Abstract
Caveolae are flask-shape membrane invaginations of the plasma membrane that have been implicated in endocytosis, transcytosis, and cell signaling. Recent years have witnessed the resurgence of studies on caveolae because they have been found to be involved in the uptake of some membrane components such as glycosphingolipids and integrins, as well as viruses, bacteria, and bacterial toxins. Accumulating evidence shows that endocytosis mediated by caveolae requires unique structural and signaling machinery (caveolin-1, src kinase), which indicates that caveolar endocytosis occurs through a mechanism which is distinct from other forms of lipid microdomain-associated, clathrin-independent endocytosis. Furthermore, a balance of glycosphingolipids, cholesterol, and caveolin-1 has been shown to be important in regulating caveolae endocytosis.
Introduction
Multiple clathrin-independent mechanisms of endocytosis have recently been described and characterized. These internalization mechanisms are distinct from clathrin-dependent endocytosis which has been more extensively studied Citation[1], Citation[2]. Clathrin-independent uptake mechanisms include caveolae-mediated endocytosis, rhoA-dependent endocytosis of the interleukin 2 receptor ß subunit (IL-2R ß), and cdc42-regulated endocytosis of GPI-anchored proteins and fluid phase markers Citation[1–7]. Each of these endocytic mechanisms has distinct biochemical sensitivities and specific requirements for certain adaptor and signaling proteins that are summarized in . For example, caveolae-mediated endocytosis is dependent on dynamin 2 (Dyn2) and src kinase but is insensitive to Clostridium difficile Toxin B, which inhibits Rho family GTPases such as RhoA and cdc42. In contrast, the endocytosis of IL-2R and fluorescent dextran is sensitive to Toxin B because of their dependence on RhoA and cdc42, respectively, but is insensitive to specific src kinase inhibitors such as PP2. A further distinction between the RhoA and cdc42-regulated mechanisms is that only the latter is independent of Dyn2. It has been proposed that caveolae and other cholesterol-dependent microdomains are internalized via a single, common pathway Citation[8]; however, several studies have shown the persistence of separate non-clathrin endocytic pathways with different cargo, protein machinery and pharmacologic sensitivities within a single cell type Citation[5–7].
Table I. Distinguishing characteristics of endocytic mechanisms. Data are compiled from Citation[3–7] and our unpublished data.
In the present review, we focus on endocytosis via caveolae. Our laboratory has used fluorescent sphingolipid (SL) analogues and the SL-binding toxin, cholera toxin subunit B (CtxB) to study the mechanism by which SLs are internalized and subsequently sorted and transported to various intracellular compartments Citation[4], Citation[9]. Glycosphingolipids (GSLs), a subgroup of SLs, have been found to be internalized predominantly by a clathrin-independent, caveolar mechanism in most cell types studied Citation[4], Citation[6], Citation[7], Citation[10]. Here, we discuss recent studies on the caveolar endocytosis of GSLs and other caveolar markers (albumin, CtxB, SV40) and highlight current studies of the molecular mechanisms regulating this endocytic process. The controversial role of caveolin-1 (cav1) in caveolae-mediated endocytosis is also discussed.
Caveolae and other lipid microdomains
Caveolae were first described in the 1950s by Palade and Yamada based on their characteristic morphology as 50–80 nm diameter flask-shape invaginations observed by electron microscopy of thin sections Citation[11], Citation[12]. In the 1990s, a series of studies provided evidence for the existence of plasma membrane (PM) microdomains which were enriched in GSLs, cholesterol, GPI-linked proteins and certain intracellular signaling proteins (e.g., src family kinases) Citation[13]. Many of these studies relied on the characteristic of insolubility in cold (4°C) detergent (Triton X-100 or CHAPS) solutions for the isolation of these microdomains Citation[14–17]. A key study in 1992 identified a major protein found in these detergent-insoluble complexes, VIP21 Citation[18], which was found to be identical to cav1, a major coat protein of caveolae Citation[19]. Cav1 was soon found to directly bind to cholesterol Citation[20]. Because early studies focused on the use of detergent insolubility to isolate lipid microdomains, it was initially suggested that these isolated microdomains were synonymous with caveolae. Additional confusion was caused by observations that GPI-linked proteins visualized using antibodies were present in caveolae Citation[16], Citation[21]. Later studies have demonstrated that cells without cav1 also possess detergent-insoluble microdomains Citation[22], Citation[23], and that most GPI-linked proteins probably reside in non-caveolar microdomains but can be sequested into caveolae when living cells are treated with crosslinking antibodies Citation[24], Citation[25]. In contrast, GM1 ganglioside and cholesterol have been shown to be concentrated in caveolae by a variety of methods Citation[26–29]. Based on a variety of studies and technologies, it has become evident that there are probably several different types of PM cholesterol-enriched microdomains besides caveolae Citation[2], Citation[30–32]. As noted above, caveolar and non-caveolar lipid microdomains are associated with distinct forms of clathrin-independent endocytosis.
