ABSTRACT
Objectives: β-Thalassemia disease is caused by mutations in the β-globin gene. This is considered as one of the common genetic disorders in Syria. The aim of this study was to identify the geographical distribution of the β-thalassemia mutations in Syria.
Methods: β-Globin gene mutations were characterized in 636 affected patients and 94 unrelated carriers using the amplification refractory mutations system-polymerase chain reaction technique and DNA sequencing.
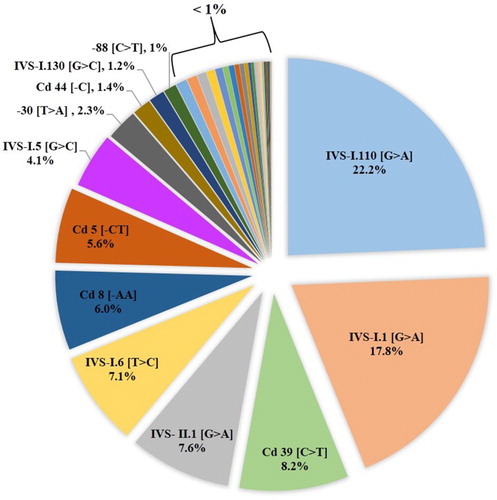
Results: The study has revealed the presence of 38 β-globin gene mutations responsible for β-thalassemia in Syria. Important differences in regional distribution were observed. IVS-I.110 [G > A] (22.2%), IVS-I.1 [G > A] (17.8%), Cd 39 [C > T] (8.2%), IVS-II.1 [G > A] (7.6%), IVS-I.6 [T > C] (7.1%), Cd 8 [−AA] (6%), Cd 5 [−CT] (5.6%) and IVS-I.5 [G > C] (4.1%) were the eight predominant mutations found in our study. The coastal region had higher relative frequencies (37.9 and 22%) than other regions. A clear drift in the distribution of the third common Cd 39 [C > T] mutation in the northeast region (34.8%) to the northwest region (2.5%) was noted, while the IVS-I.5 [G > C] mutation has the highest prevalence in north regions. The IVS-I.6 [T > C] mutation had a distinct frequency in the middle region. Ten mutations −86 [C > G], −31 [A > G], −29 [A > G], 5′UTR; +22 [G > A], CAP + 1 [A > C], Codon 5/6 [−TG], IVS-I (−3) or codon 29 [C > T], IVS-I.2 [T > A], IVS-I.128 [T > G] and IVS-II.705 [T > G] were found in Syria for the first time.
Conclusions: These data will significantly facilitate the population screening, genetic counseling and prenatal diagnosis in Syrian population.
Background
Hemoglobin disorders are defined as hereditary diseases consisting of thalassemia, a genetic disease affecting a large proportion of births [Citation1]. β-Thalassemia is a common autosomal recessive disorder. It is characterized by a reduced or absent β-globin chains in the Hb molecule. This results in the accumulation of unbound α-globin chains which precipitate in erythroid precursors in the bone marrow and in the mature erythrocytes, and therefore leading to ineffective erythropoiesis and peripheral hemolysis [Citation2,Citation3]. It results from over 350 different mutations of the β-globin gene, with the majority being single nucleotide substitutions, one or two nucleotide deletions or insertions within the β-globin gene and large deletions [Citation4,Citation5]. The disease frequents a high range in Mediterranean basin, the Middle East, Africa, Southeast Asia and the Indian subcontinent [Citation1]. Between the different ethnic populations and their geographical regions, there are a huge variation of the spectrum of β-globin gene mutations [Citation6,Citation7]. In general, each population has its own β-globin gene mutation spectrum. In Syria, because of its geographical location, hemoglobin disorders are highly prevalent. There are more than 8000 registered transfusion-dependent patients in 13 thalassemia centers in 16 provinces [Citation8]. Each year, there is a permanent increase in the number of patients.
The aims of the present study were to identify the detailed geographical distribution of the β-globin gene mutations in Syria and construct detailed frequency maps in order to perform effective genetic counseling and prenatal diagnosis in each region and extract accurate risk estimates.
Materials and methods
Patients and methods
The present study was undertaken to determine the frequencies of β-thalassemia mutations and their geographical distribution in Syria. After complete genetic counseling and recording of all hematologic values for most patients, all individuals were subjected to molecular analysis for detection of mutation. All patients participated in this study were affected with β-thalassemia, and were of Syrian origin.
