Keller P, Labrador J, Martinez-A C. Membrane raft microdomains mediate front-rear polarity in migrating cells. EMBO J 1999; 18: 6211–6220
- Martin M, Schneider H, Azouz A, Rudd CE. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J Exp Med 2001; 194: 1675–1681
- Michaely P, Mineo C, Ying Y, Anderson R. Polarized distribution of endogenous Rac1 and RhoA at the cell surface. J Biol Chem 1999; 274: 21430–21436
- Mellado M, Rodriguez-Frade JM, Manes S, Martinez AC. Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu Rev Immunol 2001; 19: 397–421
- Michel F, Mangino G, Attal-Bonnefoy G, Tuosto L, Alcover A, Roumier A, Olive D, Acuto O. CD28 utilizes Vav-1 to enhance TCR-proximal signaling and NF-AT activation. J Immunol 2000; 165: 3820–3829
- Millan J, Montoya M, Sancho D, Sanchez-Madrid F, Alonso M. Lipid rafts mediate biosynthetic transport to the T lymphocyte uropod subdomain and are necessary for uropod integrity and function. Blood 2002; 99: 978–984
- Moffett S, Brown D, Linder M. Lipid-dependent targeting of G proteins into rafts. J Biol Chem 2000; 275: 2191–2198
- Molon B, Gri G, Bettella M, Goumez-Mouton C, Lanzavechia A, Martinez-A C, Manes S, Viola A. T cell costimulation by chemokine receptors. Nature Immunol 2005; 6: 465–471
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998; 395: 82–86
- Montixi C, Langlet C, Bernar AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J 1998; 17: 5334–5348
- Nguyen D, Taub D. CXCR4 Function requires membrane cholesterol: implications for HIV infection. J Immunol 2002; 168: 4121–4126
- Nguyen D, Giri B, Collins G, Taub D. Dynamic reorganization of chemokine receptors, cholesterol, lipid rafts, and adhesion molecules to sites of CD4 engagement. Exp Cell Res 2005; 304: 559–569
- Nombela-Arrieta C, Lacalle R, Montoya M, Kunisaki K, Megías D, Marqués M, Carrera A, Mañes S, Fukui Y, Martínez-A C, Stein J. Differential requirements for DOCK2 and phosphoinositide-3-kinase gamma during T and B lymphocyte homing. Immunity 2004; 21: 429–441
- Oh P, Schnitzer JE. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas G(i) and G(s) target lipid rafts by default. Mol Biol Cell 2001; 12: 685–698
- Paccani SR, Boncristiano M, Patrussi L, Ulivieri C, Wack A, Valensin S, Hirst TR, Amedei A, Del Prete G, Telford JL, D'Elios MM, Baldari CT. Defective Vav expression and impaired F-actin reorganization in a subset of patients with common variable immunodeficiency characterized by T-cell defects. Blood 2005; 106: 626–634
- Parat M, Anand-Apte B, Fox P. Differential caveolin-1 polarization in endothelial cells during migration in two and three dimensions. Mol Biol Cell 2003; 14: 3156–3168
- Parolini I, Sargiacomo M, Lisanti MP, Peschle C. Signal transduction and glycophosphatidylinositol-linked proteins (lyn, lck, CD4, CD45, G proteins, and CD55) selectively localise in Triton-insoluble plasma membrane domains of human leukemic cell lines and normal granulocytes. Blood 1996; 87: 3783–3794
- Pierini L, Eddy R, Fuortes M, Seveau S, Casulo C, Maxfield F. Membrane lipid organization is critical for human neutrophil polarization. J Biol Chem 2003; 278: 10831–10841
- Pizzo P, Giurisato E, Tassi M, Benedetti A, Pozzan T, Viola A. Lipid rafts and TCR signalling: a critical revaluation. Eur J Immunol 2002; 32: 3082–3091
- Pizzo P, Viola A. Lymphocyte lipid rafts: structure and functions. Curr Opin Immunol 2003; 15: 255–260
- Pizzo P, Giurisato E, Bigsten A, Tassi M, Tavano R, Shaw A, Viola A. Physiological T cell activation starts and propagates in lipid rafts. Immunol Lett 2004; 91: 3–9
- Pizzo P, Viola A. Lipid-based membrane microdomains in T cell activation. Curr Immunol Reviews 2005; 1: 7–12
- Raab M, Pfister S, Rudd CE. CD28 signaling via VAV/SLP-76 adaptors: regulation of cytokine transcription independent of TCR ligation. Immunity 2001; 15: 921–933
- Rimoldi V, Reversi A, Taverna E, Rosa P, Francolini M, Cassoni P, Parenti M, Chini B. Oxytocin receptor elicits different EGFR/MAPK activation patterns depending on its localization in caveolin-1 enriched domains. Oncogene 2003; 22: 6054–6060
- Rodgers W, Rose JK. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol 1996; 135: 1515–1523
- Rodgers W, Farris D, Mishra S. Merging complexes: properties of membrane raft assembly during lymphocyte signaling. Trends Immunol 2005; 26: 97–103
