Abstract
Avian require comfortable temperatures for optimal development and heat stress is a high concern in warm weather countries. We aimed to assess the dynamics of immunoendocrine and biochemical variables responses of birds exposed to a heat stressor applied during daylight hours, during the chronic stress and the recovery periods. We hypothesize that variables involved in the birds response will be differentially and gradually modified during those periods. Female quail (n = 210) were housed in six rearing boxes. At 29 days of age, the temperature in three boxes was increased from 24 to 34 °C during the light period throughout the nine days (Stress Treatment). The other three boxes remained at 24 °C and were used as controls. The subsequent 12 days were considered as recovery period. Different sets of 12 birds/treatment were blood-sampled at 29 (basal), 32, 35, 38 (stress), 41, 44, 47, and 50 (recovery) days of age, respectively. Immunoendocrine (corticosterone, lymphoproliferation, heterophil/lymphocyte ratio (H/L), and antibody response) and biochemical (glucose, total proteins, globulins, and albumin) variables were assessed. During stress, progressive corticosterone and H/L increments, and antibody titers and lymphoproliferation decreases were detected. No clear pattern of changes was found in biochemical variables. During recovery, while corticosterone and lymphoproliferation had recovered three days after the stressor ended, H/L and antibody responses required respectively nine and 12 days to recover to their basal levels, respectively. Findings suggest that immunity is already threatened when heat stress is sustained for three or more days. However, the system appears resilient, needing six to 12 days to recover to their basal responses.
Introduction
Natural and commercial rearing environments are a source of challenges that may result in stressors such as extreme temperatures, impaired resources availability, or even diseases (Lara & Rostagno, Citation2013; McNamara, Citation2005; Roche & Guégan, Citation2011; Romero, Citation2004). Specifically, environmental temperature above the thermoneutral range represents an important stressor mainly in tropical and subtropical regions, leading to physiological responses defined as heat stress (Belhadj Slimen, Najar, Ghram, & Abdrrabba, Citation2016; Lara & Rostagno, Citation2013; Zell, Citation2004). Avian species are endothermic animals that require comfortable temperatures for their optimal development, considering that the absence of sweat glands together with many layers of feathers make birds particularly prone to heat stress and its consequences (Dai et al., Citation2011; Mashaly et al., Citation2004, Scanes, Citation2014). During rearing of birds for commercial purposes, birds are usually kept under high densities, of about 11 birds per square meter and hence heat stress can be considered almost an unavoidable condition of high relevance worldwide (Lara & Rostagno, Citation2013).
When heat stress and immune challenges occur together, birds can reach a state associated with the depression of an optimal immune response: damaged intestinal mucosa (Quinteiro-Filho et al., Citation2010), lower total white blood cell (Mashaly et al., Citation2004), diminished foreign antigen recognition, minor phagocytosis (Sandhu, Mirza, Afzal, & Mukhtar, Citation2012), and antibody production (Gasparino et al., Citation2013; Sahin et al., Citation2006). These lead to an increased exposure to antigens and bacteria, thus producing an imbalance between pro/anti-inflammatory molecules in the whole organism (Lara & Rostagno, Citation2013; Quinteiro-Filho et al., Citation2010; Song et al., Citation2013).
Much research has been conducted to clarify the effects of a wide range of stressors on biochemical and immunoendocrine responses in avian species. Nevertheless, these studies evaluated stress consequences considering mainly the pre and post-stress responses (Azad, Kikusato, Maekawa, Shirakawa, & Toyomizu, Citation2010; Mashaly et al., Citation2004; Nazar & Marin, Citation2011; Xie et al., Citation2015). Recovery has also been studied as a single point once the stress protocol is finished (Shini, Shini, & Huff, Citation2009). Consequently, insights into the dynamics of modulation of the response both during and after heat stress in subtropical avian species are still needed.
This study aimed to assess the dynamics of immunoendocrine and biochemical variables responses of birds exposed to a chronic heat stressor applied during daylight hours along nine days, during both stress exposure and the following 12 days of recovery. We hypothesized that variables involved in the birds´ immunoendocrine and biochemical response would be differentially and gradually modified during those periods. We expected this information would allow us to answer the following main questions: i) Does heat stress differentially affect the dynamics of the immunoendocrine and biochemical variables responses? and if so, ii) What is the particular dynamic of the impairment and the recovery of those variables? The information gathered can bring light to determine whether some variables are particularly susceptible to chronic heat stress and to help identify a particular point/s along chronic heat stress dynamics where vulnerability to other challenges could be increased. Results could have production as well as welfare implications if we consider that heat stress consequences can affect almost all basic principles for animal welfare (i.e. freedoms from discomfort, disease, distress and to express normal behaviors; Farm Animal Welfare Council, 2012 (http://webarchive.nationalarchives.gov.uk/20121010012427/ http://www.fawc.org.uk/freedoms.htm)).
Methods
Animals and husbandry
Female Japanese quail were only used in the present study. These birds are recognized as a useful laboratory model for avian studies (Baumgartner, Citation1994; Huss, Poynter, & Lansford, Citation2008). Husbandry was performed according to standard laboratory procedures (Guzmán, Pellegrini, Kembro, & Marin, Citation2013; Nazar, Barrios, Kaiser, Marin, & Correa, Citation2015). Four hundred and fifty newly hatched quail chicks were randomly housed in six (75 birds each) rearing pen boxes measuring 90 × 90 × 90 cm (length × width × height). Each box had one 90 cm feeder and 16 automatic nipple drinkers. A wire-mesh floor (1 cm grid) was raised 5 cm to allow the passage of excreta and a lid prevented the birds from escaping. Brooding temperature was 37 °C during the first week of life, with a decline of 3 °C per week until 25 °C was reached. For the first four weeks of life, a quail starter diet (24% CP; 2900 Kcal ME/kg) and water were provided ad libitum. At 22 days of age (DA), the birds were sexed by plumage coloration. Females were recognized by the light brown feathers with speckled black spots on their throat and chest, while males were identified by their plain golden-brown feathers on the chest. Two hundred and ten females were wing-banded for later individual identification and were randomly re-housed in groups of 35 in the same described pen boxes. Coincident with re-housing, birds were switched to a laying diet (21% protein, 2,750 kcal ME/kg) and water availability was continued. Quail were subjected to a daily cycle of 14 h light (300–320 lx) and 10 h dark during the study. Lights were turned on at 6:00am and turned off at 9:00pm.
