Abstract
Stress is a common cause of neuropsychiatric disorders, evoking multiple behavioral, endocrine and neuro-immune deficits. Animal models have been extensively used to understand the mechanisms of stress-related disorders and to develop novel strategies for their treatment. Complementing rodent and clinical studies, the zebrafish (Danio rerio) is one of the most important model organisms in biomedicine. Rapidly becoming a popular model species in stress neuroscience research, zebrafish are highly sensitive to both acute and chronic stress, and show robust, well-defined behavioral and physiological stress responses. Here, we critically evaluate the utility of zebrafish-based models for studying acute and chronic stress-related CNS pathogenesis, assess the advantages and limitations of these aquatic models, and emphasize their relevance for the development of novel anti-stress therapies. Overall, the zebrafish emerges as a powerful and sensitive model organism for stress research. Although these fish generally display evolutionarily conserved behavioral and physiological responses to stress, zebrafish-specific aspects of neurogenesis, neuroprotection and neuro-immune responses may be particularly interesting to explore further, as they may offer additional insights into stress pathogenesis that complement (rather than merely replicate) rodent findings. Compared to mammals, zebrafish models are also characterized by increased availability of gene-editing tools and higher throughput of drug screening, thus being able to uniquely empower translational research of genetic determinants of stress and resilience, as well as to foster innovative CNS drug discovery and the development of novel anti-stress therapies.
1. Introduction
Stress is a common cause of various human neuropsychiatric illnesses, especially affective disorders, such as depression, anxiety, panic, phobias, adjustment disorder and post-traumatic stress disorder (PTSD) (Cohen, Janicki-Deverts, & Miller, Citation2007; Cohen & Williamson, Citation1991; McEwen & Stellar, Citation1993). Acting via multiple physiological mechanisms, both acute and chronic types of stress affect the central nervous system (CNS) (Carlson & Rosser-Hogan, Citation1991; Johansson et al., Citation2010; Lee et al., Citation2015; Resnick, Bond, & Mueser, Citation2003), dysregulating brain neurotransmitters and hormones (Conrad, Citation2008; McGonigle, Citation2014). Acute stress evokes rapid alterations in neuronal activity, accompanied by endocrine and neurotransmitter release, quickly to reestablish physiological homeostasis (Joëls & Baram, Citation2009). In contrast, chronic stress involves repeated or prolonged action of stressor/s, inducing sustained changes in neurotransmitters, hormones and gene expression that pathologically affect multiple systems (Bisht, Sharma, & Tremblay, Citation2018; McEwen, Citation2017; Zaletel, Filipović, & Puškaš, Citation2016).
Widespread, poorly understood and treatment-resistant, stress-related neuropsychiatric disorders continue to represent an urgent unmet biomedical problem (Cohen et al., Citation2007; Cohen & Williamson, Citation1991; Lee et al., Citation2015; McEwen & Stellar, Citation1993). Because studying human stress is often limited ethically and/or methodologically, animal models become a powerful tool in stress neuroscience research (Bale et al., Citation2019; Nestler & Hyman, Citation2010). Although rodents are currently the most widely used organisms for CNS disease modeling, the zebrafish (Danio rerio) has emerged as one of popular non-rodent species in biomedicine, including translational neuroscience and CNS drug discovery (Kalueff, Stewart, & Gerlai, Citation2014; Stewart, Braubach, Spitsbergen, Gerlai, & Kalueff, Citation2014). Demonstrating multiple practical advantages and genetic homology to humans (Barbazuk et al., Citation2000), zebrafish also have analogs of most structures of the human brain, and utilize similar neurotransmitter and neuroendocrine systems for neurobehavioral modulation (Panula et al., Citation2010). Additionally, zebrafish express complex behavior spanning several major domains (e.g. locomotor activity, emotionality, reward, sociality and aggression) that are all shared with mammals and humans (Norton & Bally-Cuif, Citation2010; Stewart, et al., Citation2014).
Importantly, zebrafish possess well-expressed neuroendocrine stress axis (see further) and display robust stress-related behaviors that can be reliably assessed in various tests (). Briefly summarized in and , these tests are conceptually based on similar rodent paradigms (Stewart et al., Citation2012), with shared patterns of sensitivity to, and good predictive validity for, major classes of CNS drugs (Cachat et al., Citation2010, Citation2011; Kysil et al., Citation2017; Rihel & Schier, Citation2012; Steenbergen, Richardson, & Champagne, Citation2011). However, as zebrafish are becoming an attractive model organism for studying stress-related CNS pathogenesis, several important questions arise. For example, if zebrafish is an appropriate model for mimicking human stress disorders, do their behavioral and physiological responses differ in acute vs. chronic stress? (see (Kirsten, et al., Citation2020) for a recent discussion). And, if yes, do these commonalities and differences parallel rodent and human responses to acute vs. chronic stress? Furthermore, do zebrafish models respond to anti-stress (e.g. anxiolytic or antidepressant) pharmacotherapy in a manner similar (or different) to their mammalian counterparts? To address these and other related questions, here we critically evaluate the utility of zebrafish-based models for studying acute and chronic stress-related CNS pathogenesis, discuss their advantages and limitations, and overview their relevance for the development of novel anti-stress treatments.
Figure 1. Common behavioral tests to assess stress/anxiety in zebrafish (see for examples of potential stressors).
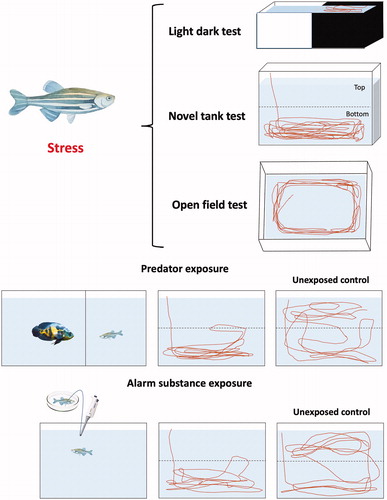
Table 1. Selected acute stress-induced behaviors assessed in zebrafish tests (see for examples).
Table 2. Comparison of behavioral and neuroendocrine effects of acute vs. chronic stress in zebrafish.
2. Stress responses in zebrafish
Zebrafish stress response is mediated by the hypothalamic-pituitary-interrenal (HPI) axis that is functionally and structurally homologous to the mammalian hypothalamic-pituitary-adrenal (HPA) axis (Alsop & Vijayan, Citation2008, Citation2009). Across taxa, catecholamines and corticosteroids are the primary facilitators of stress response. The former are released almost immediately after stress exposure (Mazeaud, Mazeaud, & Donaldson Citation1977; Pickering, Citation1998; Sumpter, Citation1997; Wendelaar Bonga, Citation1997), and the latter are activated by corticotropin-releasing factor (CRF) minutes later (Ghisleni et al., Citation2012). Regulating multiple physiological functions, cortisol is an important glucocorticoid hormone involved in stress responses in both humans and zebrafish (unlike rodents, which mainly utilize corticosterone). Cortisol acts at the glucocorticoid receptor (GR), which itself is very similar between the two species, both genetically and functionally (Schaaf et al., Citation2008). Activated GRs alter the transcription of multiple target genes to induce robust metabolic, neurobehavioral and structural responses (Joëls & Baram, Citation2009; Schaaf, Chatzopoulou, & Spaink, Citation2009).
In teleost fishes, the HPI axis includes the hypothalamus (especially, nucleus preopticus, NPO, homologous to the mammalian paraventricular nucleus, PVN) that, upon stress exposure, induces the adrenocorticotropic hormone (ACTH)-CRF cascade to stimulate synthesis and release of cortisol by the interrenal tissue (Sumpter, Pottinger, Rand-Weaver, & Campbell, Citation1994; Wendelaar Bonga, Citation1997). Despite the genome duplication event in teleost fishes, zebrafish CRF is encoded by a single gene, like other major components of the HPI axis (Alsop & Vijayan, Citation2009). CRF, released by NPO in the hypothalamus, also stimulates the release of proopiomelanocortin (POMC) in the anterior pituitary gland, reaching it via direct projections from NPO (Lederis, Fryer, Okawara, Schönrock, & Richter Citation1994). The effects of CRF are mediated by its CRFR1 receptor (a typical G protein-coupled receptor, GPCR) and modulated by the CRF binding protein, CRF-BP (Huising et al., Citation2004). CRFR1 is highly structurally conserved between both teleosts and mammals (Huising et al., Citation2004). While two (CRFR1 and CRFR2) receptors have been characterized in fish (e.g. in carp), the stress response is usually attributed to CRFR1, as its mRNA levels are reduced by stress (Huising et al., Citation2004). The function of vertebrate CRFR2 can be outside of immediate stress axis control, but may contribute to delayed post-stress anxiolysis via a homeostatic biofeedback mechanism, as shown in acutely stressed crfr2−/− mice (Bale, Lee, & Vale, Citation2002; Kishimoto et al., Citation2000). Although fish also facilitate the release of thyroid-stimulating hormone (TRH) following CRFR2 activation (De Groef, Goris, Arckens, Kuhn, & Darras, Citation2003), the impact of this receptor on their stress behaviors remains unclear. Thus, since a crfr2 orthologue occurs in zebrafish, future research may address the role of this receptor in stress-related disorders.
Another component of the CRF signaling, CRF-BP, is highly conserved, as its sequence is almost identical in all vertebrates ((Flik, Klaren, Van den Burg, Metz, & Huising, Citation2006; Huising & Flik, Citation2005). CRF and CRF-BP are most abundantly expressed in the interrenal tissue of teleost fishes (e.g. carp (Huising et al., Citation2007)), as well as in zebrafish brain (e.g. the preoptic area, including tectum, trigeminal motor nucleus and ventral telencephalon) (Alderman & Bernier, Citation2007). While the exact physiological function of CRF-BP is unclear, the presence of CRF and CRF-BP in the interrenal and brain tissue suggests a pathway homologous to the mammalian CRF system (Huising et al., Citation2007). Likely involved in fish stress responses, given its upregulation in carp hypothalamus following acute restraint stress (Huising et al., Citation2004), this protein merits further scrutiny in zebrafish models of stress.