- Round JL, Tomassian T, Zhang M, Patel V, Schoenberger SP, Miceli MC. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med 2005; 201: 419–430
- Rouquette-Jazdanian, AK, Pelassy, C, Breittmayer, JP, Aussel, C. 2005. Revaluation of the role of cholesterol in stabilizing rafts implicated in T cell receptor signaling. Cell Signal, 18: 105–122
- Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J 2005; 24: 1537–1545
- Sadra A, Cinek T, Imboden JB. Translocation of CD28 to lipid rafts and costimulation of IL-2. Proc Natl Acad Sci USA 2004; 101: 11422–11427
- Saint-Ruf C, Panigada M, Azogui O, Debey P, von Boehmer H, Grassi F. Different initiation of pre-TCR and gdTCR signalling. Nature 2000; 406: 524–527
- Salazar-Fontana LI, Barr V, Samelson LE, Bierer BE. CD28 engagement promotes actin polymerization through the activation of the small Rho GTPase Cdc42 in human T cells. J Immunol 2003; 171: 2225–2232
- Sanchez-Madrid F, del Pozo M. Leukocyte polarization in cell migration and immune interactions. EMBO J 1999; 18: 501–511
- Scheiffele P, Roth M, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J 1997; 16: 5501–5508
- Schuck S, Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci 2004; 117: 5955–5964
- Seveau S, Eddy R, Maxfield F, Pierini L. Cytoskeleton-dependent membrane domain segregation during neutrophil polarization. Mol Biol Cell 2001; 12: 3550–3562
- Subczynski WK, Kusumi A. Dynamics of raft molecules in the cell and artificial membranes: approaches by pulse EPR spin labeling and single molecule optical microscopy. Biochim. Biophys. Acta 2003; 1610: 231–243
- Tavano R, Gri G, Molon B, Marinari B, Rudd CE, Tuosto L, Viola A. CD28 and lipid rafts coordinate recruitment of Lck to the immunological synapse of human T lymphocytes. J Immunol 2004; 173: 5392–5397
- Tomas E, Chau T, Madrenas J. Clustering of a lipid-raft associated pool of ERM proteins at the immunological synapse upon T cell receptor or CD28 ligation. Immunol Lett 2002; 83: 143–147
- Tuosto L, Acuto O. CD28 affects the earliest signaling events generated by TCR engagement. Eur J Immunol 1998; 28: 2131–2142
- van Buul J, Voermans C, van Gelderen J, Anthony E, van der Schoot C, Hordijk P. Leukocyte-endothelium interaction promotes SDF-1-dependent polarization of CXCR4. J Biol Chem 2003; 278: 30302–30310
- Vasanji A, Ghosh P, Graham L, Eppell S, Fox P. Polarization of plasma membrane microviscosity during endothelial cell migration. Dev Cell 2004; 6: 29–41
- Villalba M, Bi K, Rodriguez F, Tanaka Y, Schoenberger S, Altman A. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol 2001; 155: 331–338
- Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science 1996; 273: 104–106
- Viola A, Schroeder S, Sakakibara Y, Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 1999; 283: 680–682
- Viola A. Amplification of TCR signaling by membrane dynamic microdomains. Trends Immunol 2001; 22: 322–327
- Wulfing C, Bauch A, Crabtree GR, Davis MM. The vav exchange factor is an essential regulator in actin-dependent receptor translocation to the lymphocyte-antigen-presenting cell interface. Proc Natl Acad Sci USA 2000; 97: 10150–10155
- Wysoczynski M, Reca R, Ratajczak J, Kucia M, Shirvaikar N, Honczarenko M, Mills M, Wanzeck J, Janowska-Wieczorek A, Ratajczak M. Incorporation of CXCR4 into membrane hpid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood 2005; 105: 40–48
- Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity 1998; 8: 723–732
- Xue M, Vines C, Buranda T, Cimino D, Bennett T, Prossnitz E. N-formyl peptide receptors cluster in an active raft-associated state prior to phosphorylation. J Biol Chem 2004; 279: 45175–45184
- Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 2002; 296: 913–916
- Zhang W, Trible RP, Samelson LE. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 1998; 9: 239–246
- Zhang M, Moran M, Round J, Low TA, Patel VP, Tomassian T, Hernandez JD, Miceli MC. CD45 signals outside of lipid rafts to promote ERK activation, synaptic raft clustering, and IL-2 production. J Immunol 2005; 174: 1479–1490
- Zhu M, Shen S, Liu Y, Granillo O, Zhang W. LAT localization to lipid rafts is not essential in T cell activation and development. J Immunol 2005; 174: 31–35