Evidence from video microscopy and fluorescence recovery after photobleaching (FRAP) analysis has shown that cav1-GFP is relatively immobile at the PM and that only a minority of cav1-positive vesicles actually internalize, suggesting that caveolae are rather stable structures that do not have a high basal turnover at the PM Citation[33], Citation[34]. These studies give the impression that endocytosis via caveolae is a rare event. However, much evidence has suggested that caveolar endocytosis is a regulated process that can be induced or stimulated Citation[6], Citation[35–37]. Caveolar uptake is greatly accelerated by phosphatase inhibitors and inhibited by certain kinase inhibitors Citation[4], Citation[33], Citation[35], Citation[37], Citation[38]. Also, caveolae appear to be anchored to the actin cytoskeleton, and disruption of this network leads to increased lateral mobility of cav1 and to clustering of caveolae in the plane of the PM Citation[39], Citation[40]. Finally, cargo to be internalized via caveolae has been shown to stimulate caveolar uptake. For example, SV40 virus has been shown to enter host cells by inducing its own endocytosis via caveolae, whereas caveolae devoid of the virus particles remain static at the PM in the same cells Citation[41]. Once bound to the cell surface, SV40 activates local tyrosine phosphorylation, disrupts the local actin cytoskeleton and recruits dynamin 2 Citation[40]. These signaling events subsequently trigger the internalization of the virus-containing caveolae Citation[40]. Binding of albumin to its cell surface receptor, gp60, also triggers caveolar endocytosis via a Gi-coupled src kinase-mediated pathway Citation[42]. As described below, GSLs also stimulate endocytosis via caveolae Citation[6].
Caveolae-mediated endocytosis of GSLs
As already mentioned, SLs are enriched, along with cholesterol, in membrane microdomains at the PM. In principle, SLs at the PM may be internalized by one or more endocytic mechanisms and targeted to specific intracellular destinations (e.g., lysosomes or the Golgi complex). We set out to determine the mechanism of SL internalization using a series of fluorescent SL analogues in which the naturally occurring fatty acid moiety of the SL is replaced with a short chain fatty acid labeled with boron dipyrromethene difluoride (BODIPY) Citation[43–45]. The BODIPY fluorophore exhibits a concentration-dependent shift in its fluorescence emission from green to red wavelengths as a result of excimer formation Citation[45], Citation[46]. Thus, BODIPY-labeled lipids are especially helpful in determining the rapid and dynamic changes in SL concentrations in specific intracellular locations.
The endocytosis of BODIPY labeled GSLs [e.g., BODIPY-lactosylceramide (BODIPY-LacCer) or BODIPY-globoside] was first studied in human skin fibroblast (HSFs), but similar results were later reported for rat fibroblasts (RFs) and other cell types Citation[4], Citation[7]. The uptake of these GSL analogues is insensitive to treatments that specifically inhibit clathrin-dependent endocytosis [chlorpromazine (CPZ), K + depletion, or expression of a dominant negative (DN) mutant form of EGF receptor pathway substrate 15 (Eps15)]. When HSFs are transfected with DN Rab5, which specifically inhibits the transport of clathrin-derived endocytic vesicles from the PM to sorting endosomes, the endocytosis of Tfn is inhibited 80% but BODIPY-LacCer uptake is not affected Citation[10]. These results suggest that the endocytosis of these GSL analogues is independent of clathrin.
Expression of the dynamin 2 mutant, Dyn 2 K44A, blocks BODIPY-LacCer internalization Citation[4], Citation[6], Citation[7]. Dynamin 2 has been shown to be involved in clathrin-dependent endocytosis, caveolae-mediated endocytosis and RhoA-regulated IL-2 receptor internalization, but not in cdc42-regulated pinocytosis Citation[3], Citation[5]. Also, BODIPY-LacCer shows little co-localization with fluorescent dextran (a marker for cdc42-regulated pinocytosis) Citation[7]. Furthermore, Clostridium difficile toxin B (Toxin B), a general inhibitor of the Rho family GTPases, blocks dextran uptake but has no effect on LacCer internalization Citation[6]. Together, these results suggest that LacCer is internalized via an endocytic mechanism distinct from that utilized by dextran.
Further evidence indicates that LacCer is internalized via a caveolae-related mechanism in HSFs and RFs. First, LacCer uptake is significantly inhibited by the cholesterol binding or extracting agents, nystatin and methyl-ß-cyclodextrin, respectively Citation[4], Citation[6], Citation[7], Citation[36], suggesting the involvement of lipid microdomains. Second, at early time points of endocytosis (<5 min), BODIPY-LacCer exhibits extensive overlap (>80%) with either fluorescent albumin or CtxB, but little co-localization with DiI-LDL and transferrin (Tfn), two markers for clathrin-dependent endocytosis Citation[4], Citation[7], Citation[10], Citation[36]. Although the endocytic mechanism of certain markers (such as CtxB) has been shown to be cell type-dependent, albumin and CtxB extensively overlap (>50%) with cav1-GFP or DsRed-cav1 in HSFs Citation[4], Citation[7], indicating that both are valid markers for caveolae in HSFs. Importantly, BODIPY-LacCer also shows extensive co-localization with expressed fluorescent cav1 fusion proteins Citation[6], Citation[7].