POMC is also an evolutionary conserved polypeptide found in various species, including humans (Chang, Cochet, & Cohen, Citation1980) and fishes, such as carp (Arends et al., Citation1998) and zebrafish (Gonzalez-Nunez, Gonzalez-Sarmiento, & Rodriguez, Citation2003). In stress, the POMC cleavage by prohormone convertases in cortico- and melanotropic cells of the adenohypophysis generates several important bioactive products that are essential to stress response (Metz, Peters, & Flik, Citation2006; Morash, MacDonald, Croll, & Anini, Citation2009). For example, tissue-specific convertases produce ACTH, α- and β-MSH (melanocyte-stimulating hormone) and various endorphins (Gonzalez-Nunez et al., Citation2003). Likewise, β-endorphin is implicated in the regulation of stress response via the feedback inhibition of CRF expression (Sarkar, Kuhn, Marano, Chen, & Boyadjieva, Citation2007), also inducing CRF secretion in the isolated rat hypothalamus (Buckingham, Citation1986). This function of β-endorphin raises the question of direct role of endogenous opioids in stress response, and their potential indirect role (e.g. via the modulation of the stress axis), especially in chronic stress. Supporting the translational relevance of zebrafish models for such studies, their opioidergic system shows a generally high level of conservation, with ∼61% opioid genes being homologous, in humans and zebrafish (Demin et al., Citation2018).
Like in other vertebrates, ACTH is the primary hormone responsible for stimulating cortisol biosynthesis and secretion in fish (Wendelaar Bonga, Citation1997). Zebrafish ACTH is encoded by a single gene, due to the disappearance of a prohormone convertase cleavage site in the zebrafish POMC-like peptide following a genome duplication (Alsop & Vijayan, Citation2009; Gonzalez-Nunez et al., Citation2003). While the genomes of teleost fishes and mammals include five melanocortin receptors (MC1R–MC5R), zebrafish express six of them, including two MC5Rs (Logan et al., Citation2003). In various species, MC1R regulates the synthesis of dark eumelanin, MC3R and MC4R regulate feeding behavior and energy metabolism, whereas MC5R regulates synthesis of sebum in various tissues, including skin (Chen et al., Citation1997; Healy et al., Citation2001; Logan et al., Citation2003; London & Volkoff, Citation2019; Marsh et al., Citation1999; Metz Peters, & Flik, Citation2006; Richardson et al., Citation2008; Yang, Zhang, Wen, & Tao, Citation2019). A Gs-coupled MC2R is a target for ACTH action, that controls cortisol biosynthesis by binding to MC2R expressed on adrenocortical cells (Roebuck, Jones, Robinson, Mitchell, & Thorburn, Citation1984; Schioth, Chhajlani, Muceniece, Klusa, & Wikberg Citation1996) to elevate cytoplasmic cAMP and rapidly stimulate the steroidogenic acute regulatory protein (StAR) shuttling to the inner mitochondrial membrane (Stocco & Clark, Citation1996). The second, delayed phase upregulates the transcription of steroidogenic enzymes under control of the cAMP-activated transcription factor SF-1 (Hu et al., Citation2001). The newly synthesized cortisol is released into the circulation and then transported to the target organs, bound by plasma proteins corticosteroid-binding globulin (transcortin) and albumin (Goodman, Citation2009; Hammond, Citation2016).
The tissue-specific effects of cortisol in zebrafish are mediated by MR and GR (Pippal, Cheung, Yao, Brennan, & Fuller, Citation2011; Schaaf et al., Citation2008) that translocate to the nucleus upon binding the ligand, and serve as transcription factors that recognize DNA glucocorticoid response elements (GREs) (Surjit et al., Citation2011). In various other species, multiple effects produced by cortisol involve direct recruitment of basal transcription factors (Giguere, Hollenberg, Rosenfeld, & Evans, Citation1986; Hollenberg & Evans, Citation1988), DNA-binding independent protein-protein interactions with transcription factors (e.g. NF-κB and AP-1) (Scheinman, Gualberto, Jewell, Cidlowski, & Baldwin, Citation1995; Yang-Yen et al., Citation1990) and epigenetic effects on chromatin (Deroo & Archer, Citation2001; Fryer & Archer, Citation1998; Ito, Barnes, & Adcock, Citation2000). Cortisol also inhibits glucose uptake, induces lipolysis and increases gluconeogenesis in humans and other mammals, suggesting its pro-catabolic role mobilizing the organism’s energy resources in response to a stressor (Christiansen et al., Citation2007; Djurhuus et al., Citation2002; McMahon, Gerich, & Rizza Citation1988). In teleost fishes, these effects appear to be generally similar, albeit with some interspecies variance (Mommsen, Vijayan, & Moon Citation1999; an Der Boon, Van Den Thillart, & Addink Citation1991). Although zebrafish metabolism has not been extensively studied (Mommsen et al., Citation1999), transcriptomic and metabolomic data link their upregulated gluconeogenesis- and amino acid catabolism-associated genes to increased concentration of glucose, proteolysis, liver glycogenolysis and lipolysis (Chatzopoulou et al., Citation2015).
Glucocorticoid-mediated CRF repression has multiple implications for stress and its treatment (Holsboer & Ising, Citation2008; Keck & Holsboer, Citation2001; Müller & Wurst, Citation2004). For example, although GRs are critical for cortisol effects (Chrousos & Kino, Citation2007; Holsboer & Ising, Citation2008; Keck & Holsboer, Citation2001; McQuade & Young, Citation2000), the exact mechanisms of its negative feedback on CRF expression remain unclear, and may involve both genomic and non-genomic effects. The former are likely mediated by the functional negative glucocorticoid response element (nGRE) in the CRF promoter region (Malkoski & Dorin, Citation1999), and the latter may involve presynaptic release of endocannabinoids upstream PVN (Di, Malcher-Lopes, Halmos, & Tasker, Citation2003), in line with the well-established HPA axis modulation by endocannabinoids (Barna, Zelena, Arszovszki, & Ledent, Citation2004; Cota et al., Citation2007). In zebrafish, this process is rather similar to mammals, because GRs on CRF neurons alter CRF expression in wild type, but not grs357−/− mutants (Alderman & Vijayan, Citation2012). In addition, these mutants display impaired habituation to acute stress, suggesting disrupted biofeedback mechanism that normally blocks re-initiation of stress response by CRF (Griffiths et al., Citation2012; Ziv et al., Citation2013). Moreover, as grs357−/− fish display anxiety-like behavior ameliorated by antidepressants, the relevance of zebrafish to study stress-related pathologies become clear (Griffiths et al., Citation2012; Ziv et al., Citation2013). In line with this, the zebrafish GR shows high homology to its human equivalent, and the C-terminal GR splice variant similar to human GRβ (Schaaf et al., Citation2009). These GR zebrafish mutants also have higher basal levels of the pomc transcript that fail to respond to betamethasone 17-valerate (a synthetic steroid and an analog of cortisol), indicating a direct GRE-based repression of pomc expression by GR (Griffiths et al., Citation2012). This also generally supports the highly conserved character of the stress axis in vertebrates, from zebrafish to humans, especially since the zebrafish gr shows a 98-% homology for its DNA-binding domain and a 87-% homology for its ligand-binding domain with the human GR (Brittijn et al., Citation2009; Schaaf et al., Citation2008).
Finally, there can also be transgenerational neuroendocrine mechanisms regulating zebrafish stress responses. For example, maternal cortisol may regulate the development of HPI axis in zebrafish, subsequently impacting larval stress response and its timing after acute stressor (Nesan & Vijayan, Citation2016). Indeed, the role of maternal cortisol in neurogenesis and behavior in larval zebrafish has already been reported (Best, Kurrasch, & Vijayan, Citation2017), paralleling stress regulation in humans, where maternal cortisol affects the HPA axis function of the child, and may evoke stress-related disorders later in life (Davis et al., Citation2007; Karlén, Frostell, Theodorsson, Faresjö, & Ludvigsson, Citation2013; Oberlander et al., Citation2008).
3. Stress beyond cortisol signaling
In addition to cortisol, other important neuroendocrine mechanisms regulate zebrafish stress responses. For example, unpredictable chronic stress (UCS) not only elevates cortisol levels in zebrafish, but also upregulates GRs, mineralocorticoid receptors and non-cortisol signaling via prolactin (an anterior pituitary hormone with intrinsic role in the activation of the HPI-axis) and hypocretin/orexin (the two neuropeptides regulating food intake, reward and sleep) (Pavlidis, Theodoridi, & Tsalafouta, Citation2015). Adrenal catecholamines (i.e. adrenaline and noradrenaline) also rise during stress response in adult zebrafish (Eto, Mazilu-Brown, Henderson-MacLennan, Dipple, & McCabe, Citation2014). In rodents, stress alters the biosynthesis of neuropeptide Y (NPY) in different brain regions, where the magnitude and direction of this effect vary with the duration and type of stress (Reichmann & Holzer, Citation2016). In zebrafish, NPY, isotocin, urocortin 3 and prolactin show consistent transcriptomic patterns (along with reduced stress/anxiety behavior) following fluoxetine treatment (Wong, Oxendine, & Godwin, Citation2013). Stress can also enhance the effect of opioid ligands in the development of addiction (Koob, Citation2009), implicating the HPI axis in opioid pathobiology. Zebrafish chronically exposed to morphine display anxiety-like behavior following its 24-h withdrawal (Khor, Jamil, Adenan, & Shu-Chien, Citation2011). However, these effects are reduced by mitragynine (an alkaloid mu/kappa/delta-opioid agonist isolated from kratom, Mitragyna speciose), which also normalizes cortisol levels and lowers mRNA expression of crhr1 and crhr2 (Khor, Jamil, Adenan, & Shu-Chien, Citation2011). In contrast, chronic morphine treatment alone exerts anxiolytic-like behaviors and lowers whole-body cortisol in zebrafish (Cachat, Stewart, et al., Citation2011). The endocannabinoid system also regulates stress responses in zebrafish. For example, the disruption of the cannabinoid 1 receptor gene (cnr1) potentiates, and the disruption of the endocannabinoid-degrading enzyme fatty acid amide hydrolase 2a gene (faah2a) attenuates, locomotor responses (distance traveled) to hyperosmotic stress (Krug et al., Citation2018).
