Abstract
Stigma may strain the heart health of lesbian, gay, and bisexual (LGB) individuals. To date, however, LGB-related differences in cardiovascular diagnosis, risk factors, and basal biomarkers are inconsistently reported. Using a laboratory-based stress paradigm, the current study assessed whether cardiovascular stress reactivity differs as a function of sexual orientation and disclosure status (“coming out”) in a sample of healthy young LGB and heterosexual adults. Eighty-seven participants aged 18–45 (M = 24.61 ± 0.61 SE) identifying as LGB and heterosexual (47%) were exposed to the Trier Social Stress Test, a well-validated laboratory stressor involving public speaking and mental arithmetic. Throughout a two-hour session, ambulatory recordings for heart rate and blood pressure were collected. Self-report questionnaires were also administered to assess psychosocial and demographic variables. Gay/bisexual men showed higher heart rate and lesbian/bisexual women showed marginally higher mean arterial blood pressure in response to a stressor, compared to sex- and age-matched heterosexuals. No significant differences emerged when comparing LGB individuals who had completely disclosed and those that had not completely disclosed their sexual orientation to family and friends. Compared to heterosexuals, heart rate is higher among gay/bisexual men and blood pressure is marginally higher among lesbian/bisexual women when exposed to a laboratory-based stressor. These preliminary findings contribute to small literature on sexual orientation differences in stress reactive biomarkers that requires further exploration.
In response to stress exposure in a laboratory, gay/bisexual men showed higher heart rate than heterosexual men. By contrast, lesbian/bisexual showed a non-significant tendency towards higher blood pressure than heterosexual women. These preliminary findings suggest that the heart health of LGB individuals might be strained by stigma exposure.
Lay abstract
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States (Xu, Murphy, Kochanek, & Bastian, Citation2016) and worldwide (Mozaffarian et al., Citation2015). Numerous studies have indicated that socially disadvantaged groups, such as racial/ethnic minorities, are at heightened risk for CVD relative to socially advantaged majority groups. Recently, researchers have begun to examine whether sexual minorities – that is, individuals who identify as lesbian, gay, or bisexual (LGB), who engage in same-sex behaviors, and/or who report same-sex attractions – are at increased risk for CVD outcomes compared to heterosexuals due in part to stigma and stress processes (IOM, Citation2011; Lick, Durso, & Johnson, Citation2013).
To date, studies have provided mixed evidence for LGB health disparities in CVD. Inconsistencies are due, in part, to LGB subgroup differences in CVD risk profiles (Caceres, Brody, & Chyun, Citation2016). Among sexual minority men, some studies have reported no differences in CVD risk factors (e.g. smoking, obesity, hypertension) relative to heterosexual men (Clark et al., Citation2015; Conron, Mimiaga, & Landers, Citation2010; Farmer, Bucholz, Flick, Burroughs, & Bowen, Citation2013), while others have reported increased risk factors among certain subgroups, such as bisexual men (Conron et al., Citation2010; Farmer, Bucholz, Flick, Burroughs, & Bowen, Citation2013). In a nationally representative study of blood pressure by sexual orientation, gay men showed double the risk of hypertension compared to heterosexual men, while no differences were found among women (Everett & Mollborn, Citation2013). By contrast, other nationally representative studies have identified increased CVD risk among sexual minority women compared to heterosexual women (Clark et al., Citation2015; Conron et al., Citation2010; Farmer, Jabson, Bucholz, & Bowen, Citation2013; Simoni, Smith, Oost, Lehavot, & Fredriksen-Goldsen, Citation2017). A systematic review concluded that there is an elevated risk for CVD in both sexual minority men and women that should be further assessed by combining subjective and objective measures of CVD risk (Caceres et al., Citation2017).
One approach to further study susceptibilities to CVD among LGB individuals is stress reactivity paradigms. When physical and psychosocial threats are detected by the brain, stress response systems (e.g. neuroendocrine, cardiovascular) are activated (McEwen, Citation1998). This involves the sympathetic-adrenal-medullary (SAM)-axis release of catecholamines (e.g. adrenalin) within seconds that increases heart rate and blood pressure, followed by the hypothalamic-pituitary-adrenal (HPA)-axis production of glucocorticoids (e.g. cortisol) within minutes to sustain the stress response. Chronic activation of the stress response may damage physiological integrity. It has been proposed that cumulative activation of stress responses may be compounded by stigma faced by LGB individuals who are consequently more vulnerable to stress-related diseases (Juster, bVencill, & Johnson, Citation2017; Lick et al., Citation2013).
The reactivity hypothesis (Manuck, Citation1994) states that exaggerated physiological reactivity to stressors is linked to stress-related conditions like CVD (Lovallo, Citation2010; Lovallo & Gerin, Citation2003; Schwartz et al., Citation2003). Cardiovascular stress reactivity refers to the magnitude or pattern of an individual’s hemodynamic responses to such stressors. It is believed that cardiovascular reactivity is amplified among individuals exposed to social adversities (Treiber et al., Citation2003). In addition to the magnitude of reactivity, the concept of recovery plays an important role in understanding stress-induced disease risk. Recovery represents the duration and prolongation of stress responses after the stressor ceases (Brosschot, Citation2010; Brosschot, Pieper, & Thayer, Citation2005). Stress reactivity/recovery dynamics are characterized by much individual variability both in terms of exposure to stress and in terms of recovery (Earle, Linden, & Weinberg, Citation1999; Linden, Earle, Gerin, & Christenfeld, Citation1997; Rutledge, Linden, & Paul, Citation2000).
The Trier Social Stress Test (TSST) is a classic stress reactivity paradigm used extensively to assess stress reactivity/recovery linked to stress-related disease (Allen et al., Citation2017). The TSST involves a 10-min anticipation phase, 5-min performing a mock job interview, and 5-min calculating mental arithmetic (Kirschbaum, Pirke, & Hellhammer, Citation1993). This paradigm elicits social-evaluative threat (Dickerson & Kemeny, Citation2002) that robustly activates the SAM- and HPA-axes. Stress reactivity and recovery can then be discerned by assessing objective biomarkers (e.g. cortisol, blood pressure) pre- and post-TSST.
