Abstract
The molecular events and the protein components that are involved in signalling by the T cell receptor (TCR) for antigen have been extensively studied. Activation of signalling cascades following TCR stimulation depends on the phosphorylation of the receptor by the tyrosine kinase Lck, which localizes to the cytoplasmic face of the plasma membrane by virtue of its post-translational modification. However, the precise order of events during TCR phosphorylation at the plasma membrane, remains to be defined. A current theory that describes early signalling events incorporates the function of lipid rafts, microdomains at the plasma membrane with distinct lipid and protein composition. Lipid rafts have been implicated in diverse biological functions in mammalian cells. In T cells, molecules with a key role in TCR signalling, including Lck, localize to these domains. Importantly, mutant versions of these proteins which fail to localise to raft domains were unable to support signalling by the TCR. Biochemical studies using purified detergent-resistant membranes (DRM) and confocal microscopy have suggested that upon stimulation, the TCR and Lck-containing lipid rafts may come into proximity allowing phosphorylation of the receptor. Further, there are data suggesting that phosphorylation of the TCR could depend on a transient increase in Lck activity that takes place within lipid rafts to initiate signalling. Current results and a model of how lipid rafts may regulate TCR signalling are discussed.
The T cell antigen receptor
T lymphocytes are essential components of the immune system that coordinate immune responses. Through their TCR, T cells recognize antigens presented by antigen presenting cells (APC) in the context of Major Histocompatibility Complex (MHC) proteins. The TCR is a multi-chain structure that includes a polymorphic α/β dimer that binds to antigen/MHC complexes, and the associated non-polymorphic transmembrane proteins CD3γ, CD3δ, CD3ε, and a ζ homo-dimer Citation[1]. The function of the non-polymorphic molecules is to communicate signals that are generated by antigen recognition, which ultimately lead to T cell activation and development of effector functions. A structurally conserved amino acid motif has been identified in the intracellular domain of the signal transducing chains, which is called the Immunoreceptor Tyrosine-based Activation Motif (ITAM) and contains in tandem two YXXL sequences separated by a 6–8 long amino acid spacer (Y = tyrosine, L = leucine and X = any amino acid) Citation[2]. Characteristically, each ζ chain contains three ITAMs while each of the CD3ε, CD3γ, and CD3δ proteins contains only one. The role of ITAMs in TCR signalling, are to serve as substrates for specific tyrosine kinases. When the tyrosine residues in the motif are phosphorylated they form docking sites for the recruitment of other signalling proteins and initiation of intracellular signalling cascades Citation[3].
Antigen recognition and signal transduction by the TCR is facilitated by co-receptors expressed on the T cell surface which bind to cognate ligands on APCs. Two such important co-receptors, that define distinct T cell subsets with specialized functions, are the CD4 and CD8 proteins which bind to MHC class II and MHC class I respectively. The cytoplasmic region of CD4 and CD8 interacts with Lck, thus bringing the kinase to the proximity of the TCR during antigen presentation to facilitate its phosphorylation Citation[4].
Binding of antigen to the receptor stimulates a number of signalling cascades which diverge to produce second messengers and communicate the signal to the cell interior. The co-ordinated action of second messengers, enzymes and scaffold molecules results in activation of diverse families of transcription factors, and production of new proteins which control clonal expansion and immunity Citation[5].
Signalling events proximal to the TCR
The immediate outcome of TCR ligation by the appropriate MHC/peptide complex is a rapid and transient increase in ITAM phosphorylation followed by tyrosine phosphorylation of several other protein substrates Citation[6]. ITAM phosphorylation is mediated by the Src-family of tyrosine kinases, Lck and Fyn. Studies of mutant mice deficient for either of these proteins have shown that, while Lck expression is indispensable for early tyrosine phosphorylation of the TCR Citation[7], the absence of Fyn does not impair phosphorylation and cell activation Citation[8]. The absolute requirement for Lck has also been documented in experiments using a Jurkat T cell line deficient for Lck (JCaM-1.6) Citation[9]. These results indicate that Lck is the principal tyrosine kinase in T cells that couples the antigen receptor to downstream signalling. Another molecule that is required for TCR phosphorylation is the transmembrane tyrosine phosphatase CD45. As for Lck, experiments in CD45 knock-out mice and CD45-negative cell lines have demonstrated lack of ITAM phosphorylation following TCR ligation Citation[10], Citation[11]. CD45 de-phosphorylates a carboxy-terminal (C-terminal) tyrosine in Lck. This tyrosine when phosphorylated causes the folding of the kinase into low activity conformation (see section below on the regulation of Lck) Citation[12].