A total of 1460 unrelated β-thalassemia alleles were analyzed in this study. Alleles were grouped according to their geographical origins, which included the northwest region (NWR; 80), middle region (MR; 544), coastal region (CR; 214), south region (SR; 490) and northeast region (NER; 132). Provinces covered by this survey include Damascus, Damascus Suburb, Daraa, Quneitra, Tartus, Baniyas, Latakia, Hama, Homs, Aleppo, Idlib, Deir ez-Zor and Raqqa. The Al-Hasakah, Qamishli and As-Suwayda provinces were excluded from the study because no patient was referred to the laboratory from these provinces.
All patients were informed about the project of the study and asked to write a consent for blood sampling. This study has been approved by the Institutional Review Board of the Atomic Energy Commission of Syria (AECS). All participants have provided written informed consent and that the subject’s parents provided written consent to publish the report.
DNA extraction
Genomic DNA was isolated from frozen blood samples using Mini kit QIAamp DNA Blood (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Amplification refractory mutation system-polymerase chain reaction
The eight most common β-globin gene defects in previous studies [Citation9,Citation10] were examined by the amplification refractory mutations system-polymerase chain reaction (ARMS-PCR) technique [Citation11]. ARMS primers were selected from Old [Citation12]. In cases where no mutation was identified, most common α-thal mutations in the Middle East −α4.2, −(α)20.5, −−MED and −α3.7 were screened as previously reported [Citation13].
DNA sequencing
Direct DNA sequencing (ABI 310 DNA Genetic Analyzer, Applied Biosystems, Foster City, CA, USA) for exon 1, exon 2, exon 3 and contiguous introns of the human HBB gene was done in cases where no mutation was identified or we had a doubt of ARMS assay. The BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) was used according to the manufacturer’s instructions. The sequences of the primers for amplifying the HBB gene are previously reported [Citation14].
Results
The survey resulted in the generation of detailed data on the distribution of 38 β-thalassemia mutations (26 point mutations, 2 large deletions, 3 insertions and 7 deletions) in 13 provinces comprising a total of 1460 β-thalassemia alleles ().
Table 1. β-thalassemia mutation frequency in Syrian population provinces are listed according to their relative geographical location.
Of the 38 β-globin gene mutations, the 8 most common ones among Syrian comprise nearly 78.5% of all β-thalassemia alleles. The frequency of these mutations is in decreasing order: IVS-I.110 [G > A] (22.2%), IVS-I.1 [G > A] (17.8%), Cd 39 [C > T] (8.2%), IVS-II.1 [G > A] (7.6%), IVS-I.6 [T > C] (7.1%), Cd 8 [−AA] (6%), Cd 5 [−CT] (5.6%) and IVS-I.5 [G > C] (4.1%). These mutations are then followed by four mutations with frequencies between 2.3 and 1%. Twenty six mutations were observed at a frequency of less than 1%. Ten mutations were very rare and each was found on a single allele (). All these 8 common mutations were reported in all 13 provinces included in this survey (). Ten mutations −86 [C > G], −31 [A > G], −29 [A > G], 5′UTR; +22 [G > A], CAP + 1 [A > C], Codon 5/6 [−TG], IVS-I (−3) or codon 29 [C > T], IVS-I.2 [T > A], IVS-I.128 [T > G], IVS-II.705 [T > G] were detected in Syrian population for the first time ().
Figure 2. Histogram of the relative frequencies of the eight common β-thalassemia mutations in some provinces in Syria. The eight β-globin gene mutations are as follows: IVS-I.110 [G > A], IVS-I.1 [G > A], Cd 39 [C > T], IVS-II.1 [G > A], IVS-I.6 [T > C], Cd 8 [−AA], Cd 5 [−CT] and IVS-I.5 [G > C]. The frequencies of all other mutations are summed. The number of alleles analyzed in each province is indicated in .
![Figure 2. Histogram of the relative frequencies of the eight common β-thalassemia mutations in some provinces in Syria. The eight β-globin gene mutations are as follows: IVS-I.110 [G > A], IVS-I.1 [G > A], Cd 39 [C > T], IVS-II.1 [G > A], IVS-I.6 [T > C], Cd 8 [−AA], Cd 5 [−CT] and IVS-I.5 [G > C]. The frequencies of all other mutations are summed. The number of alleles analyzed in each province is indicated in Table 1.](/cms/asset/93144a7e-7fba-4b8e-8690-0886d488db4b/yhem_a_1461291_f0002_c.jpg)
Significant differences in the frequency and distribution were observed in the five regions of Syria (NWR (includes Aleppo and Idlib), NER (includes Deir ez-Zor and Raqqa), MR (includes Hama and Homs), CR (includes Latakia, Tartus and Baniyas) and SR (includes Damascus, Damascus Suburb, Daraa and Quneitra)) ().