Recently, three international TSST studies have assessed HPA-axis functioning of LGB individuals. First, American LGB individuals (N = 74) growing up in less socially tolerant states (compared to more socially tolerant states) evidenced blunted HPA-axis production of cortisol in response to a modified TSST in which participants described an event where they experienced rejection based on their sexual orientation (Hatzenbuehler & McLaughlin, Citation2014). The authors suggest that this hypo-reactive cortisol pattern might indicate a pathophysiological profile associated with chronic stress, trauma, and/or fatigue (Fries, Hesse, Hellhammer, & Hellhammer, Citation2005). Second, a Canadian study of young adults (N = 87) showed that sexual orientation modulates cortisol reactivity to the TSST (Juster et al., Citation2015). Specifically, lesbian/bisexual women showed higher cortisol during recovery than heterosexual women, while gay/bisexual men demonstrated lower cortisol throughout testing compared to heterosexual men. Third, an Israeli study of gay and heterosexual men exposed to a modified TSST (N = 36) showed that gender atypical behavior and heterosexual sexual orientation predicted higher levels of social interaction anxiety; however, these variables were not associated with cortisol dynamics (Jacobson, Cohen, & Diamond, Citation2016).
To summarize, sexual minority men may show dampened HPA-axis reactivity, while sexual minority women appear to show both HPA-axis hypo-reactivity (Hatzenbuehler & McLaughlin, Citation2014) and hyper-reactivity (Juster et al., Citation2015). This underlines the need to conduct sex/gender-specific analyses when assessing LGB populations. In addition, other biological systems need to be assessed. Of pertinence to the present study, cardiovascular stress reactivity has yet to be reported in a TSST study of sexual orientation. To the best of our knowledge, only one American study assessed cardiovascular reactivity by exposing 27 healthy adult gay men to a psychosocial stressor that involved discussing difficulties associated with concealing one’s sexual orientation in day-to-day life (Pérez-Benitez, O'Brien, Carels, Gordon, & Chiros, Citation2007). Men with high concealment of their sexual orientation but who engaged in more disclosure during the laboratory task exhibited greater cardiovascular recovery (assessed via heart rate and stroke volume) than those men who engaged in less disclosure during the task (Pérez-Benitez et al., Citation2007). Despite low power and no sexual minority women, this study highlights the role that concealment and disclosure may have on cardiovascular reactivity and the heart health of sexual minority men. Given that the existing literature on health disparities in cardiovascular disease is mixed, further investigation of cardiovascular stress processes is warranted.
The current study assessed whether TSST-induced cardiovascular stress reactivity differs as a function of sexual orientation and disclosure status in a sample of healthy young LGB and heterosexual adults. Due to heightened exposure to stigma, we expected LGB individuals to show amplified cardiovascular stress reactivity. First, we hypothesized that sexual minority women and men would show higher cardiovascular stress reactivity to the TSST than sex- and age-matched heterosexual individuals. Second, among the LGB participants only, we hypothesized that LGB individuals who had fully disclosed their sexual orientation would evidence lower cardiovascular stress reactivity than those LGB individuals who had not yet fully disclosed and may, therefore, experience additional distress.
Methods
Participants
Eighty-seven participants aged 18–45 (M = 24.61 ± 0.61 SE) identifying as lesbian or gay (8 women and 20 men), bisexual (13 women and 5 men) or heterosexual (20 women and 21 men) were recruited from Montreal as part of a broader study. The main exclusionary criteria were medicinal use of synthetic steroid hormones, major health problems (e.g. HIV/AIDS, cancer), and severe mental illness (e.g. schizophrenia). No participant was taking medications related to cardiovascular problems (e.g. anti-hypertensives) or that could interfere with cardiovascular functioning (e.g. stimulants).
According to a recent systemic review (Simoni et al., Citation2017), two demographic variables of importance in research on physical health conditions among sexual minorities are race/ethnicity and educational attainment. Race/ethnicity was coded as “0” for white individuals (74.5%) and “1” for nonwhite individuals (25.5%). Education was coded as “0” for “no college degree attained” (53.5%) and “1” for “college degree attained” (46.5%). Both race/ethnicity and education were controlled for in our statistical analyses.
Sexual orientation
Sexual orientation was assessed and cross-validated using three methods: (a) response to separate advertisements recruiting either lesbian/gay/bisexual or heterosexual participants; (b) asking participants their identified sexual orientation in an open-ended manner during a telephone screening interview; and (c) administration of a modified 5-item Klein Sexual Orientation Scale (Klein, Sepekoff, & Wolf, Citation1990). This instrument uses a 7-point Likert scale to assess sexual attractions, sexual behavior, sexual fantasies, lifestyle preference, and sexual identity along with a continuum of sexual experiences “in your life up to now,” with low scores representing greater heterosexuality and high scores representing greater homosexuality. The sample’s responses showed near perfect internal consistency (α = 0.98). Based on the correspondence among these three methods, sexual orientation was coded as “LGB” (n = 46) or “heterosexual” (n = 41).
Disclosure status
Disclosure was measured using a four-item inventory created by our group that asked the LGB participants (n = 46) their age for four key milestones regarding same-sex sexual attractions that included self-recognition, self-identification, disclosure to friends, and disclosure to the family. Participants were coded as “disclosed” (n = 31) if they provided ages for all four items or as “non-disclosed” (n = 14) if one or more of the items were not answered completely. One LGB participant did not provide this information and was dropped from analyses assessing disclosure status among LGB participants.
Mastery
The 7-item Pearlin and Schooler Personal Mastery Scale (Pearlin & Schooler, Citation1978) were used to assess perceived mastery and control. This widely employed instrument uses a 4-point Likert scale (strongly disagree to strongly agree) to items such as “there is little I can do to change many of the important things in my life.” Reliability was good in the current sample (Cronbach’s α = 0.82). Individuals with higher mastery are believed to appraise themselves as better able to manage stressors, which may buffer their physiological arousal to threat (Roepke et al., Citation2008). Mastery was, therefore, used as a covariate based on previous literature linking its role in modulating stress processes (Chida & Hamer, Citation2008).
General protocol
This study was approved by the research ethics board of Louis-H. Lafontaine Hospital and, therefore, accords with proper ethical conduct. Upon a 15-minute study explanation and screening interview by telephone, eligible participants were scheduled for two laboratory visits. The current analysis focuses on an afternoon/evening visit scheduled between 12:00 and 19:00 (M = 14.34, SE = 0.11) that lasted two hours in which participants were exposed to the standardized psychosocial stressor (e.g. TSST). Upon debriefing, participants were compensated with $50 CAD.