Experimental design
A bi-factorial design combining the effect of Stress Treatment and Time of Sampling was used. As mentioned, at 22 DA female quail were assigned to one of the six rearing boxes. From 30–38 DA, all birds housed in half of the boxes were submitted to a nine days chronic heat stress protocol (Stress Treatment, ) and temperature in the boxes was daily increased from 24 to 34 °C starting at 9:00am. Temperature was increased during a 30 min period (about +0.3 °C per minute) and was maintained at 34 °C until 5:00pm. After 5:00pm the temperature was gradually lowered returning to 24 °C by 8:00pm. Females housed in the other boxes remained with unaltered temperature (at a comfort temperature, 24 °C during the whole study) and were used as controls. At 39 DA the comfort temperature was reestablished in the boxes submitted to the Stress Treatment and until 50 DA the recovery from the heat stress was evaluated. Thus, the stress period was applied during daylight hours along nine days and the subsequent 12 days were considered the recovery period (under continued 24 °C comfort temperature).
Figure 1. Experiment schematic time line expressed in days of age (DA). To analyze immunoendocrine system and biochemical stress induced modulations, eight sampling days were defined (#1–8). Female Japanese quail were submitted to a chronic heat stress protocol during nine days (DA 30–38) and was followed by a recovery period of 12 days (DA 39–50). Heat stress protocol: temperature in half of the boxes was daily increased from 24 to 34 °C, during the photoperiod light phase. Recovery period: temperature in stressed boxes was established at a continuous comfort temperature (24 °C). On each sampling day, 12 stressed and 12 non-stressed quail were sampled. Each bird was sampled only once.
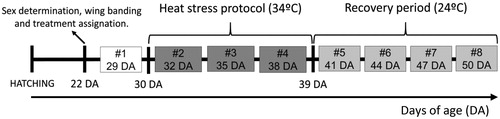
To assess immunoendocrine and biochemical variables (see below) during thermal heat stress and recovery periods, eight sampling days were defined:
Sampling day 1: basal, the day before heat stress started (29 DA);
Sampling days 2–4: during stress period (32, 35, and 38 DA);
Sampling days 5–8: during recovery period (41, 44, 47, and 50 DA).
Thus, Time of Sampling comprised eight levels (days 1–8). Every sampling day, 24 females were evaluated (four randomly assigned individuals from each of the six boxes (12 stressed and 12 control quail)). Thus, each bird was sampled only once. The use of colored wing bands allowed fast recognition of individuals and blood samples were withdrawn in less than 80 s, thus minimizing potential alterations in response to handling (Nazar et al., Citation2015; Romero & Reed, Citation2005).
Sampling
Blood samples were withdrawn (0.5–1 ml) from the brachial vein using 1 ml syringes previously treated with ethylenediaminetetraacetic acid (EDTA) to avoid blood coagulation; anesthesia was not used in line with guidance in the National Centre for the Replacement Refinement and Reduction of Animals in Research (www.nc3rs.org.uk/general-principles). Blood samples were immediately centrifuged at 2500 g for 15 min at 4 °C and the plasma was stored at −20 °C until further analysis. Blood withdrawal was performed at the same time (10:00am) every sampling day and the birds order was alternated between control and stressed groups. After sampling, each bird was immediately returned to its home box.
Hormone analysis
Plasma corticosterone concentrations were determined by enzyme immunoassay (EIA) using polyclonal antibodies, standards, and their corresponding horseradish peroxidase conjugates (anti-corticosterone CJM006, Department of Population Health and Reproduction, C. Munro, UC Davis, CA). The polyclonal CJM006 antibody cross-reacts with: corticosterone 100%, desoxycorticosterone 14.25%, progesterone 2.65%, tetrahydrocorticosterone 0.90%, testosterone 0.64%, cortisol 0.23%, prednisolone 0.07%, 11-desoxycortisol 0.03%, prednisone <0.01%, cortisone <0.01%, and oestradiol <0.01%. The assays were performed according to the general technique described by Munro & Lasley (Citation1988). Briefly, plasma samples were assayed in duplicates using flat-bottom microtiter plates. These were first coated with 50 µl of the antibody stock diluted in coating buffer (50 mM bicarbonate buffer, pH 9.6, 1: 15000) and were incubated overnight at 4 °C. Later, plates were washed to remove any unbound antibody and immediately after, 50 µl of samples, standards, and controls were added in duplicates, followed by 50 µl of horseradish peroxidase conjugate diluted in EIA buffer (1:70,000). Plates were then incubated at room temperature (21 °C) for 2 h in an orbital shaker. Following incubation, plates were washed and blotted dry, and 100 µl of substrate solution (50 mM citrate, 1.6 mM hydrogen peroxide, and 0.4 mM 2,20-azino-di-(3-ethylbenzthiazoline sulfonic acid) diammonium salt, pH 4.0) were added to each well. Absorbance was measured at 405 nm using a microplate reader (Thermo Electron Corporation, Waltham, MA). For all hormone determinations, the intra- and inter-assay coefficients of variation were <12 and 5.1%, respectively. Assay sensitivity was 0.08 ng/ml.
Immunological assays
Three immune-related variables were evaluated in order to assess the response of the immune system: (i) Cell mediated immunity was determined via percentage lymphoproliferative response to phytohemagglutinin-p (Inflammatory response to PHA-P; Moore & Siopes, Citation2005; Stadecker & Lukic, Citation1977); (ii) Heterophil/lymphocyte (H/L) ratio was determined as a commonly used hematological indicator of chronic stress (Jones, Citation1989; Nazar et al., Citation2015); and (iii) Humoral immunity was determined via the primary antibody response against sheep red blood cells (SRBC) using a microagglutination assay (Adriaansen-Tennekes, Decuypere, Parmentier, & Savelkoul, Citation2009; Sever, Citation1962).