Figure 3. The common β-thalassemia gene mutations in different regions of Syria. NWR, Northwest region; MR, Middle region; CR, Coastal region; SR, South region; NER, Northeast region. The map of Syria was generated using the free web tool Pixel Map Generator (http://pixelmap.amcharts.com).
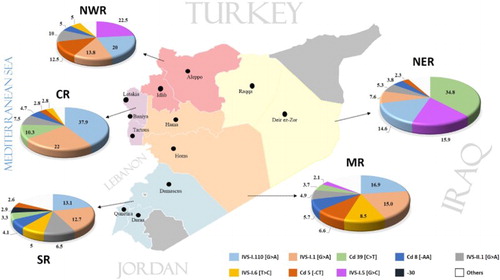
In the MR, 544 alleles were studied. IVS-I.110 [G > A] (16.9%) and IVS-I.1 [G > A] (15%) were most frequent in this region, which accounted for 31.9% of all mutations. IVS-I.6 [T > C], Cd 5 [−CT], Cd 8 [−AA], IVS-II.1 [G > A], Cd 39 [C > T], IVS-I.5 [G > C], Cd 44 [−C], −30 [T > A], IVS-I (−3) or codon 29 (C > T), IVS-I.130 [G > C], 290 bp deletion, Cds 8/9 [+G], Cd 9/10 [+T], Cd 15 [G > A], IVS-II.745 [C > A], Cd 37 [G > A], −28 [A > G], IVS-I-25 [25 bp del], −88 [C > T], −31 [G > A], −29 [A > G], 5′UTR; +22 [G > A], Codon 30 (G > C) and Cd 36/37 [−T] were the other mutations in this region. These 24 mutations increase the efficiency of the mutation panel in this region by an additional 42.1%, and the overall detection rate is about 74%. Out of 38 studied mutations, we detected 26 mutations (, ).
In the CR, 240 alleles were studied; but interestingly, the IVS-I.110 [G > A] was the dominant mutation (81 out of 214 alleles), and also, the mutation IVS-I.1 [G > A] was high frequent in about 22% of the mutations. Cd 39 [C > T], IVS-II.1 [G > A], Cd 8 [−AA], Cd 5 [−CT], IVS-I.6 [T > C], −30 [T > A], Cds 22/23/24 [7 bp del], Cd 37 [G > A], IVS-I.5 [G > C] and Cd 44 [−C] were the other mutations. Twelve β-globin gene mutations were found in this region. The mutations could not be identified in 16 of the alleles (, and ).
In the NER, a total of 132 alleles have been investigated in this region and we detected 15 mutations. Remarkably, Cd 39 [C > T] accounted for about 34.8% of the mutations in this region. IVS-I.5 [G > C], IVS-I.110 [G > A], IVS-I.1 [G > A], IVS-II.1 [G > A], Cd 8 [−AA], Cd 5 [−CT], IVS-I.130 [G > C], IVS-II-848 [C->A], Cd 9/10 [+T], Cd 15 [G > A], Cd 36/37 [−T], Codon 47 [+A], Codon 82/83 [−G] and IVS-I-25 [25 bp del] were the other mutations encountered in this region. The frequency of these mutations is about 57%. We could not determine the mutations in 11 of the alleles (, ).
Four hundred and ninety alleles were studied in the SR. IVS-I.110 [G > A] and IVS-I.1 [G > A] were the two most common mutations, contributing about 25.8% of the mutations in this region. IVS-II.1 [G > A], IVS-I.6 [T > C], Cd 8 [−AA], Cd 39 [C > T], −30 [T > A], Cd 5 [−CT], −88 [C > T], −87 [C > A], IVS-I.130 [G > C], Cd 37 [G > A], IVS-I-25 [25 bp del], Cd 15 [G > A], IVS-I.5 [G > C], Cd 44 [−C], IVS-I.116 [T > G], IVS-II.745 [C > A], −86 [C > G], −29 [A > G], CAP + 1 [A > C], Codon 5/6 [−TG], IVS-I.2 [T > A] and IVS-II.705 [T > G] were the other encountered mutations. Mutations could not be identified in 441 of alleles (, ).
