ABSTRACT
Introduction: Thalassemia-related complications are one of the main factors that increase morbidity and mortality in aging patients with thalassemia. This study was aimed to report the prevalence and clinical risk factors for the complications in thalassemia.
Methods: A multi-center prospective cohort study was conducted in patients with thalassemia aged ≥10 years old. Thalassemia-related complications were heart failure, pulmonary hypertension, extramedullary hematopoiesis, endocrine disorders, infections, thrombosis and leg ulcers. The clinical parameters significantly associated with the complications were analyzed by logistic regression methods.
Results: The prevalence of thalassemia-related complications was 60.5% in patients with transfusion-dependent thalassemia (TDT) and 43% in patients with non-transfusion-dependent thalassemia (NTDT). Splenectomy was statistically associated with complications in both TDT and NTDT patients (adjusted odds ratio (AOR) = 7.4, p-value = 0.0001 and AOR = 2.6, p-value = 0.001). Age ≥50 years old (AOR = 2.9, p-value = 0.04) and female gender (AOR = 0.5, p-value = 0.03) were statistically associated with the complications in patients with NTDT.
Conclusion: Nearly half of the patients in this cohort had disease-related complications. Splenectomy and advanced age were important factors for complication involvement. Early screening for the complications may reduce the morbidity and mortality in patients with thalassemia.
Introduction
Thalassemia syndrome is the most common autosomal recessive disorder worldwide. The disease is caused by a defect in globin genes synthesis resulting in the abnormality of amount and quality of the globin chain production. The clinical manifestations of thalassemia can be classified into three different phenotypes according to the severity of the disease that include: (1) transfusion-dependent thalassemia (TDT), (2) non-transfusion-dependent thalassemia (NTDT) and (3) thalassemia minor. TDT includes patients with severe forms of thalassemia, e.g. homozygous β0 thalassemia or hemoglobin E/β-thalassemia who have severe clinical symptoms and require regular red blood cell transfusions for survival. NTDT includes patients with moderate forms of thalassemia, e.g. hemoglobin H disease and some cases of hemoglobin E/β-thalassemia who have moderate anemia, moderate splenomegaly and require occasional red blood cell transfusions [Citation1,Citation2]. Thalassemia minor includes patients who have no clinical symptoms and do not require transfusions.
Recently, advances in understanding the pathophysiology of the thalassemia disease introduced novel treatments and improved the clinical outcomes in patients with thalassemia. Disease-related complications seem to be the challenging problem in aging patients with thalassemia who now survive longer than in the past [Citation3,Citation4]. There are many thalassemia-related complications which are found to increase morbidity and mortality of the disease that includes heart failure [Citation5–7], pulmonary hypertension [Citation8–11], extramedullary hematopoiesis [Citation12–15], osteoporosis [Citation16,Citation17], cholelithiasis, infections [Citation18–20], thrombosis [Citation21–23] and endocrinopathies [Citation24,Citation25]. Several studies in the past few years have shown the clinical risk factors for disease-related complications that include: advanced age, anemia, splenectomy, iron overload, severe thalassemia genotypes and iron chelation therapy [Citation9,Citation19,Citation26–30]. Some of these clinical risk factors had strong associations with the subgroup of thalassemia-related complications. Iron overload is commonly found to be a significant risk factor for cardiac complications, infection and endocrine disorders [Citation30–33]. Splenectomy is a strong risk factor for pulmonary hypertension; thrombosis and chronic leg ulcers and these complications are more prevalent in patients with NTDT than those patients with TDT [Citation9,Citation21,Citation23,Citation27]. Screening for all of the thalassemia-related complications in all patients with thalassemia may not be possible in a country with limited resources. Tailoring treatment based on the patients’ risk factors might be the best treatment modality in patients with thalassemia. A long-term follow-up study is needed in aging patients with thalassemia to determine the incidence, clinical risk factors and survival in the era of novel therapy. Therefore, a prospective cohort study in patients with thalassemia to determine the incidence, overall survival and clinical risk factors for disease-related complications in these patients is needed.
