ABSTRACT
Objective: This study aimed to investigate synergistic effects of recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand (rmhTRAIL) and heat-shock protein 90 (HSP90) inhibitor (geldanamycin derivative 17 -allylamino- 17-demethoxy -geldanamycin, 17-AAG) on the proliferation and apoptosis of multiple myeloma (MM) cells.
Methods: MTT assays evaluated inhibitory effects of rmhTRAIL and 17-AAG in different concentrations and treatment durations on the proliferation of RPMI8226 and U266 cells. The half maximal inhibitory concentration was calculated using OriginPro7.5. Synergistic effects of rmhTRAIL and 17-AAG on apoptosis of MM cells were detected using flow cytometry at 24 and 48 h post-treatment. To evaluate synergistic effects of rmhTRAIL and 17-AAG, the Q-value was calculated using King's formula.
Results: rmhTRAIL exhibited significant inhibitory effects on the proliferation of RPMI8226 cells in a dose- and time-dependent manner (>50%), whereas U266 cells were not sensitive to rmhTRAIL (<50%). 17-AAG inhibited the proliferation of RPMI8226 and U266 cells in a dose-dependent manner (>80%). Significant synergistic effects of rmhTRAIL and 17-AAG on the proliferation of RPMI8226 cells were revealed (Q-value > 1.15), whereas synergistic effects were not evident on the proliferation of U266 cells (Q-value < 1.15). rmhTRAIL and 17-AAG exhibited significant synergistic effects on apoptosis of RPMI8226 and U266 cells (Q-value > 1.15).
Conclusion: The combined application of rmhTRAIL and 17-AAG revealed favorable synergistic effects in the treatment of MM.
Highlights
Both rmhTRAIL and 17-AAG could inhibit the proliferation of RPMI8226 cells.
U266 cells were not sensitive to rmhTRAIL
rmhTRAIL and 17-AAG exhibited synergistic inhibitory effects on RPMI8226 cells
Introduction
Multiple myeloma (MM), characterized by clonal proliferation of malignant plasma cells, is a serious malignant tumor and accounts for 1% of all cancers and >10% of all hematological malignancies [Citation1]. Despite advances in treatments, including chemotherapy and novel autologous hematopoietic stem cell transplantation, the prognosis of MM remains poor [Citation2]. Therefore, developing novel therapeutic strategies for MM is urgently needed.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL), a cytokine belonging to the TNF family, has recently been regarded as a potential anti-cancer agent [Citation3]. TRAIL can induce apoptosis of tumor cells without damaging normal cells. As the application of wild-type TRAIL has limited stability and biological activity, recombinant mutant human TRAIL (rmhTRAIL) has been developed [Citation4]. It has been reported that rmhTRAIL can remarkably inhibit the proliferation of tumor cell lines from the lung, colon, and breast cancer [Citation5]. Because rmhTRAIL can selectively induce apoptosis in various types of tumor cells, including myeloma cells, it is a potential therapeutic drug for MM [Citation4]. However, as some tumor cells from the breast, colon, and bone marrow are not sensitive to rmhTRAIL, the clinical application of rmhTRAIL is significantly limited [Citation4,Citation6].
Heat-shock proteins (HSPs) are a group of highly conserved proteins produced in response to stressful conditions. HSPs are present in all cells of all organisms and play crucial roles in the occurrence and development of tumors [Citation7]. Among HSPs, HSP90 is a major therapeutic target for cancer treatment because it can inhibit the proliferation and induce apoptosis of tumor cells through various signaling pathways [Citation8]. Geldanamycin derivative 17-allylamino-17-demethoxygeldanamycin (17-AAG) is a mature inhibitor of HSP90 and exhibits 20–200 times greater binding affinity for HSP90 in tumor cells [Citation9]. 17-AAG can reduce ErbB2 levels and inhibit the proliferation of trastuzumab-resistant breast tumor cells [Citation10]. Furthermore, intratumor injection of 17-AAG inhibits growth and induces apoptosis of prostate cancer cells [Citation11]. Previous studies have also revealed that 17-AAG exhibits antitumor activity in MM, which could increase the sensitivity of tumor cells to apoptosis induction [Citation12,Citation13]. However, clinical effects of 17-AAG are still unsatisfactory because of poor water-solubility, drug resistance, and metastasis risk.