Inflammatory response
A day before each defined sampling day, the wing-web of each bird was measured using a digital caliper and then a 0.05 ml intradermal injection of a solution of PHA-P (Sigma Chemical, St Louis, MO) in phosphate saline buffer (PBS, pH = 7, 1 mg/ml) was given to induce a lymphoproliferative response to PHA-P. Twenty four hours later (sampling day of each group) the brachial vein from the opposite wing was punctured to obtain blood samples for the other variable determinations (see below). After blood withdrawal, the corresponding wing-web was again measured to determine percentage of inflammation as follows:
Percentage of inflammation = (Wing-web measurement (mm) 24 h prior to sampling day/Wing-web measurement (mm) on the sampling day) × 100.
H/L ratio
A blood smear was made on a slide for each sample. Smears were stained with May Grünwald Giemsa and were analyzed using a light microscope at 100 × immersion objective for a total 1000 × magnification (Fair, Hansen, & Ricklefs, Citation1999). One hundred leukocytes were counted and differentiated per blood smear. H/L ratio was calculated using the following formulae:
H/L ratio = (Number of heterophils)/(Number of lymphocytes).
Antibody response against SRBC
One week before sampling day each bird was intraperitoneally injected with 300 µl of a 10% SRBC (HEMO-G, Rafaela, Santa Fe, Argentina) suspension in order to induce a humoral immune response. Antibody titers were measured in plasma samples one week after the intraperitoneal administration of the SRBC suspension. The antibody response was assessed with a microagglutination assay, as follows: 30 µl of plasma (complement inactivated by heating at 56 °C for 30 min) was serially diluted in 30 µl of phosphate buffered saline (PBS; 1:2, 1:4, 1:8, up to 1:512). Next, 30 µl of a 2% suspension of SRBC in PBS was added to each well. Microplates were incubated at 40 °C for 45 min and hemagglutination of the plasma samples was compared to the blank (PBS only) and negative control (wells with no SRCB suspension). Antibody titers were reported as the Log2 of the highest dilution yielding agglutination.
Biochemical variables
Glucose, total proteins, total globulins, and albumin concentrations for each bird were measured in the plasma obtained after centrifugation (Nazar, Magnoli, Dalcero, & Marin, Citation2012; Oğuz, Keçeci, Birdane, Onder, & Kurtoğlu, Citation2000). Concentrations were determined with a clinical chemistry analyzer (Wiener Lab, Commercial kit: 2000, Calorimetric method for determination of total protein, albumin, and plasma transaminase) according to the manufacturer’s recommended procedure. Sensitivity for each target molecule was: Glucose = 0.54 mg/dl; Total proteins and total globulins = 0.01 g/dl; Albumin = 0.01 g/dl. Intra- and inter-assay variability for each target molecule were 1.2 and 1.77% (Glucose), 0.48 and 1% (Total proteins and total globulins), and 2.27 and 3.78% (Albumin). It is important to consider that based on the time chosen for sampling, results represented the regulatory metabolism of energetic and protein components and not the basal metabolism after fasting.
Ethical statement
Animal care was provided in adherence with Institutional animal Care and Use Committee guidelines. The experiment was approved by the ethical committee at Instituto de Investigaciones Biológicas y Tecnológicas in compliance with the legislation regarding the use of animals for experimental and other scientific purposes (Acta 27, 09/04/2015). Regarding the chronic heat stress protocol, temperatures between 34 and 41 °C have been shown to affect avian physiology with a wide spectrum of responses (Gasparino et al., Citation2013; Sahin et al., Citation2006; Sandhu et al., Citation2012). We chose 34 °C to induce a chronic stress state while minimizing potential compromise of later welfare and survival. It is important to note that no mortalities were registered during or after stress exposure. At the end of the study the birds were euthanized by cervical dislocation.
Statistical analysis
Analyses were performed using General Linear Mixed Model, which included chronic Stress Treatment (stressed vs. control birds) and Time of Sampling (days 1–8) as fixed effects and the birds boxes as a random effect. The interactions between fixed factors were also evaluated. Post-hoc treatment group comparisons were conducted using Tukey tests. A p value of <.05 was considered to represent significant differences. All statistical analyses were performed through an ‘R’ (The R Foundation for Statistical Computing) user-friendly interface implemented in InfoStat (Di Rienzo et al., Citation2016).
Results
Corticosterone
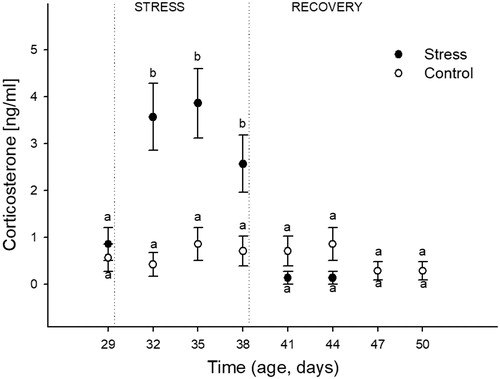
The effects of heat stress over time on plasma corticosterone concentration are shown in . Analysis showed a significant effect of Stress (F(1,176) = 17.43; p = 0.01), Time (F(7,176) = 5.50; p < 0.0001), and their interaction (F(7,176) = 5.72; p < 0.0001). Post-hoc analyses showed that control birds maintained basal values during the studied time period. Birds under the heat stress protocol showed greater corticosterone concentrations at the three sampling points and the values were similar to each other. Three days post the heat stress protocol (41 DA), the corticosterone concentrations showed similar values to the birds sampled before stress started (basal) and to their respective control counterparts. These measures remained similar until the end of the study.
Figure 2. Effects of chronic heat stress (during nineconsecutive days) and the recovery during the following 12 days on the plasma concentration of corticosterone in female Japanese quail. Points represent mean value and lines represent standard error of the mean. The age of sampling is shown in the time axis (age, days). Day 29 corresponds to the sample day before heat stress (34 °C) started. Days 32, 35, and 38 correspond to sampling during heat stress exposure. Days 41, 44, 47, and 50 correspond to sampling after the chronic heat stress protocol was finished. a, b: different letters represent significant differences between groups (p < 0.05; Tukey post-hoc test). A total of 24 birds (12 stressed and 12 control) were sampled on each of the eight sampling days (192 birds total). Each bird was sampled only once.