Visit order
The order of visits was counterbalanced randomly to manipulate experienced novelty of the testing environment (Sindi, Fiocco, Juster, Pruessner, & Lupien, Citation2013). In the first group (morning/afternoon; n = 49), participants received a blood draw (findings reported elsewhere; (Juster, Almeida et al., Citation2016; Juster, Ouelle, et al., Citation2016; Juster, Smith, Ouellet, Sindi, & Lupien, Citation2013)) in the morning during their first visit and were exposed to the TSST in the afternoon during their second visit about a week later; this was reversed for the second group (afternoon/morning; n = 37). Since the second group arrived for the first time to our laboratory when exposed to the TSST, we expected that they would be more distressed than the first group who had already familiarized themselves with the laboratory setting. Given that novelty to testing environments can be appraised as stressful (Sindi et al., Citation2013), preliminary analyses assessed whether visit order modulated cardiovascular functioning. This manipulation does not represent a circadian dissimilarity since participants were all exposed to the TSST in the afternoon (arrival time: M = 14.34, SE = 0.11; departure time: M = 16.17, SE = 0.11).
Stress reactivity paradigm
The two-hour afternoon visit involved exposure to a modified version (Andrews et al., Citation2007; Wadiwalla et al., Citation2010) of the TSST (Kirschbaum et al., Citation1993). Upon arrival, participants acclimated to the laboratory environment for approximately 40 minutes while we recorded their cardiovascular parameters every 10 mins. They were then given instructions for the TSST and asked to prepare their mock job interview speech. After this 10-minute anticipation phase, participants walked for approximately 15 seconds to another room where they were asked to deliver a 5-minute mock job interview, followed by 5 minutes of mental arithmetic in front of an unseen “behavioral expert” (who was actually a trained confederate) seated behind a one-way mirror. The participant and the “behavioral expert” communicated via an intercommunication device and the participant’s performance was recorded by a video camera. Upon completion of the TSST, participants were returned to the original room and cardiovascular recordings resumed with recordings every 10 mins. until the study concluded.
Cardiovascular measures
Auscultatory cardiovascular functioning was recorded throughout the 2-hour visit before and after the TSST. No recordings were taken during the TSST. We employed an electronic sphygmomanometer (A&D Medical: Model UA-631 V) on participants’ non-dominant arm (selection of three cuff sizes based on size). Participants were seated and asked to refrain from moving or talking while the cuff inflated. In total, we recorded heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) at 10-minute intervals at the following time-points relative to the TSST (): −40 min, −30 min, −20 min, −10 min, 0 (TSST), +10 min, +20 min, +30 min, +40 min, and +50 min.
Figure 1. Study timeline. Ten cardiovascular recordings were taken at 10-minute intervals and aggregated into phases representing acclimation, reactivity, and recovery from the Trier Social Stress Test (TSST).
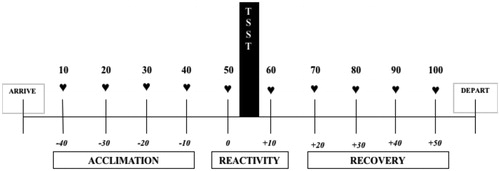
Cardiovascular recordings of HR, SBP, and DBP were averaged according to three phases (): acclimation (−40 min, −30 min, −20 min, and −10 min pre-TSST), reactivity (immediately before and after the TSST), and recovery (+10 min, +20 min, +30 min, and +40 min post-TSST). This aggregated approach enhances reliability and reduces measurement error (Kamarck, Debski, & Manuck, Citation2000). SBP and DBP were transformed into mean arterial pressure (MAP) using a standard formula: ∑ (DBP + 1/3 (SBP – DBP)).
Statistical analysis
To optimize power due to fewer lesbians and bisexual men, we combined groups of lesbian/gay and bisexual individuals within sex (20 women and 26 men) and contrasted them to sex-matched heterosexual individuals (20 women and 21 men). The direction of results was similar for lesbian and bisexual women as well as gay and bisexual men, albeit of reduced statistical strength as indicated by lower p-value when assessing differences with heterosexual individuals.
To assess group differences in descriptive information reported in , we employed univariate analysis of variance (ANOVA), Tukey’s post-hoc analysis, and chi-square tests. Our main analyses were stratified by sex, given our focus on sexual orientation differences in cardiovascular stress reactivity. Sex-disaggregation of analyses is also recommended by the National Institute of Health when assessing sex-specific phenomena (NIH, Citation2014) and sexual orientation may modulate stress reactivity in a sex-specific manner (Allen et al., Citation2017).
Table 1. Sample descriptive statistics according to sexual orientation and sex (N = 87).
Mixed-design repeated measures analysis of covariance (ANCOVA) were run with sexual orientation (LGB and heterosexual) entered as the between-subject factor and time (repeated measures of HR and MAP for acclimation, reactivity, and recovery) entered as the within-subjects factor while adjusting for covariates (race/ethnicity, education, mastery, and visit order). Greenhouse–Geisser corrections are reported whenever Mauchly’s tests denoted violations in sphericity. Significant time-by-group interaction effects were assessed using post-hoc one-way ANOVAs, and significant time effects were assessed using paired-sample t-tests. Supplemental analyses assessed SBP and DBP separately. Effect sizes are interpreted as follows: small (η2P ≌ 0.01), medium (η2P ≌ 0.06), or large (η2P ≌ 0.14) effects (Fritz, Morris, & Richler, Citation2012).
Results
Descriptive statistics
Descriptive analyses assessed sex differences by sexual orientation. As seen in , LGB respondents did not differ from heterosexual individuals with respect to a broad array of sample characteristics. Two exceptions involved significant group differences between sexual orientation and contraception. First, LGB participants reported greater propensity towards same-sex only responses on the Klein Sexual Orientation Scale (ps < .001) than heterosexuals. Second, heterosexual women were more likely to be using oral contraceptives than lesbian/bisexual women (p = .017). Note that this refers to orally administered hormonal contraceptives and not those delivered by other modes. also shows that risk factors for CVD like tobacco smoking, alcohol (over)consumption, and illicit drug use were not different between groups by sexual orientation.