The phosphorylated TCR propagates the signal by recruiting to the plasma membrane the cytosolic tyrosine kinase ZAP-70 Citation[13]. The main protein substrate for ZAP-70 is the transmembrane adapter molecule LAT (linker for activation of T cells), which becomes phosphorylated at multiple tyrosine residues. Phosphorylated LAT is the focal point for the assembly of multi-protein signalling complexes that include other scaffold molecules such as SLP-76, Grb2 and Gads, and enzymes like PLCγ1 and phosphoinositide-3 kinase (PI3K) Citation[14], Citation[15]. The LAT-induced protein assemblies at the inner leaflet of the plasma membrane are considered to be the origin from which multiple signalling cascades branch out to activate transcription factors and to re-model the actin cytoskeleton.
Lipid rafts
In the past few years there have been a large number of papers investigating how lipid rafts might regulate signal transduction by the TCR. Lipid rafts is a general term used to describe microdomains at the plasma membrane which have a lipid composition that differs from the glycerophospholipid-rich bilayer of the surrounding membrane Citation[16]. They are considered to be distinct formations that compartmentalise the plasma membrane although they may be dynamic rather than rigid structures with a rapidly changing size and organization. Lipid rafts are enriched in glycosphingolipids, sphingomyelin and cholesterol creating an environment which attracts certain proteins while excludes others Citation[17–19]. Microdomain formation has been observed in model membranes where sphingolipids group together to form distinct areas that are resistant to solubilization with non-ionic detergents Citation[20]. Addition of cholesterol stabilizes the sphingolipid assemblies suggesting that one of the roles of cellular cholesterol may be in the formation of similar microdomains in biological membranes. Likewise, extraction of mammalian cells with non-ionic detergents produces a detergent-resistant membrane (DRM) fraction Citation[16], Citation[18]. Purified DRMs have been used widely to study the properties of lipid rafts in T cells although there is some debate about how faithfully they represent lipid raft structures in live cells Citation[21]. It can not be excluded that detergent solubilization of cells could result in the formation of nonphysiological DRM structures Citation[22]. Proteomic analysis has shown the presence of protein contaminants in DRM preparations Citation[23–25], therefore in order to piece together a more complete picture of how lipid rafts function in T cells, other methods should be applied as well. Microscopy is one such complementary method that has been used extensively to visualize lipid rafts and their co-localization with components of the TCR signalling apparatus. Cholera toxin B subunit (CTB) binds specifically to ganglioside GM1, a lipid that is heavily enriched in lipid rafts and has been used as a specific marker for their detection Citation[26]. A 2-dimensional reconstruction of confocal Z series collected from a Jurkat T cell stained with CTB conjugated to fluorescein isothiocyanate (FITC) is shown in .
Figure 1. Two-dimensional reconstruction of confocal Z-series recorded from a Jurkat T cell that was stained with FITC-CTB for 15 min on ice, and fixed with 4% paraformaldehyde. Images were obtained with a Zeiss LSM510META confocal microscope. Scale bar = 10 µm.
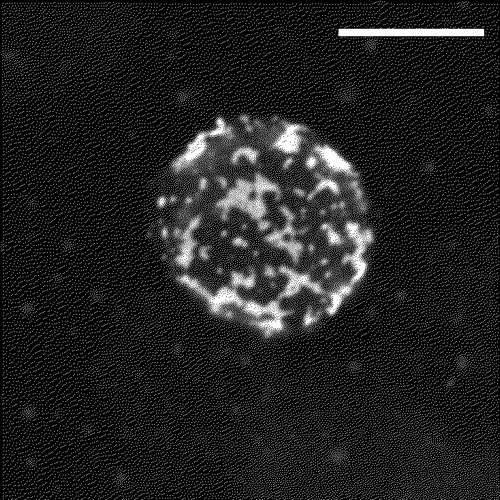
However, since CTB is a pentamer with 5 available GM1 binding sites, staining of cells could result in lipid raft aggregation and therefore the observed fluorescence may indicate raft assemblies rather than individual rafts, or CTB binding may initiate signalling and subsequent aggregation of GM1 molecules. Alternatively, if the size of individual rafts is below the resolution threshold of light microscopy then other methods will be needed to visualize rafts. Recent results obtained by single-molecule tracking of membrane proteins in live cells has shown that lipid rafts may be small (50–200 nm in size), short-lived structures in resting cells Citation[27]. In T cells, application of this technology revealed that rafts and the actin cytoskeleton play important roles in facilitating accumulation of Lck at the stimulated TCR clusters by decreasing its diffusion rate Citation[28]. Detailed descriptions of the properties, size and composition of lipid rafts in mammalian cells are presented in the accompanying articles of this issue. This review will focus on the involvement of lipid rafts in the initial steps in TCR signalling which take place at the plasma membrane. For a discussion on how lipid rafts may regulate other steps during T cell activation the reader is referred to the accompanying article in this issue by A. Viola.