Interestingly, time course studies of the endocytosis of BODIPY-LacCer and AF594-Tfn in doubly labeled cells show that although LacCer and Tfn are internalized by distinct endocytic mechanisms (little co-localization between LacCer and Tfn after 1 min internalization), these markers rapidly converge and co-localize in EEA1-positive early endosomes after 5 min internalization before further sorting to different intracellular locations Citation[10]. Similarly, fluorescent albumin, another marker for caveolar endocytosis in HSFs, also merges with Tfn-positive early endosomes Citation[10]. These results are consistent with the earlier observation of Pol et al Citation[47], Citation[48] who report the detection of cav1 in early endosomes. Recently, Pelkmans et al. Citation[49] reported cav1-positive vesicles containing SV40 or CtxB (referred to as caveosomes) also transiently interact with early endosomes to form subdomains, suggesting that merging with early endosomes might be a general phenomenon for cargo internalized via caveolae. Similarly, IL2-R alpha subunit and MHC I endocytosed by clathrin-independent endocytosis are initially internalized into vesicles which are devoid of LDL, a marker for the clathrin pathway, but at later times (e.g., 20 min) are delivered to early and then late endosomes Citation[50]. In contrast, GPI-APs internalized via the cdc42-dependent pathway are not delivered to early endosomes but are held in discrete structures (GPI-enriched early endosomal compartments; GEECs), and are later delivered to the recycling endosome Citation[5].
Structural determinants for GSL internalization via caveolae
Structurally, GSLs consist of a large lipid family in which the composition of the carbohydrate headgroup varies. We were interested in determining whether GSLs with different head groups are internalized via a similar mechanism to that utilized by BODIPY-LacCer.
We systematically varied the carbohydrate headgroup of the fluorescent GSL analogues as shown in A and examined the effect of these variations on the mechanism of analogue internalization. Surprisingly, we found that BODIPY-labeled GalCer, MalCer, globoside, GM1, and sulfatide were each internalized identically to BODIPY-LacCer Citation[7]. Second, since lipid hydrophobicity is presumed to affect its ability to partition into microdomains, we prepared a series of BODIPY-LacCer analogues in which the chain length of the sphingosine base (e.g., C12 vs. C20 sphingosine), or fluorescent fatty acid was varied (see ). However, these variations had no effect on the LacCer internalization mechanism Citation[7]. Also, replacing the BODIPY-fluorophore with NBD did not influence the caveolar internalization of the LacCer analogues Citation[7]. Thus, GSL uptake via caveolae is not selective for a specific carbohydrate headgroup, acyl chain hydrophobicity, or fluorophore substitution. We also examined the uptake of a fluorescent glycerophospholipid analogue, NBD-phosphatidylcholine (PC) (B), which has a different lipid backbone from LacCer (glycerol vs. ceramide). The endocytosis of NBD-PC in RFs is predominantly CPZ-inhibitable, suggesting that its uptake occurs largely by a clathrin-dependent mechanism Citation[7]. These studies suggest that the sphingoid backbone of the GSLs (but not the headgroup or overall hydrophobicity) may play an important role in caveolar endocytosis of GSLs.
Figure 1. Structures of fluorescent lipid analogues used to evaluate the features critical for caveolar uptake of the lipids. (A) Various headgroups (R) were attached to BODIPY-ceramide, resulting in BODIPY-GalCer, -LacCer, -MalCer, -globoside, -sulfatide, or -GM1. BODIPY-LacCer analogues were also synthesized using various chain length (C12, C16, C18, or C20) sphingosines or BODIPY-fatty acids (C3vs C5 spacer). Fluorescent LacCer bearing an NBD-fatty acid (see panel B) in place of the BODIPY-fatty acid was also synthesized. (B) Structure of the D-isomer of NBD-labeled PC, a glycerolipid. Reproduced from Citation[7] with permission from the American Society for Cell Biology.
![Figure 1. Structures of fluorescent lipid analogues used to evaluate the features critical for caveolar uptake of the lipids. (A) Various headgroups (R) were attached to BODIPY-ceramide, resulting in BODIPY-GalCer, -LacCer, -MalCer, -globoside, -sulfatide, or -GM1. BODIPY-LacCer analogues were also synthesized using various chain length (C12, C16, C18, or C20) sphingosines or BODIPY-fatty acids (C3vs C5 spacer). Fluorescent LacCer bearing an NBD-fatty acid (see panel B) in place of the BODIPY-fatty acid was also synthesized. (B) Structure of the D-isomer of NBD-labeled PC, a glycerolipid. Reproduced from Citation[7] with permission from the American Society for Cell Biology.](/cms/asset/c5e66c6f-7900-4848-b2f1-7a22ad37d4b6/imbc_a_145987_f0001_b.jpg)
Stimulation and regulation of caveolar endocytosis by GSLs and cholesterol
The regulation of endocytosis at caveolae is just beginning to be understood. Cholesterol depletion or sequestration (e.g., with filipin or nystatin) have been demonstrated to disrupt caveolae integrity and inhibit the endocytosis of SV40, CtxB, BODIPY-LacCer and albumin Citation[4], Citation[6], Citation[7], Citation[40], Citation[51], Citation[52].