In the NWRs, we had fewer individuals (80 alleles), IVS-I.5 [G > C] and IVS-I.110 [G > A] were the dominant mutations and accounted for about 42.5% of the mutations of this region. IVS-I.1 [G > A], Cd 5 [−CT], IVS-II.1 [G > A], Cd 8 [−AA], IVS-I.6 [T > C], IVS-I.130 [G > C], Cd 39 [C > T], −28 [A > G] and IVS-I.128 [T > G] were the other detected mutations. Mutation could not be identified in three alleles (, ).
The type of mutation was not identified in some affected patients by ARMS, or even β-globin gene sequencing. Since our research only covers β-thalassemia gene mutations, these unknown cases may be caused by other anemia disorders.
Discussion
Syria is located in the Middle East region and stands as a crossroads between north of Asia and Middle East. Because of its geographic location, Syria has historically been in contact with various races and ethnic groups. Syria was colonized from a number of Empires as Byzantines, Mongols and Ottomans [Citation15].
Syria is distinguished by the great diversity of its ethnicities and religions, and most Syrian populations are of Arab origin. The heterogeneity of the population is reflected in the 38 different β-globin gene mutations detected in this study.
This study presents the geographical distribution of β-globin gene mutations in Syria. Distribution of these mutations is not identical throughout all regions in Syria. There were previously published studies on the spectrum of β-globin gene mutations in the Syrian population, the authors did not indicate the distribution of the mutations to different parts of the country because the number of studied alleles was insufficient for mapping the distribution of mutations along the country [Citation9,Citation10,Citation16,Citation17].
In this study, we have tried to map the mutations in different regions of Syria. Although the number of patients from NWR was low, in most other studied regions the sample size was adequate to indicate a reliable frequency.
The order and frequency of the dominant common mutations IVS-I.110 [G > A], IVS-I.1 [G > A], Cd 39 [C > T] and IVS-II.1 [G > A] in these pooled data were very similar to those of our previous published data [Citation9].
The IVS-I.110 [G > A] mutation was the first base substitution identified in a β-thalassemia gene [Citation18]. It is a severe β-thalassemia mutation commonly encountered in Arabs. One of every five Arabs β-thalassemia alleles carries IVS-I.110 mutation [Citation19]. This mutation was the most common mutation found in our study (22.2% of all β-thalassemia alleles). The coastal region had higher relative frequency than other regions. IVS-I.110 [G > A] still remained the predominant mutation in the Syrian population. High frequency for this mutation was also reported in Cyprus and Greece [Citation20], Turkey [Citation21]. Among Arab populations, this mutation is most frequent in Lebanon and Egypt, Iraq and Jordon [Citation22–25]. IVS 1.110 is seldom observed in Gulf countries [Citation19]. Recently published reports regarding Restriction Fragment Length Polymorphism (RFLP) and haplotypes associated with IVS-I.110 mutation suggested that the Mediterranean region is the origin of this mutation, and it was maybe introduced into Arab world by population migrations [Citation19,Citation26,Citation27].
IVS-I.1 [G > A] mutation seems to have a higher overall distribution in all regions in Syria (about 17.8%). High prevalence of this mutation was observed in four regions, while it was presented in the fourth order in NER (7.6%). This mutation had low frequency among Arab populations, and the most of the alleles originated from Iraq (13%), Lebanon (13%), Egypt (12%) and Algeria (10%) [Citation19]. In Czech Republic, the prevalence rate of IVS-I.1 [G > A] is 53% [Citation28] and in Spain 30% [Citation29]. The diversity of RFLP haplotypes is associated with IVS-I.1 [G > A] in Portugal, Morocco and Algeria, and the homogeneity of the IVS-I.1 [G > A] RFLP background in Lebanon hints for western Mediterranean origin for the mutation [Citation19,Citation27,Citation30].