To avoid drug resistance, the combined application of drugs has become a hot topic in the treatment of tumors. In this study, the combined application of rmhTRAIL and 17-AAG was performed on MM cells. Our findings reveal synergistic effects of rmhTRAIL and 17-AAG on the proliferation and apoptosis of MM cells and provide an attractive strategy for treating MM.
Materials and methods
Cell culture
Human MM cell lines RPMI8226 and U266 were provided by Shadongb Biological Technology Co., Ltd (Beijing, China). RPMI8226 and U266 cells were cultured in RPMI 1640 medium containing 10% and 15% fetal bovine serum, respectively. These cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Logarithmic growth phase cells were used in the following assays.
Cell proliferation assays
MTT colorimetric assays were performed for detecting inhibitory effects of rmhTRAIL and 17-AAG on the proliferation of MM cells. RPMI8226 and U266 cells (5.0 × 105) were inoculated in 96-well plates (50 μl/well) and treated with different concentrations of rmhTRAIL (15.625, 62.5, 250, and 1000 ng ml−1) and 17-AAG (312.5, 625, 1250, 2500, 5000, and 10,000 ng ml−1) for 24, 48, 72, and 96 h. Four hours before the end of rmhTRAIL and 17-AAG treatment, 20 μl MTT was added to each well. Following 4 h of incubation, 100 μl acidified lysis buffer was added, and the plate was shaken until the crystals were solubilized. Finally, optical density (OD) at 570/460 nm was measured using an ultraviolet spectrophotometer (Bio-Rad, CA, USA). Each experiment was repeated in triplicate, and RPMI 1640 medium without rmhTRAIL and 17-AAG was used as the control. The inhibitory rate was calculated using the following formula: Inhibitory rate = 1 − (ODexperiment/ODcontrol) × 100%. The half maximal inhibitory concentration (IC50) was calculated using OriginPro7.5 software (OriginLab, MA, USA). To evaluate synergistic effects of rmhTRAIL and 17-AAG, the Q-value was calculated using King's formula.
Cell apoptosis assays
Synergistic effects of rmhTRAIL and 17-AAG on apoptosis of MM cells were detected using flow cytometry. RPMI8226 and U266 cells (1.0 × 106) were inoculated in 96-well plates and treated with rmhTRAIL and 17-AAG (combined application of 5.625 ng ml−1 rmhTRAIL and 625 ng ml−1 17-AAG for RPMI8226 cells and combined application of 1000 ng ml−1 rmhTRAIL and 625 ng ml−1 17-AAG for U266cells) for 24 and 48 h, respectively. These cells were subsequently stained with 50 μl PI and incubated in the dark for 30 min. Apoptosis rates of these samples were determined by the intensity of red fluorescence detected using flow cytometry.
Statistical analyses
All data were expressed as mean ± Standard Deviation. Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, USA). Comparisons between different groups were determined using t-tests. A p-value of <0.05 was considered to be statistically significant.
Results
Inhibitory effects of rmhTRAIL on the proliferation of MM cells
Inhibitory effects of rmhTRAIL on the proliferation of MM cells were evaluated by MTT using different concentrations of rmhTRAIL. Results showed that the inhibitory rate of proliferation of RPMI8226 cells significantly increased with increasing concentrations of rmhTRAIL, indicating a dose-dependent relationship (p < 0.05). When the concentration of rmhTRAIL reached 1000 ng ml−1, the inhibitory rate was 30.02% ± 2.29%, 52.14% ± 1.36%, and 77.91% ± 1.20% at 24, 48, and 72 h post-treatment, respectively. At a given concentration of rmhTRAIL, the inhibitory rate of proliferation of RPMI8226 cells was significantly increased with an increase in treatment duration, indicating a time-dependent relationship (p < 0.05) (). At 72 h post-treatment with 1000 ng ml−1 rmhTRAIL, IC50 was 34.64 ± 1.11 ng ml−1.
