Abstract
The ATP binding cassette (ABC) transporter Atm1p of the mitochondrial inner membrane performs crucial roles in both the biogenesis of cytosolic/nuclear iron-sulfur proteins and cellular iron homeostasis. Since the function of the mitochondrial iron-sulfur cluster (ISC) assembly machinery is also required for these two processes, Atm1p is thought to translocate a still unknown product of this pathway to the cytosol. Here, we provide a detailed in vitro characterization of Atm1p in order to better understand its function. Atm1p was purified using an expression system in E. coli. The detergent-solubilised protein exhibits a stable ATPase activity. Reconstitution of Atm1p into proteoliposomes allowed us to determine the biochemical characteristics of the ATPase such as: (i) the strong inhibition by the transition state analogue vanadate, (ii) a Km value of 0.1 mM, and (iii) a turnover number of 127 min−1. The ATPase activity of ABC transporters is generally stimulated by their specific substrate. We used this property to define the chemical properties of the substrate transported by Atm1p. ATPase hydrolysis by Atm1p-containing proteoliposomes was specifically increased 3–5-fold by thiol-containing compounds, in particular by micromolar concentrations of cysteine thiol groups in peptides, even though Atm1p is not a general peptide transporter such as yeast Mdl1p or mammalian TAP which share sequence similarity with Atm1p. We speculate that the physiological substrate of Atm1p may contain multiple sulfhydryl groups in a peptidic environment.
Introduction
ATP binding cassette (ABC) transporters comprise a large family of proteins which translocate a broad spectrum of compounds such as sugars, lipids, peptides, proteins and hydrophobic agents across biological membranes (Higgins [Citation1992], Holland et al. [Citation2003]). Members of this protein family contain two hydrophobic trans-membrane domains and two ABC domains which protrude into the soluble phase. Transport is highly specific for the substrate, is driven by the hydrolysis of ATP, and usually occurs from the side of the ABC domains to the other face of the membrane. ABC transporters are remarkably conserved in prokaryotic and eukaryotic cells. In bacteria, they are involved in, e.g., the extrusion of antibiotics or in the uptake of nutritional compounds. In the latter case they co-operate with periplasmic binding proteins which leads to reversed transport towards the side of the ABC domain. Eukaryotic ABC transporters have been detected in many cellular membranes and translocate a wide variety of compounds such as phospholipids, fatty acids, peptides, glutathione conjugates, and hydrophobic compounds (Holland et al. [Citation2003]).
The yeast ABC transporter Atm1p is located in the mitochondrial inner membrane with its ABC domains facing the matrix space (Leighton & Schatz [Citation1995], Kispal et al. [Citation1997], Lill & Kispal [Citation2001], Lill & Kispal [Citation2003]). On the basis of sequence comparisons Atm1p belongs to the MDR/TAP subfamily of ABC transporters (subfamily B; Dean & Allikmets [Citation2001]). Some members of this subfamily transport peptides such as yeast Ste6p, yeast Mdl1p or mammalian TAP1/2 (Kuchler et al. [Citation1989], McGrath & Varshavsky [Citation1989], Young et al. [Citation2001], Townsend & Trowsdale [Citation1993], Abele & Tampe [Citation2004]). Others such as human MDR1/P-glycoprotein are involved in the membrane extrusion of hydrophobic, xenobiotic compounds (Gottesman & Pastan [Citation1993]). Proteins with sequence similarity to Atm1p have been found throughout the eukaryotic kingdom including fungi, mammals and plants. Despite the bacterial origin of mitochondria, only few bacterial genomes contain a close sequence homologue of Atm1p. Expression of the two human counterparts of Atm1p designated ABCB7 and ABCB6 (previously termed MTABC3) as well as of the mitochondrial protein Sta1 from Arabidopsisthaliana have been shown to reverse the phenotypes associated with deletion of the yeast ATM1 gene, i.e. the strong growth defect, the cytosolic iron-sulfur (Fe/S) protein deficiency and the iron accumulation in mitochondria (Csere et al. [Citation1998], Mitsuhashi et al. [Citation2000], Kushnir et al. [Citation2001]). Thus, these eukaryotic proteins might perform an orthologous function in mitochondria. In keeping with this notion, patients with mutations in the ABCB7 gene accumulate iron inside mitochondria (ring sideroblasts) and develop the rare neurodegenerative disorder X-linked sideroblastic anaemia and cerebellar ataxia (XLSA/A) (Allikmets et al. [Citation1999], Bekri et al. [Citation2000]).
A combination of cell biological and biochemical studies has gained initial insights into the potential cellular function of yeast Atm1p. These studies documented two, not necessarily independent, roles of Atmp1. First, the transporter is involved in cellular iron homeostasis (Kispal et al. [Citation1997]). ATM1 deletion cells (▵atm1 strain) accumulate 20–30-fold higher amounts of iron in mitochondria relative to wild-type organelles. Recently, these studies were extended by the finding that the transcriptional activators of cellular iron uptake, Aft1p and Aft2p, respond to the signal transported by Atm1p to the cytosol (Rutherford et al. [Citation2005]). Second, Atm1p function is required for the biogenesis of cytosolic and nuclear Fe/S proteins (Kispal et al. [Citation1999], Balk et al. [Citation2004]). Depletion of Atm1p in the regulatable strain Gal-ATM1 leads to strong defects in cytosolic Fe/S protein activities and to impaired incorporation of 55Fe into cytosolic and nuclear Fe/S proteins while mitochondrial Fe/S proteins are unaffected. Conspicuously, similar phenotypes of both mitochondrial iron accumulation and defects in cytosolic/nuclear Fe/S protein biogenesis were observed upon depletion of components of the Fe/S cluster (ISC) assembly machinery of the mitochondrial matrix (Kispal et al. [Citation1999], Schilke et al. [Citation1999], Lange et al. [Citation2000], Li et al. [Citation2001], for review see Lill & Mühlenhoff [Citation2005]). These data suggest that the ISC assembly machinery generates a compound which is exported by mitochondrial Atm1p and then may be used for both iron uptake regulation and Fe/S protein assembly outside mitochondria. The compound, however, may not be generic and hence the substrate(s) transported by Atm1p has remained elusive so far.
In this communication, we describe our work to characterize Atm1p in more detail. First, the protein was purified using a novel heterologous expression approach in E. coli and then reconstituted into proteoliposomes. This allowed us to perform a biochemical characterization of its endogenous ATPase activity. Finally, we used the fact that the ATPase activity of many ABC transporters is stimulated by specific substrates in order to identify functional groups which affect the Atm1p ATPase and hence may possess chemical properties resembling the specific substrate of Atm1p.