Cd 39 [C > T] was the third most common mutation found in our study (8.2%). It was determined as the most frequently observed β-globin gene mutation in the North-Eastern region (34.8%). It is considered as one of the first nonsense mutations to be characterized and extensively studied [Citation31,Citation32]. On the other hand, Cd 39 [C > T] is the second common β-thalassemia allele among Arabs; nearly one of every eight Arab β-thalassemia alleles carries the mutation [Citation19]. The geographic distribution of this mutation demonstrates a more widespread dispersal in the region mainly in Maghreb countries [Citation33]. In the Arabian Peninsula, Cd 39 [C > T] mutation generally occurs at lower frequencies [Citation25]. In Sardinia, Cd 39 [C > T] attains high frequency (95%) [Citation34]. In Italy, Cd 39 [C > T] is the second common β-thalassemia mutation [Citation35]. High frequency for the Cd 39 [C > T] mutation is also noticed in southern Brazil [Citation36]. The analysis of RFLP and haplotypes supports the hypothesis of the Roman origin of the Cd 39 [C > T] mutation and few millennia earlier, the mutation could have been introduced into the Maghreb region by the Roman Empire [Citation27]. Cd 39 [C > T] reached probably the eastern coasts of the Arabian Peninsula at the beginning of the sixteenth century, as a result of the Portuguese occupation in this region [Citation37–39]. High frequency of Cd 39 [C > T] mutation in the NER of Syria could be interpreted by the social relations existed between the Arab tribes in this region and the tribes in the Arabian Peninsula.
IVS-I.5 [G > C] frequency was increased from middle (2.1%) to southeast (15.9%) and southwest (22.5%) regions in Syria. In contrast to the geographical distribution of IVS-I.110 [G > A] and Cd 39 [C > T] β-globin gene mutations, that of IVS-I.5 [G > C] in the Arab world is concentrated in some Arab countries such as Oman (67%), the UAE (57%) and Qatar (36%) [Citation25]. World data indicate a Southern Asian origin of the IVS-I.5 [G > C] with high frequencies in India (64%), Iran (69%) and Pakistan (38%) [Citation27,Citation40–42].
A similar relative frequency of the IVS-II.1 [G > A] mutation was observed in the SR, CR and NWR (6.5, 7.5 and 10%, respectively). Likewise, IVS- II.1 [G > A] is reported as a Mediterranean mutation. It is frequent in Arab countries including Yemen (27%), Saudi Arabia (19%), Kuwait (26%) and Iraq (24%) [Citation25]. In Lebanon, IVS-II.1 [G > A] shows a high frequency (24%) [Citation30]. In Asia, this mutation is frequent among Kurdish patients in Azerbaijan (22%) and Iran [Citation43,Citation44]. Association of IVS-II.1 [G > A] with multiple backgrounds in Asia and Mediterranean region hints that the Eastern Mediterranean is the origin for this mutation [Citation19,Citation27].
However, the Mediterranean mutations include IVS-I.6 [T > C] and Cd 5 [−CT] have been remarkably distributed in the middle and SRs for the IVS-I.6 [T > C] mutation and middle and NERs for the Cd 5 [−CT]. These mutations were also reported in most Arab countries, with highest rates in Palestinian territories [Citation45], Lebanon, Iraq and Egypt [Citation25].
Cd 8 [−AA] mutation is considered to be a Turkish mutation [Citation46]. In our study, this mutation was found in all regions of Syria and it was ranked fifth or sixth in the order of mutations. This frameshift mutation has also been detected in Iraq, Kuwait and Morocco [Citation25].
Like any other population, Syria has rare mutations. These mutations were very rare and each of those was found on a single allele (). The −86 [C > G] mutation had also low frequency in Thailand and China [Citation47,Citation48]. Furthermore, −31 [A > G] mutation was just reported in Thai and Japanese populations [Citation49]. While −29 [A > G] mutation was common among blacks patients [Citation50]. On the other hand, 5′UTR; +22 [G > A] mutation was observed as a rare mutation in Erbil province of Iraqi Kurdistan and in a Mazandaran province in Iran [Citation51,Citation52]. The other two rare mutations codon 29 [C > T] and IVS-I.2 [T > A] were reported in a low frequency in Lebanon and Algeria, respectively [Citation53,Citation54]. Most of these rare mutations were probably brought to Syria through population migration.
In this study, we did not have any case that, no mutation was detected in both alleles, while, in some cases, just only one mutation was detected. The results of screening for the most common α-thalassemia mutations in the Middle East indicated that none of these mutations was found. These cases are maybe carriers of β-thalassemia and are not affected, due to their inaccurate hematologic values. More studies must be done for those cases to detect the second mutation which may be present in δ-globin gene.
In summary, the present study provides information on the geographical distribution of the β-thalassemia mutations in Syria. Moreover, 10 known β-globin mutations were reported in the Syrian population for the first time. The application of the knowledge in the detection of most frequent mutations in each region would help to reduce the screening cost and also facilitate early and better genetic counseling of β-thalassemia in families and in high-risk couples.