Methods
This is the initial analyses of the baseline clinical characteristics data from the thalassemia registry titled ‘Epidemiologic Study of Major Complications in Adult and Adolescent Patients with Thalassemia in Northeastern Thailand’, designated as the E-SAAN study. The E-SAAN study is a multi-center prospective cohort study in patients with thalassemia conducted at Srinagarind Hospital, Khon Kaen University and Udonthani Hospital that started in December 2012. This study was divided into two phases, phase I and phase II. This phase I study was aimed to describe the prevalence and clinical risk factors for major complications in patients with thalassemia. The phase II study will be aimed to determine the incidence of the major complications, the clinical risk factors and overall survival of patients with thalassemia enrolled in the long-term study. Eligible participants were patients aged ≥10 years old with a diagnosis of thalassemia according to genotype data. All participants were evaluated for a history of disease-related complications, the number of red blood cell transfusions, iron chelation therapy and clinical risk factors for the disease-related complications, including age, gender, genotype groups by hemoglobin typing and DNA analysis, age at first diagnosis, age at first blood transfusion, physical examination, complete blood count, liver function tests, renal function tests, nucleated red blood cell counts, serum ferritin, serum lactate dehydrogenase, hepatitis profiles, ultrasonography, echocardiography, bone mineral density (if clinically indicated), computed tomography scan (CT scan) (if clinically indicated) and magnetic resonance imaging (MRI) (if clinically indicated).
Disease-related complications are the sequelae of thalassemia syndromes including;
Heart failure that was defined as the presence or the history of signs and symptoms of heart failure according to the Framingham criteria [Citation34].
Pulmonary hypertension risk defined as a peak tricuspid regurgitation velocity >2.9 m/s by trans-thoracic echocardiography (AlokaProSound F75 sonographic system; Hitachi-Aloka Medical, Ltd, Tokyo, Japan) [Citation35].
Extramedullary hematopoiesis defined as the presence of clinical symptoms or evidence of extramedulaary hematopoiesis by ultrasonography, CT scan or MRI.
Cholelithiasis was defined as the presence of gallstones in the gall bladder by ultrasonography.
Diabetes mellitus was defined as the fasting plasma glucose ≥126 mg/dl [Citation36].
Hypothyroidism was defined by the presence of elevation of serum thyroid stimulating hormone (TSH) more than the upper limit and the level of free T4 that was lower than normal range.
Osteoporosis was defined as the presence of pathological fracture or the bone mineral density T-score <2.5 SD [Citation37].
Thrombosis was defined by the presence of clinical signs and symptoms of thrombosis or the evidence of thrombosis by venography, angiography, doppler ultrasonography, computed tomography angiogram (CT angiogram), CT scan or MRI.
Infection was defined as the history or the presence of clinical signs and symptoms of viral infection, bacterial infection, fungal infection or parasitic infections which were confirmed by the isolation of pathogens from blood, pus, stool, cerebrospinal fluid or other body fluids.
Leg ulcers were defined by the presence of chronic venous ulcers on the legs.
Genotype group classification including;
β-thalassemia/hemoglobin E defined as the presence of β-gene mutation(s) and hemoglobin E by hemoglobin type testing and or the DNA analysis.
Combined α and β-thalassemia defined as the group of patients with Hb H disease, compound heterozygous Hb H and heterozygous Hb E (EABart’s disease), compound heterozygous Hb H and homozygous Hb E (EFBart’s disease), compound heterozygous Hb H with Hb CS and heterozygous Hb E (EABart’s disease with Hb CS), compound heterozygous Hb H with Hb CS and homozygous Hb E (EFBart’s disease with Hb CS), compound heterozygous Hb H with Hb Pakséand heterozygous Hb E (EABart’s disease with Hb Paksé).
α-thalassemia defined as the group of patients with hemoglobin H (Hb H) disease with hemoglobin Constant Spring (Hb CS), Hb H disease with hemoglobin Paksé (Hb Paksé).
TDT defined as a group of patients with thalassemia major who require regular blood transfusions every 2–6 weeks for survival.
NTDT defined as a group of patients with thalassemia who do not require lifelong regular blood transfusions for survival, but may require occasional blood transfusions in certain clinical conditions, e.g. infection, pregnancy and surgery [Citation1].
All participants gave consent, and the research protocol was approved by the Ethics Review Board of the Faculty of Medicine, Khon Kaen University and Udonthani Hospital.