Preliminary analysis
Potential confounders of heart rate (HR) and mean arterial pressure (MAP) were scrutinized in preliminary analyses using repeated-measures ANOVA as a function of visit order (morning/afternoon: n = 49; afternoon/morning group: n = 37), self-reported menstrual cycle status (follicular: n = 20; luteal: n = 20), and oral contraceptive use (users: n = 14; non-users: n = 26). For visit order, no between-subject differences were detected for HR (p = .45) and MAP (p=.30). By contrast, time-by-visit order interaction effects were significant for HR (F(6.88,578.13) = 2.73, p = .009, η2P = .032) and marginal for MAP (F(6.15,516.4) = 1.891, p = .079, η2P = .022). Those coming to the laboratory for the first time showed greater cardiovascular reactivity than those coming for the second time. Among women, no between-group differences in HR and MAP were found as a function of the menstrual cycle (respectively, p = .998 and p = .952) or oral conceptive use (respectively, p = .415 and p = .804). Therefore, only visit order is controlled for in the main analyses.
Sexual orientation differences in cardiovascular stress reactivity
The following mixed-design repeated-measures ANCOVAs assessed HR and MAP as a function of sexual orientation while adjusting for race/ethnicity, education, mastery, and visit order split by sex.
For HR among women (), only a within-subjects time effect was detected (F(1.56,53.13) = 31.72, p < .001, η2P =.483): HR increased between acclimation and reactivity (p < .001) and decreased from reactivity to recovery (p < .001). No group difference was detected as a function of sexual orientation (p = .494, η2P=.014).
Figure 2. Estimated mean (±SE) heart rate (A–B) and mean arterial pressure (C–D) in response to the Trier Social Stress Test as a function of sexual orientation among women (n = 40) and men (n = 46) adjusted for race/ethnicity, education, mastery, and visit order. Note: *: p < .05; †: p < .10.
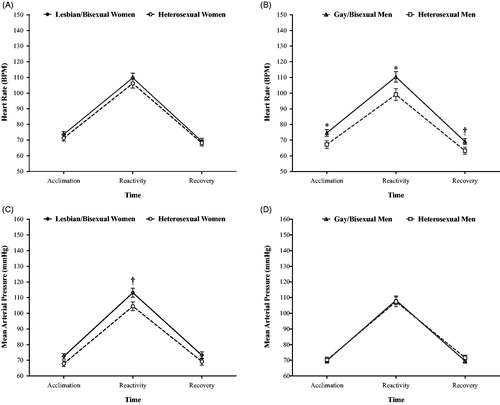
For HR among men (), a within-subjects time effect was also detected (F(1.41,56.47) = 9.01, p = .001, η2P = .184): HR increased between acclimation and reactivity (p<.001) and decreased from reactivity to recovery (p < .001). In addition, a time-by-sexual orientation interaction effect was detected (F(1.41,56.47) = 3.56, p = .050, η2P = .082). Post-hoc analysis revealed that gay/bisexual men had higher HR during acclimation (p = .029, η2P = .114) and reactivity (p = .034, η2P = .108), and marginally during recovery (p = .055, η2P = .089), compared to heterosexual men. Between-subjects effects were significant for sexual orientation (F(1,40) = 4.85, p = .033, η2P = .108), whereby gay/bisexual men showed higher overall HR across the entire visit than heterosexual men.
For MAP among women (), a within-subjects time effect was significant (F(1.56,53.94) = 20.47, p < .001, η2P = .376): MAP increased from acclimation to reactivity (p < .001) and decreased from reactivity to recovery (p < .001). A marginally significant time-by-sexual orientation interaction effect was detected (F(1.56,53.16) = 3.30, p = .056, η2P = .089). Post-hoc analysis revealed that lesbian/bisexual women had marginally higher MAP than heterosexual women during the reactivity phase (p = .054, η2P = .105), but not during acclimation or recovery. The between-subjects effect for sexual orientation was marginally significant (F(1,34) = 3.12, p = .086, η2P = .084), whereby lesbian/bisexual women showed higher overall MAP than heterosexual women. In supplemental analyses assessing blood pressure separately, between-group differences were marginally higher among lesbian/bisexual women in contrast to heterosexual women for DBP (p = .076, η2P = .090) and were non-significant for SBP (p = .195, η2P = .049).
For MAP among men (), a within-subjects time effect was significant (F(1.42,56.77) = 25.93, p < .001, η2P = .393): MAP increased from acclimation to reactivity (p < .001) and decreased from reactivity to recovery (p < .001). No group difference was found between gay/bisexual men and heterosexual men in MAP (p = .978, η2p < .001) nor in supplemental analyses assessing SBP and DBP separately.
Disclosure status in relation to cardiovascular stress reactivity among sexual minorities
The following mixed-design repeated-measures ANCOVAs re-assessed HR and MAP as a function of disclosure status and sex while adjusting for race/ethnicity, education, mastery, and visit order in the full sample. Here, we did not disaggregate analyses by sex but rather entered sex as a covariate/moderator to preserve power and to ascertain if sex differences were present among the LGB individuals. The groups were divided as follows for those who had completely disclosed (men: n = 19, women: n = 12) and those who had not fully disclosed (men: n = 7, women: n = 7) their sexual orientation to family and friends.
For HR, no between-group differences were found for HR by disclosure status (p = .239, η2P = .037), sex (p = .656, η2P = .005), or disclosure status X sex interaction (p = .931, η2P < .001).
For MAP, we detected a time X disclosure status X sex interaction effect (F(1.42,52.42) = 4.13, p = .034, η2P = .100); however, follow-up analyses did not attain significance. While non-significant, sexual minority women who had not disclosed showed a tendency towards higher MAP during reactivity. No between-group differences were found for MAP by disclosure status (p = .401, η2P = .019), sex (p = .973, η2P<.001), or disclosure status X sex interaction (p = .651, η2P = .006). Results remained non-significant when assessing SBP and DBP separately.
Discussion
The current study provides preliminary evidence that cardiovascular stress reactivity may differ by sexual orientation. Using a well-established psychosocial stress paradigm (Allen et al., Citation2017), we found that gay/bisexual men showed higher HR compared to heterosexual men. By contrast, lesbian/bisexual women showed marginally higher MAP compared to heterosexual women; however, this result was only at the trend level. Those LGB individuals who had not completely disclosed their sexual orientation to family and friends did not show significant differences in cardiovascular parameters during the acclimation, reactivity, and recovery phases. This study contributes to small literature on stress reactive biomarkers among LGB individuals, a health disparities population. All the same, we must be cautious in our interpretations, given marginal group differences and the number of non-significant findings which may alternatively suggest that there are, in fact, no sexual orientation differences in cardiovascular stress reactivity.