Melatonin is another important non-steroid hormone involved in acute and chronic stress responses in various species, including zebrafish (Genario, de Abreu, Giacomini, Demin, & Kalueff Citation2019; Kopp, Vogel, Rettori, Delagrange, & Misslin, Citation1999; Zisapel, Citation2018). For example, stress, such as net chasing and high stocking density, inhibits melatonin synthesis in the pineal gland of fish (e.g. rainbow trout), decreasing its nocturnal levels (López-Patiño, Gesto, Conde-Sieira, Soengas, & Míguez, Citation2014). The hypocretin/orexin (HCRT) neuropeptides are also involved in these processes, as HCRT perfusion of cultured zebrafish pineal cells induces melatonin release (Appelbaum et al., Citation2009). Notably, melatonin consistently reduces anxiety-like behaviors in various zebrafish models, paralleling its effects in humans and in various rodent models of stress (Genario, Giacomini, et al., Citation2019; Kumari, Choo, Shaikh, & Othman, Citation2019; Nirwane, Sridhar, & Majumdar, Citation2016).
4. Behavioral effects of acute stress in zebrafish
Complementing molecular effects of stress, studying various behaviors is important for probing complex stress-related CNS phenotypes. The exhaustive classification of zebrafish behaviors is currently presented in the Zebrafish Behavior Catalog (ZBC) (Kalueff et al., Citation2013) that summarizes multiple endpoints assessed experimentally (Cachat, Stewart, et al., Citation2011). Zebrafish anxiety-like behavior parallels that of human and rodents in its bidirectional sensitivity to anxiogenic and anxiolytic factors (Bencan, Sledge, & Levin, Citation2009; Cachat, Stewart, et al., Citation2011; Champagne, Hoefnagels, de Kloet, & Richardson, Citation2010). Briefly summarized in , the phenotypic and behavioral phenomena observed in anxiety-like states in zebrafish can be divided into two categories: (1) emergent in anxiety, such as freezing, erratic movements, thigmotaxis (staying close to walls when exploring an novel environment), bottom-dwelling, geotaxis (diving), hyperactivity, lethargy, and (2) modified in anxiety (e.g. exploration, foraging, scototaxis (preference for dark environments), shoaling, habituation, feeding behavior, aggression and memory acquisition) (Kalueff et al., Citation2013). Thus, the effects of stress on zebrafish behavior can be evaluated by measuring fear-related and anxiety-like behaviors, and assessing how animals explore novel environments and habituate to them (Cachat, Stewart, et al., Citation2011; Kysil et al., Citation2017; Stewart, Gaikwad, Kyzar, Green, et al., Citation2012). Common zebrafish stress-related tests include the novel tank test to analyze novelty exploration as a measure of time in top vs. bottom parts of the apparatus, and the light-dark box test to evaluate anxiety as a measure of dark zone avoidance (), conceptually similar to the rodent open field and light-dark tests; also see the aquatic open field test in this figure (Cachat, Stewart, et al., Citation2011; Maximino, Marques de Brito, Dias, Gouveia, & Morato, Citation2010; Stewart, Gaikwad, Kyzar, & Kalueff, Citation2012).
In zebrafish, acute stress can be triggered by various stimuli, such as novelty, alarm pheromone or predator exposure (), handling, crowding, social isolation, air exposure, changing water parameters (e.g. pH, salinity and temperature) or bright light exposure (Abreu et al., Citation2015; Barcellos et al., Citation2007; Egan et al., Citation2009; Piato, Rosemberg, et al., Citation2011; Ramsay et al., Citation2009). In response to acute stressors, zebrafish may display fear/anxiety-like behaviors, such as altered locomotion (e.g. increased distance and average speed in the tank) (Cachat, Stewart et al., Citation2011; Champagne et al., Citation2010; Giacomini et al., Citation2016), erratic movements (Egan et al., Citation2009), freezing (Cachat, Canavello, et al., Citation2010), avoidance of lit/bright areas, (Champagne et al., Citation2010) and thigmotaxis (Champagne et al., Citation2010; Egan et al., Citation2009), as well as memory deficits (e.g. reduced cognitive performance following alarm pheromone or natural predator, Indian leaf-fish Nandus nandus, exposure) (Gaikwad et al., Citation2011). Following an acute stress, zebrafish cortisol levels rise sharply within 15 min, and gradually dissipate within 60–90 min (Ramsay et al., Citation2009). Supporting the role of monoamines in zebrafish stress regulation, fluoxetine (a selective serotonin reuptake inhibitor, SSRI) reduces anxiety-like bottom-dwelling in the novel tank (Giacomini et al., Citation2016). However, various types of acute stressors may differentially modulate the fish serotoninergic system, given unaltered serotonin following an acute net chasing stress, and increased serotonin following a 24-h social isolation (Shams, Seguin, Facciol, Chatterjee, & Gerlai, Citation2017).
Interestingly, zebrafish display some resilience to stress by increasing the levels of brain-derived neurotrophic factor (BDNF) (Pavlidis et al., Citation2015), an important neurotrophin involved in neuroplasticity and neurogenesis, thus likely representing a target for further studies on potential stress-protection in zebrafish. Other regulators of stress behaviors in zebrafish include the noradrenergic, dopaminergic and gamma-aminobutyric acid (GABA)-ergic systems. For zebrafish increase locomotion following exposure to a monoaminergic psychostimulant D-amphetamine (Irons, MacPhail, Hunter, & Padilla, Citation2010), reduce anxiety after treatment with GABA-ergic positive modulators (e.g. ethanol, diazepam) (Egan et al., Citation2009) and adrenergic agents (e.g. dexmedetomidine, an α-2 adrenergic receptor agonist) (Ruuskanen, Peitsaro, Kaslin, Panula, & Scheinin, Citation2005), but increase anxiety-like behavioral and cortisol responses following “stressogenic” ethanol and morphine withdrawal (Cachat, Canavello, et al., Citation2010).
5. Effects of chronic stress on zebrafish behavior and physiology
Although chronic stress can be caused in zebrafish by constant exposure to stimuli evoking a strong, innate response (e.g. natural predator), frequent exposure of some stressors can gradually suppress the response, causing habituation (Piato, Capiotti, et al., Citation2011). This justifies the wide use of various unpredictable (e.g. UCS) paradigms to model stress disorders in zebrafish (Chakravarty et al., Citation2013; Fulcher, Tran, Shams, Chatterjee, & Gerlai, Citation2017; Piato, Capiotti, et al., Citation2011). Initially designed for rodent models (D'Aquila, Brain, & Willner, Citation1994; Mineur, Belzung, & Crusio, Citation2006; Yalcin, Belzung, & Surget, Citation2008), UCS has been recently adapted for zebrafish (Pavlidis et al., Citation2015; Piato, Capiotti, et al., Citation2011). To increase translatability while avoiding the likely desensitizing effects of milder UCS protocols (Piato, Capiotti, et al., Citation2011), a zebrafish model of rigorous prolonged strong 5-week UCS has also been developed (Song et al., Citation2018).
In general, chronically stressed zebrafish exhibit hypoactivity, anxiety-like bottom-dwelling and freezing (Chakravarty et al., Citation2013; Marcon et al., Citation2016, Citation2018; Piato, Capiotti, Citation2011). However, the effects of UCS on shoal cohesion vary between studies, including nonlinear relationship with UCS duration (Piato, Capiotti, et al., Citation2011) or increased shoaling (Chakravarty et al., Citation2013). Increased aggression has also been reported in chronically stressed male, but not female, zebrafish (Rambo et al., Citation2017), whereas UCS inhibits memory retention in zebrafish inhibitory avoidance learning (Manuel et al., Citation2014; Piato, Capiotti, et al., Citation2011).
In humans and rodents, chronic stress has long been known to affect neuroendocrine, immune and metabolic pathways (Bisht et al., Citation2018; Foster, Rinaman, & Cryan, Citation2017; Lupien, Juster, Raymond, & Marin, Citation2018). Similar findings are also seen in zebrafish UCS (Marcon et al., Citation2016; Song et al., Citation2018). For example, implicated in pathogenesis of human affective disorders (Claes, Citation2004; Keck & Holsboer, Citation2001), CRF modulates stress-related behaviors in zebrafish (Manuel et al., Citation2014; Pavlidis et al., Citation2015; Piato, Capiotti, et al., Citation2011). Chronic stress also alters monoamine signaling in zebrafish, as 90-day chronic social isolation lowers serotonin, but not dopamine, levels (Shams, Chatterjee, & Gerlai, Citation2015), while UCS alters dopamine levels in developmentally isolated fish (Fulcher et al., Citation2017). Like acute stress, UCS increases BDNF levels in zebrafish (Song et al., Citation2018), but not in rodents (Radahmadi, Alaei, Sharifi, & Hosseini, Citation2015), and this effect is normalized in fish by an SSRI fluoxetine. Furthermore, in adult zebrafish brain, UCS increases the expression of the mineralocorticoid receptor (mr), GR (grα and grβ)), serotonin receptor 1ab (htr1ab) and the bdnf genes (Manuel et al., Citation2014; Piato, Capiotti, et al., Citation2011), and downregulates the gene of 11-β-hydroxysteroid dehydrogenase 2 (hsd11b2), a neuroprotective enzyme (Huang, Butler, & Lubin, Citation2019). UCS upregulates the expression of genes of pro-inflammatory cytokines interleukin (IL) IL-1β and TNF-α, the anti-inflammatory cytokine IL-10, and reduces the c-Fos gene expression (Kirsten et al., Citation2020). UCS also increases brain expression of pro-inflammatory biomarker genes of cyclooxygenase 2 (cox-2) and IL-6 (il-6), the effect prevented by some anxiolytic (e.g. bromazepam) and antidepressant (e.g. fluoxetine and nortriptyline) drugs (Marcon et al., Citation2016; Song et al., Citation2018). Finally, UCS also alters brain proteome profile, implicating mitochondrial dysfunction in chronically stressed zebrafish (Chakravarty et al., Citation2013).