Localization and function of key signalling proteins in T cell lipid rafts
Lck
Lck, like all the Src-family kinases, has conserved domains that perform distinct functions Citation[29]. At the N-terminus is a membrane targeting motif in which a myristic and two palmitic acid groups are attached at glycine 2 and cysteines 3 and 5 respectively, to form a hydrophobic anchor that attaches the protein to the cytoplasmic leaflet of the plasma membrane Citation[30–32]. Further downstream is a Src homology (SH) 3 domain followed by an SH2 and a catalytic domain. Both SH3 and SH2 domains are involved in binding to other proteins but they also participate in intramolecular interactions which determine the tertiary structure and catalytic activity of the enzyme. The activity of Lck, and of all Src-family kinases, is regulated by the phosphorylation status of two tyrosine (Y) residues, one located within the catalytic domain and the other at the C-terminus of the molecule Citation[33]. The C-terminal tyrosine (Y505 in murine Lck) plays an inhibitory role when phosphorylated because it interacts with the SH2 domain causing the folding of the enzyme into a low activity state Citation[34]. In contrast, auto-phosphorylation of the tyrosine residue within the catalytic domain (Y394) leads to the unfolding of the protein allowing the enzyme to assume an ‘extended’ configuration with a substantially higher activity Citation[35].
The conversion between the ‘closed’ and ‘extended’ conformation of Lck is regulated by the action of other proteins. The tyrosine kinase Csk (C-terminal Src kinase) phosphorylates the C-terminal tyrosine and reduces the activity of Lck Citation[36]. In contrast, the transmembrane tyrosine phosphatase CD45, which is expressed in cells of the haematopoietic lineage, dephosphorylates this tyrosine and maintains the kinase in its ‘extended’ configuration Citation[12], Citation[37]. However, CD45 can also dephosphorylate Y394 and possibly downstream substrates of Lck, therefore it can play both a positive and a negative role in Lck-mediated signal transduction Citation[38]. It is unclear how the positive and negative actions of CD45 are balanced during the early stages of TCR signalling. It is possible that the role of CD45 is dictated by its subcellular compartmentalization. For example, there are experiments showing that during the formation of the immunological synapse, CD45 is excluded from the core of the synapse where the TCR and other signalling molecules are concentrated Citation[39]. Exclusion of CD45 may temporarily shift the balance towards tyrosine phosporylation and signal transmission. Moreover, different splice isoforms of the extracellular part of the protein have been linked with differential ability of the phosphatase to support T cell activation suggesting that this region of the molecule also has a functional role in T cells Citation[37].
Lipidation of Lck in its N-terminal membrane-anchoring motif targets the protein to lipid rafts Citation[30–32]. Comparing detergent-soluble and -insoluble lysates from Jurkat T cells it was found that approximately 25–50% of total Lck co-purifies with the DRM fraction Citation[40]. A mutant form of Lck that was integrated to the plasma membrane via a transmembrane domain but which was excluded from DRMs, was unable to phosphorylate the stimulated TCR Citation[32]. Furthermore, reduction in TCR signalling was seen in T cells treated with 13-oxypalmitic acid, a less hydrophobic analogue of palmitic acid, which preserves membrane-attachment of Lck but reduces its affinity for lipid rafts Citation[41]. Taken together these results suggest that Lck localization in raft microdomains is required for its function to couple the TCR to downstream signalling.
Adapter proteins
Another group of proteins that partition into DRMs are transmembrane adapters that contain a C-X-X-C motif (C = cysteine and X = any amino acid) that immediately follows their transmembrane domain. S-acylation of the cysteines contributes to membrane attachment of the protein and its association with lipid rafts. The prototype member of this group of proteins is LAT Citation[14]. A mutant form of LAT where the two membrane-proximal cysteines were substituted failed to localise to lipid rafts and was unable to support signalling downstream of ITAM phosphorylation Citation[42]. It is worth noting, however, that a recent study suggested that while membrane attachment of LAT is required for its function, lipid raft localization is not essential Citation[43]. It will be essential in future experiments to conclusively document whether incorporation of LAT into lipid rafts determines its function in TCR signalling.
Two other members of this group of adapters that are expressed in T cells are PAG/Cbp (protein associated with GEMs/Csk binding protein) Citation[44], Citation[45], and the recently identified LIME (Lck-interacting molecule) Citation[46], Citation[47]. PAG/Cbp is constitutively phosphorylated on tyrosine in resting T cells. One of the phospho-tyrosines (317 in the human protein) is a specific binding site for the SH2 domain of Csk. The fact that PAG/Cbp is constitutively phosphorylated in resting T cells could suggest that this molecule has an important role in the homeostatic regulation of Lck. Hence in DRMs prepared from resting cells, Lck was found to be heavily phosphorylated on Y505 compared with the Lck pool in the soluble lysate fraction Citation[48–51]. The study of PAG/Cbp knock-out mice should provide important information regarding the function of this protein.