Compared to heterosexual men, sexual minority men evidenced higher HR not only throughout the TSST reactivity phases but also during the acclimation phase of the 2-hour laboratory visit. The magnitude of this effect is medium to large and this provides the first report of cardiovascular stress reactivity differences as a function of sexual orientation in men. Increased HR via the autonomic nervous system among gay/bisexual men is a pattern consistent with a large body of literature among racial/ethnic minority populations that likewise report an increased cardiovascular reactivity/recovery due to discrimination (Lick et al., Citation2013; Williams & Mohammed, Citation2009). Future research using more sophisticated measures of autonomic nervous system functioning (e.g. heart rate variability) is warranted. Moreover, continuous measures of high-frequency cardiovascular activity during specific tasks would allow a more nuanced assessment of stress experienced during the job talk versus mathematics phases of the TSST. In contrast to HR, we found no evidence for differences between men for MAP, which is inconsistent with a report of a two-fold increased risk for hypertension among gay men (Everett & Mollborn, Citation2013). It is noteworthy that past research assessing CVD risk among LGB individuals used measures like diagnosis, self-reports, and basal biomarkers that cannot be easily compared to our use of biomarkers representing acclimation, reactivity, and recovery.
In contrast to sexual minority men, sexual minority women appear to exhibit marginally amplified MAP reactivity relative to heterosexuals, but otherwise, no differences in HR. Specifically, lesbian/bisexual women showed marginally elevated overall and reactive MAP compared to heterosexual women, a finding of medium effect magnitude and at trend level. As our study is the first to report on cardiovascular stress reactivity in sexual minority women, we can only compare our findings to previous research on basal biomarkers related to CVD, which showed no sexual orientation differences in hypertension for women (Everett & Mollborn, Citation2013). The current findings provide preliminary evidence that sexual minority women may show marginally higher blood pressure in a stress reactivity paradigm than heterosexual women. As this result was at a trend level, we reserve caution in concluding that blood pressure differs by sexual orientation for women. This will need to be replicated and compared with other physiological systems.
Our analyses did not identify differences in cardiovascular stress reactivity according to disclosure. We must be cautious about this conclusion, however, given our limited power and sample heterogeneity. Since LGB individuals who conceal their sexual orientation are inhibiting a wide range of complex behaviors (Pachankis, Citation2007), they are likely allocating resources to this inhibition at a potential physiological price (Juster et al., Citation2013; Pérez-Benitez et al., Citation2007). On the other hand, past research indicates that disclosure may not always be associated with better health outcomes for sexual minorities (Doyle and Molix, Citation2016). For example, increased harassment, victimization, distress, HPA-axis production, and suicidality can occur as part of disclosing (D'Augelli, Citation2002; Huebner, Rebchook, & Kegeles, Citation2004; Igartua, Gill, & Montoro, Citation2003; McGregor et al., Citation2001; Oetjen and Rothblum, Citation2000; Waldo, Citation1999). Therefore, it is important to consider broader contextual factors and individual differences that may modify the salutogenic and/or pathogenic effects of disclosure processes. Lastly, sexual identity formation and disclosure are complex processes that were crudely captured in our binary classification that would benefit from more refined measurement in future work.
Limitations
Despite medium to large effect sizes, our results are derived from a small convenience sample measured at one-time point. Additional psychosocial variables (e.g. relationship factors, health behaviors) should be considered to fully understand CVD risk among LGB individuals (Frech, Lynch, & Barr, Citation2016). CVD is influenced by numerous health behaviors like diet, smoking, alcohol consumption, exercise, and sleep that may function as moderators. In addition, variables like mastery that we included as a covariate might be involved in causal pathways that link sexual orientation (and minority stigma) to cardiovascular reactivity, since it is possible that having a history of being stigmatized might result in increased reactivity to social stressors in a laboratory setting. Larger sample sizes would allow the future researcher to test mediation or moderation effects of such health behaviors and psychosocial profiles.
In addition, CVD risk factors may cluster differently among subgroups of the LGB community (IOM, Citation2011). Moreover, inconsistencies in how CVD risk factors, biomarkers, and disorders are measured in the literature might lead to discrepancies that future research should consider (Caceres, et al., Citation2016). Lastly, our study did not include transgender people that evidence stress biomarker profiles that appear to increase CVD risk (Dubois, Citation2012; DuBois, Powers, Everett, & Juster, Citation2017). Finally, the sex of participants interacts with the sex of TSST behavioral judges (Goodman, Janson, & Wolf, Citation2017); however, 87% of our participants interacted with a female TSST behavioral judge, a distribution that should be better balanced in future studies. Despite these limitations, our preliminary study contributes to small literature on sexual orientation differences in cardiovascular health and points to several areas for future research on stress reactivity within the context of sexual orientation.
Future directions
This preliminary study provides several directions for future research. First, the current cardiovascular stress reactivity findings among men (i.e. gay/bisexual men show amplified HR throughout the TSST session compared to heterosexual men) complement our previous report that showed low HPA-axis reactivity among gay/bisexual men in this same sample (Juster et al., Citation2015). These findings collectively suggest a counter-regulation of HPA-axis and cardiovascular functioning among sexual minority men. It is not, however, clear whether these profiles can be considered maladaptive. Noteworthy as well in this sample is that other risk factors for CVD (BMI, triglycerides) were actually lower among sexual minority men (Juster et al., Citation2013). The compiled findings using this sample suggest that changes in one physiological system examined individually may draw an incomplete picture of the underlying stress processes involved. Instead, attending to coordinated change across multiple systems and their dynamic alignment using multi-systemic approaches is ideally suited to evaluating the correlates of social adversities (Lucas et al., Citation2017). We suggest that future studies employ more rigorous measurement of these systems with more frequent sampling (e.g. heart rate variability).
Second, life course perspectives must be considered when assessing the stigma-related determinants of CVD trajectories among LGB populations. However, the current study did not measure sources of stigma experienced throughout the life course, specifically related to one’s sexual orientation other than our crude measure of the disclosure. As these sources of sexual minority stigma can have long-term effects on CVD (Lick et al., Citation2013), future prospective research (Katz-Wise et al., Citation2017a) is required to determine how stigma “gets under the skin” of LGB individuals across life and across generations. It has been recommended that measures of victimization should use standard timeframes (e.g. lifetime reports, past year reports, before/after “coming out” reports) to better understand developmental variation and age trends (Katz-Wise & Hyde, Citation2012). The timing of sexual orientation development is also important to consider (Katz-Wise et al., Citation2017b). For example, those with earlier timing of same-sex/gender sexual experiences are at greater risk of mental health problems than those who experience such experiences at a later age when more emotionally and cognitively mature (Katz-Wise et al., Citation2017a).