6. Moving beyond the anxiety deficits
In addition to anxiety (caused by anticipation of danger, ), stress also elicits human or animal fear – a rapid response to immediate danger, that is transitory and dissipates when the danger passes (Davis, Walker, Miles, & Grillon, Citation2010). Conceptually, large-scale behavioral screening may help differentiate fear- vs. anxiety-like stress-induced states in zebrafish (Jesuthasan, Citation2012). For example, larval zebrafish sense the movement of water generated by the predator (via the lateral line) and display escape behaviors (McHenry, Feitl, Strother, & Van Trump, Citation2009). A fear-like escape response can also be reliably elicited in adult zebrafish by alarm pheromone (excreted by the skin of injured conspecifics, ) (Jesuthasan & Mathuru, Citation2008; Speedie & Gerlai, Citation2008), the electric shock (Kenney, Scott, Josselyn, & Frankland, Citation2017), or exposure to an animated (moving) bird silhouette (Luca & Gerlai, Citation2012).
Another important pathology influenced by stress is PTSD, a severely debilitating clinical disorder that develops following one or more traumatic events and causes hypervigilance, sleep disturbances, social and cognitive degradation and flashbacks (American Psychiatric Association, Citation2013). PTSD is often comorbid with anxiety, depression, substance abuse, altered sleep patterns, decreased cognitive abilities and memory impairment (Danckwerts & Leathem, Citation2003). Zebrafish display several phenotypes relevant to modeling PTSD states (e.g. anxiety, learning, social interaction) in the presence of a stressor, and also exhibit individual and sex differences that would be ancillary to modeling the clinical complexity of PTSD (Caramillo, Khan, Collier, & Echevarria, Citation2015; Stewart, Yang, Nguyen, & Kalueff, Citation2014).
Depression, another critical stress-related psychiatric condition that affects millions globally, is generally characterized by low/alternating mood, anhedonia, social withdrawal and other severely debilitating psychiatric symptoms (James et al., Citation2018). Animal models, including zebrafish, emerge as an important tool for studying depression pathogenesis and its potential treatments (Nguyen, Stewart, & Kalueff, Citation2014). The common phenotype likely reflecting zebrafish depression-like states is associated with elevated anxiety and basal cortisol, reduced shoaling, motor deficits/retardation and anhedonia (de Abreu et al., Citation2018; Nguyen, Stewart, & Kalueff, Citation2014). In general, our current understanding of zebrafish behavioral models and affective circuits (Kalueff, Stewart, et al., Citation2014; Stewart, Braubach, Spitsbergen, Gerlai, & Kalueff, Citation2014) does not allow to firmly support the presence of clear-cut depression- vs. anxiety-like states in zebrafish. Therefore, further experimental dissection is needed to clarify whether depression in zebrafish can be separated from other stress-evoked phenotypes. For example, while zebrafish anxiety is a specific condition that can be evoked acutely (Egan et al., Citation2009), we do not know whether chronic stress evokes pathological generalized anxiety, depression or their comorbidity (). Likewise, the use of conventional antidepressants (e.g. fluoxetine) may not solve this problem experimentally, since even after short treatment they may evoke robust anxiolytic effects in zebrafish (Egan et al., Citation2009). Thus, novel experimental tests and models may be needed to better separate these two states, if they exist in zebrafish (de Abreu et al., Citation2018). One such approach can involve targeting specific 'core' depression-related phenotypic domains, such as anhedomia, motor retardation, social withdrawal or despair (learned helplessness), in zebrafish. For instance, a novel behavioral paradigm, the zebrafish tail immobilization (ZTI) test, has been recently suggested as a potential tool to assess fish despair-like behavior (Demin et al., Citation2020). Conceptually similar to rodent 'despair' models (e.g. the forced swim and the tail suspension tests), the ZTI protocol involves immobilizing the caudal half of the fish body for 5 min (leaving the cranial part to hang vertically and move freely in the small beaker with water), to assess the number of mobility episodes, time spent moving, total distance moved and other activity measures of the cranial part of the body, using video-tracking (Demin et al., Citation2020). While the acute stressors (e.g. electric shock and alarm pheromone exposure) decrease ZTI activity and alter monoamine neurotransmission, several antidepressants increase fish ZTI activity, while an anxiolytic phenazepam remains inactive, collectively suggesting the ZTI test as a potentially useful tool to assess stress/despair-related behaviors, relevant to CNS drug screening and behavioral phenotyping of zebrafish (Demin et al., Citation2020).
Epigenetics has recently provided novel insights into CNS functioning in stress, as DNA methylation, histone modifications and microRNA activity mediate dynamic molecular adaptations of the brain to acute and chronic stressors (Stankiewicz, Swiergiel, & Lisowski, Citation2013). Zebrafish are becoming a useful tool for epigenetic studies of CNS mechanisms (Lakstygal, de Abreu, & Kalueff, Citation2018). For example, acute morphine exposure to zebrafish embryos downregulates the epigenetic factor miR-212 and further decreases BDNF expression (Jimenez-Gonzalez et al., Citation2016), thereby negatively affecting zebrafish neural plasticity. Environmental epigenetics also merits further scrutiny in this fish model. For example, exposure to chemical stressors affects DNA methylation in zebrafish (Cavalieri & Spinelli, Citation2017) and the expression of several epigenetic genes (Dorts et al., Citation2016). Zebrafish exposure to fluoxetine modifies pathways associated with cortisol synthesis, thus, affecting cortisol levels and reducing novelty exploration in several generations of fish (Vera-Chang, Moon, & Trudeau, Citation2019; Vera-Chang et al., Citation2018). Although this area of research is only emerging, due to their high physiological and genetic homology to humans, zebrafish may markedly improve our understanding of epigenetic mechanisms underlying stress pathogenesis.
7. Stress, neuro-immune and gut-brain axis modulation
Neuro-immune interactions play a key role in stress response (Costa-Pinto & Palermo-Neto, Citation2010; Dantzer, Citation2018; Deak et al., Citation2015), and both acute stress and stress-related disorders (e.g. PTSD and depression) involve aberrant neuro-immune mechanisms (Engelsma et al., Citation2002; Hodes, Kana, Menard, Merad, & Russo, Citation2015). Acute stress generally elevates circulating interleukins (IL) IL-6, IL-1β, IL-10, TNF-α and interferon-γ (Arango Duque & Descoteaux, Citation2014; Marsland, Walsh, Lockwood, & John-Henderson, Citation2017), whereas clinical PTSD is associated with higher levels of pro-inflammatory cytokines IL-6 and IL-8, and lower levels of the regulatory cytokine TGF-β (Cohen et al., Citation2011). In teleost fishes, cortisol inhibits the innate immune response, decreases phagocytosis (e.g. in tilapia, common carp and goldfish) (Law, Chen, Song, Dufour, & Chang, Citation2001; Wang & Belosevic, Citation1995) and reduces pro-inflammatory cytokine secretion (e.g. in Atlantic salmon, rainbow trout and carp) (Fast, Hosoya, Johnson, & Afonso, Citation2008; Huising et al., Citation2005; Stolte et al., Citation2008). Cortisol also lowers leukocyte counts due to antiproliferative/proapoptotic effects on monocytes/macrophages in rainbow trout (Pagniello, Bols, & Lee, Citation2002) and exerts an antiapoptotic action on neutrophils in seabream (Esteban, Rodriguez, Ayala, & Meseguer, Citation2004). During adaptive immune responses, cortisol has an apoptotic action on carp B lymphocytes, in line with zebrafish leukogram data in acute stress (Grzelak et al., Citation2017). However, to modulate the HPI axis functioning, complex regulatory networks are required for cortisol effects (Wendelaar Bonga, Citation1997). For instance, 11-β-hydroxysteroid dehydrogenase II, an enzyme oxidizing cortisol into cortisone (a metabolite inactive at GR) (Alderman & Vijayan, Citation2012), and 20-β-hydroxysteroid dehydrogenase that also generates a GR-inactive excretable metabolite (Tokarz, Norton, Möller, Hrabé de Angelis, & Adamski, Citation2013) are both induced by cortisol in zebrafish, creating a rapid feedback loop that quickly eliminated cortisol after an acute stress.
As already mentioned, zebrafish UCS elevates pro-inflammatory cytokines IL-1β and IL-6, whole-body cortisol and pro-inflammatory markers COX-2, as well as consistently upregulates BDNF (Chakravarty et al., Citation2013; De Felice et al., Citation2014; Manuel et al., Citation2014; Marcon et al., Citation2016; Pavlidis et al., Citation2015; Song et al., Citation2018). Social stress also alters brain BDNF expression, increasing it in the telencephalon of defeated (loser) zebrafish (Teles, Cardoso, & Oliveira, Citation2016). Zebrafish also present genetic models to assess the role of stress in the neuro-immune interplay. For example, the zebrafish GR mutant (gr−/−) strain displays higher levels of whole-body cortisol and basal crh and pomca transcripts (Facchinello et al., Citation2017). Importantly, pro-inflammatory reaction induced by dextran sodium sulfate, a chemical colitogen with anticoagulant properties, upregulates IL-1β, IL-8 and IL-6 genes in the wild-type, but not the gr−/−, fish (Facchinello et al., Citation2017). Likewise, zebrafish subjected to social “crowding” stress display higher cortisol levels (Sallin & Jaźwińska, Citation2016) and lower neutrophil activity (Palić, Andreasen, Ostojić, Tell, & Roth, Citation2007). Acute social isolation, a well-studied psychological stressor in this highly social species, elevates anxiety and cortisol levels in zebrafish (Kalueff, Echevarria, et al., Citation2014; Tea, Alderman, & Gilmour, Citation2019), but blunts cortisol responses to acute stress (e.g. net chasing) (Giacomini et al., Citation2015). In contrast, chronic social isolation in zebrafish does not affect baseline cortisol but lowers it during net chasing stress, accompanied by reduced serum lysozyme activity (Forsatkar, Safari, & Boiti, Citation2017). Psychosocial stress can also be induced by treating zebrafish embryos with cortisol during early development (Hartig, Zhu, King, & Coffman, Citation2016), which elevates basal cortisol in larvae and adults, as well as upregulates some immune genes (e.g. immunoresponsive gene 1-like, irg1l and suppressor of cytokine signaling 3a, socs3a) in adults (Palić et al., Citation2007).