Statistical analyses
Baseline clinical characteristics were reported as means and standard deviations (SD) for continuous variables. Categorical variables were reported as numbers and percentages. Clinical risk factors for thalassemia-related complications were evaluated using the univariate logistic regression and multivariate logistic regression methods. All statistical analyses were performed using STATA statistical software version 10 (StataCorp, College Station, TX, U.S.A.). A p-value of less than 0.05 was considered statistically significant.
Results
A total of 380 patients (240 females and 140 males) were enrolled in the phase I cohort study. Nearly half of the patients had previous disease-related complications (180 patients, 47.4%). Baseline characteristics of the 380 patients with thalassemia are summarized in . The mean ages of patients with TDT and NTDT at the time of study enrollment were 19.5 and 28 years. Splenectomy was found in 44 patients with TDT (48.4%) and 71 patients with NTDT (24.6%). Disease-related complications were found in 55 patients with TDT (60.5%) and 124 patients with NTDT (43%). The mean serum ferritin was higher in patients with TDT than patients with NTDT (2091 vs. 1490 ng/ml). All of the patients with TDT were β-thalassemia/Hb E. The most common genotype in the patients with NTDT was also β-thalassemia/Hb E (139 patients, 48.1%) followed by EABart’s disease with Hb CS (44 patients, 15.2%), and Hb H with Hb CS (42 patients, 14.6%). The prevalence of disease-related complications in patients with thalassemia is shown in . The most common disease-related complication in this cohort was cholelithiasis (20%) followed by infection (11.5%) and pulmonary hypertension (5.8%). All of the disease-related complications were more prevalent in the patients with TDT than the patients with NTDT. shows the prevalence and number of disease-related complications in the patients with thalassemia by age group. The prevalence of disease-related complications increased with age. The peak prevalence of the disease-related complications in patients with thalassemia was in the third decade until the fourth decade. Univariate analysis of the clinical risk factors for disease-related complications in patients with TDT is shown in . Splenectomy was the only clinical risk factor that was statistically significantly associated with the disease-related complications in patients with TDT (odds ratio, OR = 6.1, 95% CI 2.4–15.8 and p-value = 0.0001). Univariate analysis of the clinical risk factors for disease-related complications in patients with NTDT is shown in . There were two clinical risk factors including: (1) splenectomy (OR = 2.5, 95% CI 1.5–4.1 and p-value = 0.00031) and (2) female gender (OR = 0.5, 95% CI 0.3–0.9 and p-value = 0.02) that were statistically significantly associated with the disease-related complications in patients with NTDT. In the multivariate analysis of clinical risk factors for disease-related complications in patients with TDT (), splenectomy remained statistically proven to be associated with the disease-related complications (adjusted odds ratio (AOR) = 7.4, 95% CI 2.4–22.1 and p-value = 0.0001). The multivariate analysis of clinical risk factors for disease-related complications in patients with NTDT is shown in . There were three significant clinical risk factors including: (1) splenectomy (AOR = 2.6, 95% CI 1.4–4.4 and p-value = 0.001), (2) female gender (AOR = 0.5, 95% CI 0.3–0.9 and p-value = 0.03) and (3) age more than 50 years old (AOR = 2.9, 95% CI 1.1–8.7 and p-value = 0.04). These factors remained the independent risk factors for the disease-related complications in those patients with NTDT.
Figure 1. Prevalence and numbers of disease-related complications in 380 patients with thalassemia by age group.
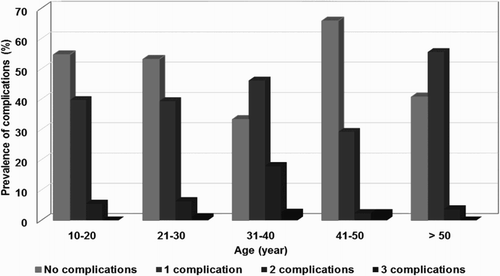
Table 1. Baseline clinical characteristics of 380 patients with thalassemia.
Table 2. Prevalence of disease-related complications between patients with TDT and NTDT.
Table 3. Univariate analysis of risk factors for disease-related complications in 91 patients with TDT.
Table 4. Univariate analysis of risk factors for disease-related complications in 289 patients with NTDT.
Table 5. Multivariate analyses of risk factors for major complications in 91 patients with TDT.
Table 6. Multivariate analyses of risk factors for major complications in 289 patients with non-transfusion-dependent thalassemia.