Table 1. Inhibitory effects of rmhTRAIL on the proliferation of RPMI8226 and U266 cells.
As observed with RPMI8226 cells, rmhTRAIL also inhibited the proliferation of U266 cells in a dose-dependent manner. The inhibitory rate of the proliferation of U266 cells was significantly higher at 72 h post-treatment, compared with that at 48 h post-treatment (p < 0.05) under same dose of rmhTRAIL. However, no significant differences were detected in the inhibitory rate of the proliferation of U266 cells between 72 and 96 h post-treatment (p > 0.05). Despite a high concentration and long treatment duration, the inhibitory effect of rmhTRAIL on the proliferation of U266 cells was relatively low (<50%; ). Therefore, U266 cells were considered to not be sensitive to rmhTRAIL.
Inhibitory effects of 17-AAG on the proliferation of MM cells
To evaluate inhibitory effects of 17-AAG on the proliferation of MM cells, MTT assays were performed in RPMI8226 and U266 cells treated with different concentrations of 17-AAG. As shown in , at 72 h, inhibitory rates of the proliferation of both RPMI8226 and U266 cells were significantly increased with increased concentrations of 17-AAG in a dose-dependent manner (p < 0.05). When the concentration of 17-AAG reached 10,000 ng ml−1, the inhibitory rate of the proliferation of RPMI8226 cells was 81.96% ± 11.53% and IC50 was 1304.48 ± 53.39 ng ml−1. Meanwhile, the inhibitory rate of the proliferation of U266 cells was 96.26% ± 1.97% and IC50 was 631.20 ± 14.86 ng ml−1.
Table 2. Inhibitory effects of 17-AAG on the proliferation of RPMI8226 and U266 cells following 72 h of treatment with different concentrations of 17-AAG.
Combination effects of rmhTRAIL and 17-AAG on the proliferation of MM cells
Combination effects of rmhTRAIL and 17-AAG on the proliferation of MM cells were evaluated using the median IC50 at 72 h post-treatment. As shown in , a relatively high inhibitory rate of the proliferation of RPMI8226 cells was exhibited by the combined application of 15.625 ng ml−1 rmhTRAIL and 625 ng ml−1 17-AAG; this was significantly greater than that exhibited by the combined application of 15.625 ng ml−1 rmhTRAIL and 312.5 ng ml−1 17-AAG (53.08% ± 1.79% vs. 47.38 ± 1.25%). Meanwhile, the Q-value showed that combination effects of rmhTRAIL and 17-AAG on the proliferation of RPMI8226 cells were greater than the effects of rmhTRAIL or 17-AAG alone (1.24 ± 0.06 and 1.77 ± 0.09, respectively).
Table 3. Combination effects of rmhTRAIL and 17-AAG on the proliferation of RPMI8226 and U266 cells at 72 h post-treatment.
Because our results indicated that U266 cells were not sensitive to rmhTRAIL, a high concentration of 1000 ng ml−1 was used. The combined application of 1000 ng ml−1 rmhTRAIL and 625 ng ml−1 17-AAG exhibited a relatively high inhibitory rate of the proliferation of U266 cells (72.09% ± 3.20%). However, when treated by 1000 ng ml−1 rmhTRAIL and 312.5 ng ml−1 17-AAG (42.68% ± 2.17%), the inhibitory rate of the proliferation of U266 cells was still less than 50%. The Q-values showed that combination effects of rmhTRAIL and 17-AAG were simply an additive effect of rmhTRAIL and 17-AAG(1.056 ± 0.07 and 1.012 ± 0.10, respectively) ().