Pang et al. Citation[53] report that CtxB internalization is sensitive to cholesterol depletion in high-GM1-expressing cells, but not in low-GM1 cells, and addition of exogenous GM1 to the PM enhances the cholesterol-dependent delivery of CtxB to the Golgi apparatus. As mentioned above, there is evidence that endocytosis via caveolae can be stimulated in various ways. First, endocytosis of caveolae can be stimulated by phosphatase inhibitors such as okadaic acid and sodium vanadate Citation[33], Citation[35]. In addition, caveolar endocytosis can be stimulated by the presence of cargo (e.g., SV40 virus, albumin) Citation[37], Citation[41]. The stimulation of caveolar endocytosis is accompanied by increased activation of src and phosphorylation of cav1 and dynamin Citation[37], Citation[40], Citation[54], suggesting that stimulation occurs via a triggering of increased kinase activities.
In a preliminary experiment in our laboratory, CtxB internalization was found to be very robust in cells co-labeled with BODIPY-LacCer, but was much lower in the absence of BODIPY-LacCer. Further study showed that acute treatment of HSFs with non-fluorescent natural LacCer or GM1 ganglioside, or with a synthetic short-chain C8-LacCer selectively stimulates the caveolar uptake of BODIPY-LacCer, whereas the internalization of markers internalized by fluid phase or clathrin-dependent endocytosis are not affected Citation[6]. The caveolar internalization of albumin and ß1-integrin is also greatly enhanced in cells pretreated with non-fluorescent GSLs or cholesterol Citation[6], Citation[36]. These results provide a partial interpretation to our previous observation that BODIPY-LacCer is internalized through caveolae within seconds Citation[6], Citation[10], which is in apparent contradiction to studies of some other caveolar markers in which an hour or more is required for significant internalization to occur Citation[40], Citation[41], Citation[52]. Thus, it is likely that BODIPY-LacCer may stimulate its own internalization. Furthermore, when the cellular cholesterol level of HSFs is increased (by culturing in the presence of excess of LDL, or acute treatment with a methyl-β-cyclodextrin (mβ-CD)/cholesterol complex, the internalization of albumin and BODIPY-LacCer is significantly increased, but no effect is seen on the endocytosis of Tfn Citation[6], suggesting that addition of exogenous cholesterol also can selectively stimulate caveolar uptake.
Biochemical inhibitors and dominant negative proteins (see ) were used to determine that exogenous GSLs or cholesterol did not induce endocytosis by an alternate mechanism rather than via caveolae Citation[6]. In addition, the endocytosis of both BODIPY-LacCer and albumin was significantly inhibited by cav1 siRNA under both stimulated and unstimulated conditions (Citation[36], and our unpublished studies). Finally, at very short times (30 sec) of BODIPY-LacCer internalization, this lipid was shown to co-localize with expressed cav1-mRed in stimulated and unstimulated HSFs Citation[6]. Thus, the endocytic pathway stimulated by exogenous GSLs and cholesterol retained identical properties to those of caveolar endocytosis in unstimulated cells. Importantly, addition of C8-LacCer or mß-CD/cholesterol to cells results in an 8–10 fold increase in src kinase activity and transient cav1 phosphorylation, similar to findings for stimulation of albumin in endothelial cells Citation[37], Citation[54]. Thus, different stimuli evoke caveolar uptake via the same signaling cascade.
Two models have been proposed concerning the mechanism by which addition of GSLs to the PM stimulates caveolar uptake Citation[6]. In the first model, addition of GSLs to the outer leaflet of the PM may cause a specific interaction of the GSL with a particular PM protein, which in turn could initiate a signaling cascade resulting in stimulation of caveolar endocytosis. However, since exogenous cholesterol can elicit similar effects on caveolar endocytosis, this model seems unlikely. Another model is that GSLs (or cholesterol) added to the outer leaflet of the PM bilayer could change the organization of lipids in the PM, thereby inducing the clustering and activation of a transmembrane protein and/or signaling proteins on the inner leaflet of the PM (). Support for this hypothesis comes from recent work in our laboratory which takes advantage of the concentration-dependent spectral properties of BODIPY fluorescence Citation[36]. We examined the PM distribution and organization of BODIPY-LacCer incubated with HSFs at low temperature (10°C). In control HSFs, the PM is uniformly labeled with BODIPY-LacCer and emitted only green fluorescence. However when cells were treated with C8-LacCer or mβ-CD/cholesterol, small “patches” of BODIPY-LacCer with increased red emission (shown as yellow/orange areas in green/red overlay) were observed at the PM (A), suggesting the formation or coalescence of GSL (BODIPY-LacCer)-enriched membrane microdomains.
Figure 2. Model for GSL-initiated clustering of plasma membrane microdomains. (1) In untreated cells, PM microdomains are too small or transient to be visualized. Certain transmembrane proteins (e.g., ß1-integrins) are dispersed in the membrane. (2) Addition of exogenous GSL or cholesterol to the membrane causes the formation or coalescence of GSL-enriched microdomains. (3) Certain transmembrane proteins and intracellular signaling proteins (e.g., src kinases) become clustered in GSL-enriched microdomains. Clustering leads to protein activation and intracellular signaling.