LIME was described recently as an adapter molecule that is phosphorylated by Lck following stimulation of surface receptors Citation[46], Citation[47]. Interestingly, Lck itself can associate with phospho-LIME via its SH2 domain and one of the studies showed that Csk also interacts with phospho-LIME raising the possibility that in addition to PAG/Cbp, this adapter also plays a role in the regulation of Lck in lipid rafts Citation[46]. Furthermore, it was found that LAT can associate with the ‘extended’, but not the ‘closed’, form of Lck in DRMs Citation[51]. The physiological significance of this interaction is unknown at present.
Expression of Lck in T cells is also regulated by ubiquitination. Prolonged stimulation of the TCR results in a reduction in the total level of Lck protein which, at least in part, is due to its ubiquitination and proteasomal degradation Citation[52]. Interestingly, loss of Lck expression was also seen in cells cultured at high cell density, where initial de-phosphorylation of Y505 was followed by ubiquitination and loss of protein Citation[53]. Taken together, these results demonstrate that the regulation of Lck in T cells is complex. Future work will undoubtedly shed more light in the biology of this key signalling molecule in T cells.
TCR signalling in lipid rafts
As mentioned above, Lck that co-purifies with DRMs prepared from 1% TritonX-100 cell lysates, is phosphorylated on Y505 and is predominantly in the ‘closed’ conformation Citation[48–51]. It is plausible that in lipid rafts the ‘closed’ conformation of Lck is maintained by the action of the PAG/Cbp-Csk inhibitory complex, although other molecules such as LIME may also play a role. In resting T cells, PAG/Cbp is phosphorylated by Fyn which is active in DRMs Citation[49], Citation[54], and possibly by active Lck which moves into lipid rafts from the surrounding membrane Citation[51]. As a result of PAG/Cbp phosphorylation and Csk recruitment, Lck is phosphorylated on Y505 and becomes inactive. Such an auto-regulatory mechanism will be able to keep Lck in a low activity state in lipid rafts, which, however, could change if under certain conditions a phosphatase, like CD45, associates with the raft ().
Figure 2. A model describing the initial steps that lead to phosphorylation of the TCR in lipid rafts. In resting T cells, the TCR may weakly interact with lipid rafts. Also, Lck in these domains has low activity due to the action of the PAG/Cbp-Csk inhibitory complex. Antigen presentation by APCs strengthens the association of the TCR with lipid rafts and induces a transient redistribution of CD45 to these domains. Dephosphorylation of Lck-Y505 and of PAG/Cbp by CD45 and dissociation of Csk, increases the activity of Lck resulting in ITAM phosphorylation and signalling. This Figure is reproduced in colour in Molecular Membrane Biology online.
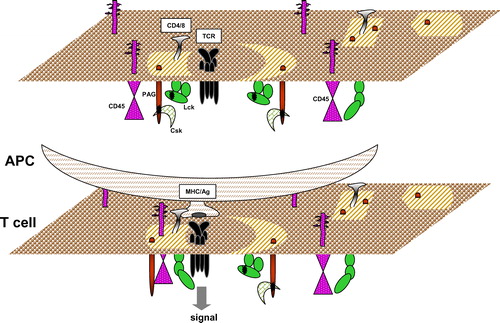
A number of reports have shown that stimulation of the TCR induces its association with TritonX-100 DRMs. Hence, tyrosine phosphorylated ζ and ε chains were detected in DRMs following stimulation of the TCR with mitogenic antibodies or super-antigen Citation[55–57]. Based on these results, it has been suggested that upon stimulation the TCR translocates to lipid rafts where it becomes phosphorylated by resident Lck. However, it is unclear how stimulation increases the affinity of the TCR for lipid raft domains, which according to this model should be independent of ITAM phosphorylation. Also, as discussed earlier, Lck in rafts has low catalytic activity. Therefore, TCR association with lipid rafts may not be sufficient for initiation of signalling and a transient increase in the activity of raft-associated Lck may be required. In this scenario, a tyrosine phosphatase must be involved capable of de-phosphorylating PAG/Cbp resulting in the shedding Csk, and/or de-phosphorylating the inhibitory Y505. These changes will allow phosphorylation of the ITAMs and signal transmission until re-phosphorylation of PAG/Cbp, possibly by Lck, will cause the re-attachment of Csk and cessation of signal transduction (). A number of reports support this model of TCR activation in lipid rafts. Firstly, stimulation of the TCR in human peripheral blood T cells induced the transient de-phosphorylation of PAG/Cbp and release of Csk from lipid rafts Citation[44], Citation[58]. Secondly, over-expression of wild type or a phosphorylation-defective mutant of PAG/Cbp in murine T cells demonstrated that this adapter, via its association with Csk, regulates membrane-proximal signalling by the TCR Citation[59]. Thirdly, over-expression of Csk dominant-negative mutants in Jurkat T cells augmented TCRζ phosphorylation and NFAT production Citation[58], Citation[60]. In future studies, down-regulation of PAG/Cbp expression should provide further insights into the function of this adapter.