Combination effects of rmhTRAIL and 17-AAG on the apoptosis of MM cells
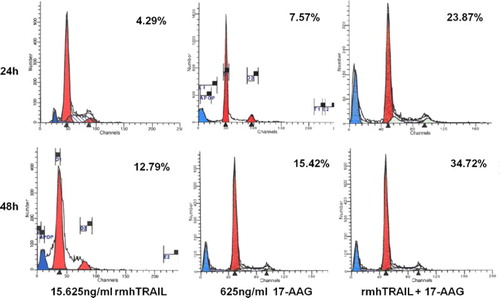
According to the proliferation results, combination effects of rmhTRAIL and 17-AAG on the apoptosis of RPMI8226 cells were evaluated using 5.625 ng ml−1 rmhTRAIL and 625 ng ml−1 17-AAG. At 24 and 48 h post-treatment, the apoptosis rates of RPMI8226 cells treated by the combined application of rmhTRAIL and 17-AAG were significantly higher than those of RPMI8226 cell treated by rmhTRAIL or 17-AAG alone (24 h: 23.87% vs. 4.29% and 7.57%, p < 0.05 and 48 h: 34.72% vs. 12.79% and 15.42%, p < 0.05). In addition, significantly higher apoptosis rates were exhibited in RPMI8226 cells at 48 h than at 24 h post-treatment (p < 0.05). Q-values of 2.07 at 24 h and 1.32 at 48 h illustrate a synergistic effect of rmhTRAIL and 17-AAG on the apoptosis of RPMI8226 cells ().
Figure 1. Combination effects of rmhTRAIL and 17-AAG on the apoptosis of RPMI8226 cells at 24 and 48 h post-treatment.
For the apoptosis of U266 cells, combination effects of 1000 ng ml−1 rmhTRAIL and 625 ng ml−1 17-AAG were evaluated. As shown in , apoptosis rates of RPMI8226 cells treated by the combined application of rmhTRAIL and 17-AAG at both 24 and 48 h post-treatment were significantly higher than those of RPMI8226 cells treated by rmhTRAIL or 17-AAG alone (24 h: 20.25% vs. 0.54% and 6.96%, p < 0.05 and 48 h: 31.87% vs. 3.81% and 19.83%, p < 0.05). Meanwhile, apoptosis rates were significantly higher in RPMI8226 cells at 48 h than those at 24 h post-treatment (p < 0.05). The Q-values of 2.71 at 24 h and 1.57 at 48 h illustrate the synergistic effect of rmhTRAIL and 17-AAG on the apoptosis of U266 cells.
Discussion
TRAIL has recently been regarded as an anti-cancer agent that can induce apoptosis of various tumors, such as liver cancer, lung cancer, ovarian cancer, acute lymphoblastic leukemia, acute myeloid leukemia, and MM [Citation14]. In this study, rmhTRAIL exhibited significant inhibitory effects on the proliferation of RPMI8226 cells in a dose- and time-dependent manner, which illustrated antitumor effects of rmhTRAIL on MM. In tumors, the expression of DcR1 and DcR2 is relatively low. TRAIL can activate caspase-8 by forming a TRAIL-DR4/DR5-FADD-procaspase-8/death-inducing signaling complex (DISC) with DR4 and DR5 in the cell membrane, thereby promoting apoptosis signal transduction [Citation15]. However, TRAIL-induced apoptosis is inhibited by the presence of DcR1 and DcR2 in normal cells. Therefore, TRAIL an induce the apoptosis of MM cells but not of normal cells.
Previous research revealed that some tumor cells, including MM cells, are resistant to TRAIL-induced apoptosis. In this study, rmhTRAIL exhibited a relatively low inhibitory rate on the proliferation of U266 cells despite a high concentration and long treatment duration. This phenomenon indicates that U266 cells are not sensitive to rmhTRAIL. To date, underlying mechanisms of TRAIL resistance in tumors remain unclear. Cell surface expression of DR4 and DR5 is higher in TRAIL-sensitive colon cancer cells than in benign tumor cells [Citation16]. Reduced cell surface expression of DR4 and DR5 significantly correlates with plasma cell leukemia cells insensitive to TRAIL [Citation17]. The overexpression of cFLIP can block apoptosis signal transduction through the inhibition of caspase-8, thereby inducing TRAIL resistance [Citation18]. Defection of Bax/Bak and overexpression of Bcl-xL/Bcl-2 are contributors to TRAIL resistance [Citation19]. Despite these findings, further research is needed for elucidating the underlying mechanism of TRAIL resistance in U266 cells.