Materials and methods
Materials
Peptides including γ-glutamyl-seryl-glycine were synthesized by Dr M. Krause (Institut for Molecular Biology and Cancer Research, Marburg) and purified by HPLC. The peptide sequences were CQI KMS KDI DGI R (designated P1-C1), SQI KMS KDI DGI R (P1-S1), CGD AVA EEV KKI LA (P2-C1), FWL RFT (P3-C0), KLC EGG CIA CGA CGG W (P4-C4) (Mulholland et al. [Citation1999]) and KLS EGG SIA SGA SGG W (P4-S4). An Ellman assay was performed for estimating the amount of SH-containing groups. For instance, 2.4 nmol of reactive thiol per µl of a 1 mM peptide solution were found for P4-C4. This is somewhat lower than the theoretical value of 4 nmol/µl assuming that no disulfide bridges were formed within the peptides. No thiol compound could be detected in a 1 mM solution of, e.g., peptide P4-S4. This indicates that sulfhydryl-containing contaminants were efficiently removed during purification of the peptides by HPLC. There was also no thiol reactivity detectable in a 5 mM solution of oxidised glutathione (GSSG). The apo- and holoforms of the [2Fe-2S] ferredoxin PetF from Synechococcus elongatus (now designated as Thermosynechococcus elongatus) were purified as described (Floss et al. [Citation1997]).
Cloning, expression and purification of Atm1p
The ATM1 gene lacking its first 26 codons was amplified by PCR using vector pYES-Su9-His8-ATM1 and inserted into vector pASK-IBA1 (IBA Göttingen, Germany). The fusion gene contained the coding sequence of the OmpA signal peptide, an octahistidinyl-tag, the ATM1 gene lacking its presequence, and a Strep-tag II (see A). The resulting plasmid was verified by DNA sequencing. The ATM1 fusion gene was expressed in E. coli BL21 cells that were freshly transformed. Cells were grown at 37°C in LB medium plus ampicillin to an OD600 of 0.8, induced by addition of 200 µg/l anhydrotetracycline and incubated for an additional 3 h. Cells were harvested and frozen at −80°C.
Figure 1. Expression and purification of Atm1p. (A) A fusion protein construct (OmpA-His8-ATM1-Strep) was used for heterologous synthesis of Atm1p in E. coli. The fusion protein consisted of an OmpA signal sequence, an eight codon long N-terminal His-tag, the ATM1 gene lacking the first 26 codons, and a C-terminal Strep-tag II. The corresponding DNA construct was cloned into the vector pASK-IBA1 (IBA Göttingen). The E. coli derived OmpA signal sequence facilitates targeting and insertion of the protein into the bacterial inner membrane. (B) E. coli BL21 cells carrying the plasmid described in A were grown in the presence or absence of anhydrotetracycline (Tet) to induce gene expression of the ATM1 fusion gene. Cell extracts were analysed by immunostaining for Atm1p or the Strep-tag using specific antibodies. A similar analysis was performed for synthesis of an Atm1p fusion protein lacking the bacterial OmpA signal sequence. C) Atm1p purification from E. coli BL21 after induction of gene expression by anhydrotetracycline. E. coli inner membranes were isolated and solubilized with the detergent n-dodecylmaltoside. The protein was purified on a StrepTactin Macroprep column. Fractions of each isolation step were subjected to SDS-PAGE, stained with Coomassie Brilliant Blue (lower panel), or electroblotted and immunodecorated with an Atm1p-specific antibody (upper panel). Lanes 1 and 2: Whole cell extracts without and with induction by anhydrotetracycline (50 µg protein); lane 3: solubilized membranes of induced E. coli (50 µg protein); lane 4: column flow-through (50 µg); lane 5: wash fraction; lane 6: eluted, purified protein (4.1 µg). (D) Purified Atm1p was applied to a gelfiltration column (Shodex Protein KW-804 column) and two peaks with molecular masses of 58 and 260 kDa were eluted.
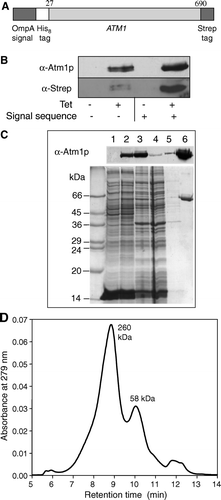
For purification of the Atm1p fusion protein E. coli membranes were isolated (Chang et al. [Citation1978]). Cells were suspended in TS buffer (50 mM Tris-HCl pH 8.0, 75 mM NaCl), incubated with 1mg/ml lysozyme and 10 mM EDTA for 30 min on ice and broken by sonicating twice for 3 min. Cell debris was removed by centrifugation for 30 min at 3,000 xg. The supernatant was centrifuged again at 150,000 xg to isolate the membrane fraction. The pellet was resuspended in solubilization buffer (20 mM MOPS-KOH pH 6.5, 200 mM NaCl, 20% sorbitol), homogenized in a Potter-Elvehjem homogeniser and brought to a protein concentration of 3 mg/ml. The detergent n-dodecylmaltoside (critical micellar concentration = 0.008%) was added from a 10% stock solution to reach a final concentration of 0.5% (w/v). The suspension was incubated for 30 min on ice and centrifuged at 150,000×g for 1 h. The supernatant containing the solubilized Atm1p fusion protein was diluted 1:1 with purification buffer PB (100 mM Tris-HCl pH 8.0, 150 mM NaCl, 1mM EDTA, 10% sorbitol, 0.025% n-dodecylmaltoside) and the Atm1p fusion protein was purified on a StrepTactin column (IBA Göttingen, Germany). The resin was washed with buffer PB, until no protein eluted anymore. Atm1p was recovered from the resin with buffer PB containing 2.5 mM desthiobiotin.
Mass spectrometry and gelfiltration of purified Atm1p
Mass spectrometry of Atm1p was performed using a Bruker Daltonics AutoflexTM mass spectrometer equipped with a nitrogen laser (laser 337 nm, 3ns pulse width and 50 Hz repetition rate). The spectra were acquired after an external calibration using reference protein standards trypsinogen, protein A, Albumin-Bovine, Protein A 2+ and Albumin-Bovine 2+ (Protein calibration standard II, Bruker Daltonics). Mass spectra were acquired in the linear positive mode with a pulsed extraction using approximately 100 laser shots and the masses assigned and processed using BiotoolsTM and FlexAnalysisTM software (Bruker Daltonics). Gelfiltration analysis of purified Atm1p was performed in buffer G (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10% glycerol, 0.03% dodecylmaltoside) using a Shodex HW804 (Waters) column and a Waters 2487 HPLC system (flow rate 0.5 ml/min). Molecular mass standards from Sigma were used for calibration of the column.