Finally, metabolic factors and gut microbiota play an important role in stress pathobiology. For example, in rodents, ingestion of particular probiotics reduces anxiety and stress-associated inflammation cytokines (Liu et al., Citation2016). In humans, disruption of the serotonergic and kynurenine routes of tryptophan metabolism (as a part of dysbiosis) can contribute to the pathogenesis of dementia, Alzheimer’s and Huntington’s diseases (Ruddick et al., Citation2006). In zebrafish, tryptophan supplementation alleviates neuroendocrine and behavioral responses to stress (Giacomini et al., Citation2020), collectively reinforcing the bidirectional modulation of the gut-brain axis (Martin, Osadchiy, Kalani, & Mayer, Citation2018). From this point of view, zebrafish may indeed be a promising animal model to assess the impact of stress on gut-brain interactions (de Abreu, Giacomini, Sysoev, et al., Citation2019), especially since characteristic responses to an acute stressor are obliterated in microbe-free larvae (Davis, Bryda, Gillespie, & Ericsson, Citation2016). Moreover, feeding frequency in zebrafish may also affect their anxiety-like behavior, which is higher when feeding once vs. twice per day (Dametto et al., Citation2018).
8. Limitations of zebrafish stress models and concluding remarks
Although the use of zebrafish in translational stress research is steadily increasing, they do possess certain limitations as a model, meriting further consideration. For example, unlike mice and humans, zebrafish are poikilothermic organisms, and studying their stress-thermogenesis interplay (e.g. mediated via the β-adrenergic system) is therefore limited (Seth, Stemple, & Barroso, Citation2013). The small size of zebrafish also impedes some types of experiments, since intraperitoneal and intracerebroventricular injections in zebrafish, although doable, are technically challenging and time-consuming (Seth et al., Citation2013). Other factors to consider, albeit not fish-specific, involve problems with appropriate description of methods used and the resultant overall replicability of behavioral studies and protocols. For instance, acute net chasing procedure is usually only briefly described in terms of duration, while the intensity and velocity of stimulus presentation are usually neither evaluated nor described in the literature. Likewise, the chronic stress batteries (e.g. 5-week chronic stress), although sufficiently described in the literature (e.g. Song et al., Citation2018), typically involve complex in-house manipulations (e.g. predator or conspecific exposure) that are difficult to standardize and, hence, replicate between and within the laboratories.
When testing potential stress-modulating drugs in zebrafish, the quantification of chemical compounds entering the fish CNS is not precise, and can be difficult to assume unless specific studies (e.g. mass spectrometry) are performed. Moreover, the aquatic zebrafish models are well-suited for testing water-soluble drugs, whereas other compounds may need organic solvents or, in some cases, systemic injections. However, using the solvents may per se affect the experimental procedure (Deutsch-Feldman, Picetti, Seip-Cammack, Zhou, & Kreek, Citation2015), whereas the injection and related to it handling procedure may also introduce unwanted additional stress (de Abreu, Giacomini, Echevarria, & Kalueff, Citation2019). Even when the drug is water-soluble, the treatment procedure may require exposing individual fish to a drug solution, or using a group exposure. In the former case, even a single short social isolation may markedly increase zebrafish stress (Abreu, Giacomini, Koakoski, Piato, & Barcellos, Citation2017). In the latter case, if a CNS drug impacts fish behavior, its effects on other members of the exposed cohort (shoal) may indirectly influence the target fish behavior, thus potently (and uncontrollably) affecting the behavioral outcomes assessed. Likewise, using some water-soluble drugs can also result in irritation to skin, eyes and gills, as well as affect vision and other sensory functions, eventually nonspecifically compromising zebrafish behavioral data.
In contrast to mammals, zebrafish exhibit a much greater proliferative potential (Adolf et al., Citation2006; Schmidt, Strähle, & Scholpp, Citation2013; Zupanc, Hinsch, & Gage, Citation2005), demonstrating nearly 20 different proliferating regions in the brain, including the regions equivalent to the mammalian subventricular and subgranular zones (Adolf et al., Citation2006; Schmidt et al., Citation2013). Adult zebrafish brain consists of approximately 10 million cells, with nearly 6000 cells born every 30 min (Hinsch & Zupanc, Citation2007). On the one hand, such increased neurogenesis may imply a faster CNS adaptation and/or improved resilience to stress. On the other hand, although widespread neurogenic activity makes zebrafish an attractive model to study adult neurogenesis and its impact on stress responses, such findings may be problematic to translate into rodent and human data, whose neurogenesis is less extensive. Lastly, zebrafish brain has a different structure from human brain, including the lack of neocortex, lower white matter/gray matter ratio and differing limbic system complexity (Mueller, Citation2012). Another point to consider here is the high genetic diversity observed between individual zebrafish (Balik-Meisner, Truong, Scholl, Tanguay, & Reif, Citation2018), even within the same strain (Coe et al., Citation2009), which complicates the evaluation of the effects of stress on gene expression and the standardization of the experimental procedures used.
Nevertheless, the evidence presented here () strongly supports the growing utility of zebrafish models in stress research. The effects acute and chronic stress in zebrafish behavioral and physiological phenotypes reveal some common aspects of stress action, such as altered monoamine levels, albeit in sex- and context-specific manner (e.g. aggression, ). Thus, in addition to the utility of zebrafish in behavioral studies, this model organism is particularly valuable for probing neuroendocrine mechanisms of stress responses and their therapy. For example, exposure to UCS increases brain BDNF levels in zebrafish (Song et al., Citation2018), but generally reduces them in rodents (Radahmadi et al., Citation2015), indicating that the zebrafish is not just a “small mouse” model in stress research, but a useful organism in its own right, able to fostering in-depth comparative studies of both protective and harmful impacts of stress in vertebrate CNS.
Table 3. Selected examples of pharmacological modulation of zebrafish cortisol levels in acute and chronic stress.
As common in biomedicine, the models’ limitations may also be their strengths, which can be used advantageously depending on specific research goals (Stewart, Braubach, et al., Citation2014). For example, zebrafish display overt behavioral and physiological strain differences, and this variance may complicate the detection and dissection of stress-specific phenotypes in this model. However, the availability of such strains may also help dissect both distinct and shared mechanisms of the HPI axis pathobiology in zebrafish. Indeed, the AB and Tupfel long-fin (TL) strains differ in the HPI axis activity (e.g. AB fish display higher brain crf, gr-beta, bdnf, pcna, neurod1, cart4, igf1, soc3a, mpeg 1.1 and 1.2 expression) in both larvae and adult zebrafish (Gorissen et al., Citation2015; van den Bos et al., Citation2017). Domesticated for many generations, such laboratory strains also differ markedly in behavior and stress responses from wild-caught or wild-derived (e.g. WIK) zebrafish (Collier, Kalueff, & Echevarria, Citation2017; Kalueff et al., Citation2016). Likely reflecting domestication, some laboratory strains appear to have diminished response to vital stressors, such as conspecific alarm pheromone (Ogwang, Citation2008). Moreover, genetic mutations may also impact zebrafish behavior, as the leopard and albino zebrafish strains demonstrate higher anxiety than the wild-type fish (Egan et al., Citation2009). Thus, different strains may be used as genetic tools to further probe zebrafish stress behaviors and their molecular and genetic mechanisms (Kalueff et al., Citation2016).
However, using different zebrafish laboratory strains may result in completely different results because the HPI axis reactivity and behavioral responses to behavioral tests (e.g. the novel-tank test) differ greatly between strains (Egan et al., Citation2009). Therefore, inter-strain differences in behavioral and neuroendocrine stress responses must be considered carefully when interpreting results from zebrafish studies. In addition, overt sex differences have also been reported in zebrafish behavioral models (Genario, de Abreu, et al., Citation2020; Rambo et al., Citation2017). In some cases, analyses of such sex differences can be even more complicated in stress studies, since the two sexes may respond differently in different zebrafish strains (Genario, de Abreu, et al., Citation2020; Genario et al., Citation2020; Vital & Martins, Citation2011). Thus, such factors must be carefully considered in various stress models utilizing this species, at least in adult zebrafish.
Furthermore, there is a growing recognition of the importance of social stress in animal and human affective phenotypes (Schmidt et al., Citation2010; Spencer, Citation2017; Wood, Walker, Valentino, & Bhatnagar, Citation2010). There is also a large body of evidence on sociality and social stress in teleost species (Backström & Winberg, Citation2017; Fox, White, Kao, & Fernald, Citation1997), including zebrafish (Forsatkar et al., Citation2017; Tea et al., Citation2019). For example, zebrafish are a highly social species, spending most of their time swimming together in groups (shoals) (Saverino & Gerlai, Citation2008; Wright, Ward, Croft, & Krause, Citation2006). Thus, various social factors, such as isolated vs. group housing, can modulate zebrafish stress behaviors experimentally. Indeed, zebrafish kept in low densities display higher aggression and develop strong hierarchies (Dahlbom, Lagman, Lundstedt-Enkel, Sundström, & Winberg, Citation2011; Larson, O’Malley, & Melloni, Citation2006). Thus, such type of social stress can be an interesting model for stress-related disorders, especially since socially subordinate fish have no control over their environment, and are subjected to strong unpredictable social stressors. Moreover, zebrafish housed individually display blunted cortisol response to an acute stressor, unlike their group-housed counterparts (Giacomini et al., Citation2015), thereby suggesting an interaction between social and nonsocial stressors in this model organism. Likewise, subordinate male zebrafish exhibit elevated plasma cortisol following a social interaction, compared to dominant or group-housed control males (Tea et al., Citation2019). Collectively, this implicates a complex interplay between sex, sociality and stress responses in zebrafish models, warranting further in-depth studies. Finally, stress itself potently modulates zebrafish social behavior, since, for instance, acute stress increases anxiety-like shoaling cohesion in zebrafish (Kleinhappel, Pike, & Burman, Citation2019), whereas chronic unpredictable stress may disrupt shoaling (e.g. decrease shoal cohesion), likely representing depression-like “social withdrawal” responses (Piato, Capiotti, et al., Citation2011).