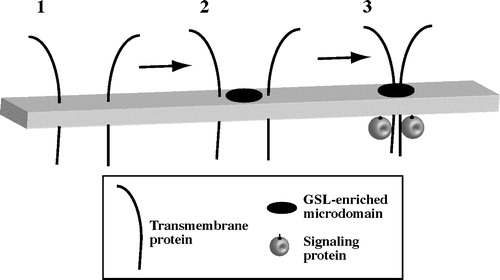
Figure 3. C8-LacCer and cholesterol induce clustering of β1-integrins within GSL-enriched microdomains. (A) Visualization of PM domains after induction by various treatments. Human skin fibroblasts were incubated with BODIPY-LacCer for 30 min at 10°C, washed, and then incubated in buffer alone (untreated) or with C8-LacCer, Mβ-CD/cholesterol complex, or ß1-integrin IgG for 30 min at 10°C. In the far right panel, cells were preincubated with 5 mM mß-CD to deplete cholesterol and then treated with BODIPY-LacCer and ß1-integrin IgG. The samples were then washed and observed by fluorescence microscopy at green and red BODIPY emission wavelengths. Samples were maintained at 10°C at all times to prevent endocytosis. Micrographs shown are overlays of red and green images. Areas outlined with white rectangles are further magnified in insets. Yellow orange patches indicate regions with the highest red signal, indicating enrichment of BODIPY-LacCer in these regions of the PM. Bar, 5 µm. (B) β1-integrin clusters are localized to PM lipid domains. Cells were co-labeled with BODIPY-LacCer and Alexa647 anti-β1-integrin Fab fragments for 30 min at 10°C. Samples were then further incubated for 30 min at 10°C±C8-LacCer, washed, and viewed by fluorescence microscopy. Images were acquired at red and green (for BODIPY as above) and at far red wavelengths for AF647-ß1-integrin Fab labeling. Note the overlap of integrin staining (shown in blue) with the enriched (red/orange) PM domains of BODIPY-LacCer. Bar, 2 µm. Reproduced from Citation[36] with permission from the American Association for Cancer Research.
![Figure 3. C8-LacCer and cholesterol induce clustering of β1-integrins within GSL-enriched microdomains. (A) Visualization of PM domains after induction by various treatments. Human skin fibroblasts were incubated with BODIPY-LacCer for 30 min at 10°C, washed, and then incubated in buffer alone (untreated) or with C8-LacCer, Mβ-CD/cholesterol complex, or ß1-integrin IgG for 30 min at 10°C. In the far right panel, cells were preincubated with 5 mM mß-CD to deplete cholesterol and then treated with BODIPY-LacCer and ß1-integrin IgG. The samples were then washed and observed by fluorescence microscopy at green and red BODIPY emission wavelengths. Samples were maintained at 10°C at all times to prevent endocytosis. Micrographs shown are overlays of red and green images. Areas outlined with white rectangles are further magnified in insets. Yellow orange patches indicate regions with the highest red signal, indicating enrichment of BODIPY-LacCer in these regions of the PM. Bar, 5 µm. (B) β1-integrin clusters are localized to PM lipid domains. Cells were co-labeled with BODIPY-LacCer and Alexa647 anti-β1-integrin Fab fragments for 30 min at 10°C. Samples were then further incubated for 30 min at 10°C±C8-LacCer, washed, and viewed by fluorescence microscopy. Images were acquired at red and green (for BODIPY as above) and at far red wavelengths for AF647-ß1-integrin Fab labeling. Note the overlap of integrin staining (shown in blue) with the enriched (red/orange) PM domains of BODIPY-LacCer. Bar, 2 µm. Reproduced from Citation[36] with permission from the American Association for Cancer Research.](/cms/asset/bf6e1faa-d340-4bbf-856a-9099a6289522/imbc_a_145987_f0003_b.jpg)
One possible class of proteins that might mediate the stimulatory effect of GSL and cholesterol on caveolar uptake is the integrins. Integrins are a family of αβ heterodimeric integral membrane proteins at the PM that are responsible for many types of cell adhesion and migration events Citation[55], Citation[56]. Binding of extracellular matrix (ECM) proteins and extracellular ligands to integrins induces a series of signaling cascades including activation of src kinase, phosphorylation of focal adhesion kinase, and elevation of intracellular calcium Citation[55], Citation[57]. Upon binding of ligands or crosslinking antibodies, some integrins are activated and redistributed into lipid microdomains Citation[58–60]. GSLs have been shown to modulate integrin-based cell attachment. For example, gangliosides (sialic acid-terminated GSLs) are reported to enhance binding of integrins to the ECM or collagen in a variety of cell lines Citation[61], Citation[62].
We recently found that the addition of C8-LacCer or cholesterol to HSFs at low temperature causes the clustering and activation of β1-integrin within GSL-enriched PM microdomains (B and Citation[36]). The same effect was observed by crosslinking of integrins with integrin antibodies which is an established method for integrin activation Citation[60], Citation[63]. Furthermore, β1-integrins are rapidly internalized via caveolae upon warming to 37°C in cells treated with C8-LacCer or cholesterol, whereas little endocytosis of β1-integrin is seen in untreated cells Citation[36]. This is consistent with the previous observation that GSLs and cholesterol stimulate caveolar uptake Citation[6]. A series of integrin-associated, downstream signaling events are also observed upon integrin clustering, including src activation, increased cav1 phosphorylation and reorganization of the actin cytoskeleton which precedes integrin internalization, RhoA movement away from the PM and transient cell detachment after integrin internalization Citation[36]. Results from these studies suggest the possibility that aberrant levels of GSLs found in cancer cells may influence cell attachment by modulating integrin clustering and internalization.