Discussion
Disease-related complications were common in aging patients with thalassemia. The disease-related complications were more prevalent in the patients with TDT than those patients with NTDT (60.5 vs. 43%). Splenectomy was the significant risk factor for disease-related complications in patients in both TDT and NTDT. The prevalence and the numbers of disease-related complications increased with age in patients with thalassemia. The disease-related complications were strikingly increased at the third decade until the fourth decade; this finding is similar to the previous reports of the complications in patients with thalassemia [Citation27,Citation38]. An interesting finding was that the prevalence of disease-related complications in this cohort decreased after the fourth decade of life. This result may be explained by two main reasons: (1) this cohort study recruited both transfusion-dependent and NTDT patients. There were a wide variety of the genotypes and phenotypes of the patients. The patients who were diagnosed later in life were more likely to be milder cases, and may not have presented with disease-related complications and (2) some of the patients who had several thalassemia-related complications might have died after the third decade of life.
Almost all of the disease-related complications were more prevalent in patients with TDT than the patients with NTDT. Cardiomyopathy, endocrinopathies and infections are more prevalent in the patients with TDT than those patients with NTDT. These disease-related complications are believed to be associated with transfusion-related iron loading in these patients [Citation6,Citation19,Citation31,Citation33]. Pulmonary hypertension, thrombosis and leg ulcers are the unique complications in the patients with NTDT and rarely seen in those patients with TDT. These findings are similar to the previous study by Taher et al. [Citation2]. They found that extramedullary hematopoiesis, leg ulcers, gallstones and thrombophilia were more common in the patients with NTDT than those patients with TDT.
Splenectomy is well established as a clinical risk factor for several disease-related complications in thalassemia, for example, pulmonary hypertension [Citation9,Citation11,Citation29,Citation35], thrombosis [Citation21–23] and infection [Citation18,Citation19,Citation39]. Taher et al. also found that splenectomy is an important risk factor for almost all disease-related complications in patients with thalassemia intermedia [Citation27]. This finding suggested that the splenectomized patients were at a high risk for developing disease-related complications. The splenectomized patients, therefore, should be regularly screened for thalassemia-related complications.
Advancing age is one of the important risk factors for disease-related complications in patients with thalassemia in many studies [Citation26,Citation27,Citation29,Citation35,Citation38]. In this study, advanced age was a significant risk factor for disease-related complications in the patients with NTDT. In patients with TDT, age was not found to be a significant risk factor. This finding may be explained by two main reasons: (1) the patients with TDT had shorter survival than those patients with NTDT. The peak incidence of disease-related complications in patients with TDT was in the third decade of life and (2) there were a small number of the patients with TDT in this cohort.
An interesting finding is that in females there was a decrease in the risk for disease-related complications in patients with NTDT. These results may be due to the nature of long-term follow-up in patients with NTDT. Female patients came more regularly for scheduled follow-ups than male patients.
Iron overload is one of the important risk factors for most of the major complications in patients with thalassemia. Musallam et al. demonstrated that high serum ferritin levels increased the morbidity risk in patients with thalassemia. Their study showed that patients with multiple thalassemia-related morbidities had higher mean overall serum ferritin levels compared to the patients who did not have any related morbidities [Citation40]. In this current study, however, iron overload was not found to be a significant risk factor for disease-related complications in neither patients with TDT nor NTDT.
The limitation of this study is that the gold standards for the diagnosis of the disease-related complications were not performed in all patients. This is the initial analyses of the baseline clinical characteristics of the patients in the prospective cohort study. Therefore, most of the diagnoses were based on the clinical signs and symptoms of the complications. However, the patients were and will be followed up in the study. The long-term follow-up data will show more strong evidence of the clinical risk factors and the treatment outcomes in the patients with thalassemia.
In conclusion, disease-related complications were found in almost half of the aging patients with thalassemia. Splenectomy and advanced age were the main risk factors for disease-related complications in these patients. Regular screening for the disease-related complications should be performed in patients with thalassemia, particularly in the high-risk patients to improve the treatment outcomes and reduce the morbidity and mortality in patients with thalassemia.