The above model implies that upon stimulation the TCR up-regulates the activity, or changes the location, of a protein tyrosine phosphatase which then acts to increase the activity of Lck. The identity of this phosphatase and how it is regulated by the antigen receptor is unknown. A potential candidate is CD45, since studies in CD45-deficient cell lines and mice have documented that in its absence ITAM phosphorylation is defective. It is widely accepted that the constitutive action of CD45 maintains a pool of Lck at the plasma membrane primed and available to support signalling by stimulated antigen receptors. However, it can not be excluded that the TCR can change the localization of a pool of CD45 on demand, to transiently up-regulate the activity of Lck in rafts. Initial studies that addressed the question of whether CD45 can partition into lipid rafts suggested that the phosphatase is excluded from such domains Citation[40], Citation[61]. However, recent reports have suggested that a small fraction is present in DRMs and that the ectodomain of the molecule has a role in determining its membrane distribution Citation[62], Citation[63]. One report, using CD45 chimeras that differed in the length of the extracellular domain, found a correlation between the levels of the phosphatase detected in DRMs and the strength of the TCR signal Citation[62]. Pertinent to this hypothesis is the finding that in T cells isolated from patients with the autoimmune disease systemic lupus erythematosus, which manifest a ‘hyper-active’ phenotype, a larger fraction of CD45 associates with raft domains Citation[64–66]. As mentioned earlier, CD45 can have a negative role in signalling as well, and following its initial positive role in priming Lck for action, CD45 exclusion from multi-protein signalling complexes may be critical for sustained signal transduction. Accordingly, CD45 was found to be excluded from the mature immunological synapse Citation[39]. Therefore, the levels and kinetics of CD45 distribution to lipid rafts may be a critical factor in determining the threshold for T cell activation during physiological immune responses but also in auto-immunity.
An alternative theory regarding localization of the TCR to lipid rafts has been proposed. In intact cells cross-linking of GM1-containing microdomains with CTB/anti-CTB, induced co-patching of the TCR. The GM1/TCR co-patching was sensitive to extraction with TritonX-100 suggesting that the receptor may constitutively associate with lipid rafts in the absence of stimulation, this association, however, is sensitive to TritonX-100 extraction Citation[67]. Furthermore, DRMs prepared from unstimulated cells that were lysed in Brij 98, a detergent considered to be milder than TritonX-100, contained components of the TCR which became tyrosine phosphorylated after stimulation Citation[68]. Based on these results, it has been proposed that at least a fraction of the TCR constitutively resides in microdomains in the proximity of Lck, and it becomes signalling active following its stimulation Citation[68]. In this model, however, it is unclear what prevents the phosphorylation of the TCR by Lck in unstimulated cells, since both molecules are already close to each other, assuming that they do not localize to different microdomains but are randomly distributed among existing lipid rafts. An explanation may have to do with the activity of Lck, which as in the first scenario, is increased following antigen stimulation to phosphorylate ITAMs and initiate signalling.
The CD4 co-receptor partitions to lipid rafts as well due to its interaction with Lck but also because it is S-acylated on two membrane-proximal cysteines Citation[69], Citation[70]. Stimulation of CD4 enhanced signalling by the TCR, in part, because it induced aggregation of lipid rafts and the formation of molecular assemblies at the site of the immunological synapse Citation[70], Citation[71]. Interestingly, stimulation of the CD8 co-receptor in the CD8+ T cell subset did not induce similar polarization of lipid rafts, suggesting that there are differences in the way that CD4+ and CD8+ T cells become activated Citation[72]. FRET analysis with labelled antibodies showed an increase in TCR/CD4 interactions after TCR stimulation Citation[73], and in addition, during antigen presentation by APCs, it was found that CD4 and CD28 receptors cooperated to induce activation of Lck and its recruitment to the T cell/APC interface Citation[74]. Therefore, the co-receptors must play a major role in early TCR signalling, although their mechanism of action may differ between the two T cell subsets.