Cav1 and caveolae endocytosis
There are three members of the caveolin family, cav1, cav2 and cav3. Cav1 and cav2 are co-expressed in most cell types, whereas cav3 is expressed only in muscle cells Citation[64]. Cav1 is the defining protein component of caveolae, and is essential for caveolae biogenesis in most cells, although cav3 plays a similar role in muscle cells Citation[64], Citation[65]. In nonmuscle cells which do not express cav-1, such as normal lymphocytes, some transformed cell lines, and cells from cav1 knockout mice, there are no morphologically identifiable caveolae Citation[22], Citation[23], Citation[66–68]. Conversely, overexpression of cav-1 in caveolin-deficient cell lines results in the formation of recombinant caveolae vesicles Citation[68], Citation[69].
Despite the need for cav1 expression in the formation of caveolae, the role of cav1 in caveolae-mediated endocytosis has been controversial. A number of studies have shown that cav1 knockdown or expression of dominant negative cav1 decreases endocytosis via caveolae Citation[36], Citation[41], Citation[70], Citation[71]. In addition, we have shown that BODIPY-LacCer internalization is dramatically reduced in cell types with low levels of cav1, and could be stimulated in cell types with low cav1 levels by overexpression of cav1 Citation[6], Citation[7]. Although some studies have demonstrated an increase in endocytosis via caveolae upon over-expression of wild type cav1 Citation[71], Citation[72], other groups have reported that over-expression of cav1 negatively regulates caveolar endocytosis of albumin, CtxB, and autocrine motility factor receptors Citation[42], Citation[73]. This apparent discrepancy might be a result of cell type differences in the levels of cav1 and caveolar lipids (GSLs and cholesterol) that regulate caveolar endocytosis. For example, albumin uptake in HeLa cells is inhibited by cav1 over-expression, but when cav1 over-expressing cells are also treated with C8-LacCer, albumin uptake is stimulated Citation[6]. Thus, the balance between cav1, GSLs, and cholesterol may affect whether cav1 expression has a positive or negative impact on endocytosis via caveolae.
Another confounding factor in the study of the role of cav1 in endocytosis is the possibility that the same endocytic cargo may be internalized by different mechanisms in different cell types or even switch pathways in a single cell type under different experimental conditions. For example, CtxB is internalized via caveolae in some cells which express abundant cav1 and exhibit morphological caveolae, but is internalized through other endocytic mechanisms in cells with little or no cav1 Citation[7], Citation[52], Citation[74], Citation[75]. A possible limitation of some of these studies is that the criteria used to determine endocytosis via caveolae have not always excluded the possibility of internalization via other non-clathrin, non-caveolar mechanisms. For example, the use of cholesterol depletion or sequestration could affect other mechanisms of internalization apart from caveolar uptake Citation[7], Citation[52], Citation[74], Citation[75].
Studies of cells from cav1 knockout mice have only partially addressed the question of the role of cav1 in endocytosis. Endothelial cells from cav1 knock-out mice show defects in the uptake and transport of albumin in vivo, confirming the role of caveolae in the transcytosis of albumin Citation[76]. However, two recent studies carried out using murine embryonic fibroblasts (MEFs) from cav1 knockout and wild type mice, have shown that CtxB and SV40, which internalize via caveolae in many cell types, could be internalized via cholesterol-dependent, but clathrin- and cav1-independent mechanisms, in both the cav1 knockout and the wild type MEFs Citation[77], Citation[78]. These studies are in agreement with others which showed that CtxB can be internalized by non-caveolar mechanisms in cells with little or no cav1 Citation[52], Citation[75], Citation[79]. However, since the process of caveolar endocytosis could not be demonstrated (using either SV40 or CtxB) in wild type MEFs which do express cav1, no conclusion can be made concerning the role of cav1 in endocytosis in this cell type.
Conclusions
Studies from our laboratory and others have begun to characterize the process of caveolar endocytosis and shown it to be distinct from other clathrin-independent endocytic mechanisms. GSLs are selectively internalized via caveolae and this selectivity may occur because of the capability of GSLs to enhance microdomain formation at the PM. However, the precise mechanism by which GSLs stimulate domain formation, and the possible role of transmembrane proteins, requires further study. Although GSLs and other materials (e.g., SV40 virus) are internalized via caveolae, it is not clear if these occur by identical process since they differ in their kinetics and the intracellular compartments that are eventually targeted. For example, endocytosed BODIPY-LacCer is first visualized in small endocytic vesicles and then rapidly moves to EEs, without obvious occurrence in caveosomes, such as those seen upon SV40 uptake. Furthermore, the possibility that there are multiple forms of caveolar endocytosis has not been ruled out. While it is clear that cav1 expression is a prime requirement for the existence of morphological caveolae, the role of cav1 in the endocytic process is still in question. Is cav1 a stabilizer of the caveolae structure and thus an inhibitor of internalization from the membrane? Do stimulatory lipids such as GSLs and cholesterol initiate the phosphorylation of cav1 and thus its movement away from caveolae, allowing for a destabilization of the caveolae coat? The answers to these questions will require multiple cell biological, biochemical, and molecular approaches.
This paper was first published online on prEview on 27 January 2006.
This work was supported by USPHS grant GM-22942 to REP and an NRSA award to ZJC.