Third, stress reactivity paradigms are inherently limited in their lab-to-life generalizability. It has been argued that cardiovascular reactivity in the laboratory is at best only moderately generalizable to non-laboratory situations, however, psychosocial stress paradigms (e.g. TSST) may be more representative of daily life stressors than cognitive or physical tasks routinely used in the cardiovascular reactivity literature (Schwartz et al., Citation2003). Lastly, future research should assess genetic, environmental, and behavioral risk factors together when assessing cardiovascular endophenotypes (Collaboration, Citation2017; Schwartz et al., Citation2003) to broaden our understanding of key mechanisms. In addition, we encourage future studies to consider measuring both sex-based and gender-based variables to refine understanding of subgroup differences that cannot be detected by focusing solely on male/female distinctions.
Conclusions
Due to increased exposure to stigma, it has been proposed that sexual minorities may be at increased risk of cardiovascular disease. To date, the literature supporting this proposal has been mixed. Using a stress reactivity paradigm among a small convenience sample, we conclude that gay/bisexual men evidence a higher heart rate during acclimation and reactivity phases compared to heterosexual men. Marginal differences were found for lesbian/bisexual women showing higher blood pressure than heterosexual women. These preliminary findings will need to be replicated in future studies that use more comprehensive approaches to assess the heart health of sexual and gender minority populations.
Acknowledgments
Robert Paul Juster held a Doctoral scholarship from the Institute of Aging of the Canadian Institutes of Health Research [SIA 95402] during data collection of this manuscript. Current support during manuscript preparation is thanks to the Canadian Institutes of Health Research Banting postdoctoral fellowship. We wish to thank our participants for their commitment to this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Robert-Paul Juster
Dr. Juster is an Assistant Research Professor in the Department of Psychiatry and Addiction at the University of Montreal. His research focuses on nuancing sex and gender factors that influence vulnerability or resilient to stress-related disease.
David Matthew Doyle
Dr. Doyle is a Lecturer in Social Psychology at University of Exeter. His interdisciplinary program of research focuses on social identities, relationships, and health, with the aim of remediating social disparities.
Mark L. Hatzenbuehler
Dr. Hatzenbuehler is Assistant Professor in Sociomedical Sciences and Sociology at Columbia University. His research focuses on: 1) documenting the role of structural stigma in shaping adverse health outcomes among members of socially disadvantaged populations, and 2) identifying biopsychosocial mechanisms linking stigma and health.
Bethany G. Everett
Dr. Everett is an Assistant Professor in the Department of Sociology at the University of Utah. Her research focuses on the impact of minority stress, both interpersonal and structural, on sexual and gender minority health.
L. Zachary DuBois
Dr. DuBois is Assistant Professor in the Department of Anthropology at the Univeristy of Oregon. His research aims to improve understanding of how social inequality and stigma become embodied and contribute to health disparities among trans and gender diverse people.
Jennifer J. McGrath
Dr. McGrath is an Associate Professor in the Department of Psychology at Concordia University. Her research examines the pathogenesis of subclinical disease markers (cardiovascular reactivity, metabolic functioning, cardiac structural changes) across childhood and adolescence as mediated by potential behavioral, environmental, and psychological mechanisms.
References
- Allen, A.P., Kennedy, P.J., Dockray, S., Cryan, J.F., Dinan, T.G., & Clarke, G. (2017). The Trier Social Stress Test: Principles and practice. Neurobiology of Stress, 6, 113–126. doi:10.1016/j.ynstr.2016.11.001
- Andrews, J., Wadiwalla, M., Juster, R.P., Lord, C., Lupien, S.J., & Pruessner, J.C. (2007). Effects of manipulating the amount of social-evaluative threat on the cortisol stress response in young healthy men. Behavioral Neuroscience, 121, 871–876. doi:2007-13974-006.
- Brosschot, J.F. (2010). Markers of chronic stress: Prolonged physiological activation and (un)conscious perseverative cognition. Neuroscience and Biobehavioural Review, 35, 46–50. doi:S0149-7634(10)00005-9
- Brosschot, J.F., Pieper, S., & Thayer, J.F. (2005). Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology, 30, 1043–1049. doi:10.1016/j.psyneuen.2005.04.008
- Caceres, B.A., Brody, A., & Chyun, D. (2016). Recommendations for cardiovascular disease research with lesbian, gay and bisexual adults. Journal of Clinical Nursing, 25, 3728–3742. doi:10.1111/jocn.13415
- Caceres, B.A., Brody, A., Luscombe, R.E., Primiano, J.E., Marusca, P., Sitts, E.M., & Chyun, D. (2017). A Systematic review of cardiovascular disease in sexual minorities. American Journal of Public Health, 107, 570. doi:10.2105/AJPH.2016.303630a.
- Chida, Y., & Hamer, M. (2008). Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin, 134, 829–885. doi:2008-14745-003
- Clark, C.J., Borowsky, I.W., Salisbury, J., Usher, J., Spencer, R.A., Przedworski, J.M., … Everson-Rose, S.A. (2015). Disparities in long-term cardiovascular disease risk by sexual identity: The National Longitudinal Study of Adolescent to Adult Health. Prev Med, 76, 26–30. doi:10.1016/j.ypmed.2015.03.022
- Collaboration, N.C.D.R.F. (2017). Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet, 389, 37–55. doi:10.1016/S0140-6736(16)31919-5
- Conron, K.J., Mimiaga, M.J., & Landers, S.J. (2010). A population-based study of sexual orientation identity and gender differences in adult health. American Journal of Public Health, 100, 1953–1960. doi:10.2105/AJPH.2009.174169
- D'Augelli, A.R. (2002). Mental health problems among lesbian, gay, and bisexual youths ages 14 to 21. Clinical Child Psychology and Psychiatry, 7, 433–456. doi:10.1177/1359104502007003039
- Dickerson, S.S., & Kemeny, M.E. (2002). Acute stressors and cortisol reactivity: a meta-analytic review. Psychosomatic Medicine, 54, 105–123.