Reconstitution of Atm1p into proteoliposomes
For reconstitution of Atm1p a protocol described earlier was adapted (Knol et al. [Citation1996], Knol et al. [Citation1998], Lambert et al. [Citation1998]). A chloroform solution containing 41 mol% 1,2-dioleoyl-sn-glycero-3 phosphatidylcholine (DOPC), 39 mol% 1,2- dioleoyl-sn-glycero-3 phosphatidylethanolamine (DOPE), 2 mol% L-α- phosphatidylinositol (PI; all from Avanti Polar Lipids) and 18 mol% cardiolipin (CL, Sigma) was prepared. Chloroform was evaporated using a water-jet vacuum pump and lipid films were further dried in a desiccator. The lipid films were rehydrated by adding buffer B (100 mM Tris-HCl, pH 8.0, 50 mM NaCl), stirring with a glass marble and freeze-thawing of the suspension for several times in liquid nitrogen and water. The liposomes were made unilamellar by 20 passes through an extruder (200 nm membrane) and equilibrated with a small amount of n-dodecylmaltoside (1 mol per mol lipid) for 45 min at 30°C. Purified Atm1p was added and the suspension was incubated for 1 h at room temperature. The detergent was removed by adsorption to BioBeads SM2 and stirring for 1 h at room temperature. To improve removal of the detergent, treatment with the BioBeads and stirring for 2 h at 4°C was repeated twice.
Flotation of Atm1p in proteoliposomes
The insertion of Atm1p into the lipid bilayer was tested by flotation centrifugation (45 min, 140,000×g) of the proteoliposomes through a sucrose gradient consisting of a 45%, 40%, 35% and 0% sucrose prepared in buffer B (Mayer et al. [Citation1995]). The proteoliposomes were harvested from the 35%/0% sucrose interphase.
Other methods
ATPase was measured using a dye assay (Lill et al. [Citation1990]). Protein concentrations of E. coli membranes were determined using the BCA assay (Pierce). Protein content of purified Atm1p in lipid mixtures, in proteoliposomes, and in samples of was measured by subjecting aliquots to SDS-PAGE, subsequent Coomassie staining and densitometric analysis. The typical Atm1p protein concentration in the ATPase assay was 0.3–1.4 µg/ml (=5–20 nmol/l).
Results
Heterologous expression and purification of Atm1p
Atm1p of Saccharomyces cerevisiae was produced in E.coli BL21 making use of the plasmid pASK-IBA1. To enhance membrane targeting of the protein the coding sequence of ATM1 was inserted at the 3′-end of a DNA piece encoding the OmpA signal sequence which directs translocation across the E. coli inner membrane (A). To facilitate subsequent purification of Atm1p we attached an affinity tag (Strep-tag II) to the C-terminus of the protein and a His-tag to the N-terminus. Plasmids containing this OmpA-His8-ATM1-Strep fusion gene gave rise to the synthesis of significantly higher levels of Strep-tagged Atm1p compared to plasmids without the encoded signal sequence (B), even though the protein still could not be detected by staining with the Coomassie dye in whole cell extracts (C, compare lanes 1 and 2). To further optimize the expression level, several E. coli strains were tested. The fusion protein was only produced in detectable amounts in E. coli B strains lacking the major E. coli proteases Lon and OmpT (not shown). The fusion protein was insensitive to protease digestion in spheroplasts suggesting that it had inserted into the bacterial membrane with its ABC domain facing the cytosol (not shown).
For purification of the Atm1p fusion protein, we first isolated E. coli inner membranes. No soluble form of Atm1p was detectable in the cytosolic extracts and Atm1p did not give rise to inclusion bodies (not shown). The isolated membranes were dissolved in buffer containing the non-ionic detergent n-dodecylmaltoside. The extract was clarified by centrifugation and Atm1p was purified to apparent homogeneity by affinity chromatography on a StrepTactin column (C). No DnaK or GroEL (exhibiting similar molecular masses) could be detected in the purified fractions by immunostaining with specific antibodies. The yield of purified protein was 0.4–1 mg/l cell culture. This was much higher than the amount of Atm1p that could be isolated after overproduction in yeast, where only 0.5 µg/l cell culture was obtained for a Strep-tagged Atm1p fusion protein (not shown). The purified recombinant protein appeared as a homogeneous peak of 68,207±250 Da according to MALDI-TOF mass spectrometry. This mass is smaller than the calculated value of 75,604 Da for the plasmid-encoded protein. Apparently, the purified protein was proteolytically processed after integration into E. coli inner membranes. Since the C-terminal Strep-tag was still present on the purified protein and the His-tag was not detectable (see B, and data not shown), the cleavage obviously had occurred at the N-terminus. Residual molecular mass fits perfectly to a starting point of the protein at amino acid residue 93 (±2) of the Atm1p protein sequence, i.e., the OmpA signal sequence, the His-tag and part of the (non-conserved) mature portion of Atm1p were proteolytically removed.
The oligomerization properties of Atm1p were analysed by gelfiltration. A major peak of 260 kDa and a minor peak of 58 kDa eluted from the column (D). This indicates that, in addition to the monomeric form of Atm1p, the majority of the protein is in an oligomeric, possibly tetrameric state. The ratio of the two forms depended on the isolation procedure, but both forms gave rise to active protein (see below).
Purified solubilised Atm1p exhibits an ATPase activity
Purified Atm1p in n-dodecylmaltoside-containing buffer showed an ATPase activity (A). The activity was temperature-dependent, as a higher ATPase activity was found at 30°C compared to 20°C. The ATPase activity was linear indicating that Atm1p was stable over time. In keeping, we noted that no major loss of activity was seen upon incubation of the protein at 0°C overnight (not shown). However, incubation of the protein with other detergents such as octylglucoside and Hecameg resulted in the rapid destruction of the ATPase activity (not shown). Therefore, the detergent n-dodecylmaltoside was preferred for purification and also used for the reconstitution process described below.
Figure 2. Purified, solubilized Atm1p has an ATPase activity that is stimulated by lipids. (A) Purified Atm1p was incubated at 20°C and 30°C in 100 mM Tris-HCl pH 8.0 containing 0.025% n-dodecylmaltoside and 1 mM ATP. ATPase activity was followed by phosphate release using a colorimetric assay (Lill et al. [Citation1989]). (B) The four most abundant phospholipids of the mitochondrial inner membrane and purified Atm1p (in 0.025% n-dodecylmaltoside solution) were mixed and incubated at 30°C for 30 min. Aliquots containing 0.14 µg Atm1p and 7.4 µg lipid were tested for their ATPase activity. Composition of lipid mixtures: PC/PE/PI: 45% DOPC, 37% DOPE, 18% PI; CL/PC/PE/PI 18% cardiolipin, 41% DOPC, 39% DOPE, 2% PI. Bars represent the standard errors of three independent experiments.