Inflammatory response
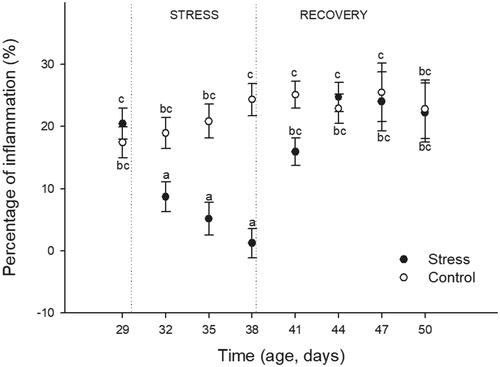
The effects of heat stress and time on the percentage of inflammation are shown in . The analysis revealed a significant effect of Stress (F(1,176) = 6.59; p = 0.05), Time (F(7,176) = 5.01; p < 0.0001), and their interaction (F(7,176) = 5.71; p < 0.0001). Post-hoc analyses showed that control birds maintained their inflammation responses during the time period evaluated. However, birds exposed to heat stress showed lower percentages of inflammation during the stress protocol. The lower responses were observed on the day 9 of heat stress exposure (38 DA). Three days after the heat stress protocol ended (41 DA), the inflammatory response showed similar values to birds sampled before stress started (basal) and to their respective control counterparts. These responses remained similar until the end of the study.
Figure 3. Effects of chronic heat stress exposure (during nine consecutive days) and the recovery during the following 12 days on the percentage of inflammation induced by the injection of phytohemagglutinin-p in female Japanese quail. Percentage of Inflammation = (Wing-web measurement (mm) 24 h prior to sampling day/Wing-web measurement (mm) on the sampling day) x 100. Points represent mean value and lines represent standard error of the mean. The age of sampling is shown in the time axis (age, days). Day 29 correspond to the sample day before heat stress (34 °C) started. Days 32, 35, and 38 correspond to sampling during heat stress exposure. Days 41, 44, 47, and 50 correspond to sampling after the chronic heat stress protocol was finished. a–c: different letters represent significant differences between groups (p < 0.05; Tukey post-hoc test). A total of 24 birds (12 stressed and 12 control) were sampled on each of the eight sampling days (192 birds total). Each bird was sampled only once.
H/L ratio
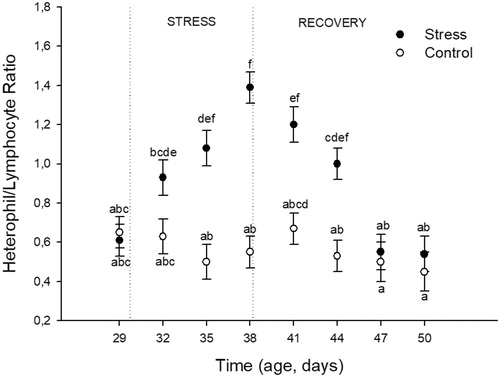
The effects of heat stress on the H/L ratio over time are shown in . The analysis revealed a significant effect of Stress (F(1,176) = 66.18; p < 0.0001), Time (F(7,176) = 7.91; p < 0.0001), and their interaction (F(7,176) = 6.62; p < 0.0001). Post-hoc analyses showed that control birds maintained similar values throughout the studied time period. Birds under the heat stress protocol showed increased H/L ratio values during the heat stress period with the highest value reached on the day 9 of heat stress exposure (38 DA). After the stress protocol ended, H/L values returned to basal levels (similar to control birds) on the day 9 of recovery (47 DA).
Figure 4. Effects of chronic heat stress (during nine consecutive days) and recovery during the following 12 days on heterophil to lymphocyte ratio in female Japanese quail. Points represent mean value and lines represent standard error of the mean. The age of sampling is shown in the time axis (age, days). Day 29 correspond to the sample day before heat stress (34 °C) started. Days 32, 35, and 38 correspond to sampling during heat stress exposure. Days 41, 44, 47, and 50 correspond to sampling after chronic heat stress protocol was finished. (a–f) different letters represents significant differences between groups (p < 0.05; Tukey post-hoc test). A total of 24 birds (12 stressed and 12 control) were sampled on each of the eight sampling days (192 birds total). Each bird was sampled only once.
Antibody response against SRBC
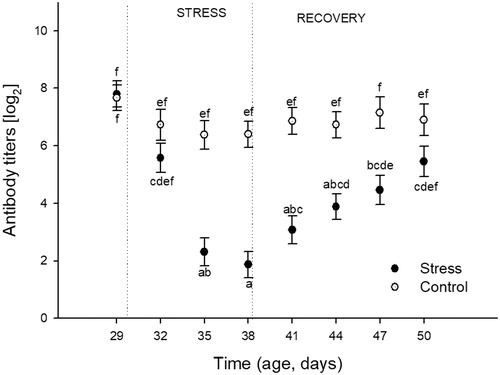
The effects of heat stress over time on induced SRBC antibody titers are shown in . The analysis revealed significant effects of Stress (F(1,176) = 59.56; p < 0.0001), Time F(7,176) = 12.13; p < 0.0001), and their interaction (F(7,176) = 5.70; p < 0.0001). Post-hoc analyses showed that control birds maintained similar values through the studied time period. The exposure to heat stress decreased the mentioned humoral response in birds after the day 6 of the stress protocol (35 DA), with the minimum numerical value registered post nine days (38 DA) of heat stress exposure. During the recovery period, titer values of previously stressed birds continually increased and reached values not significantly different from the control birds on the 1 day 12 of recovery (50 DA).
Figure 5. Effects of chronic heat stress (during nine consecutive days) and recovery during the following 12 days on antibody titers against sheep red blood cells (SRBC) in female Japanese quail. One week before each sampling day, the corresponding birds were immunized against SRBC. Points represent mean value and lines represent standard error. The age of sampling is shown in the time axis (age, days). Day 29 correspond to the sample day before heat stress (34 °C) started. Days 32, 35, and 38 correspond to sampling during heat stress exposure. Days 41, 44, 47, and 50 correspond to sampling after chronic heat stress protocol was finished. (a–f) different letters represent significant differences between groups (p < 0.05; Tukey post-hoc test). A total of 24 birds (12 stressed and 12 control) were sampled on each of the eight sampling days (192 birds total). Each bird was sampled only once.