Following the initial steps that lead to TCR phosphorylation, a number of signalling proteins were found to translocate to lipid rafts and to co-purify with DRMs Citation[40], Citation[55], Citation[75], Citation[76]. Transiently recruited proteins are the enzymes ZAP-70 and PLCγ1 Citation[55], Citation[56], Citation[67], Citation[77], the adapters SLP-76 and Grb2 Citation[78], Citation[79] and the θ isoform of protein kinase C (PKCθ) Citation[80] among others. The accumulation of signalling proteins in DRMs/lipid rafts after TCR stimulation led to the proposal that these domains may represent signalling ‘hot spots’ at the plasma membrane with a role not only in the initial steps of TCR activation but also in downstream events.
Considerations and concluding remarks
In the past few years, a lot of effort has been devoted to understanding the function of plasma membrane microdomains during T cell activation, which is reflected in the large volume of papers that have appeared in the literature. Identification of signalling proteins that co-purify with DRMs, experiments that address protein localization and function, and sophisticated imaging techniques that report on the co-localization of proteins and lipids in live cells have provided the bulk of the evidence to suggest a role for lipid rafts in TCR signalling. Nonetheless, the exact sequence of biochemical events in these domains, and the topological arrangement of the TCR in relation to lipid rafts remains a matter of debate. Furthermore, not all published evidence concurs with a role for lipid rafts in T cell activation and the accuracy of the methods used to argue for the existence of lipid microdomains in mammalian cells has been questioned Citation[21]. Recent reports cast doubts on whether lipid rafts preferentially accumulate at sites of T cell stimulation Citation[15], or at the site of the immunological synapse Citation[81]. FRET analysis between GPI-linked proteins and CTB in Jurkat T cells, failed to detect accumulation of lipid rafts in the area of stimulated TCR complexes Citation[81]. Another recent report, using mutagenesis and single-molecule tracking technology, questions the view that lipid rafts play a key role in mediating the assembly of functional signalling complexes Citation[82]. Rather, the report suggests it is specific protein-protein interactions that determine the lateral mobility of a protein and its inclusion or exclusion from signalling complexes in T cells Citation[82]. Collectively, these studies argue against the concept that raft microdomains are the critical component in the clustering and immobilization of molecules in the T cell plasma membrane. It should be noted, however, that the importance of protein-protein interactions is well established over the years and does not exclude a parallel role for lipid rafts in TCR signalling. A fundamental property of lipid rafts is their ability to compartmentalize proteins at the plasma membrane thus preventing unwanted protein interactions that can lead to deregulated signalling, nevertheless such interactions may take place after receptor stimulation.
Unanswered questions and in some instances the opposing results reported by different groups regarding the involvement of lipid rafts, most likely will continue to dominate research in this area of immunology. Continuing work to understand the function of proteins that participate in TCR signalling, the importance of their sub-membrane distribution, and the application of more sensitive and sophisticated techniques that reliably detect lipid rafts in live cells, will help us in gradually reaching a consensus as to the role of these domains in TCR signalling. Advances in this area will shape our thinking of how other cell surface receptors are signalling as well. Note added in proof: After this review went to press, PAG/Cbp knock out mice were reported (M-W Dobenecker et al., Mol. Cell. Biol., 2005; 25: 10533–10542) in which T cell development and function, and recruitment of Csk to lipid rafts were normal, suggesting that additional adapter molecules are involved in this process.
This paper was first published online on prEview on 27 January 2006.
This work was supported by the Arthritis Research Campaign (UK) and the Wellcome Trust. I would like to thank Ulrike Meltzer for help with confocal microscopy, and Yuti Chernajovsky, Rizgar Mageed and David Gould for critically reading the manuscript. The author apologizes to colleagues whose work is not cited in this review owing to space limitation.