Given valid concerns regarding behavioral data reproducibility in general (Kafkafi et al., Citation2018) and in zebrafish studies (Gerlai, Citation2019), care should also be paid to methodological details of stress models and behaviors assessed. For example, a widely used method of inducing acute stress in zebrafish, commonly referred to in the literature as “net stress”, often involves completely different procedures, in some studies including net chasing in the water for 2 min (de Abreu et al., Citation2014), and in others – a brief episode of netting fish out and suspending in the net above water (Aponte & Petrunich-Rutherford, Citation2019), the procedure also described as “air exposure” in some other studies (Abreu et al., Citation2015). Clearly confusing the literature, such terminological and methodological discrepancies may further complicate our understanding of acute stress responses in zebrafish, especially since distinct neurobehavioral responses may be elicited by the seemingly identical (based on their “labeling” in the literature) stressors. In a similar vein, it is also possible that manipulations described as seemingly distinct would have little impact on stress responses. For example, comparing net stress in young zebrafish, a brief 30-s air exposure was deemed ineffective in inducing behavioral or cortisol responses (Aponte & Petrunich-Rutherford, Citation2019), at odds with earlier findings with this stressor in adult fish (Tran, Chatterjee, & Gerlai, Citation2014; Tran & Gerlai, Citation2015). However, the difference between netting naïve control fish for behavioral testing vs. similar netting of experimental fish with an added 30-s air exposure stress in the same net (Aponte & Petrunich-Rutherford, Citation2019), instead of placing stressed subjects in a social isolation in order for stress response to develop (e.g. Tran et al., Citation2014; Tran & Gerlai, Citation2015), may reflect only minimal procedural distinction between the groups labeled as “stressed” and “unstressed”, thus yielding the lack of otherwise expected and well-reproduced phenotypic responses (Tran et al., Citation2014; Tran & Gerlai, Citation2015).
Other differences in zebrafish stress responses heavily depend on the nature of stress stimuli. For instance, physical stressors (e.g. net chasing or pain) may cause a stronger anxiogenic response than chemical cues (e.g. exposure to alarm pheromone), and this difference can impact drug effects as well (e.g. fluoxetine blunts cortisol release in response to physical stress (Abreu et al., Citation2017), see for details). Zebrafish also possess an external development with the production of hundreds of eggs, transparent embryos and fast development, which together with the availability of multiple genetic tools (Rieger, Wang, & Sagasti, Citation2011) may help assess the role of genetic and pharmacological modulation of stress-related states. As many outstanding questions remain open in this field (), this collectively calls for further translational neurobehavioral research utilizing zebrafish models of stress.
Table 4. Selected open questions related to zebrafish models of stress-related disorders.
In summary, the zebrafish emerges as a powerful model organism highly sensitive to both acute and chronic stress (), whose behavioral phenotypes and physiological responses are generally evolutionarily conserved and shared with mammals and humans. However, some zebrafish-specific features, especially in the domains of neurogenesis, neuroprotection and neuro-immune responses, can be particularly interesting to assess further, as they may offer additional insights into stress pathogenesis, thereby complementing (rather than merely replicating) rodent findings. Compared to mammals, there is an increased availability of zebrafish gene-editing tools (Cheresiz et al., Citation2020; Clark, Boczek, & Ekker, Citation2011), thus empowering translational research into genetic determinants of stress and resilience. Finally, unlike traditional mammalian systems, zebrafish have a remarkable “higher-throughput” capability of CNS drug screening (Kokel et al., Citation2010; Kokel & Peterson, Citation2008; Stewart, Gerlai, & Kalueff, Citation2015), and may therefore uniquely foster innovative drug discovery and the development of novel anti-stress therapies.
Acknowledgements
The research was supported by the Russian Science Foundation grant 19‐15‐00053. KAD is supported by the President of Russia Graduate Fellowship and the Special Rector's Productivity Fellowship for SPSU PhD Students. His research is supported by the Russian Foundation for Basic Research (RFBR) grant 18‐34‐00996. AVK is the President of the International Stress and Behavior Society (ISBS, www.stress-and-behavior.com) and Chair of the International Zebrafish Neuroscience Research Consortium (ZNRC) that coordinated this collaborative multi-laboratory project. His research is supported by the Southwest University Zebrafish Platform Construction Fund. The funders had no role in the design, analyses and interpretation of the submitted study, or decision to publish. The authors thank Mrs. Lyudmyla E. Kalueva for her assistance with the graphic design for this paper.
Disclosure statement
The authors declare no conflict of interests.
Additional information
Funding
References
- Abreu, M.S., Giacomini, A., Koakoski, G., Piato, A.L.S., & Barcellos, L.J.G. (2017). Divergent effect of fluoxetine on the response to physical or chemical stressors in zebrafish. PeerJ, 5, e3330. doi:10.7717/peerj.3330
- Abreu, M.S., Giacomini, A.C.V., Koakoski, G., Oliveira, T.A., Gusso, D., Baldisserotto, B., & Barcellos, L.J.G. (2015). Effects of waterborne fluoxetine on stress response and osmoregulation in zebrafish. Environmental Toxicology and Pharmacology, 40, 704–707. doi:10.1016/j.etap.2015.09.001
- Adolf, B., Chapouton, P., Lam, C.S., Topp, S., Tannhäuser, B., Strähle, U., … Bally-Cuif, L. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Developmental Biology, 295, 278–293. doi:10.1016/j.ydbio.2006.03.023
- Alderman, S.L., & Bernier, N.J. (2007). Localization of corticotropin‐releasing factor, urotensin I, and CRF‐binding protein gene expression in the brain of the zebrafish, Danio rerio. The Journal of Comparative Neurology, 502, 783–793. doi:10.1002/cne.21332
- Alderman, S.L., & Vijayan, M.M. (2012). 11β-Hydroxysteroid dehydrogenase type 2 in zebrafish brain: A functional role in hypothalamus-pituitary-interrenal axis regulation. Journal of Endocrinology, 215, 393–402. doi:10.1530/JOE-12-0379
- Alsop, D., & Vijayan, M. (2009). The zebrafish stress axis: Molecular fallout from the teleost-specific genome duplication event. General and Comparative Endocrinology, 161, 62–66. doi:10.1016/j.ygcen.2008.09.011
- Alsop, D., & Vijayan, M.M. (2008). Development of the corticosteroid stress axis and receptor expression in zebrafish. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 294, R711–R719. doi:10.1152/ajpregu.00671.2007
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: Author.
- Aponte, A., & Petrunich-Rutherford, M.L. (2019). Acute net stress of young adult zebrafish (Danio rerio) is not sufficient to increase anxiety-like behavior and whole-body cortisol. PeerJ, 7, e7469. doi:10.7717/peerj.7469
- Appelbaum, L., Wang, G.X., Maro, G.S., Mori, R., Tovin, A., Marin, W., … Mourrain, P. (2009). Sleep–wake regulation and hypocretin–melatonin interaction in zebrafish. Proceedings of the National Academy of Sciences of the United States of America, 106, 21942–21947. doi:10.1073/pnas.906637106
- Arango Duque, G., & Descoteaux, A. (2014). Macrophage cytokines: Involvement in immunity and infectious diseases. Frontiers in Immunology, 5, 491. https://www.frontiersin.org/article/10.3389/fimmu.2014.00491
- Arends, R.J., Vermeer, H., Martens, G.J.M., Leunissen, J.A.M., Wendelaar Bonga, S.E., & Flik, G. (1998). Cloning and expression of two proopiomelanocortin mRNAs in the common carp (Cyprinus carpio L.). Molecular and Cellular Endocrinology, 143, 23–31. doi:10.1016/S0303-7207(98)00139-7
- Backström, T., & Winberg, S. (2017). Serotonin coordinates responses to social stress – What we can learn from fish. Frontiers in Neuroscience, 11, 595. doi:10.3389/fnins.2017.00595
- Bale, T.L., Abel, T., Akil, H., Carlezon, W.A., Moghaddam, B., Nestler, E.J., … Thompson, S.M. (2019). The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology, 44, 1349–1353. doi:10.1038/s41386-019-0405-9
- Bale, T.L., Lee, K.F., & Vale, W.W. (2002). The role of corticotropin-releasing factor receptors in stress and anxiety. Integrative and Comparative Biology, 42, 552–555. doi:10.1093/icb/42.3.552
- Balik-Meisner, M., Truong, L., Scholl, E.H., Tanguay, R.L., & Reif, D.M. (2018). Population genetic diversity in zebrafish lines. Mammalian Genome, 29, 90–100. doi:10.1007/s00335-018-9735-x
- Barbazuk, W.B., Korf, I., Kadavi, C., Heyen, J., Tate, S., Wun, E., … Johnson, S.L. (2000). The syntenic relationship of the zebrafish and human genomes. Genome Research, 10, 1351–1358. doi:10.1101/gr.144700
- Barcellos, H.H.D., Kalichak, F., da Rosa, J.G.S., Oliveira, T.A., Koakoski, G., Idalencio, R., … Barcellos, L.J.G. (2016). Waterborne aripiprazole blunts the stress response in zebrafish. Scientific Reports, 6, 37612. doi:10.1038/srep37612
- Barcellos, L.J.G., Ritter, F., Kreutz, L.C., Quevedo, R.M., da Silva, L.B., Bedin, A.C., … Cericato, L. (2007). Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture, 272, 774–778. doi:10.1016/j.aquaculture.2007.09.002
- Barna, I., Zelena, D., Arszovszki, A.C., & Ledent, C. (2004). The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: In vivo and in vitro studies in CB1 receptor knockout mice. Life Sciences, 75, 2959–2970. doi:10.1016/j.lfs.2004.06.006
- Bencan, Z., Sledge, D., & Levin, E.D. (2009). Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology Biochemistry and Behavior, 94, 75–80. doi:10.1016/j.pbb.2009.07.009
- Best, C., Kurrasch, D.M., & Vijayan, M.M. (2017). Maternal cortisol stimulates neurogenesis and affects larval behaviour in zebrafish. Scientific Reports, 7, 40905. doi:10.1038/srep40905
- Bisht, K., Sharma, K., & Tremblay, M.-È. (2018). Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiology of Stress, 9, 9–21. doi:10.1016/j.ynstr.2018.05.003
- Brittijn, S.A., Duivesteijn, S.J., Belmamoune, M., Bertens, L.F.M., Bitter, W., Debruijn, J.D., … Richardson, M.K. (2009). Zebrafish development and regeneration: New tools for biomedical research. The International Journal of Developmental Biology, 53, 835–850. doi:10.1387/ijdb.082615sb
- Buckingham, J.C. (1986). Stimulation and inhibition of corticotrophin releasing factor secretion by beta endorphin. Neuroendocrinology, 42, 148–152. doi:10.1159/000124266
- Cachat, J., Canavello, P., Elegante, M., Bartels, B., Hart, P., Bergner, C., … Kalueff, A.V. (2010). Modeling withdrawal syndrome in zebrafish. Behavioural Brain Research, 208, 371–376. doi:10.1016/j.bbr.2009.12.004
- Cachat, J., Kyzar, E.J., Collins, C., Gaikwad, S., Green, J., Roth, A., … Kalueff, A.V. (2013). Unique and potent effects of acute ibogaine on zebrafish: The developing utility of novel aquatic models for hallucinogenic drug research. Behavioural Brain Research, 236, 258–269. doi:10.1016/j.bbr.2012.08.041
- Cachat, J., Stewart, A., Grossman, L., Gaikwad, S., Kadri, F., Chung, K.M., … Kalueff, A.V. (2010). Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nature Protocols, 5, 1786–1799. doi:10.1038/nprot.2010.140
- Cachat, J., Stewart, A., Utterback, E., Hart, P., Gaikwad, S., Wong, K., … Kalueff, A.V. (2011). Three-dimensional neurophenotyping of adult zebrafish behavior. PLoS One, 6, e17597. doi:10.1371/journal.pone.0017597
- Cachat, J.M., Canavello, P.R., Elegante, M.F., Bartels, B.K., Elkhayat, S.I., Hart, P.C., … Mohnot, S. (2011). Modeling stress and anxiety in zebrafish. In A. V. Kalueff & J. Cachat (Eds.), Zebrafish models in neurobehavioral research (pp. 73–88). Totowa, NJ: Humana Press.