![Figure 2. Purified, solubilized Atm1p has an ATPase activity that is stimulated by lipids. (A) Purified Atm1p was incubated at 20°C and 30°C in 100 mM Tris-HCl pH 8.0 containing 0.025% n-dodecylmaltoside and 1 mM ATP. ATPase activity was followed by phosphate release using a colorimetric assay (Lill et al. [Citation1989]). (B) The four most abundant phospholipids of the mitochondrial inner membrane and purified Atm1p (in 0.025% n-dodecylmaltoside solution) were mixed and incubated at 30°C for 30 min. Aliquots containing 0.14 µg Atm1p and 7.4 µg lipid were tested for their ATPase activity. Composition of lipid mixtures: PC/PE/PI: 45% DOPC, 37% DOPE, 18% PI; CL/PC/PE/PI 18% cardiolipin, 41% DOPC, 39% DOPE, 2% PI. Bars represent the standard errors of three independent experiments.](/cms/asset/380c9b3b-d162-4b4a-984e-71dafc70f3b7/imbc_a_147346_f0002_b.gif)
Incubation of detergent-solubilized Atm1p with various phospholipids resulted in a stimulation of its ATPase activity. A particularly pronounced effect was found for the acidic phospholipid cardiolipin, which yielded a 5-fold stimulation of the ATPase activity (B). This may indicate a specific interaction of Atm1p with phospholipids, in particular negatively charged lipids such as cardiolipin.
Reconstitution of Atm1p into proteoliposomes
The purified ABC transporter Atm1p was reconstituted into pre-formed liposomes adapting a procedure described earlier (Knol et al. [Citation1996], Lambert et al. [Citation1998]). The four most abundant phospholipids of the mitochondrial inner membrane (Zinser et al. [Citation1991]) were chosen for reconstitution to mimic the natural environment of Atm1p. The protein content and the ATPase activity were followed throughout the reconstitution procedure to optimize each step (A). Our standard reconstitution procedure involved incubation of pre-formed liposomes with a small amount of n-dodecylmaltoside (1 mol/mol lipid) for 45 min at 30°C, addition of purified solubilized Atm1p (1/50 of lipid mass; fraction A1 in A), incubation for 1 h at room temperature (fraction A2) and removal of the detergent by two incubations with the detergent adsorbent BioBeadsSM2 (fractions A3 and A4). The ATPase activity remained similar during these treatments confirming that purified Atm1p is a stable protein. Successful insertion of Atm1p into the lipid bilayer was tested by floating the proteoliposomes on a discontinuous sucrose gradient with steps of 45%, 40%, 35% and 0% sucrose concentrations. A high amount of the protein (between 20% and 60%) was recovered after flotation from the 35%/0% sucrose interface demonstrating that a fair amount of the protein was inserted into the lipid bilayer. The specific activity of Atm1p was lowered by 40% during the flotation centrifugation (fraction A5 in A). This is best explained by removal of residual detergent, since re-addition of minute amounts of detergent increased the ATPase activity (not shown). The specific activity of reconstituted and floated Atm1p in proteoliposomes was found to be 2.5-fold higher compared to solubilized Atm1p in detergent-containing solution (fraction Con in A) suggesting functional integration of the ABC transporter into the membranes.
Figure 3. Reconstitution of Atm1p into proteoliposomes. (A) The reconstitution of purified Atm1p was carried out by detergent removal as described in Materials and Methods. After each reconstitution step an aliquot (A1–A5) of the suspension was removed and tested for ATPase activity and protein content. The aliquots were chosen as follows: A1, mixture of lipid and protein; A2, same after 1 h incubation at room temperature; A3 and A4, samples after first and second detergent removal steps; A5, floated sample obtained after flotation centrifugation (see Materials and Methods). A control sample (Con) containing only purified, solubilized Atm1p and no lipids was mock-treated in 0.025% n-dodecylmaltoside-containing buffer at the same temperatures as the reconstitution samples. Specific activities of the Atm1p ATPase are given for four independent experiments (error bars represent the standard deviation). (B) Membrane orientation of reconstituted Atm1p. Floated proteoliposomes (equivalent to sample A5) were treated with proteinase K (PK) and Triton X-100 (Tx-100) as indicated. Atm1p was analysed by silver staining or detected by the Strep-tag (α-Strep-tag) affinity detection system.
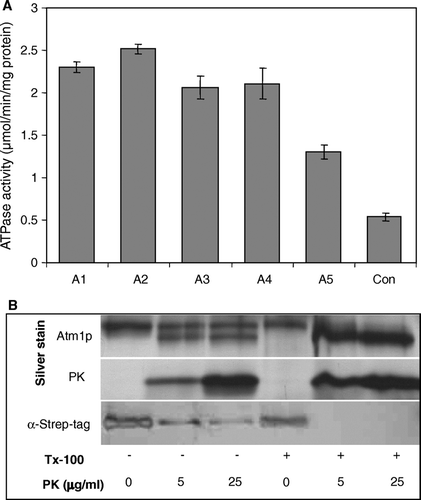
The orientation of Atm1p after insertion into the proteoliposomes was determined by a protease protection assay. For this purpose we monitored the proteolytic accessibility of the C-terminal Strep-tag of the Atm1p fusion protein in floated proteoliposomes (B). Proteinase K treatment yielded, in addition to uncleaved Atm1p, a shorter form which could not be detected by the Strep-tag staining procedure indicating proteolytic removal of this part of the protein. This notion was confirmed by treatment of detergent-lysed proteoliposomes with proteinase K. Under these conditions only the shorter fragment was observed (B). The difference in molecular mass of the long and short forms of Atm1p fit nicely to the mass of the Strep-tag. We therefore conclude that the C-terminal Strep-tag was removed from the protease-resistant Atm1p by the proteinase K treatment. In proteoliposomes the shorter form was generated in a 1:1 ratio over the intact Atm1p fusion protein (B) suggesting that Atm1p was inserted in a random orientation into the proteoliposomes.
Characterization of the ATPase activity of reconstituted Atm1p
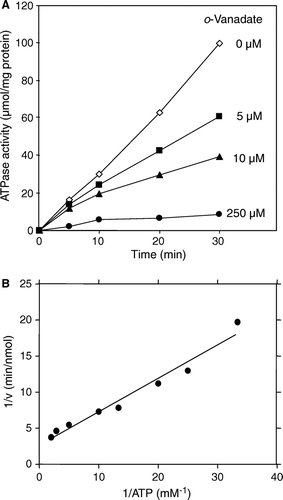
We next analysed the ATPase activity of reconstituted Atm1p. A linear ATPase activity over time was found (A, compare also A). This indicates that Atm1p is stable in proteoliposomes. A characteristic property of ABC transporters is the inhibition of their ATPase activity by the transition state analogue ortho-vanadate (Senior et al. [Citation1995], Chen et al. [Citation2001]). We therefore tested the effects of vanadate on the ATPase activity of reconstituted Atm1p. Addition of vanadate diminished the ATPase activity of Atm1p proteoliposomes in a concentration-dependent fashion (A). At a concentration of 250 µM vanadate the ATPase was inhibited almost quantitatively (92%). We next determined the enzymatic properties of the reconstituted Atm1p ATPase. The Km- and Vmax-values for ATP were estimated to be 0.13 mM and 1.9 nmol phosphate/min/µg, respectively (B). The turnover number was calculated to be 127 min–1. These values are comparable to other reconstituted ABC transporters (Senior et al. [Citation1995], Sun et al. [Citation1999], Ketchum et al. [Citation2001], Holland et al. [Citation2003]). The numbers differ, however, from the biochemical parameters determined for the soluble ABC domain of S. Pombe Atm1p which exhibits only a weak and slow ATPase (Km of 0.5 mM, turnover number 0.055 min-1; Chen & Cowan [Citation2003]).