Because drug resistance considerably hindered the clinical application of TRAIL, drug combinations have been considered as an effective strategy with broad application prospects. It has been reported that the combined application of TRAIL with etoposide [Citation20], arsenious acid [Citation21], bortezomib [Citation22], and mabthera [Citation23] all exhibited significant synergistic effects in tumor treatment. In this study, combination effects of rmhTRAIL and 17-AAG were evaluated, and significant synergistic effects on the proliferation and apoptosis of RPMI8226 cells were revealed. Consistent with the results of previous studies, this result further illustrated antitumor effects of 17-AAG. As a mature inhibitor of HSP90, 17-AAG can specifically bind to the conserved region of HSP90 and then inhibit the metabolism of ATP enzymes by inducing the folding of HSP90 effector proteins, thereby promoting apoptosis and inhibiting proliferation of tumor cells [Citation12]. It has been reported that 17-AAG can promote apoptosis of U266 cells through downregulation of anti-apoptotic proteins NF-κB, Bcl-2, and Bcl-xL [Citation24]. The inhibitory role of 17-AAG on the proliferation of MM cells is related to the down-expression of cell surface receptors IGF-1R and IL-6R [Citation25]. As detailed mechanisms of action of 17-AAG in tumors remain unclear, further research is needed.
17-AAG was also reported to recover the sensitivity of prostate cancer cells to TRAIL [Citation26]. In fact, an apparent synergistic effect of rmhTRAIL and 17-AAG on the apoptosis of TRAIL-insensitive U266 cells was also revealed in this study. In MM, 17-AAG may inhibit signal transduction of the caspase-related apoptosis pathway by downregulation of various downstream signaling molecules, such as Akt, NF-κB, Bcl-2, and Bcl-xL, leading to the recovery of TRAIL sensitivity. However, synergistic effects of rmhTRAIL and 17-AAG on the proliferation of U266 cells were not significant and reflected a simple addition effect. This phenomenon is explained by the possibility that in U266 cells, induction effects of rmhTRAIL and 17-AAG on apoptosis were stronger than inhibition effect of proliferation . Meanwhile, this difference may also correlate with IL-6 sensitivity [Citation25].
In conclusion, rmhTRAIL could significantly inhibit the proliferation of MM cells and 17-AAG could enhance this effect. Significant synergistic effects of rmhTRAIL and 17-AAG on the apoptosis of MM cells were revealed. However, the clinical application of rmhTRAIL combined with 17-AAG still needs to be studied.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;3(1):1–15.
- Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp Hematol. 2015;43(8):732–741. doi: https://doi.org/10.1016/j.exphem.2015.04.010
- Kuijlen JM, Bremer E, Mooij JJA, et al. Review: on TRAIL for malignant glioma therapy? Neuropathol Appl Neurobiol. 2010;36(3):168–182. doi: https://doi.org/10.1111/j.1365-2990.2010.01069.x
- Jian Y, Chen Y, Geng C, et al. Target and resistance-related proteins of recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand on myeloma cell lines. Biomed Rep. 2016;4(6):723–727. doi: https://doi.org/10.3892/br.2016.650
- Fang F. Antitumor activity of a novel recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand. Acta Pharmacol Sin. 2005;26(11):1373–1381. doi: https://doi.org/10.1111/j.1745-7254.2005.00206.x
- Lincz LF, Yeh TX, Spencer A. TRAIL-induced eradication of primary tumour cells from multiple myeloma patient bone marrows is not related to TRAIL receptor expression or prior chemotherapy. Leukemia. 2001;15(10):1650–1657. doi: https://doi.org/10.1038/sj.leu.2402251
- Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther. 2004;101(3):227–257. doi: https://doi.org/10.1016/j.pharmthera.2003.11.004