Acknowledgments
We thank Prof. Ibrahim Othman, the Director General of AECS, and the head of Molecular Biology & Biotechnology Department for the support. We also thank Dr Nyazieh Halloum, Dr Khawla Al-keba and Dr Ahlam Al-ali for their help in collecting samples of patients.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Hossam Murad http://orcid.org/0000-0003-2980-7759
References
- Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bull. World Health Organ. 2001;79(8):704–712.
- Weatherall DJ, Clegg JB. Thalassemia – a global public health problem. Nat Med. 1996;2(8):847–849. doi: https://doi.org/10.1038/nm0896-847
- Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010;5(1):11. doi: https://doi.org/10.1186/1750-1172-5-11
- Thein SL. The molecular basis of beta-thalassemia. Cold Spring Harb Perspect Med. 2013;3(5):a011700. doi: https://doi.org/10.1101/cshperspect.a011700
- Cao A, Galanello R. Beta-Thalassemia. Genet Med. 2010;12(2):61–76. doi: https://doi.org/10.1097/GIM.0b013e3181cd68ed
- Sanctis V D, Kattamis C, Canatan D, et al. β-thalassemia distribution in the old world: a historical standpoint of an ancient disease. Mediterr J Hematol Infect Dis. 2016;9(1):e2017018. doi: https://doi.org/10.4084/mjhid.2017.018
- Rezaee AR, Banoei MM, Khalili E, et al. Beta-thalassemia in Iran: new insight into the role of genetic admixture and migration. Scientific World J. 2012;2012:1–7. doi: https://doi.org/10.1100/2012/635183
- Al-Zir KN. Prevention of hemoglobinopathies in Syria. Hemoglobin. 2009;33(Suppl 1):S25–S27. doi: https://doi.org/10.3109/03630260903346460
- Jarjour RA, Murad H, Moasses F, et al. Molecular update of beta-thalassemia mutations in the Syrian population: identification of rare beta-thalassemia mutations. Hemoglobin. 2014;38(4):272–276. doi: https://doi.org/10.3109/03630269.2014.912661
- Murad H, Moassas F, Jarjour R, et al. Prenatal molecular diagnosis of β-thalassemia and sickle cell anemia in the Syrian population. Hemoglobin. 2014;38(6):390–393. doi: https://doi.org/10.3109/03630269.2014.978455
- Newton CR, Graham A, Heptinstall LE, et al. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989;17(7):2503–2516. doi: https://doi.org/10.1093/nar/17.7.2503
- Old JM. Screening and genetic diagnosis of haemoglobin disorders. Blood Rev. 2003;17(1):43–53. doi: https://doi.org/10.1016/S0268-960X(02)00061-9
- Liu YT, Old JM, Miles K, et al. Rapid detection of alpha-thalassaemia deletions and alpha-globin gene triplication by multiplex polymerase chain reactions. Br J Haematol. 2000;108(2):295–299. doi: https://doi.org/10.1046/j.1365-2141.2000.01870.x
- Sirdah MM, Sievertsen J, Al-Yazji MS, et al. The spectrum of β-thalassemia mutations in Gaza Strip, Palestine. Blood Cells Mol Dis. 2013;50(4):247–251. doi: https://doi.org/10.1016/j.bcmd.2012.12.004
- Beshara A. The origins of Syrian nationhood: histories, pioneers and identity. London: Routledge, Taylor & Francis Group; 2011, p. 1–384.
- El-Hazmi MA, Warsy AS, Al-Swailem AR. The frequency of 14 β-thalassemia mutations in the Arab populations. Hemoglobin. 1995;19(6):353–360. doi: https://doi.org/10.3109/03630269509005827
- Kyriacou K, Al Quobaili F, Pavlou E, et al. Molecular characterization of β-thalassemia in Syria. Hemoglobin. 2000;24(1):1–13. doi: https://doi.org/10.3109/03630260009002268
- Spritz RA, Jagadeeswaran P, Choudary PV, et al. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci. 1981;78:2455–2459. doi: https://doi.org/10.1073/pnas.78.4.2455
- Tadmouri GO, Gulen RI. Deniz: the electronic database for beta-thalassemia mutations in the Arab world. Saudi Med J. 2003 Nov;24(11):1192–1198.