Figure 4. Characterization of the ATPase activity of reconstituted Atm1p. (A) The ATPase activity of Atm1p in proteoliposomes (corresponding to sample A4 in A) is inhibited by vanadate. Reconstituted Atm1p was incubated at 30°C in 100 mM Tris pH 8.0, 50 mM NaCl, 2 mM MgCl2, 1 mM ATP in the presence of the sodium vanadate concentrations as indicated. The ATPase activity was estimated as in . (B) The Km- and Vmax-values for ATP of reconstituted Atm1p in proteoliposomes were calculated as 0.13 mM (± 0.03) and 1.9 (± 0.4) nmol phosphate/min/µg, respectively using WordPadPrism™ non-linear regression analysis. For each ATP concentration chosen the initial rate of the reaction was determined by measurement of the ATPase activity at five time points. The data are presented according to Lineweaver-Burk.
Stimulation of the ATPase activity by thiol-containing compounds
For many ABC transporters it is known that their endogenous ATPase activity is specifically stimulated by interaction with the transported substrate (see, e.g., Sun et al. [Citation1999], Ketchum et al. [Citation2001], Gorbulev et al. [Citation2001]). We therefore used the Atm1p-containing proteoliposomes in an attempt to search for potential effectors of the ATPase activity and to get an idea about the chemical character of the Atm1p-specific substrate. Previously, we observed an accumulation of the thiol compound glutathione in Δatm1 cells (Kispal et al. [Citation1997]). Recently, a specific requirement of this compound for cytosolic Fe/S protein biogenesis was shown (Sipos et al. [Citation2002]). These observations prompted us to test whether glutathione or any other thiol compound might stimulate the ATPase activity of reconstituted Atm1p. Different thiol compounds including the monothiols glutathione, cysteine and cysteinyl-glycine, as well as the dithiol DTT were tested. For comparison, the respective oxidised forms of these reagents were included in the assay. A 3.5-5 fold stimulation of the ATPase activity was observed for the reduced forms of glutathione, cysteine, cysteinyl-glycine and DTT at mM concentrations (A). The stimulatory effect was saturable and showed a characteristic concentration-dependence for each component (B). The half maximal effect of cysteine was reached around 30 µM, while for glutathione it was 20-fold higher. No significant stimulatory effect was seen with cystine and the oxidised forms of cysteinyl-glycine and DTT as well as for the non-thiol reducing agent ascorbic acid as a control. The specificity of the stimulatory effect for thiol groups is further supported by the observation that the reduced, but not oxidized form of other thiol-containing compounds such as lipoic acid and coenzyme A stimulated the Atm1p ATPase (not shown). As the only exception, oxidized glutathione (GSSG) increased the ATPase activity, yet at a rather weak efficiency similar to reduced glutathione (A and data not shown). Control experiments using Ellman's assay confirmed that the oxidized thiol reagents (including GSSG) did not contain relevant amounts of free thiol groups (not shown). We further tested thiol derivatives of glutathione. S-Methyl glutathione or the non-thiol derivatives γ-glutamyl-seryl-glycine and ophthalmic acid (γ-glutamyl-α-aminobutyryl-glycine) did not stimulate the ATPase activity of Atm1p suggesting that the free thiol group or the disulfide group of GSSG are critical for the stimulatory effect (not shown).
Figure 5. Stimulation of the Atm1p ATPase activity by thiol compounds. (A) Atm1p-containing proteoliposomes were floated on a sucrose gradient and tested for their ATPase activity in the presence of different thiol-containing compounds (1 mM final concentration). The figure shows the mean of three independent determinations, relative to the value obtained without further addition. GSH, reduced glutathione; GSSG, oxidized glutathione; DTT ox, oxidized DTT. (B) The ATPase activity of floated Atm1p proteoliposomes was measured in the presence of increasing concentrations of cysteine, DTT, GSH, and of the reducing agents Tris(2-carboxyethyl)phosphine (TCEP) and Tris(2-cyanoethyl)phosphine (TCP). For the latter two reagents, no stimulatory effect was seen, whereas the thiol-containing compounds increased the ATPase activity in a concentration-dependent fashion.
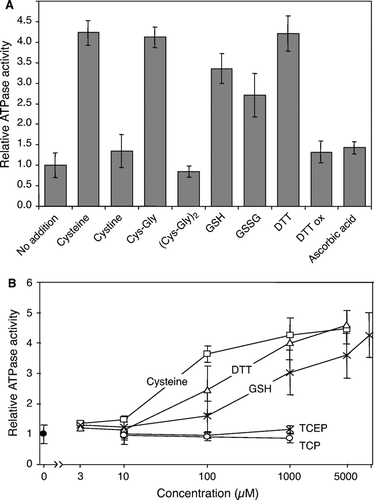
The Atm1p polypeptide chain contains three (non-conserved) cysteine residues raising the possibility that the ATPase activity of Atm1p was stimulated by a conformational change associated with the opening of a disulfide bridge. To test this idea the non-thiol compounds Tris(2-cyanoethyl)phosphine (TCP) and Tris(2-carboxyethyl)phosphine (TCEP) were analysed for their effects on the ATPase activity of Atm1p in proteoliposomes. Both reagents efficiently reduce disulfide bonds (Burns et al. [Citation1991]). However, no effect of both reagents was seen on the ATPase activity of Atm1p, even when applied at high (1 mM) concentration (B). These data make it unlikely that the ATPase stimulation could be caused by the opening of a disulfide bridge in Atm1p.
To further substantiate this notion, purified detergent-solubilized Atm1p was first treated with cysteine (to open putative disulfide bridges) and then incubated either anaerobically or with a high amount of N-ethylmaleimide (NEM) to prevent re-oxidation. After reconstitution of Atm1p into proteoliposomes and flotation, the ATPase activity could still be fully stimulated by cysteine and other thiol compounds (not shown). In conclusion, the increase of the ATPase activity by reduced thiol compounds is caused by specific interaction of Atm1p with these components, and is not due to the opening of disulfide bonds within Atm1p.
Efficient stimulation of the ATPase activity of Atm1p by cysteine-containing peptides
Since Atm1p belongs to the class of ABC transporters encompassing the peptide transporters Ste6p and Mdl1p of yeast and TAP of mammals (Ketchum et al. [Citation2001], Young et al. [Citation2001], Gorbulev et al. [Citation2001]), we asked whether peptides may affect the ATPase activity of reconstituted Atm1p, and whether the cysteine content in the peptides may influence the response. We found that peptides lacking cysteine residues do not modulate the Atm1p ATPase activity (A, 6B and data not shown). In marked contrast, peptides containing cysteine residues stimulated the ATPase activity at low peptide concentrations (<30 µM) 3- to 5-fold. A strikingly low effective concentration (half maximal stimulation <3 µM) was found for a 16mer containing four cysteine residues (P4-C4 in B; Mulholland et al. [Citation1999]) indicating a particularly high affinity of this peptide for Atm1p. When the four cysteine residues were replaced by serine, the 16mer peptide (P4-S4) did not exhibit any stimulatory effect on the ATPase activity of Atm1p demonstrating that the thiol groups were critical for the effect. We conclude that the ATPase activity of Atm1p is not altered by peptides, unless they contain cysteine residues. Thus, Atm1p most likely is not a general peptide transporter, but is specifically modulated by thiol groups.