- Doyle, D.M., & Molix, L. (2016). Minority stress and inflammatory mediators: covering moderates associations between perceived discrimination and salivary interleukin-6 in gay men. Journal of Behavioral Medicine, 39, 782–792. doi:10.1007/s10865-016-9784-0
- Dubois, L.Z. (2012). Associations between transition-specific stress experience, nocturnal decline in ambulatory blood pressure, and C-reactive protein levels among transgender men. American Journal of Human Biology, 24, 52–61. doi:10.1002/ajhb.22203
- DuBois, L.Z., Powers, S., Everett, B.G., & Juster, R.P. (2017). Stigma and diurnal cortisol among transitioning transgender men. Psychoneuroendocrinology, 82, 59–66. doi:10.1016/j.psyneuen.2017.05.008
- Earle, T.L., Linden, W., & Weinberg, J. (1999). Differential effects of harassment on cardiovascular and salivary cortisol stress reactivity and recovery in women and men. Journal of Psychosomatic Research, 46, 125–141. doi:S00223999980007501
- Everett, B., & Mollborn, S. (2013). Differences in hypertension by sexual orientation among U.S. young adults. Journal of Community Health, 38, 588–596. doi:10.1007/s10900-013-9655-3
- Farmer, G.W., Bucholz, K.K., Flick, L.H., Burroughs, T.E., & Bowen, D.J. (2013). CVD risk among men participating in the National Health and Nutrition Examination Survey (NHANES) from 2001 to 2010: differences by sexual minority status. Journal of Epidemiology and Community Health, 67, 772–778. doi:10.1136/jech-2013-202658
- Farmer, G.W., Jabson, J.M., Bucholz, K.K., & Bowen, D.J. (2013). A population-based study of cardiovascular disease risk in sexual-minority women. American Journal of Public Health, 103, 1845–1850. doi:10.2105/AJPH.2013.301258
- Frech, A., Lynch, J.L., & Barr, P. (2016). Health consequences of same and opposite-sex unions: partnership, parenthood, and cardiovascular risk among young adults. Journal of Behavioral Medicine, 39, 13–27. doi:10.1007/s10865-015-9673-y
- Fries, E., Hesse, J., Hellhammer, J., & Hellhammer, D.H. (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30, 1010–1016. doi:S0306-4530(05)00089-2
- Fritz, C.O., Morris, P.E., & Richler, J.J. (2012). Effect size estimates: Current use, calculations, and interpretation. Journal of Experimental Psychology: General, 141, 2–18. doi:10.1037/a0024338
- Goodman, W.K., Janson, J., & Wolf, J.M. (2017). Meta-analytical assessment of the effects of protocol variations on cortisol responses to the Trier Social Stress Test. Psychoneuroendocrinology, 80, 26–35. doi:10.1016/j.psyneuen.2017.02.030
- Hatzenbuehler, M.L., & McLaughlin, K.A. (2014). Structural stigma and hypothalamic-pituitary-adrenocortical axis reactivity in lesbian, gay, and bisexual young adults. Annals of Behavioral Medicine, 47, 39–47. doi:10.1007/s12160-013-9556-9
- Huebner, D.M., Rebchook, G.M., & Kegeles, S.M. (2004). Experiences of harassment, discrimination, and physical violence among young gay and bisexual men. American Journal of Public Health, 94, 1200–1203. doi:10.2105/AJPH.94.7.1200
- Igartua, K.J., Gill, K., & Montoro, R. (2003). Internalized homophobia: A factor in depression, anxiety, and suicide in the gay and lesbian population. Canadian Journal of Community Mental Health, 22, 15–30. doi:10.7870/cjcmh-2003-00111
- IOM. (2011). The health of lesbian, gay, bisexual, and transgender people: Building a foundation for better understanding. Washinton, DC: The National Academies Press.
- Jacobson, R., Cohen, H., & Diamond, G.M. (2016). Gender atypicality and anxiety response to social interaction stress in homosexual and heterosexual men. Archives of Sexual Behavior, 45, 713–723. doi:10.1007/s10508-015-0528-y
- Juster, R.-P., Almeida, D., Cardoso, C., Raymond, C., Johnson, P.J., Pfaus, J.G., … Lupien, S.J. (2016). Gonads and strife: Sex hormones vary according to sexual orientation for women and stress indices for both sexes. Psychoneuroendocrinology, 72, 119–130. doi:10.1016/j.psyneuen.2016.06.011
- Juster, R.P., bVencill, J.A., & Johnson, P.J. (2017). Impact of stress and strain on current LGBT health disparities. In K. Eckstrand & J. Potter (Eds.), Trauma, resilience, and health promotion for lgbt patients: what every healthcare provider should know (Vol. 1, pp. 35–48). New York: Springer Press.
- Juster, R.-P., Hatzenbuehler, M.L., Mendrek, A., Pfaus, J.G., Smith, N.G., Johnson, P.J., … Pruessner, J.C. (2015). Sexual orientation modulates endocrine stress reactivity. Biological Psychiatry, 77, 668–676. doi:10.1016/j.biopsych.2014.08.013
- Juster, R.P., Ouellet, E., Lefebvre-Louis, J.P., Sindi, S., Johnson, P.J., Smith, N.G., & Lupien, S.J. (2016). Retrospective coping strategies during sexual identity formation and current biopsychosocial stress. Anxiety Stress Coping, 29, 119–138. doi:10.1080/10615806.2015.1004324
- Juster, R.P., Smith, N.G., Ouellet, E., Sindi, S., & Lupien, S.J. (2013). Sexual orientation and disclosure in relation to psychiatric symptoms, diurnal cortisol, and allostatic load. Psychosomatic Medicine, 75, 103–116. doi:10.1097/PSY.0b013e3182826881
- Kamarck, T.W., Debski, T.T., & Manuck, S.B. (2000). Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology, 37, 533–542. doi:10.1111/1469-8986.3740533
- Katz-Wise, S.L., & Hyde, J.S. (2012). Victimization experiences of lesbian, gay, and bisexual individuals: A meta-analysis. Journal of Sex Research, 49, 142–167. doi:10.1080/00224499.2011.637247
- Katz-Wise, S.L., Rosario, M., Calzo, J.P., Scherer, E.A., Sarda, V., & Austin, S.B. (2017a). Associations of timing of sexual orientation developmental milestones and other sexual minority stressors with internalizing mental health symptoms among sexual minority young adults. Archives of Sexual Behavior, 46, 1441–1452. doi:10.1007/s10508-017-0964-y
- Katz-Wise, S.L., Rosario, M., Calzo, J.P., Scherer, E.A., Sarda, V., & Austin, S.B. (2017b). Endorsement and timing of sexual orientation developmental milestones among sexual minority young adults in the growing up today study. Journal of Sex Research, 54, 172–185. doi:10.1080/00224499.2016.1170757
- Kirschbaum, C., Pirke, K.M., & Hellhammer, D.H. (1993). The 'Trier Social Stress Test'–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28, 76–81. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8255414 doi:119004.