Acknowledgements
The authors would like to thank Emeritus Professor James A Will University of Wisconsin–Madison for a critical review and valuable suggestions on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Weatherall DJ. The definition and epidemiology of non-transfusion-dependent thalassemia. Blood Rev. 2012;26(Suppl 1):S3–S6. doi: 10.1016/S0268-960X(12)70003-6
- Taher A, Isma’eel H, Cappellini MD. Thalassemia intermedia: revisited. Blood Cells Mol Dis. 2006;37(1):12–20. doi: 10.1016/j.bcmd.2006.04.005
- Zurlo MG, De Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassaemia major. Lancet. 1989;334(8653):27–30. doi: 10.1016/S0140-6736(89)90264-X
- Borgna-Pignatti C, Cappellini MD, De Stefano P, et al. Survival and complications in thalassemia. Ann N Y Acad Sci. 2005;1054:40–47. doi: 10.1196/annals.1345.006
- Aessopos A. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood. 2001;97(11):3411–3416. doi: 10.1182/blood.V97.11.3411
- Aessopos A, Farmakis D, Deftereos S, et al. Thalassemia heart disease: a comparative evaluation of thalassemia major and thalassemia intermedia. Chest. 2005;127(5):1523–1530. doi: 10.1378/chest.127.5.1523
- Aessopos A, Kati M, Farmakis D. Heart disease in thalassemia intermedia: a review of the underlying pathophysiology. Haematologica. 2007;92(5):658–665. doi: 10.3324/haematol.10915
- Aessopos A, Farmakis D. Pulmonary hypertension in β-thalassemia. Ann N Y Acad Sci. 2005;1054:342–349. doi: 10.1196/annals.1345.041
- Atichartakarn V, Likittanasombat K, Chuncharunee S, et al. Pulmonary arterial hypertension in previously splenectomized patients with β-thalassemic disorders. Int J Hematol. 2003;78(2):139–145. doi: 10.1007/BF02983382
- Aessopos A, Stamatelos G, Skoumas V, et al. Pulmonary hypertension and right heart failure in patients with β-thalassemia intermedia. Chest. 1995;107(1):50–53. doi: 10.1378/chest.107.1.50
- Chueamuangphan N, Wongtheptien W, Nawarawong W, et al. Clinical indicators for pulmonary arterial hypertension in thalassemia. J Med Assoc Thai. 2012;95(1):16–21.
- Tantawy AAG, Adly AAM, Mahdy SAR, et al. Spinal cord compression and extramedullary hematopoiesis in young Egyptian β-thalassemia patients. Hemoglobin. 2009;33(6):448–462. doi: 10.3109/03630260903337451
- Haidar R, Mhaidli H, Taher AT. Paraspinal extramedullary hematopoiesis in patients with thalassemia intermedia. Eur Spine J. 2010;19(6):871–878. doi: 10.1007/s00586-010-1357-2
- Intragumtornchai T, Arjhansiri K, Posayachinda M, et al. Obstructive uropathy due to extramedullary haematopoiesis in β thalassaemia/haemoglobin E. Postgrad Med J. 1993;69(807):75–77. doi: 10.1136/pgmj.69.807.75
- Aessopos A, Tassiopoulos S, Farmakis D, et al. Extramedullary hematopoiesis-related pleural effusion: the case of β-thalassemia. Ann Thorac Surg. 2006;81(6):2037–2043. doi: 10.1016/j.athoracsur.2006.01.026
- Jensen CE, Tuck SM, Agnew JE, et al. High incidence of osteoporosis in thalassaemia major. J Pediatr Endocrinol Metab. 1998;11(Suppl 3):975–977.