- Georgiou I, Makis A, Chaidos A, et al. Distribution and frequency of beta-thalassemia mutations in northwestern and central Greece. Eur J Haematol. 2003;70(2):75–78. doi: https://doi.org/10.1034/j.1600-0609.2003.00017.x
- Tadmouri GO, Tuzmen S, Ozcelik H, et al. Molecular and population genetic analyses of β-thalassemia in Turkey. Am J Hematol. 1998;57(3):215–220. doi: https://doi.org/10.1002/(SICI)1096-8652(199803)57:3<215::AID-AJH6>3.0.CO;2-Y
- Soliman OE, Yahia S, Shouma A, et al. Reverse hybridization Strip Assay detection of β-thalassemia mutations in northeast Egypt. Hematology. 2010;15(3):182–186. doi: https://doi.org/10.1179/102453310X12583347010214
- Zahed L, Demont J, Bouhass R, et al. Origin and history of the IVS-I-110 and codon 39 [beta]-thalassemia mutations in the Lebanese population. Hum Biol. 2002;74(6):837–847. doi: https://doi.org/10.1353/hub.2003.0013
- Al-Allawi NA, Al-Mousawi BM, Badi AI, et al. The spectrum of beta-thalassemia mutations in Baghdad, Central Iraq. Hemoglobin. 2013;37(5):444–453. doi: https://doi.org/10.3109/03630269.2013.810641
- Hamamy HA, Al-Allawi NA. Epidemiological profile of common haemoglobinopathies in Arab countries. J Community Genet. 2012;4(2):147–167. doi: https://doi.org/10.1007/s12687-012-0127-8
- Tadmouri GO, Garguier N, Demont J, et al. History and origin of beta-thalassemia in Turkey: sequence haplotype diversity of beta-globin genes. Hum Biol. 2001;73(5):661–674. doi: https://doi.org/10.1353/hub.2001.0075
- Zahed L. The spectrum of β-thalassemia mutations in the Arab populations. J Biomed Biotechnol. 2001;1(3):129–132. doi: https://doi.org/10.1155/S1110724301000298
- Indrak K, Brabec V, Indrakova J, et al. Molecular characterization of β-thalassemia in Czechoslovakia. Hum Genet. 1992;88(4):399–404. doi: https://doi.org/10.1007/BF00215673
- Villegas A, Ropero P, Gonzalez FA, et al. The thalassemia syndromes: molecular characterization in the Spanish population. Hemoglobin. 2001;25(3):273–283. doi: https://doi.org/10.1081/HEM-100105220
- Makhoul NJ, Wells RS, Kaspar H, et al. Genetic heterogeneity of beta thalassemia in Lebanon reflects historic and recent population migration. Ann Hum Genet. 2005;69(Pt 1):55–66. doi: https://doi.org/10.1046/j.1529-8817.2004.00138.x
- Humphries RK, Ley TJ, Anagnou NP, et al. B0-39 thalassemia gene: a premature termination codon causes b-mRNA deficiency without affecting cytoplasmic b-mRNA stability. Blood. 1984;64:23–32.
- Takeshita K, Forget BG, Scarpa A, et al. Intranuclear defect in b-globin mRNA accumulation due to a premature translation termination codon. Blood. 1984;64:13–22.