Figure 6. Efficient stimulation of Atm1p ATPase activity by cysteine-containing peptides or proteins. Atm1p was reconstituted into proteoliposomes, floated on a sucrose gradient and tested for ATPase activity in presence of different concentrations of peptides (A and B) and proteins (C). Part A shows two cysteine-containing peptides (P1-C1, P2-C1) and two peptides without a cysteine residue (P1-S1, P3-C0). Part B documents the efficient stimulation of the Atm1p-ATPase activity by a peptide containing four cysteine residues (P4-C4), but not by its derivative, where the cysteine residues were replaced by serine residues (P4-S4). In part C the apo- and holoforms of the S. elongatus ferredoxin PetF were incubated with Atm1p-containing proteoliposomes and the ATPase activity was recorded as above. Note that the preparation contains about 15% apoform. For comparison the ATPase activity of the proteoliposomes was recorded in the presence of cysteine. All data are presented relative to the value obtained without further additions. Data are the mean of three independent experiments; the bars represent the standard deviation.
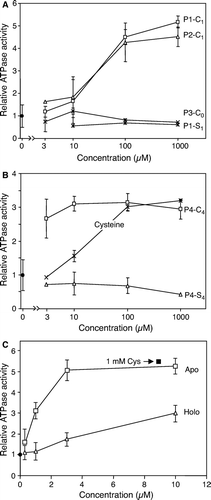
We finally tested whether proteins would affect the ATPase activity of Atm1p in proteoliposomes. To analyse the influence of free thiol groups, we employed the apo- and holoforms of the [2Fe-2S] ferredoxin PetF from S.elongatus (containing five cysteines among 98 residues; Floss et al. [Citation1997]). The apoform strongly increased the ATPase activity of Atm1p with a half-maximal stimulation as low as 1 µM (C). In contrast, the holoform was far less effective. This stimulatory effect is fully explained by the 15% contamination of the holoprotein preparation with the apoform suggesting that the holoform did not appreciably affect the ATPase activity of Atm1p. In conclusion, any component containing sulfhydryl groups was able to stimulate the ATPase activity of Atm1p in floated proteoliposomes. A particularly strong effect was seen with thiol-containing peptides and proteins.
Discussion
In our current study we present a biochemical characterization of the mitochondrial ABC transporter Atm1p. We first used a novel expression strategy to synthesize yeast Atm1p in E. coli in yields high enough to readily purify the protein to apparent homogeneity and to allow subsequent biochemical studies. For expression, we attached an N-terminal bacterial signal sequence to Atm1p which largely increased the protein amount as compared to standard expression. The addition of a bacterial signal sequence may have facilitated more efficient targeting of Atm1p to the plasma membrane and/or facilitate better membrane integration thus preventing aggregation. Purification of Atm1p was eased by a C-terminal Strep-tag which allowed a one step affinity purification using isolated E. coli plasma membranes as a starting material. The yields of purified Atm1p (0.4–1 mg/l E. coli cell culture) are higher than those reported for other ABC transporters (see, e.g., Ketchum et al. [Citation2001], Gorbulev et al. [Citation2001], Sun et al. [Citation1999]). Therefore, our protocol may provide a general strategy for the recombinant production of other ABC transporters in high yields, particularly for mitochondrial ABC transporters which are not glycosylated.
The purified, detergent-solubilized Atm1p appeared mainly as an oligomer, possibly a tetramer. Previously, Atm1p was found to form a homodimer in an ATP-dependent fashion, but higher order structures were not excluded in this study (Chloupkova et al. [Citation2004]). Solubilized Atm1p in the non-ionic detergent n-dodecylmaltoside exhibited an ATPase activity which was stable over time. Other detergents such as octylglucoside rapidly destroyed the ATPase activity indicating the importance of the detergent for the purification procedure. The ATPase activity of soluble Atm1p was increased by the addition of lipids, mainly negatively charged phospholipids such as cardiolipin. We conclude that in the lipid bilayer Atm1p may specifically interact with phospholipids such as cardiolipin.
For a detailed functional characterization, we chose to reconstitute purified Atm1p into proteoliposomes. Reconstituted Atm1p exhibited a stable ATPase activity which was strongly inhibited by the transition state analogue o-vanadate. Further, we determined the kinetic parameters of the ATP hydrolysis reaction with Km=0.13 mM, Vmax=1.9 nmol phosphate/min/µg, and kcat=127 min–1. These numbers compare well to reports for a number of eukaryotic ABC transporters that have been purified and biochemically characterized such as Ste6p, ABCR, and TAP (Ketchum et al. [Citation2001], Sun et al. [Citation1999], Gorbulev et al. [Citation2001]). In summary, our biochemical data on Atm1p fit well to the overall properties of ABC transporters, classify the protein as a bona fide representative of this family, and hence show that our reconstitution system is well suited for the molecular analysis of Atm1p function.
The substrate of Atm1p likely is a (direct or indirect) product of the mitochondrial ISC assembly machinery, but so far has remained elusive, mainly because it may not be a generic compound (for discussion see Balk & Lill [Citation2004], Lill & Mühlenhoff [Citation2005]). Therefore, one of the goals of this study was to define chemical properties of the potential substrate by identifying functional groups that stimulate the ATPase of Atm1p. It is well-known that physiological substrates of ABC transporters usually stimulate their ATPase activity (Holland et al. [Citation2003]). Since Atm1p belongs to the group of peptide transporters (subgroup B) and shares sequence similarity with S. pombe Hmt1p which translocates glutathione-derived compounds into vacuoles (Ortiz et al. [Citation1995]), we were particularly interested in testing the effects of peptides and glutathione-like molecules. We used proteoliposomes obtained by flotation centrifugation for these experiments to assure that the entire population of Atm1p is membrane-integrated. A wide variety of components was assayed and as a striking consensus it was found that any compound containing free sulfhydryl groups was able to stimulate the ATPase activity of Atm1p by a factor of 3.5–5. The components include amino acids (cysteine), short peptides (Gly-Cys and GSH), cofactors (lipoic acid and coenzyme A), and organic substances (DTT). The half-maximal concentration for stimulation was ranging from 30 µM for cysteine to 300 µM for glutathione. The effect of the thiol compounds was only seen for proteoliposomes or when lipids were present. Several lines of arguments argue against the possibility that this stimulatory effect was due to the reduction of intra- or intermolecular disulfide bonds within Atm1p. First, Atm1p does not contain conserved cysteine residues. Second, the redox potential of the compounds did not match their efficacy in the ATPase stimulation. Third, reduction by various reagents such as ascorbic acid, TCP, and TCEP which are known to efficiently open disulfide bridges did not significantly alter the ATPase activity of Atm1p. Finally, treatment of Atm1p with DTT and subsequent reaction of the free thiol groups with alkylating reagents (NEM) did not affect the stimulatory effect of sulfhydryl-containing compounds on the ATPase activity of Atm1p. Together, these data demonstrate the specificity of the ATPase stimulation for thiol groups, and make it unlikely that the increased ATPase activity may be caused by the opening of a disulfide bridge and a subsequent conformational change affecting the ATPase reaction.