Biochemical variables
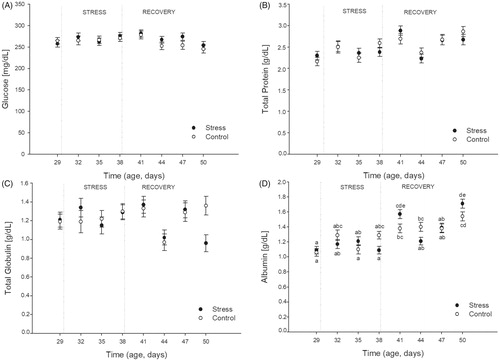
The effects of stress and time on plasma concentrations of glucose, total proteins, total globulins, and albumin are shown in (panel A–D, respectively). A significant effect of Time of Sampling was found for all the metabolic variables: glucose (F(7,176) = 2.06; p = 0.05), total proteins (F(7,176) = 8.13; p < 0.001), total globulins (F(7,176) = 3.09; p = 0.004), and albumin (F(7,176) = 20.22; p = 0.0001). However, no effects of stress (p > 0.17 in all cases) and no interactions between stress and time (p > 0.2 for glucose, total proteins, and total globulins) were found. Although plasma albumin concentration was the only metabolic variable showing an interactive effect between stress and time of sampling (F(7,176) = 3.63; p = 0.001), post-hoc analysis showed no differences between control and stressed birds either during the stress or during the recovery period.
Figure 6. Effects of chronic heat stress (during nine consecutive days) and recovery during the following 12 days on plasma concentrations of glucose (panel A), total proteins (panel B), total globulins (Panel C), and albumin (Panel D) in female Japanese quail. Points represent mean value and lines represent standard error of the mean. The age of sampling is shown in the time axis (age, days). Day 29 correspond to the sample day before heat stress (34 °C) started. Days 32, 35, and 38 correspond to sampling during heat stress exposure. Days 41, 44, 47, and 50 correspond to sampling after chronic heat stress protocol was finished. (a–e) Different letters represents significant differences between groups (p < 0.05; Tukey post-hoc test). A total of 24 birds (12 stressed and 12 control) were sampled on each of the eight sampling days (192 birds total). Each bird was sampled only once.
Discussion
The present study evaluated the dynamics of biochemical and immunoendocrine response modulation in female Japanese quail during nine consecutive days of chronic heat stress exposure and their subsequent recovery during the following 12 days. Previous studies on chronic heat stress have provided results considering a comparison between the pre- and post-stress situation: increment in synthesis of corticosterone (Shini & Kaiser, Citation2009; Shini, Kaiser, Shini, & Bryden, Citation2008) decreased concentration of thyroid hormones T3 and T4, lower levels of IGF-I (Willemsen et al., Citation2011), and insulin (Dai et al., Citation2011; Donkoh, Citation1989). Some other metabolic and nutritional aspects, such as blood metabolites, circulating amino acids, weight gain, and food conversion alterations have also been reported (Sahin et al., Citation2006, Citation2011; Sahin, Orhan, Tuzcu, Tuzcu, & Sahin, Citation2012; Xie et al., Citation2015) but those studies did not inform about the temporal changes through the exposure.
There are many different types of environmental pathogens continually threatening survival of animals. Once pathogens overcome the body’s physical, chemical, or behavioral barriers, they enter into the organism and trigger an innate immune system response (Murphy, Citation2009; Schat, Kaspers, & Kaiser, Citation2012). This allows the recruitment of phagocytic cells and antigen-presenting cells, thereby activating the acquired component of the immune system: B cells produce antibodies and/or T cells produce activated cytotoxic cells (Buehler, Tieleman, & Piersma, Citation2010; Murphy, Citation2009; Schat et al., Citation2012). Taken together, innate and acquired components complement each other, offering the benefit of neutralizing the intruding pathogen. However, developing immune responses implies a cost (Colditz, Citation2008; Hanssen, Citation2006; Hanssen, Hasselquist, Folstad, & Erikstad, Citation2004). The free access to vital resources (food and water) offered to the quail in the present study, avoids the investment of energy in the search for these resources, hence energy was theoretically available as in the control groups for the development of an optimal immune response. However, the pressure in the form of an environmental challenge by the chronic heat stress exposure, had consequences in the modulation of the immunoendocrine system which indicate an altered energy assignment. Indeed, the immunoendocrine parameters analyzed in our study were differently affected by the chronic heat stress exposure and each of them showed a particular recovery dynamic following stress. Focusing on the period of elevated environmental temperature, plasma corticosterone concentration, and inflammatory response to PHA-P were the first variables altered, with a sudden increase and decrease, respectively, after three days of heat stress. Values remained similarly altered until the end of the chronic heat stress protocol. Antibody production in response to SRBC challenge instead, required more than three days to show significantly reduced titers, reaching the minimum values after nine days of chronic heat stress exposure. However, the H/L ratio showed increasing values along the stress protocol, reaching also a maximum alteration after nine days of stress. These results are consistent with studies reported by Mashaly et al. (Citation2004); Shini et al (Citation2008) and Nazar and Marin (Citation2011) which showed similar alterations of immunological parameters when determined at the end of a chronic stress challenge. These authors used different stressors to induce a chronic stress response, such as exposure to corticosterone in drinking water (Shini et al., Citation2008), brief mechanical restraint (Nazar & Marin, Citation2011), or elevation of environmental temperature (Mashaly et al., Citation2004). Nevertheless, independently from the stressor applied, it seems that the non-specificity of the stress response allowed us to replicate the overall phenomena when strictly analyzed by comparing with before and post stress activities.
Stress signals are perceived in the brain and the stress response is triggered (Dhabhar, Citation2009; Dohms & Metz, Citation1991; Siegel, Citation1995), ending in the production and release of glucocorticoids, especially corticosterone (De Kloet, Citation2003; Mumma, Thaxton, Vizzier-Thaxton, & Dodson, Citation2006). In this study, heat stress strongly increased plasma corticosterone concentrations, which remained high until the last day of the stress protocol. Hence the hypothalamic-pituitary-adrenal (HPA) axis was evidently continually activated, with the birds showing no endocrine indicators of habituation (Shini et al., Citation2008). When the heat stress protocol ended, three days without the influence of the stressor were sufficient to reestablish basal activity similar to that in the controls. These results indicate that the heat stress protocol used is a valid and robust stressor.