- Caramillo, E.M., Khan, K.M., Collier, A.D., & Echevarria, D.J. (2015). Modeling PTSD in the zebrafish: Are we there yet? Behavioural Brain Research, 276, 151–160. doi:10.1016/j.bbr.2014.05.005
- Carlson, E.B., & Rosser-Hogan, R. (1991). Trauma experiences, posttraumatic stress, dissociation, and depression in Cambodian refugees. The American Journal of Psychiatry, 148, 1548–1551. doi:10.1176/ajp.148.11.1548
- Cavalieri, V., & Spinelli, G. (2017). Environmental epigenetics in zebrafish. Epigenetics & Chromatin, 10, 46. doi:10.1186/s13072-017-0154-0
- Chakravarty, S., Reddy, B.R., Sudhakar, S.R., Saxena, S., Das, T., Meghah, V., … Idris, M.M. (2013). Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: Altered brain proteome profile implicates mitochondrial dysfunction. PLoS One, 8, e63302. doi:10.1371/journal.pone.0063302
- Champagne, D.L., Hoefnagels, C.C.M., de Kloet, R.E., & Richardson, M.K. (2010). Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behavioural Brain Research, 214, 332–342. doi:10.1016/j.bbr.2010.06.001
- Chang, A.C., Cochet, M., & Cohen, S.N. (1980). Structural organization of human genomic DNA encoding the pro-opiomelanocortin peptide. Proceedings of the National Academy of Sciences of the United States of America, 77, 4890–4894. doi:10.1073/pnas.77.8.4890
- Chatzopoulou, A., Roy, U., Meijer, A.H., Alia, A., Spaink, H.P., & Schaaf, M.J.M. (2015). Transcriptional and metabolic effects of glucocorticoid receptor α and β signaling in zebrafish. Endocrinology, 156, 1757–1769. doi:10.1210/en.2014-1941
- Chen, W., Kelly, M.A., Opitz-Araya, X., Thomas, R.E., Low, M.J., & Cone, R.D. (1997). Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell, 91, 789–798. doi:10.1016/S0092-8674(00)80467-5
- Cheresiz, S.V., Volgin, A.D., Kokorina Evsyukova, A., Bashirzade, A.A.O., Demin, K.A., de Abreu, M.S., … Kalueff, A.V. (2020). Understanding neurobehavioral genetics of zebrafish. Journal of Neurogenetics. doi:10.1080/01677063.2019.1698565
- Christiansen, J.J., Djurhuus, C.B., Gravholt, C.H., Iversen, P., Christiansen, J.S., Schmitz, O., … Møller, N. (2007). Effects of cortisol on carbohydrate, lipid, and protein metabolism: Studies of acute cortisol withdrawal in adrenocortical failure. The Journal of Clinical Endocrinology & Metabolism, 92, 3553–3559. doi:10.1210/jc.2007-0445
- Chrousos, G.P., & Kino, T. (2007). Glucocorticoid action networks and complex psychiatric and/or somatic disorders. Stress, 10, 213–219. doi:10.1080/10253890701292119
- Claes, S. (2004). Corticotropin‐releasing hormone (CRH) in psychiatry: From stress to psychopathology. Annals of Medicine, 36, 50–61. doi:10.1080/07853890310017044
- Clark, K.J., Boczek, N.J., & Ekker, S.C. (2011). Stressing zebrafish for behavioral genetics. Reviews in the Neurosciences, 22, 49–62. doi:10.1515/rns.2011.007
- Coe, T.S., Hamilton, P.B., Griffiths, A.M., Hodgson, D.J., Wahab, M.A., & Tyler, C.R. (2009). Genetic variation in strains of zebrafish (Danio rerio) and the implications for ecotoxicology studies. Ecotoxicology, 18, 144–150. doi:10.1007/s10646-008-0267-0
- Cohen, M., Meir, T., Klein, E., Volpin, G., Assaf, M., & Pollack, S. (2011). Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. The International Journal of Psychiatry in Medicine, 42, 117–131. doi:10.2190/PM.42.2.b
- Cohen, S., Janicki-Deverts, D., & Miller, G.E. (2007). Psychological stress and disease. JAMA, 298, 1685–1687. doi:10.1001/jama.298.14.1685
- Cohen, S., & Williamson, G.M. (1991). Stress and infectious disease in humans. Psychological Bulletin, 109, 5–24. doi:10.1037/0033-2909.109.1.5
- Collier, A.D., Kalueff, A.V., & Echevarria, D.J. (2017). Zebrafish models of anxiety-like behaviors. In A. V. Kalueff (Ed.), The rights and wrongs of zebrafish: Behavioral phenotyping of zebrafish (pp. 45–72). Cham: Springer International Publishing.
- Conrad, C.D. (2008). Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Reviews in the Neurosciences, 19, 395–412. doi:10.1515/REVNEURO.2008.19.6.395
- Costa-Pinto, F.A., & Palermo-Neto, J. (2010). Neuroimmune interactions in stress. Neuroimmunomodulation, 17, 196–199. doi:10.1159/000258722
- Cota, D., Steiner, M.-A., Marsicano, G., Cervino, C., Herman, J.P., Grübler, Y., … Pagotto, U. (2007). Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology, 148, 1574–1581. doi:10.1210/en.2005-1649
- D’Aquila, P.S., Brain, P., & Willner, P. (1994). Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiology & Behavior, 56, 861–867. doi:10.1016/0031-9384(94)90316-6
- Dahlbom, S.J., Lagman, D., Lundstedt-Enkel, K., Sundström, L.F., & Winberg, S. (2011). Boldness predicts social status in zebrafish (Danio rerio). PLoS One, 6, e23565. doi:10.1371/journal.pone.0023565
- Dametto, F.S., Fior, D., Idalencio, R., Rosa, J.G.S., Fagundes, M., Marqueze, A., … Barcellos, L.J.G. (2018). Feeding regimen modulates zebrafish behavior. PeerJ, 6, e5343. doi:10.7717/peerj.5343
- Danckwerts, A., & Leathem, J. (2003). Questioning the link between PTSD and cognitive dysfunction. Neuropsychology Review, 13, 221–235. doi:10.1023/B:NERV.0000009485.76839.b7
- Dantzer, R. (2018). Neuroimmune interactions: From the brain to the immune system and vice versa. Physiological Reviews, 98, 477–504. doi:10.1152/physrev.00039.2016
- Davis, D.J., Bryda, E.C., Gillespie, C.H., & Ericsson, A.C. (2016). Microbial modulation of behavior and stress responses in zebrafish larvae. Behavioural Brain Research, 311, 219–227. doi:10.1016/j.bbr.2016.05.040
- Davis, E.P., Glynn, L.M., Schetter, C.D., Hobel, C., Chicz-Demet, A., & Sandman, C.A. (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46, 737–746. doi:10.1097/chi.0b013e318047b775
- Davis, M., Walker, D.L., Miles, L., & Grillon, C. (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35, 105–135. doi:10.1038/npp.2009.109
- de Abreu, M.S., Friend, A.J., Demin, K.A., Amstislavskaya, T.G., Bao, W., & Kalueff, A.V. (2018). Zebrafish models: Do we have valid paradigms for depression? Journal of Pharmacological and Toxicological Methods, 94, 16–22. doi:10.1016/j.vascn.2018.07.002
- de Abreu, M.S., Giacomini, A.C.V.V., Echevarria, D.J., & Kalueff, A.V. (2019). Legal aspects of zebrafish neuropharmacology and neurotoxicology research. Regulatory Toxicology and Pharmacology, 101, 65–70. doi:10.1016/j.yrtph.2018.11.007
- de Abreu, M.S., Giacomini, A.C.V.V., Sysoev, M., Demin, K.A., Alekseeva, P.A., Spagnoli, S.T., & Kalueff, A.V. (2019). Modeling gut-brain interactions in zebrafish. Brain Research Bulletin, 148, 55–62. doi:10.1016/j.brainresbull.2019.03.003
- de Abreu, M.S., Koakoski, G., Ferreira, D., Oliveira, T.A., Rosa, J.G.S.D., Gusso, D., … Barcellos, L.J.G. (2014). Diazepam and fluoxetine decrease the stress response in zebrafish. PLoS One, 9, e103232. doi:10.1371/journal.pone.0103232
- De Felice, E., Porreca, I., Alleva, E., De Girolamo, P., Ambrosino, C., Ciriaco, E., … Sordino, P. (2014). Localization of BDNF expression in the developing brain of zebrafish. Journal of Anatomy, 224, 564–574. doi:10.1111/joa.12168
- De Groef, B., Goris, N., Arckens, L., Kuhn, E.R., & Darras, V.M. (2003). Corticotropin-releasing hormone (CRH)-induced thyrotropin release is directly mediated through CRH receptor type 2 on thyrotropes. Endocrinology, 144, 5537–5544. doi:10.1210/en.2003-0526
- Deak, T., Quinn, M., Cidlowski, J.A., Victoria, N.C., Murphy, A.Z., & Sheridan, J.F. (2015). Neuroimmune mechanisms of stress: Sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress, 18, 367–380. doi:10.3109/10253890.2015.1053451
- Demin, K.A., Lakstygal, A.M., Chernysh, M.V., Krotova, N.A., Taranov, A.S., Ilyin, N.P., … Kalueff, A.V. (2020). The zebrafish tail immobilization (ZTI) test as a new tool to assess stress-related behavior and a potential screen for drugs affecting despair-like states. Journal of Neurosci Methods, 108637. doi:10.1016/j.jneumeth.2020.108637
- Demin, K.A., Meshalkina, D.A., Kysil, E.V., Antonova, K.A., Volgin, A.D., Yakovlev, O.A., … Kalueff, A.V. (2018). Zebrafish models relevant to studying central opioid and endocannabinoid systems. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 86, 301–312. doi:10.1016/j.pnpbp.2018.03.024
- Deroo, B.J., & Archer, T.K. (2001). Glucocorticoid receptor-mediated chromatin remodeling in vivo. Oncogene, 20, 3039–3046. doi:10.1038/sj.onc.1204328
- Deutsch-Feldman, M., Picetti, R., Seip-Cammack, K., Zhou, Y., & Kreek, M.J. (2015). Effects of handling and vehicle injections on adrenocorticotropic and corticosterone concentrations in Sprague-Dawley compared with Lewis rats. Journal of the American Association for Laboratory Animal Science: JAALAS, 54, 35–39.