References
- Johannes L, Lamaze C. Clathrin-dependent or not: is it still the question?. Traffic 2002; 3: 443–451
- Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta 2005; 1745: 273–286
- Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell 2001; 7: 661–671
- Puri V, Watanabe R, Singh RD, Dominguez M, Brown JC, Wheatley CL, Marks DL, Pagano RE. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J Cell Biol 2001; 154: 535–547
- Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Develop Cell 2002; 2: 411–423
- Sharma DK, Brown JC, Choudhury A, Peterson TE, Holicky E, Marks DL, Simari R, Parton RG, Pagano RE. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell 2004; 15: 3114–3122
- Singh RD, Puri V, Valiyaveettil JT, Marks DL, Bittman R, Pagano RE. Selective caveolin-1-dependent endocytosis of glycosphingolipids. Mol Biol Cell 2003; 14: 3254–3265
- Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol 2003; 161: 673–677
- Choudhury A, Sharma DK, Marks DL, Pagano RE. Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit Rab4 and perturb membrane recycling. Mol Biol Cell 2004; 15: 4500–4511
- Sharma DK, Choudhury A, Singh RD, Wheatley CL, Marks DL, Pagano RE. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J Biol Chem 2003; 278: 7564–7572
- Palade, GE. Fine structure of blood capillaries. J Appl Physics 1953;24:1424, (abstract).
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol 1955; 1: 445–458
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387: 569–572
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 1992; 68: 533–544
- Moss DJ, White CA. Solubility and posttranslational regulation of GP130/F11 – a neuronal GPI-linked cell adhesion molecule enriched in the neuronal membrane skeleton. Eur J Cell Biol 1992; 57: 59–65
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol 1993; 122: 789–807
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosyntheic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol 1996; 133: 247–256
- Kurzchalia TV, Dupree P, Parton RG, Kellner R, Virta H, Lehnert M, Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol 1992; 118: 1003–1014
- Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell 1992; 68: 673–682
- Murata M, Peränen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA 1995; 92: 10339–10343
- Rothberg KG, Ying YS, Kamen BA, Anderson RG. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol 1990; 111: 2931–2938
- Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem 1994; 269: 30745–30748
- Gorodinsky A, Harris DA. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol 1995; 129: 619–627
- Fujimoto T. GPI-anchored proteins, glycosphingolipids, and sphingomyelin are sequestered to caveolae only after crosslinking. J Histochem Cytochem 1996; 44: 929–941
- Wu M, Fan J, Gunning W, Ratnam M. Clustering of GPI-anchored folate receptor independent of both cross-linking and association with caveolin. J Membr Biol 1997; 159: 137–147
- Fujimoto T, Hayashi M, Iwamoto M, Ohno-Iwashita Y. Crosslinked plasmalemmal cholesterol is sequestered to caveolae: Analysis with a new cytochemical probe. J Histochem Cytochem 1997; 45: 1197–1205
- Parton RG. Ultrastructural localization of gangliosides; GM1 is concentrated in caveolae. J Histochem Cytochem 1994; 42: 155–166
- Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science 1995; 269: 1435–1439
- Pitto M, Brunner J, Ferraretto A, Ravasi D, Palestini P, Masserini M. Use of a photoactivable GM1 ganglioside analogue to assess lipid distribution in caveolae bilayer. Glycoconj J 2000; 17: 215–222
- Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 2002; 296: 1821–1825
- Hakomori S. Structure, organization, and function of glycosphingolipids in membrane. Curr Opin Hematol 2003; 10: 16–24
- Sharma P, Varma R, Sarasij RC, Ira, Gousset K, Krishnamoorthy G, Rao M, Mayor S. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 2004; 116: 577–589
- Tagawa A, Mezzacasa A, Hayer A, Longatti A, Pelkmans L, Helenius A. Assembly and trafficking of caveolar domains in the cell: caveolae as stable, cargo-triggered, vesicular transporters. J Cell Biol 2005; 170: 769–779
- Thomsen P, Roepstorff K, Stahlhut M, van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell 2002; 13: 238–250
- Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol 1994; 127: 1199–1215
- Sharma DK, Brown JC, Cheng Z, Holicky EL, Marks DL, Pagano RE. The glycosphingolipid, lactosylceramide, regulates beta1-integrin clustering and endocytosis. Cancer Res 2005; 65: 8233–8241
- Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem 1997; 272: 25968–25975
- Mineo C, Anderson RG. Potocytosis. Robert Feulgen Lecture. Histochem Cell Biol 2001; 116: 109–118
- Mundy DI, Machleidt T, Ying YS, Anderson RG, Bloom GS. Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. J Cell Sci 2002; 115: 4327–4339
- Pelkmans L, Püntener D, Helenius A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 2002; 296: 535–539
- Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nature Cell Biol 2001; 3: 473–483
- Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol 2000; 150: 1057–1070
- Martin OC, Pagano RE. Internalization and sorting of a fluorescent analog of glucosylceramide to the Golgi apparatus of human skin fibroblasts: utilization of endocytic and nonendocytic transport mechanisms. J Cell Biol 1994; 125: 769–781