Assessing the dynamics of the stress effects on the immune system, inflammatory responses were the first to be disturbed, indicating an increased susceptibility to pathogens that find this stress condition beneficial. Examples of pathogens could be viruses or bacteria that are cleared by normal inflammatory responses (Schat et al., Citation2012). Later, the H/L ratio and antibody production started to show alterations, reaching the more severe state of immunosuppression (maximum alteration registered considering the three parameters studied) at day 9 of chronic heat stress exposure. In this context, any pathogen could severely affect the chances of survival (Dohms & Metz, Citation1991; Stier et al., Citation2009). Interestingly, within the recovery period, the inflammatory response to PHA-P was also the first variable to recover control values. That phenomenon was observed three days post the stressor protocol (first sampling point within the recovery period). The H/L ratio values showed a partial recovery on the days 3 and 6 without high temperatures and reached similar values to controls on the day 9 of recovery (47 DA). Antibody production was the last variable altered during the heat stress period and also was the last variable to recover control-like values (after 12 days of thermoneutral environment; 50 DA). While inflammation requires cells that already exist to migrate from the blood into a tissue in response to a chemoattractant gradient, antibody titer elevation implies the activation, proliferation, and differentiation of B cells (Cupps, Gerrard, Falkoff, Whalen, & Fauci, Citation1985; Murphy, Citation2009; Schat et al., Citation2012). Accordingly, our results are consistent with a faster recovery of the inflammatory capacity compared with the required de novo synthesis of immunoglobulins.
Regarding metabolic variables, no effects of stress and no interactions between stress and time were found for plasma glucose, total proteins, and total globulins. Although an interaction between heat stress treatment and time was found for albumin concentrations, the analysis did not show significant differences between control and stressed birds either during the stress or during the recovery period. Plasma glucose and total proteins and globulins did show some changes on different sample days during the study (within stress and recovery periods), both in the control and stressed groups. These changes with time might just reflect metabolic adjustments during ontogeny. These results are consistent with Xie et al. (Citation2015), who reported no changes in blood glucose and protein metabolism after a chronic heat stress protocol. However, Ma et al. (Citation2014) studied the effects of chronic heat stress in ducks reporting alteration in the content of energy and protein, with lowest values in ducks that were submitted to 34 °C at the end of the day 28 of heat stress. These differences between studies may be based on the duration of heat stress exposure, the chosen temperature, or a combined effect of both factors. However, increments in blood glucose after an acute heat stress have been reported by Xie et al. (Citation2015). The absence of chronic stress-induced changes in glucose concentrations in our study is consistent with adequate coping during the sustained high environmental temperatures. Such coping may have been facilitated by allowing birds to regain environmental thermoneutral temperatures during the night phase.
The corticosterone concentration dynamics can be related to the alteration of the immune parameters rather than the biochemical variables. Consequently, sustained higher corticosterone concentrations were associated with a progressive state of immuno-suppression along the nine consecutive days of exposure to the stressor. Once the stress protocol was ended, the HPA axis reestablished its basal activity, promoting survival (Huff, Huff, & Balog, Citation2005; Puvadolpirod & Thaxton, Citation2000; Romero, Dickens, & Cyr, Citation2009; Shini et al., Citation2008). The change in H/L ratio and antibody production is a consequence of the increased circulating concentrations of the stress response mediators during and after stress (Romero et al., Citation2009). Plausibly these immune responses take longer to recover than the corticosterone concentrations or inflammatory responses.
Taken together, the findings in our study indicate that heat stress represents a threat to birds with a major impact on immunoendocrine responses rather than the several metabolic variables evaluated. The response to the heat stressor was characterized by a different temporal modulation of each of the immune components studied and an increasing severity as days under heat stress went by. Immunoendocrine responses appeared already threatened when heat stress was sustained for only three days, potentially compromising bird health and welfare. In poultry production, three days of heat stress should be taken as a serious condition, strongly activating stress hormone secretion and suppressing immune defenses: management practices should be aimed at minimizing such heat and associated pathogen exposure. Nonetheless, the immune and endocrine systems appear resilient, but take six to 12 days post heat stress to recover to normal basal activity.
Acknowledgements
The authors wish to thank the technical assistance of Julia Ortiz and Dario C. Arbelo during the development of this study. We are also very thankful to the anonymous reviewers and the editor for the comments and suggested changes to a previous version of this manuscript.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Adriaansen-Tennekes, R., Decuypere, E., Parmentier, H.K., & Savelkoul, H.F.J. (2009). Chicken lines selected for their primary antibody response to sheep red blood cells show differential hypothalamic-pituitary-adrenal axis responsiveness to mild stressors. Poultry Science, 88, 1879–1882. doi:10.3382/ps.2009-00150
- Azad, M.K., Kikusato, M., Maekawa, T., Shirakawa, H., & Toyomizu, M. (2010). Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comparative, Environmental and Evolutionary Physiology. Part A: Molecular and Integrative Physiology, 155, 401–406. doi:10.1016/j.cbpa.2009.12.011
- Baumgartner, J. (1994). Japanese quail production, breeding and genetics. World’s Poultry Science Journal, 50, 227–235.
- Belhadj Slimen, I., Najar, T., Ghram, A., & Abdrrabba, M. (2016). Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. Journal of Animal Physiology and Animal Nutrition, 100, 401–412. doi:10.1111/jpn.12379
- Buehler, D.M., Tieleman, B.I., & Piersma, T. (2010). How do migratory species stay healthy over the annual cycle? A conceptual model for immune function and for resistance to disease. Integrative and Comparative Biology, 50, 346–357. doi:10.1093/icb/icq055
- Colditz, I.G. (2008). Six costs of immunity to gastrointestinal nematode infections. Parasite Immunology, 30, 63–70. doi:10.1111/j.1365-3024.2007.00964.x
- Cupps, T.R., Gerrard, T.L., Falkoff, R.J., Whalen, G., & Fauci, A.S. (1985). Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation. The Journal of Clinical Investigation, 75, 754–761. doi:10.1172/JCI111757
- Dai, S.F.F., Gao, F., Zhang, W.H.H., Song, S.X.X., Xu, X.L.L., & Zhou, G.H.H. (2011). Effects of dietary glutamine and gamma-aminobutyric acid on performance, carcass characteristics and serum parameters in broilers under circular heat stress. Animal Feed Science Technology, 168, 51–60. doi:10.1016/j.anifeedsci.2011.03.005
- De Kloet, E.R. (2003). Hormones, brain and stress. Endocrine Regulations, 37, 51–68.