The high specificity of the ATPase stimulation for thiol compounds is demonstrated by the observation that the respective oxidised forms did not affect the ATPase activity of Atm1p. The only notable exception was the oxidised form of glutathione (GSSG) which was found almost as effective as reduced glutathione (with the relatively weak half maximal stimulation around 600 µM). The reason for this finding is unknown, but it appears that the peptide backbone also exhibits a stimulatory effect. At least in part, this may be explained by the fact that glutathione plays a critical role in the same pathways as Atm1p. Its depletion in yeast causes a specific defect in the maturation of cytosolic Fe/S proteins and strongly induces the iron regulon via the transcription factors Aft1p/Aft2p (Sipos et al. [Citation2002], Rutherford et al. [Citation2005]). The precise function of glutathione in these processes is still unknown and awaits the functional reconstitution of the Atm1p-driven export process with isolated mitochondria in vitro. It should be noted in this context that ABC transporters of the MRP family respond to glutathione-S derivatives like S-methyl glutathione and ophthalmic acid, yet the Atm1p ATPase was not stimulated by these compounds (Holland et al. [Citation2003]; not shown).
Our study excludes that Atm1p is a general peptide transporter, since peptides did not stimulate its ATPase activity unless they contained cysteine residues. In that sense, Atm1p behaves differently than other peptide-translocating members of the subgroup B of ABC transporters such as Ste6p, Mdl1p, and TAP (Ketchum et al. [Citation2001], Young et al. [Citation2001], Gorbulev et al. [Citation2001]). Overproduction of Mdl1p, another mitochondrial ABC transporter, has been shown to functionally complement an ATM1 deletion mutant (Chloupkova et al. [Citation2003]) suggesting that these two mitochondrial ABC proteins can, at least in part, perform the same function. The partial adoption of the Atm1p function by Mdl1p may also explain why the former is not essential in yeast, as are numerous components of Fe/S protein biogenesis (Lill & Mühlenhoff [Citation2005]). A comparatively potent stimulation of the Atm1p ATPase was detected, when the peptides contained multiple cysteine residues. The half-maximal stimulation for the two peptides/proteins tested, a 16mer with four cysteine residues and an apo-ferredoxin from S. elongatus (five cysteines in 98 residues), was as low as 1 µM. Even when we consider the molar amount of these cysteines, the stimulatory effect was achieved at much lower thiol concentrations than with compounds containing single sulfhydryl groups (5 versus 30 µM half-maximal effect). This finding suggests that the physiological substrate of Atm1p may contain multiple sulfhydryl groups in a peptidic environment.
In our study, we have determined important biochemical properties that result in the activation of the Atm1p ATPase function and thus may be crucial hints for the identification of the native substrate. The idea that Atm1p may transport a peptidic compound with multiple thiol groups is intriguing for an additional reason. Another member of the ISC export machinery, Erv1p in the intermembrane space, is known to oxidise sulfhydryl groups to form disulfide bonds (Lee et al. [Citation2000], Farrell & Thorpe [Citation2005], Mesecke et al. [Citation2005]). It is therefore tempting to speculate that the putative free thiol groups translocated by Atm1p are oxidised by Erv1p. This reaction may stabilise the compound in the intermembrane space and facilitate its functional participation in cytosolic and nuclear Fe/S protein maturation by interaction with the CIA machinery and by activation of the Aft1p/Aft2p transcription factors for the regulation of cellular iron uptake. The ATPase stimulation assay described here should now open the way to purify the physiological substrate of Atm1p, e.g., from isolated mitochondria in which Atm1p has been inactivated.
This paper was first published online on prEview on 27 January 2006.
We thank Dr. J. Nyalwidhe for performing the mass spectroscopy analysis. Our work was supported by grants of the Sonderforschungsbereiche 593 and TR1, Deutsche Forschungsgemeinschaft (Gottfried-Wilhelm Leibniz program), the European Commission (MitEURO), and Fonds der chemischen Industrie.
References
- Abele R, Tampe R. The ABCs of immunology: structure and function of TAP, the transporter associated with antigen processing. Physiology (Bethesda) 2004; 19: 216–224
- Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM. Mutation of a putative mitochondrial iron transporter gene (ABC7) in X-linked sideroblastic anemia and ataxia (XLSA/A). Hum Mol Gen 1999; 8: 743–749
- Balk J, Lill R. The cell's cookbook for iron-sulfur clusters: recipes for fool's gold?. ChemBioChem 2004; 5: 1044–1049
- Balk J, Pierik AJ, Aguilar Netz D, Mühlenhoff U, Lill R. The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron-sulphur proteins. EMBO J 2004; 23: 2105–2115
- Bekri S, Kispal G, Lange H, Fitzsimons E, Tolmie J, Lill R, Bishop DF. Human ABC7 transporter: gene structure and mutation causing X-linked sideroblastic anemia with ataxia (XLSA/A) with disruption of cytosolic iron-sulfur protein maturation. Blood 2000; 96: 3256–3264
- Burns JA, Butler JC, Moran J, Whitesides GM. Selective reduction of disulfides by Tris(2-carboxyethyl)phosphine. J Org Chem 1991; 56: 2648–2650
- Chang CN, Blobel G, Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Nat Acad Sci USA 1978; 75: 361–365
- Chen CA, Cowan JA. Characterization of the soluble domain of the ABC7 type transporter Atm1. J Biol Chem 2003; 278: 52681–52688
- Chen J, Sharma S, Quiocho FA, Davidson AL. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc Natl Acad Sci USA 2001; 98: 1525–1530
- Chloupkova M, LeBard LS, Koeller DM. MDL1 is a high copy suppressor of ATM1: evidence for a role in resistance to oxidative stress. J Mol Biol 2003; 331: 155–165
- Chloupkova M, Reaves SK, LeBard LM, Koeller DM. The mitochondrial ABC transporter Atm1p functions as a homodimer. FEBS Lett 2004; 569: 65–69
- Csere P, Lill R, Kispal G. Identification of a human mitochondrial ABC transporter, the functional orthologue of yeast Atm1p. FEBS Lett 1998; 441: 266–270
- Dean M, Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr 2001; 33: 475–479
- Farrell SR, Thorpe C. Augmenter of liver regeneration: a flavin-dependent sulfhydryl oxidase with cytochrome c reductase activity. Biochemistry 2005; 44: 1532–1541
- Floss B, Igloi GL, Cassier-Chauvat C, Mühlenhoff U. Molecular characterization and overexpression of the petF gene from Synechococcus elongatus: evidence for a second site of electrostatic interaction between ferredoxin and the PS I-D subunit. Photosynthesis Res 1997; 54: 63–71
- Gorbulev S, Abele R, Tampe R. Allosteric crosstalk between peptide-binding, transport, and ATP hydrolysis of the ABC transporter TAP. Proc Natl Acad Sci USA 2001; 98: 3732–3737
- Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Ann Rev Biochem 1993; 62: 385–427
- Higgins CF. ABC transporters: From microorganisms to man. Annu Rev Cell Biol 1992; 8: 67–113
- BI Holland, Cole, SPC, Kuchler, K, Higgins, CF. (eds.). 2003. ABC proteins: from bacteria to man. Amsterdam: Academic Press.