- Lemsaddek W, Picanco I, Seuanes F, et al. The β-thalassemia mutation/haplotype distribution in the Moroccan population. Hemoglobin. 2004;28(1):25–37. doi: https://doi.org/10.1081/HEM-120028884
- Rosatelli C, Leoni GB, Tuveri T, et al. Beta thalassaemia mutations in Sardinians: implications for prenatal diagnosis. J Med Genet. 1987;24(2):97–100. doi: https://doi.org/10.1136/jmg.24.2.97
- Rigoli L, Meo A, Miceli MR, et al. Molecular analysis of beta-thalassaemia patients in a high incidence area of southern Italy. Clin Lab Haematol. 2001;23(6):373–378. doi: https://doi.org/10.1046/j.1365-2257.2001.00367.x
- Reichert VC, de Castro SM, Wagner SC, et al. Identification of β thalassemia mutations in south Brazilians. Ann Hematol. 2008;87(5):381–384. doi: https://doi.org/10.1007/s00277-007-0418-z
- Gomes MP, da Costa MG, Braga LB, et al. Beta-thalassemia mutations in the Portuguese population. Hum Genet. 1988;78(1):13–15. doi: https://doi.org/10.1007/BF00291226
- Jassim N, Merghoub T, Pascaud O, et al. Molecular basis of beta-thalassemia in Bahrain: an epicenter for a Middle East specific mutation. Ann NY Acad Sci. 1998;850:407–409. doi: https://doi.org/10.1111/j.1749-6632.1998.tb10505.x
- Al-Ali AK, Al-Ateeq S, Imamwerdi BW, et al. Molecular bases of β-thalassemia in the Eastern Province of Saudi Arabia. J Biomed Biotechnol. 2005;2005(4):322–325. doi: https://doi.org/10.1155/JBB.2005.322
- Agarwal S, Pradhan M, Gupta UR, et al. Geographic and ethnic distribution of β-thalassemia mutations in Uttar Pradesh, India. Hemoglobin. 2000;24(2):89–97. doi: https://doi.org/10.3109/03630260009003427
- Karimi M, Haghpanah S, Farhadi A, et al. Genotype–phenotype relationship of patients with β-thalassemia taking hydroxyurea: a 13-year experience in Iran. Int J Hematol. 2012;95(1):51–56. doi: https://doi.org/10.1007/s12185-011-0985-6
- Khan SN, Riazuddin S. Molecular characterization of β-thalassemia in Pakistan. Hemoglobin. 1998;22(4):333–345. doi: https://doi.org/10.3109/03630269809071528
- Kuliev AM, Rasulov IM, Dadasheva T, et al. Thalassaemia in Azerbaijan. J Med Genet. 1994;31(3):209–212. doi: https://doi.org/10.1136/jmg.31.3.209
- Najmabadi H, Karimi-Nejad R, Sahebjam S, et al. The β-thalassemia mutation spectrum in the Iranian population. Hemoglobin. 2001;25(3):285–296. doi: https://doi.org/10.1081/HEM-100105221
- El-Latif MA, Filon D, Rund D, et al. The β+-IVS-I-6 (T-->C) mutation accounts for half of the thalassemia chromosomes in the Palestinian populations of the mountain regions. Hemoglobin. 2002;26(1):33–40. doi: https://doi.org/10.1081/HEM-120002938
- Orkin SH, Goff SC. Nonsense and frameshift mutations in beta 0-thalassemia detected in cloned beta-globin genes. J Biol Chem. 1981;256(19):9782–9784.
- Yamsri S, Singha K, Prajantasen T, et al. A large cohort of β(+)-thalassemia in Thailand: molecular, hematological and diagnostic considerations. Blood Cells Mol Dis. 2015;54(2):164–169. doi: https://doi.org/10.1016/j.bcmd.2014.11.008
- He S, Qin Q, Yi S, et al. First description of a β-thalassemia mutation, −86 (C > G) (HBB: c.−136C > G), in a Chinese family. Hemoglobin. 2015;39(6):448–450. doi: https://doi.org/10.3109/03630269.2015.1070734
- Lerttham W, Fucharoen G, Yamsri S, et al. Two independent genetic origins of β+- thalassemia Due to -31 A to G mutation in Thai and Japanese populations. Int J Hum Genet. 2015;15(4):191–198. doi: https://doi.org/10.1080/09723757.2015.11886267
- Antonarakis SE, Irkin SH, Cheng TC, et al. Beta-thalassemia in American blacks: novel mutations in the ‘TATA’ box and an acceptor splice site. Proc Natl Acad Sci USA. 1984;81(4):1154–1158. doi: https://doi.org/10.1073/pnas.81.4.1154
- Shamoon RP, Al-Allawi NA, Cappellini MD, et al. Molecular basis of β-thalassemia intermedia in Erbil Province of Iraqi Kurdistan. Hemoglobin. 2015;39(3):178–183. doi: https://doi.org/10.3109/03630269.2015.1032415
- Mahdavi MR, Karami H, Akbari MT, et al. Detection of rare beta globin gene mutation [+22 5UTR(G>A)] in an infant, despite prenatal screening. Case Rep Hematol. 2013;2013:1–3. doi: https://doi.org/10.1155/2013/906292
- Chehab FF, Der Kaloustian V, Khouri FP, et al. The molecular basis of beta-thalassemia in Lebanon: application to prenatal diagnosis. Blood. 1987;69(4):1141–1145.
- Perrin P, Bouhassa R, Mselli L, et al. Diversity of sequence haplotypes associated with β-thalassaemia mutations in Algeria: implications for their origin. Gene. 1998;213(1-2):169–177. doi: https://doi.org/10.1016/S0378-1119(98)00200-5