- Dhabhar, F.S. (2009). Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation, 16, 300–317. doi:10.1159/000216188
- Di Rienzo, J.A., Casanoves, F., Balzarini, M.G., Gonzalez, L., Tablada, M., & Robledo, C.W. (2016). Infostat. (G. InfoStat, Ed.). Cordoba, Argentina: FCA, Universidad Nacional de Córdoba.
- Dohms, J.E., & Metz, A. (1991). Stress–mechanisms of immunosuppression. Veterinary Immunology and Immunopathology, 30, 89–109. doi:10.1016/0165-2427(91)90011-Z
- Donkoh, A. (1989). Ambient temperature: A factor affecting performance and physiological response of broiler chickens. International Journal of Biometeorology, 33, 259–265. doi:10.1007/BF01051087
- Fair, J.M., Hansen, E.S., & Ricklefs, R.E. (1999). Growth, developmental stability and immune response in juvenile Japanese quails (Coturnix coturnix japonica). Proceedings. Biological sciences Royal Society (Great Britain), 266, 1735–1742. doi:10.1098/rspb.1999.0840
- Gasparino, E., Voltolini, D.M., Del Vesco, A.P., Guimarães, S.E.F., Nascimento, C.S., & de Oliveira Neto, A.R. (2013). IGF-I, GHR and UCP mRNA expression in the liver and muscle of high- and low-feed-efficiency laying Japanese quail at different environmental temperatures. Livestock Science, 157, 339–344. doi:10.1016/j.livsci.2013.06.028
- Guzmán, D.A., Pellegrini, S., Kembro, J.M., & Marin, R.H. (2013). Social interaction of juvenile Japanese quail classified by their permanence in proximity to a high or low density of conspecifics. Poultry Science, 92, 2567–2575. doi:10.3382/ps.2013-03206
- Hanssen, S.A. (2006). Costs of an immune challenge and terminal investment in a long-lived bird. Ecology, 87, 2440–2446.
- Hanssen, S.A., Hasselquist, D., Folstad, I., & Erikstad, K.E. (2004). Costs of immunity: Immune responsiveness reduces survival in a vertebrate. Proceedings. Biological Sciences, 271, 925–930. doi:10.1098/rspb.2004.2678
- Huff, G.R., Huff, W.E., & Balog, J.M. (2005). Stress response differences and disease susceptibility reflected by heterophil to lymphocyte ratio in turkeys selected for increased body weight. Poultry Science, 84, 709–717.
- Huss, D., Poynter, G., & Lansford, R. (2008). Japanese quail (Coturnix japonica) as a laboratory animal model. Lab Animal), 37, 513–519. doi:10.1038/laban1108-513
- Jones, R.B. (1989). Chronic stressors, tonic immobility and leucocytic responses in the domestic fowl. Physiology & Behavior, 46, 439–442. doi:10.1016/0031-9384(89)90017-6
- Lara, L., & Rostagno, M. (2013). Impact of heat stress on poultry production. Animals : An Open Access Journal from Mdpi, 3, 356–369. doi:10.3390/ani3020356
- Ma, X., Lin, Y., Zhang, H., Chen, W., Wang, S., Ruan, D., & Jiang, Z. (2014). Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Animal Reproduction Science, 145, 182–190. doi:10.1016/j.anireprosci.2014.01.002
- Mashaly, M.M., Hendricks, G.L., Kalama, M.A., Gehad, A.E., Abbas, A.O., & Patterson, P.H. (2004). Effect of heat stress on production parameters and immune responses of commercial laying hens. Poultry Science, 83, 889–894. doi:10.1093/ps/83.6.889
- McNamara, J.M. (2005). Stress, resource allocation, and mortality. Behavioral Ecology, 16, 1008–1017. doi:10.1093/beheco/ari087
- Moore, C.B., & Siopes, T.D. (2005). Enhancement of cellular and humoral immunity following embryonic exposure to melatonin in turkeys (Meleagris gallopavo). General and Comparative Endocrinology, 143, 178–183.
- Mumma, J.O., Thaxton, J.P., Vizzier-Thaxton, Y., & Dodson, W.L. (2006). Physiological stress in laying hens. Poultry Science, 85, 761–769.
- Munro, C.J., & Lasley, B.L. (1988). Non-radiometric Assays: Technology and Application in Polypeptide and Steroid Hormone Detection. In B.D. Albertson, F.P. Haseltine, (Eds.), Non-radiometric methods for immunoassay of steroid hormones. New York: Alan R. Liss.
- Murphy, K. (2009). Inmunobiología de Janeway (7th ed). Mexico: M. Graw-Hill, Ed., Interamericana de México.