- Klein, F., Sepekoff, B., & Wolf, T.J. (1990). Sexual orientation: A multivariable dynamic process. In T. Geller (Ed.), Bisexuality: A reader and sourcebook. (Vol. 33, pp. 1915–1920). Ojai, CA: Times Change Press.
- Lick, D.J., Durso, L.E., & Johnson, K.L. (2013). Minority stress and physical health among sexual minorities. Perspectives on Psychological Science, 8, 521–548. doi:10.1177/1745691613497965
- Linden, W., Earle, T.L., Gerin, W., & Christenfeld, N. (1997). Physiological stress reactivity and recovery: Conceptual siblings separated at birth? Journal of Psychosomatic Research, 42, 117–135. doi:S0022399996002401
- Lovallo, W.R. (2010). Cardiovascular responses to stress and disease outcomes: A test of the reactivity hypothesis. Hypertension, 55, 842–843. doi:AHA.110.149773
- Lovallo, W.R., & Gerin, W. (2003). Psychophysiological reactivity: Mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine, 65, 36–45. doi:10.1097/01.PSY.0000033128.44101.C1
- Lucas, T., Wegner, R., Pierce, J., Lumley, M.A., Laurent, H.K., & Granger, D.A. (2017). Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosomatic Medicine, 79, 293–305. doi:10.1097/PSY.0000000000000406
- Manuck, S.B. (1994). Cardiovascular reactivity in cardiovascular disease: "once more unto the breach". International Journal of Behavioral Medicine, 1, 4–31. doi:10.1207/s15327558ijbm0101_2
- McEwen, B.S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. doi:10.1111/j.1749-6632.1998.tb09546.x
- McGregor, B.A., Carver, C.S., Antoni, M.H., Weiss, S., Yount, S.E., & Ironson, G. (2001). Distress and internalized homophobia among lesbian women treated for early stage breast cancer. Psychology of Women Quarterly, 25, 1–8. doi:10.1111/1471-6402.00001
- Mozaffarian, D., Benjamin, E.J., Go, A.S., Arnett, D.K., Blaha, M.J., Cushman, M., … Stroke Statistics, S. (2015). Heart disease and stroke statistics–2015 update: A report from the American Heart Association. Circulation, 131, e29–322. doi:10.1161/CIR.0000000000000152
- NIH. (2014). Monitoring adherance to the NIH policy on the inclusion of women and minorities as subjects in clinical research: Tracking on human subjects research as reported in the fiscal year 2009 and fiscal year 2010. Bethesda: National Institutes of Health. Retrieved from http://orwh.od.nih.gov/resources/policyreports/index.asp.
- Oetjen, H., & Rothblum, E.D. (2000). When lesbians aren't gay: Factors affecting depression among lesbians. Journal of Homosexuality, 39, 49–73. doi:10.1300/J082v39n01_04
- Pachankis, J.E. (2007). The psychological implications of concealing a stigma: a cognitive-affective-behavioral model. Psychological Bulletin, 133, 328–345. doi:2007-02367-008
- Pearlin, L.I., & Schooler, C. (1978). The structure of coping. Journal of Health and Social Behaviour, 19, 2–21. doi:10.2307/2136319
- Pérez-Benitez, C.O., O'Brien, W.H., Carels, R.A., Gordon, A.K., & Chiros, C.E. (2007). Cardiovascular correlates of disclosing homosexual orientation. Stress and Health, 23, 141–152. doi:10.1002/smi.1123
- Roepke, S.K., Mausbach, B.T., Aschbacher, K., Ziegler, M.G., Dimsdale, J.E., Mills, P.J., … Grant, I. (2008). Personal mastery is associated with reduced sympathetic arousal in stressed Alzheimer caregivers. The American Journal of Geriatric Psychiatry, 16, 310–317. doi:10.1097/JGP.0b013e3181662a80
- Rutledge, T., Linden, W., & Paul, D. (2000). Cardiovascular recovery from acute laboratory stress: Reliability and concurrent validity. Psychosomatic Medicine, 62, 648–654. doi:10.1097/00006842-200009000-00008
- Schwartz, A.R., Gerin, W., Davidson, K.W., Pickering, T.G., Brosschot, J.F., Thayer, J.F., … Linden, W. (2003). Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosomatic Medicine, 65, 22–35. doi:10.1097/01.PSY.0000046075.79922.61
- Simoni, J.M., Smith, L., Oost, K.M., Lehavot, K., & Fredriksen-Goldsen, K. (2017). Disparities in physical health conditions among lesbian and bisexual women: A systematic review of population-based studies. Journal of Homosexuality, 64, 32–44. doi:10.1080/00918369.2016.1174021
- Sindi, S., Fiocco, A.J., Juster, R.P., Pruessner, J., & Lupien, S.J. (2013). When we test, do we stress? Impact of the testing environment on cortisol secretion and memory performance in older adults. Psychoneuroendocrinology, 38, 1388–1396. doi:10.1016/j.psyneuen.2012.12.004
- Treiber, F.A., Kamarck, T., Schneiderman, N., Sheffield, D., Kapuku, G., & Taylor, T. (2003). Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine, 65, 46–62. doi:10.1097/00006842-200301000-00007
- Wadiwalla, M., Andrews, J., Lai, B., Buss, C., Lupien, S.J., & Pruessner, J.C. (2010). Effects of manipulating the amount of social-evaluative threat on the cortisol stress response in young healthy women. Stress, 13, 214–220. doi:10.3109/10253890903277561
- Waldo, C.R. (1999). Working in a majority context: A structural model of heterosexism as minority stress in the workplace. Journal of Couseling Psychology, 46, 218–232. doi:10.1037/0022-0167.46.2.218
- Williams, D.R., & Mohammed, S.A. (2009). Discrimination and racial disparities in health: evidence and needed research. Journal of Behavioral Medicine, 32, 20–47. doi:10.1007/s10865-008-9185-0
- Xu, J.Q., Murphy, S.L., Kochanek, K.D., & Bastian, B.A. (2016). Deaths: Final data for the 2013, (Vol. 16, pp. 1–119). Hyattsville, MD: National Vital Statistics Report.