- Toumba M, Skordis N. Osteoporosis syndrome in thalassaemia major: an overview. J Osteoporos. 2010: 1–7. doi: 10.4061/2010/537673
- Sakran W, Levin C, Kenes Y, et al. Clinical spectrum of serious bacterial infections among splenectomized patients with hemoglobinopathies in Israel: a 37-year follow-up study. Infection. 2012;40(1):35–39. doi: 10.1007/s15010-011-0178-5
- Rahav G, Volach V, Shapiro M, et al. Severe infections in thalassaemic patients: prevalence and predisposing factors. Br J Haematol. 2006;133(6):667–674. doi: 10.1111/j.1365-2141.2006.06082.x
- Vento S, Cainelli F, Cesario F. Infections and thalassaemia. Lancet Infect Dis. 2006;6(4):226–233. doi: 10.1016/S1473-3099(06)70437-6
- Taher AT, Otrock ZK, Uthman I, et al. Thalassemia and hypercoagulability. Blood Rev. 2008;22(5):283–292. doi: 10.1016/j.blre.2008.04.001
- Cappellini MD, Musallam KM, Poggiali E, et al. Hypercoagulability in non-transfusion-dependent thalassemia. Blood Rev. 2012;26(Suppl 1):S20–S23. doi: 10.1016/S0268-960X(12)70007-3
- Taher AT, Musallam KM, Karimi M, et al. Splenectomy and thrombosis: the case of thalassemia intermedia. J Thromb Haemost. 2010;8(10):2152–2158. doi: 10.1111/j.1538-7836.2010.03940.x
- De Sanctis V, Eleftheriou A, Malaventura C. Prevalence of endocrine complications and short stature in patients with thalassaemia major: a multicenter study by the thalassaemia international federation (TIF). Pediatr Endocrinol Rev. 2004;2(Suppl 2):249–255.
- Perera NJ, Lau NS, Mathews S, et al. Overview of endocrinopathies associated with β-thalassaemia major. Intern Med J. 2010;40(10):689–696. doi: 10.1111/j.1445-5994.2010.02254.x
- Taher AT, Musallam KM, El-Beshlawy A, et al. Age-related complications in treatment-naïve patients with thalassaemia intermedia. Br J Haematol. 2010;150(4):486–489.
- Taher AT, Musallam KM, Karimi M, et al. Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115(10):1886–1892. doi: 10.1182/blood-2009-09-243154
- Cappellini MD, Robbiolo L, Bottasso BM, et al. Venous thromboembolism and hypercoagulability in splenectomized patients with thalassaemia intermedia. Br J Haematol. 2000;111(2):467–473. doi: 10.1046/j.1365-2141.2000.02376.x
- Teawtrakul N, Ungprasert P, Pussadhamma B, et al. Effect of genotype on pulmonary hypertension risk in patients with thalassemia. Eur J Haematol. 2014;92(5):429–434. doi: 10.1111/ejh.12261
- Teawtrakul N, Jetsrisuparb A, Sirijerachai C, et al. Severe bacterial infections in patients with non-transfusion-dependent thalassemia: prevalence and clinical risk factors. Int J Infect Dis. 2015;39:53–56. doi: 10.1016/j.ijid.2015.09.001
- Jensen CE, Tuck SM, Old J, et al. Incidence of endocrine complications and clinical disease severity related to genotype analysis and iron overload in patients with β-thalassaemia. Eur J Haematol. 1997;59(2):76–81. doi: 10.1111/j.1600-0609.1997.tb00729.x
- Skordis N, Michaelidou M, Savva SC, et al. The impact of genotype on endocrine complications in thalassaemia major. Eur J Haematol. 2006;77(2):150–156. doi: 10.1111/j.1600-0609.2006.00681.x
- Fung EB, Harmatz PR, Lee PDK, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006;135(4):574–582. doi: 10.1111/j.1365-2141.2006.06332.x
- Ho KK, Pinsky JL, Kannel WB, et al. The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol. 1993;22(4 Suppl A):6A–13A. doi: 10.1016/0735-1097(93)90455-A
- Teawtrakul N, Pussadhamma B, Ungprasert P, et al. A risk score for predicting pulmonary hypertension in patients with non-transfusion-dependent thalassemia in northeastern Thailand: the E-SAAN score. Hematology. 2015;20(7):416–421. doi: 10.1179/1607845414Y.0000000211
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–S90.
- Kanis JA, Melton LJ, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):1137–1141. doi: 10.1002/jbmr.5650090802
- Teawtrakul N, Chansung K, Sirijerachai C, et al. The impact and disease burden of thalassemia in Thailand: a population-based study in 2010. J Med Assoc Thai. 2012;95(Suppl 7):S211–S216.
- Wang S-C, Lin K-H, Chern JPS, et al. Severe bacterial infection in transfusion-dependent patients with thalassemia major. Clin Infect Dis. 2003;37(7):984–988. doi: 10.1086/378062
- Musallam KM, Cappellini MD, Daar S, et al. Serum ferritin level and morbidity risk in transfusion-independent patients with β-thalassemia intermedia: the ORIENT study. Haematologica. 2014;99(11):e218–e221. doi: 10.3324/haematol.2013.097220