- Nazar, F.N., Barrios, B.E., Kaiser, P., Marin, R.H., & Correa, S.G. (2015). Immune neuroendocrine phenotypes in Coturnix coturnix: Do avian species show Lewis/Fischer-like profiles? PLoS One, 10, e0120712. doi:10.1371/journal.pone.0120712
- Nazar, F.N., Magnoli, A.P., Dalcero, A.M., & Marin, R.H. (2012). Effect of feed contamination with aflatoxin B1 and administration of exogenous corticosterone on Japanese quail biochemical and immunological parameters. Poultry Science, 91, 47–54. doi: 10.3382/ps.2011-01658
- Nazar, F.N., & Marin, R.H. (2011). Chronic stress and environmental enrichment as opposite factors affecting the immune response in Japanese quail (Coturnix coturnix japonica). Stress, 14, 166–173. doi:10.3109/10253890.2010.523093
- Nazar, F.N., Marin, R.H., Liste, G., Campderrich, I., & Estevez, I. (2015). Manipulation of the phenotypic appearance of individuals in groups of laying hens: effects on stress and immune-related variables. Stress, 18, 710–717. doi:10.3109/10253890.2015.1078306
- Oğuz, H., Keçeci, T., Birdane, Y.O., Onder, F., & Kurtoğlu, V. (2000). Effect of clinoptilolite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. i Research in Veterinary Science, 69, 89–93. doi: 10.1053/rvsc.2000.0395
- Puvadolpirod, S., & Thaxton, J.P. (2000). Model of physiological stress in chickens 1. Response parameters. Poultry Science, 79, 363–369. doi: 10.1093/ps/79.3.363
- Quinteiro-Filho, W.M., Ribeiro, A., Ferraz-De-Paula, V., Pinheiro, M.L., Sakai, M., Sá, L.R.M., … Palermo-Neto, J. (2010). Heat stress impairs performance parameters, induces intestinal injury, and decreases macrophage activity in broiler chickens. Poultry Science, 89, 1905–1914. doi:10.3382/ps.2010-00812
- Roche, B., & Guégan, J.F. (2011). Ecosystem dynamics, biological diversity and emerging infectious diseases. Comptes Rendus Biologies, 334, 385–392. doi: 10.1016/j.crvi.2011.02.008
- Romero, L.M. (2004). Physiological stress in ecology: Lessons from biomedical research. Trends in Ecology & Evolution, 19, 249–255. doi:10.1016/j.tree.2004.03.008
- Romero, L.M., Dickens, M.J., & Cyr, N.E. (2009). The reactive scope model: A new model integrating homeostasis, allostasis, and stress. Hormones and Behavior, 55, 375–389. doi:10.1016/j.yhbeh.2008.12.009
- Romero, L.M., & Reed, J.M. (2005). Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comparative, Environmental and Evolutionary Physiology. Part A: Molecular and Integrative Physiology, 140, 73–79. doi:10.1016/j.cbpb.2004.11.004
- Sahin, K., Onderci, M., Sahin, N., Gursu, M.F., Khachik, F., & Kucuk, O. (2006). Effects of lycopene supplementation on antioxidant status, oxidative stress, performance and carcass characteristics in heat-stressed Japanese quail. Journal of Thermal Biology, 31, 307–312. doi:10.1016/j.jtherbio.2005.12.006
- Sahin, K., Orhan, C., Akdemir, F., Tuzcu, M., Ali, S., & Sahin, N. (2011). Tomato powder supplementation activates Nrf-2 via ERK/Akt signaling pathway and attenuates heat stress-related responses in quails. Animal Feed Science and Technology, 165, 230–237. doi:10.1016/j.anifeedsci.2011.03.003
- Sahin, K., Orhan, C., Tuzcu, Z., Tuzcu, M., & Sahin, N. (2012). Curcumin ameloriates heat stress via inhibition of oxidative stress and modulation of Nrf2/HO-1 pathway in quail. Food and Chemical Toxicology : An International Journal Published for the British Industrial Biological Research Association, 50, 4035–4041. doi: 10.1016/j.fct.2012.08.029
- Sandhu, M.A., Mirza, F.Q., Afzal, F., & Mukhtar, N. (2012). Effect of heat stress on cellular and humoral immunity and its cure with ??-tocopherol in meat type birds. Livestock Science, 148, 181–188. doi:10.1016/j.livsci.2012.06.005
- Scanes, C.G. (2014). Sturkie’s avian physiology. London, UK: Elsevier.
- Schat, K.A., Kaspers, B., & Kaiser, P. (2012). Avian Immunology. San Diego, CA: Elsevier Science.
- Sever, J. (1962). Application of a microtechnique to viral serological investigations. Journal of Immunology, 88, 320–329.
- Shini, S., & Kaiser, P. (2009). Effects of stress, mimicked by administration of corticosterone in drinking water, on the expression of chicken cytokine and chemokine genes in lymphocytes. Stress, 12, 388–399. doi:10.1080/10253890802526894
- Shini, S., Kaiser, P., Shini, A., & Bryden, W.L. (2008). Biological response of chickens (Gallus gallus domesticus) induced by corticosterone and a bacterial endotoxin. Comparative, Environmental and Evolutionary Physiology. Part A: Molecular and Integrative Physiology, 149, 324–333. doi:10.1016/j.cbpb.2007.10.003
- Shini, S., Shini, A., & Huff, G.R. (2009). Effects of chronic and repeated corticosterone administration in rearing chickens on physiology, the onset of lay and egg production of hens. Physiology & Behavior, 98, 73–77. doi:10.1016/j.physbeh.2009.04.012
- Siegel, H.S. (1995). Gordon Memorial Lecture. Stress, strains and resistance. British Poultry Science, 36, 3–22. doi:10.1080/00071669508417748
- Song, J., Jiao, L.F.F., Xiao, K., Luan, Z.S.S., Hu, C.H.H., Shi, B., & Zhan, X.A.A. (2013). Cello-oligosaccharide ameliorates heat stress-induced impairment of intestinal microflora, morphology and barrier integrity in broilers. Animal Feed Science and Technology, 185, 175–181. doi:10.1016/j.anifeedsci.2013.08.001
- Stadecker, M., & Lukic, M. (1977). The cutaneous basophil response to phytohemagglutinin in chickens. Journal of Immunology, 118, 1564–1568.
- Stier, K.S., Almasi, B., Gasparini, J., Piault, R., Roulin, A., & Jenni, L. (2009). Effects of corticosterone on innate and humoral immune functions and oxidative stress in barn owl nestlings. The Journal of Experimental Biology, 212, 2085–2091. doi: 10.1242/jeb.024406
- Willemsen, H., Swennen, Q., Everaert, N., Geraert, P.A., Mercier, Y., Stinckens, A., … Buyse, J. (2011). Effects of dietary supplementation of methionine and its hydroxy analog DL-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poultry Science, 90, 2311–2320. doi:10.3382/ps.2011-01353
- Xie, J., Tang, L., Lu, L., Zhang, L., Lin, X., Liu, H.-C., … Luo, X. (2015). Effects of acute and chronic heat stress on plasma metabolites, hormones and oxidant status in restrictedly fed broiler breeders. Poultry Science, 94, 1635–1644. doi:10.3382/ps/pev105
- Zell, R. (2004). Global climate change and the emergence/re-emergence of infectious diseases. International Journal of Medical Microbiology, 293, 16–26. doi:10.1016/S1433-1128(04)80005-6