References
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol 1998; 16: 523–544
- Irving BA, Chan AC, Weiss A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J Exp Med 1993; 177: 1093–1103
- Shinkai Y, Ma A, Cheng H-L, Alt FW. CD3ε and CD3ζ cytoplasmic domains can independently generate signals for T cell development and function. Immunity 1995; 2: 401–411
- Veillette A, Bookman MA, Horak EM, Bolen JB. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 1988; 55: 301–308
- Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol 1996; 14: 259–274
- Latour S, Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol 2001; 13: 299–306
- Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A. Profound block in thymocyte development in mice lacking p56lck. Nature 1992; 357: 161–164
- Stein PL, Lee H-M, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell 1992; 70: 741–750
- Straus DB, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 1992; 70: 585–593
- Kishihara K, Penninger J, Wallace VA, Kundig TM, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi PS, Thomas ML, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell 1993; 74: 143–156
- Koretzky GA, Picus J, Thomas ML, Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyinositol pathway. Nature 1990; 346: 66–68
- Mustelin T, Altman A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45). Oncogene 1990; 5: 809–813
- Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell 1992; 71: 649–662
- Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: The ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 1998; 92: 83–92
- Harder T, Kuhn M. Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J Cell Biol 2000; 151: 199–208
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572
- Edidin M. Lipid microdomains in cell surface membranes. Curr Opin Struct Biol 1997; 7: 528–532
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 1998; 14: 111–136
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 2000; 1: 31
- Brown RE. Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J Cell Sci 1998; 111: 1–9
- Munro S. Lipid rafts: elusive or illusive?. Cell 2003; 115: 377–388
- Heerklotz H. Triton promotes domain formation in lipid raft mixtures. Biophys J 2002; 83: 2693–2701
- Foster LJ, De Hoog CL, Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA 2003; 100: 5813–5818
- von Haller PD, Donohoe S, Goodlett DR, Aebersold R, Watts JD. Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics 2001; 1: 1010–1021
- Razzaq TM, Ozegbe P, Jury EC, Sembi P, Blackwell NM, Kabouridis PS. Regulation of T-cell receptor signalling by membrane microdomains. Immunology 2004; 113: 413–426
- Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol 2001; 19: 375–396
- Murase K, Fujiwara T, Umemura Y, Suzuki K, Iino R, Yamashita H, Saito M, Murakoshi H, Ritchie K, Kusumi A. Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys J 2004; 86: 4075–4093
- Ike H, Kosugi A, Kato A, Iino R, Hirano H, Fujiwara T, Ritchie K, Kusumi A. Mechanism of Lck recruitment to the T-cell receptor cluster as studied by single-molecule-fluorescence video imaging. Chemphyschem 2003; 4: 620–626
- Sicheri F, Kuriyan J. Structures of Src-family tyrosine kinases. Curr Opin Struct Biol 1997; 7: 777–785
- Shenoy-Scaria AM, Gauen LKT, Kwong J, Shaw AS, Lublin DM. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol 1993; 13: 6385–6392
- Rodgers W, Crise B, Rose JK. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol 1994; 14: 5384–5391
- Kabouridis PS, Magee AI, Ley SC. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J 1997; 16: 4983–4998
- Reynolds PJ, Hurley TR, Sefton BM. Functional analysis of the SH2 and SH3 domains of the lck tyrosine protein kinase. Oncogene 1992; 7: 1949–1955
- Xu W, Doshi A, Lei M, Eck MJ, Harrison SC. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell 1999; 3: 629–638
- Yamaguchi H, Hendrickson WA. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature 1996; 384: 484–489
- Bergman M, Mustelin T, Oetken C, Partanen J, Flint NA, Amrein KE, Autero M, Burn P, Alitalo K. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J 1992; 11: 2919–2924
- Penninger JM, Irie-Sasaki J, Sasaki T, Oliveira-dos-Santos AJ. CD45: New jobs for an old acquaintance. Nat Immunol 2001; 2: 389–396
- D'Oro U, Ashwell JD. Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol 1999; 162: 1879–1883
- Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, Zaller DM, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol 2002; 3: 911–917
- Janes PW, Ley SC, Magee AI, Kabouridis PS. The role of lipid rafts in T cell antigen receptor (TCR) signalling. Sem Immunol 2000; 12: 23–34
- Hawash IY, Hu XE, Adal A, Cassady JM, Geahlen RL, Harrison ML. The oxygen-substituted palmitic acid analogue, 13-oxypalmitic acid, inhibits Lck localization to lipid rafts and T cell signaling. Biochim Biophys Acta 2002; 1589: 140–150
- Zhang W, Trible RP, Samelson LE. LAT palmitoylation: Its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 1998; 9: 239–246
- Zhu M, Shen S, Liu Y, Granillo O, Zhang W. Cutting edge: Localization of linker for activation of T cells to lipid rafts is not essential in T cell activation and development. J Immunol 2005; 174: 31–35
- Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, Drbal K, Kuramitsu Y, Kornacker B, Horejsi V, Schraven B. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med 2000; 191: 1591–1604
- Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature 2000; 404: 999–1003
- Brdickova N, Brdicka T, Angelisova P, Horvath O, Spicka J, Hilgert I, Paces J, Simeoni L, Kliche S, Merten C, Schraven B, Horejsi V. LIME: A new membrane raft-associated adaptor protein involved in CD4 and CD8 coreceptor signaling. J Exp Med 2003; 198: 1453–1462
- Hur EM, Son M, Lee OH, Choi YB, Park C, Lee H, Yun Y. LIME, a novel transmembrane adaptor protein, associates with p56lck and mediates T cell activation. J Exp Med 2003; 198: 1463–1473
- Rodgers W, Rose JK. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol 1996; 135: 1515–1523
- Kabouridis PS, Janzen J, Magee AL, Ley SC. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol 2000; 30: 954–963
- Kosugi A, Sakakura J, Yasuda K, Ogata M, Hamaoka T. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity 2001; 14: 669–680
- Kabouridis PS. Selective interaction of LAT (linker of activated T cells) with the open-active form of Lck in lipid rafts reveals a new mechanism for the regulation of Lck in T cells. Biochem J 2003; 371: 907–915
- Rao N, Miyake S, Reddi AL, Douillard P, Ghosh AK, Dodge IL, Zhou P, Fernandes ND, Band H. Negative regulation of Lck by Cbl ubiquitin ligase. Proc Natl Acad Sci USA 2002; 99: 3794–3799
- Ozegbe P, Chernajovsky Y, Kabouridis PS. Regulation of expression and function of Lck tyrosine kinase by high cell density. Mol Membr Biol 2005; 22: 363–372
- Yasuda K, Nagafuku M, Shima T, Okada M, Yagi T, Yamada T, Minaki Y, Kato A, Tani-Ichi S, Hamaoka T, Kosugi A. Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J Immunol 2002; 169: 2813–2817
- Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, Chauvin JP, Pierres M, He HT. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J 1998; 17: 5334–5348
- Xavier R, Brennan T, Li Q, McCormack C, Seed B. Membrane compartmentation is required for efficient T cell activation. Immunity 1998; 8: 356–360
- Pizzo P, Giurisato E, Bigsten A, Tassi M, Tavano R, Shaw A, Viola A. Physiological T cell activation starts and propagates in lipid rafts. Immunol Lett 2004; 91: 3–9
- Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Horejsi V, Schraven B, Rolstad B, Mustelin T, Tasken K. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem 2001; 276: 29313–29318
- Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol 2003; 23: 2017–2028
- Mustelin T, Tasken K. Positive and negative regulation of T-cell activation through kinases and phosphatases. Biochem J 2003; 371: 15–27
- van der Merwe PA. The TCR triggering puzzle. Immunity 2001; 14: 665–668
- Irles C, Symons A, Michel F, Bakker TR, van der Merwe PA, Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol 2003; 4: 189–197
- Edmonds SD, Ostergaard HL. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol 2002; 169: 5036–5042
- Jury EC, Kabouridis PS, Flores-Borja F, Mageed RA, Isenberg DA. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest 2004; 113: 1176–1187
- Krishnan S, Nambiar MP, Warke VG, Fisher CU, Mitchell J, Delaney N, Tsokos GC. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol 2004; 172: 7821–7831
- Jury EC, Kabouridis PS. T lymphocyte signalling in systemic lupus erythematosus: A lipid raft perspective. Lupus 2004; 13: 413–422
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol 1999; 147: 447–461
- Drevot P, Langlet C, Guo XJ, Bernard AM, Colard O, Chauvin JP, Lasserre R, He HT. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO J 2002; 21: 1899–1908
- Foti M, Phelouzat MA, Holm A, Rasmusson BJ, Carpentier JL. p56Lck anchors CD4 to distinct microdomains on microvilli. Proc Natl Acad Sci USA 2002; 99: 2008–2013
- Fragoso R, Ren D, Zhang X, Su MW, Burakoff SJ, Jin YJ. Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhance CD3 signaling. J Immunol 2003; 170: 913–921
- Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J Immunol 2004; 172: 5887–5892
- Kovacs B, Maus MV, Riley JL, Derimanov GS, Koretzky GA, June CH, Finkel TH. Human CD8+ T cells do not require the polarization of lipid rafts for activation and proliferation. Proc Natl Acad Sci USA 2002; 99: 15006–15011
- Mittler RS, Goldman SJ, Spitalny GL, Burakoff SJ. T-cell receptor-CD4 physical association in a murine T-cell hybridoma: induction by antigen receptor ligation. Proc Natl Acad Sci USA 1989; 86: 8531–8535
- Holdorf AD, Lee KH, Burack WR, Allen PM, Shaw AS. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat Immunol 2002; 3: 259–264
- Xavier R, Seed B. Membrane compartmentation and the response to antigen. Curr Opin Immunol 1999; 11: 265–269
- Viola A. The amplification of TCR signaling by dynamic membrane microdomains. Trends Immunol 2001; 22: 322–327
- Veri MC, DeBell KE, Seminario MC, DiBaldassarre A, Reischl I, Rawat R, Graham L, Noviello C, Rellahan BL, Miscia S, Wange RL, Bonvini E. Membrane raft-dependent regulation of phospholipase Cgamma-1 activation in T lymphocytes. Mol Cell Biol 2001; 21: 6939–6950
- Boerth NJ, Sadler JJ, Bauer DE, Clements JL, Gheith SM, Koretzky GA. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J Exp Med 2000; 192: 1047–1058
- Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity 1998; 9: 617–626
- Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ, Altman A. Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nat Immunol 2001; 2: 556–563
- Glebov OO, Nichols BJ. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat Cell Biol 2004; 6: 238–243
- Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 2005; 121: 937–950