- Ketchum CJ, Schmidt WK, Rajendrakumar GV, Michaelis S, Maloney PC. The yeast a-factor transporter Ste6p, a member of the ABC superfamily, couples ATP hydrolysis to pheromone export. J Biol Chem 2001; 276: 29007–29011
- Kispal G, Csere P, Guiard B, Lill R. The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett 1997; 418: 346–350
- Kispal G, Csere P, Prohl C, Lill R. The mitochondrial proteins Atm1p and Nfs1p are required for biogenesis of cytosolic Fe/S proteins. EMBO J 1999; 18: 3981–3989
- Knol J, Sjollema K, Poolman B. Detergent-mediated reconstitution of membrane proteins. Biochemistry 1998; 37: 16410–16415
- Knol J, Veenhoff L, Liang WJ, Henderson PJ, Leblanc G, Poolman B. Unidirectional reconstitution into detergent-destabilized liposomes of the purified lactose transport system of Streptococcus thermophilus. J Biol Chem 1996; 271: 15358–15366
- Kuchler K, Sterne RE, Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J 1989; 8: 3973–3984
- Kushnir S, Babiychuck E, Storozhenko S, Davey MW, Papenbrock J, De Rycke R, Engler G, Stephan UW, Lange H, Kispal G, Lill R, Van Montagu M. A mutation of the mitochondrial ABC transporter Sta1 leads to dwarfism and chlorosis in the Arabidopsis mutant starik. Plant Cell 2001; 13: 89–100
- Lambert O, Levy D, Ranck JL, Leblanc G, Rigaud JL. A new ‘gel-like’ phase in dodecyl maltoside-lipid mixtures: implications in solubilization and reconstitution studies. Biophys J 1998; 74: 918–930
- Lange H, Kispal G, Kaut A, Lill R. A mitochondrial ferredoxin is essential for biogenesis of intra- and extra-mitochondrial Fe/S proteins. Proc Natl Acad Sci USA 2000; 97: 1050–1055
- Lee JE, Hofhaus G, Lisowsky T. Erv1p from Saccharomyces cerevisiae is a FAD-linked sulfhydryl oxidase. FEBS Lett 2000; 477: 62–66
- Leighton J, Schatz G. An ABC transporter in the mitochondrial inner membrane is required for normal growth of yeast. EMBO J 1995; 14: 188–195
- Li J, Saxena S, Pain D, Dancis A. Adrenodoxin reductase homolog (Arh1p) of yeast mitochondria required for iron homeostasis. J Biol Chem 2001; 276: 1503–1509
- Lill R, Cunningham K, Brundage LA, Ito K, Oliver D, Wickner W. SecA protein hydrolyses ATP and is an essential component of the protein translocation ATPase of E. coli. EMBO J 1989; 8: 961–966
- Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipids, SecY, and the leader and mature domains of precursor proteins. Cell 1990; 60: 271–280
- Lill R, Kispal G. Mitochondrial ABC transporters. Res Microbiol 2001; 152: 331–340
- Lill R, Kispal G. ABC transporters in mitochondria. ABC proteins: From bacteria to man, BI Holland, SPC Cole, K Kuchler, CF Higgins. Academic Press, Amsterdam 2003; 515–531
- Lill R, Mühlenhoff U. Iron-sulfur protein biogenesis in eukaryotes. Trends Biochem Sci 2005; 30: 133–141
- Mayer A, Driessen A, Neupert W, Lill R. Purified and protein-loaded mitochondrial outer membrane vesicles for functional analysis of preprotein transport. Methods Enzymol 1995; 260: 252–263
- McGrath JP, Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature 1989; 340: 400–404
- Mesecke N, Terziyska N, Kozany C, Baumann F, Neupert W, Hell K, Herrmann JM. A disulfide relay system in the intermembrane space of mitochondria that mediates protein import. Cell 2005; 121: 1059–1070
- Mitsuhashi N, Miki T, Senbongi H, Yokoi N, Yano H, Miyazaki M, Nakajima N, Iwanaga T, Yokoyama Y, Shibata T, Seino S. MTABC3, a novel mitochondrial ATP-binding cassette protein involved in iron homeostasis. J Biol Chem 2000; 275: 17536–17540
- Mulholland SE, Gibney BR, Rabanal F, Dutton PL. Determination of nonligand amino acids critical to [4Fe-4S]2 + /+ assembly in ferredoxin maquettes. Biochemistry 1999; 38: 10442–10448
- Ortiz DF, Ruscitti T, McCue KF, Ow DW. Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein. J Biol Chem 1995; 270: 4721–4728
- Rutherford JC, Ojeda L, Balk J, Mühlenhoff U, Lill R, Winge DR. Activation of the iron-regulon by the yeast Aft1/Aft2 transcription factors depends on mitochondrial, but not cytosolic iron-sulfur protein biogenesis. J Biol Chem 2005; 280: 10135–10140
- Schilke B, Voisine C, Beinert H, Craig E. Evidence for a conserved system for iron metabolism in the mitochondria of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 1999; 96: 10206–10211
- Senior AE, Al-Shawi MK, Urbatsch IL. The catalytic cycle of P-glycoprotein. FEBS Lett 1995; 377: 285–289
- Sipos K, Lange H, Fekete Z, Ullmann P, Lill R, Kispal G. Maturation of cytosolic iron-sulfur proteins requires glutathione. J Biol Chem 2002; 277: 26944–26949
- Sun H, Molday RS, Nathans J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem 1999; 274: 8269–8281
- Townsend A, Trowsdale J. The transporters associated with antigen presentation. Semin Cell Biol 1993; 4: 53–61
- Young L, Leonhard K, Tatsuta T, Trowsdale J, Langer T. Role of the ABC transporter Mdl1 in peptide export from mitochondria. Science 2001; 291: 2135–2138
- Zinser E, Sperka-Gottlieb CD, Fasch EV, Kohlwein SD, Paltauf F, Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol 1991; 173: 2026–2034