- Pagano R, Chen C. Use of BODIPY-labeled sphingolipids to study membrane traffic along the endocytic pathway. Ann NY Acad Sci 1998; 845: 152–160
- Pagano RE, Martin OC, Kang HC, Haugland RP. A novel fluorescent ceramide analogue for studying membrane traffic in animal cells: accumulation at the Golgi apparatus results in altered spectral properties of the sphingolipid precursor. J Cell Biol 1991; 113: 1267–1279
- Chen CS, Martin OC, Pagano RE. Changes in the spectral properties of a plasma membrane lipid analog during the first seconds of endocytosis in living cells. Biophys J 1997; 72: 37–50
- Pol A, Calvo M, Lu A, Enrich C. The ‘early-sorting’ endocytic compartment of rat hepatocytes is involved in the intracellular pathway of caveolin-1 (VIP-21). Hepatology 1999; 29: 1848–1857
- Pol A, Lu A, Pons M, Peiro S, Enrich C. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J Biol Chem 2000; 275: 30566–30572
- Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 2004; 118: 767–780
- Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell 2004; 15: 3542–3552
- Anderson HA, Chen Y, Norkin LC. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol Biol Cell 1996; 7: 1825–1834
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol 1998; 141: 905–915
- Pang H, Le PU, Nabi IR. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J Cell Sci 2004; 117: 1421–1430
- Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem 2004; 279: 20392–20400
- ffrench-Constant C, Colognato H. Integrins: versatile integrators of extracellular signals. Trends Cell Biol 2004; 14: 678–686
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 2002; 110: 673–687
- Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest 2002; 109: 999–1006
- Leitinger B, Hogg N. The involvement of lipid rafts in the regulation of integrin function. J Cell Sci 2002; 115: 963–972
- Mitchell JS, Kanca O, McIntyre BW. Lipid microdomain clustering induces a redistribution of antigen recognition and adhesion molecules on human T lymphocytes. J Immunol 2002; 168: 2737–2744
- Upla, P, Marjomaki, V, Kankaanpaa, P, Ivaska, J, Hyypia, T, vand der Goot, FG, Heino, J. Clustering induces a lateral redistribution of α2β1 integrin from membrane rafts to caveolae and subsequent PKC-dependent internalization. Mol Biol Cell 2004;15:625–636.
- Kazarian T, Jabbar AA, Wen FQ, Patel DA, Valentino LA. Gangliosides regulate tumor cell adhesion to collagen. Clin Exp Metastasis 2003; 20: 311–319
- Zheng M, Fang H, Tsuruoka T, Tsuji T, Sasaki T, Hakomori S. Regulatory role of GM3 ganglioside in alpha 5 beta 1 integrin receptor for fibronectin-mediated adhesion of FUA169 cells. J Biol Chem 1993; 268: 2217–2222
- Hogg N, Henderson R, Leitinger B, McDowall A, Porter J, Stanley P. Mechanisms contributing to the activity of integrins on leukocytes. Immunol Rev 2002; 186: 164–171
- Engelman JA, Zhang X, Galbiati F, Volonte D, Sotgia F, Pestell RG, Minetti C, Scherer PE, Okamoto T, Lisanti MP. Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer disease, and muscular dystrophy. Am J Hum Genet 1998; 63: 1578–1587
- Li R, Blanchette-Mackie EJ, Ladisch S. Induction of endocytic vesicles by exogenous C6-ceramide. J Biol Chem 1999; 274: 21121–21127
- Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001; 293: 2449–2452
- Razani B, Lisanti MP. Caveolins and caveolae: molecular and functional relationships. Exp Cell Res 2001; 271: 36–44
- Engelman JA, Wykoff CC, Yasuhara S, Song KS, Okamoto T, Lisanti MP. Recombinant expression of caveolin-1 in oncogenically transformed cells abrogates anchorage-independent growth. J Biol Chem 1997; 272: 16374–16381
- Fra AM, Williamson E, Simons K, Parton RG. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci USA 1995; 92: 8655–8659
- Galvez BG, Matias-Roman S, Yanez-Mo M, Vicente-Manzanares M, Sanchez-Madrid F, Arroyo AG. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell 2004; 15: 678–687
- Sottile J, Chandler J. Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol Biol Cell 2005; 16: 757–768
- Lobie PE, Sadir R, Graichen R, Mertani HC, Morel G. Caveolar internalization of growth hormone. Exp Cell Res 1999; 246: 47–55
- Le PU, Guay G, Altschuler Y, Nabi IR. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem 2002; 277: 3371–3379
- Massol RH, Larsen JE, Fujinaga Y, Lencer WI, Kirchhausen T. Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol Biol Cell 2004; 15: 3631–3641
- Torgersen ML, Skretting G, van Deurs B, Sandvig K. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci 2001; 114: 3737–3742
- Schubert W, Frank PG, Razani B, Park DS, Chow C-W, Lisanti MP. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J Biol Chem 2001; 276: 48619–48622
- Damm EM, Pelkmans L, Kartenbeck J, Mezzacasa A, Kurzchalia T, Helenius A. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J Cell Biol 2005; 168: 477–488
- Kirkham M, Fujita A, Chadda R, Nixon SJ, Kurzchalia TV, Sharma DK, Pagano RE, Hancock JF, Mayor S, Parton RG. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol 2005; 168: 465–476
- Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nature Cell Biol 2002; 4: 374–378