- Goetz MP. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol. 2003;14(8):1169–1176. doi: https://doi.org/10.1093/annonc/mdg316
- Kamal A, Thao L, Sensintaffar J, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425(6956):407–410. doi: https://doi.org/10.1038/nature01913
- Zsebik B, Citri A, Isola J, et al. Hsp90 inhibitor 17-AAG reduces ErbB2 levels and inhibits proliferation of the trastuzumab resistant breast tumor cell line JIMT-1. Immunol Lett. 2006;104(104):146–155. doi: https://doi.org/10.1016/j.imlet.2005.11.018
- Williams CR, Tabios R, Linehan WM, et al. Intratumor injection of the Hsp90 inhibitor 17AAG decreases tumor growth and induces apoptosis in a prostate cancer xenograft model. J Urol. 2007;178(1):1528–1532. doi: https://doi.org/10.1016/j.juro.2007.05.120
- Mitsiades CS. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2005;107(3):1092–1100. doi: https://doi.org/10.1182/blood-2005-03-1158
- Ma Y. Sensitization of TRAIL-resistant cells by inhibition of heat shock protein 90 with low-dose geldanamycin. Mol Cancer Ther. 2006;5(5):170–178. doi: https://doi.org/10.1158/1535-7163.MCT-05-0129
- Secchiero P, Vaccarezza M, Gonelli A. TNF-related apoptosis-inducing ligand (TRAIL): a potential candidate for combined treatment of hematological malignancies. Curr Pharm Des. 2004;10(29):3673–3681. doi: https://doi.org/10.2174/1381612043382747
- Duiker EW, Mom CH, de Jong S, et al. The clinical trail of TRAIL. Eur J Cancer. 2006;42(14):2233–2240. doi: https://doi.org/10.1016/j.ejca.2006.03.018
- Jin Z, Dicker DT, El-Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem. 2004;279(34):35829–35839. doi: https://doi.org/10.1074/jbc.M405538200
- Blum A, Chaperot L, Molens J-P, et al. Mechanisms of TRAIL-induced apoptosis in leukemic plasmacytoid dendritic cells. Exp Hematol. 2006;34(12):1655–1662. doi: https://doi.org/10.1016/j.exphem.2006.08.002
- Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008;8(1):37–46. doi: https://doi.org/10.2174/156800908783497087
- Siegelin MD, Gaiser TA. Myricetin sensitizes malignant glioma cells to TRAIL-mediated apoptosis by down-regulation of the short isoform of FLIP and bcl-2. Cancer Lett. 2009;283(2):230–238. doi: https://doi.org/10.1016/j.canlet.2009.04.002
- Wen J, Ramadevi N, Nguyen D, et al. Antileukemic drugs increase death receptor 5 levels and enhance Apo-2L-induced apoptosis of human acute leukemia cells. Blood. 2000;96(12):3900–3906.
- Liu Q, Hilsenbeck S, Gazitt Y. Arsenic trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and synergy with APO2/TRAIL. Blood. 2003;101(10):4078–4087. doi: https://doi.org/10.1182/blood-2002-10-3231
- Balsas P, López-Royuela N, Galán-Malo P, et al. Cooperation between Apo2L/TRAIL and bortezomib in multiple myeloma apoptosis. Biochem Pharmacol. 2009;77(5):804–812. doi: https://doi.org/10.1016/j.bcp.2008.11.024
- Yee L, Fanale M, Dimick K, et al. A phase IB safety and pharmacokinetic (PK) study of recombinant human Apo2L/TRAIL in combination with rituximab in patients with low-grade non-Hodgkin lymphoma. J Clin Oncol. 2007;25:8078.
- Duus J, Bahar HI, Venkataraman G, et al. Analysis of expression of heat shock protein-90 (HSP90) and the effects of HSP90 inhibitor (17-AAG) in multiple myeloma. Leuk Lymphoma. 2006;47(7):1369–1378. doi: https://doi.org/10.1080/10428190500472123
- Hallek M, Neumann C, Schäffer M, et al. Signal transduction of interleukin-6 involves tyrosine phosphorylation of multiple cytosolic proteins and activation of Src-family kinases Fyn, Hck, and Lyn in multiple myeloma cell lines. Exp Hematol. 1998;25(13):1367–1377.
- Chaurasia AR. Fertility transition in India – evidence from DLHS 2007-08. J Fam Welfare. 2013;59(1):12–32.