- Di, S., Malcher-Lopes, R., Halmos, K.C., & Tasker, J.G. (2003). Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. The Journal of Neuroscience, 23, 4850–4857. doi:10.1523/JNEUROSCI.23-12-04850.2003
- Djurhuus, C.B., Gravholt, C.H., Nielsen, S., Mengel, A., Christiansen, J.S., Schmitz, O.E., & Møller, N. (2002). Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. American Journal of Physiology-Endocrinology and Metabolism, 283, E172–E177. doi:10.1152/ajpendo.00544.2001
- Dorts, J., Falisse, E., Schoofs, E., Flamion, E., Kestemont, P., & Silvestre, F. (2016). DNA methyltransferases and stress-related genes expression in zebrafish larvae after exposure to heat and copper during reprogramming of DNA methylation. Scientific Reports, 6, 34254. doi:10.1038/srep34254
- Egan, R.J., Bergner, C.L., Hart, P.C., Cachat, J.M., Canavello, P.R., Elegante, M.F., … Kalueff, A.V. (2009). Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behavioural Brain Research, 205, 38–44. doi:10.1016/j.bbr.2009.06.022
- Engelsma, M.Y., Huising, M.O., van Muiswinkel, W.B., Flik, G., Kwang, J., Savelkoul, H.F.J., & Verburg-van Kemenade, B.M.L. (2002). Neuroendocrine–immune interactions in fish: A role for interleukin-1. Veterinary Immunology and Immunopathology, 87, 467–479. doi:10.1016/S0165-2427(02)00077-6
- Esteban, M.Á., Rodrı́guez, A., Ayala, A.G., & Meseguer, J. (2004). Effects of high doses of cortisol on innate cellular immune response of seabream (Sparus aurata L.). General and Comparative Endocrinology, 137, 89–98. doi:10.1016/j.ygcen.2004.02.006
- Eto, K., Mazilu-Brown, J.K., Henderson-MacLennan, N., Dipple, K.M., & McCabe, E.R.B. (2014). Development of catecholamine and cortisol stress responses in zebrafish. Molecular Genetics and Metabolism Reports, 1, 373–377. doi:10.1016/j.ymgmr.2014.08.003
- Facchinello, N., Skobo, T., Meneghetti, G., Colletti, E., Dinarello, A., Tiso, N., … Dalla Valle, L. (2017). nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Scientific Reports, 7, 4371. doi:10.1038/s41598-017-04535-6
- Fast, M.D., Hosoya, S., Johnson, S.C., & Afonso, L.O. (2008). Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish & Shellfish Immunology, 24, 194–204. doi:10.1016/j.fsi.2007.10.009
- Flik, G., Klaren, P.H.M., Van den Burg, E.H., Metz, J.R., & Huising, M.O. (2006). CRF and stress in fish. General and Comparative Endocrinology, 146, 36–44. doi:10.1016/j.ygcen.2005.11.005
- Forsatkar, M.N., Safari, O., & Boiti, C. (2017). Effects of social isolation on growth, stress response, and immunity of zebrafish. Acta Ethologica, 20, 255–261. doi:10.1007/s10211-017-0270-7
- Foster, J.A., Rinaman, L., & Cryan, J.F. (2017). Stress and the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 7, 124–136. doi:10.1016/j.ynstr.2017.03.001
- Fox, H.E., White, S.A., Kao, M.H.F., & Fernald, R.D. (1997). Stress and dominance in a social fish. The Journal of Neuroscience, 17, 6463–6469. doi:10.1523/JNEUROSCI.17-16-06463.1997
- Fryer, C.J., & Archer, T.K. (1998). Chromatin remodelling by the glucocorticoid receptor requires the BRG1 complex. Nature, 393, 88–91. doi:10.1038/30032
- Fulcher, N., Tran, S., Shams, S., Chatterjee, D., & Gerlai, R. (2017). Neurochemical and behavioral responses to unpredictable chronic mild stress following developmental isolation: The zebrafish as a model for major depression. Zebrafish, 14, 23–34. doi:10.1089/zeb.2016.1295
- Gaikwad, S., Stewart, A., Hart, P., Wong, K., Piet, V., Cachat, J., & Kalueff, A.V. (2011). Acute stress disrupts performance of zebrafish in the cued and spatial memory tests: The utility of fish models to study stress–memory interplay. Behavioural Processes, 87, 224–230. doi:10.1016/j.beproc.2011.04.004
- Genario, R., de Abreu, M.S., Giacomini, A.C.V.V., Demin, K.A., & Kalueff, A.V. (2020). Sex differences in behavior and neuropharmacology of zebrafish. European Journal of Neuroscience, 714, 134548. doi:10.1111/ejn.14438
- Genario, R., Giacomini, A.C.V.V., de Abreu, M.S., Marcon, L., Demin, K.A., & Kalueff, A.V. (2020). Sex differences in adult zebrafish anxiolytic-like responses to diazepam and melatonin. Neuroscience Letters, 714, 134548. doi:10.1016/j.neulet.2019.134548
- Genario, R., Giacomini, A.C.V.V., Demin, K.A., dos Santos, B.E., Marchiori, N.I., Volgin, A.D., … Kalueff, A.V. (2019). The evolutionarily conserved role of melatonin in CNS disorders and behavioral regulation: Translational lessons from zebrafish. Neuroscience & Biobehavioral Reviews, 99, 117–127. doi:10.1016/j.neubiorev.2018.12.025
- Gerlai, R. (2019). Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacology Biochemistry and Behavior, 178, 30–38. doi:10.1016/j.pbb.2018.02.005
- Ghisleni, G., Capiotti, K.M., Da Silva, R.S., Oses, J.P., Piato, Â.L., Soares, V., … Bonan, C.D. (2012). The role of CRH in behavioral responses to acute restraint stress in zebrafish. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 36, 176–182. doi:10.1016/j.pnpbp.2011.08.016
- Giacomini, A.C.V.V., Abreu, M.S., Giacomini, L.V., Siebel, A.M., Zimerman, F.F., Rambo, C.L., … Barcellos, L.J.G. (2016). Fluoxetine and diazepam acutely modulate stress induced-behavior. Behavioural Brain Research, 296, 301–310. doi:10.1016/j.bbr.2015.09.027
- Giacomini, A., de Abreu, M.S., Koakoski, G., Idalencio, R., Kalichak, F., Oliveira, T.A., … Barcellos, L.J.G. (2015). My stress, our stress: Blunted cortisol response to stress in isolated housed zebrafish. Physiology & Behavior, 139, 182–187. doi:10.1016/j.physbeh.2014.11.035
- Giacomini, A.C.V.V., Piassetta, A.S., Genario, R., Bonan, C.D., Piato, A., Barcellos, L.J.G., & de Abreu, M.S. (2020). Tryptophan alleviates neuroendocrine and behavioral responses to stress in zebrafish. Behavioural Brain Research, 378, 112264. doi:10.1016/j.bbr.2019.112264
- Giguere, V., Hollenberg, S.M., Rosenfeld, M.G., & Evans, R.M. (1986). Functional domains of the human glucocorticoid receptor. Cell, 46, 645–652. doi:10.1016/0092-8674(86)90339-9
- Gonzalez-Nunez, V., Gonzalez-Sarmiento, R., & Rodriguez, R.E. (2003). Identification of two proopiomelanocortin genes in zebrafish (Danio rerio). Brain Research. Molecular Brain Research, 120, 1–8. doi:10.1016/j.molbrainres.2003.09.012
- Goodman, H.M. (2009). Chapter 4 – Adrenal glands. In H. M. Goodman (Ed.), Basic medical endocrinology (4th ed., pp. 61–90). San Diego, CA: Academic Press.
- Gorissen, M., Manuel, R., Pelgrim, T.N.M., Mes, W., de Wolf, M.J.S., Zethof, J., … van den Bos, R. (2015). Differences in inhibitory avoidance, cortisol and brain gene expression in TL and AB zebrafish. Genes, Brain and Behavior, 14, 428–438. doi:10.1111/gbb.12220
- Griffiths, B., Schoonheim, P., Ziv, L., Voelker, L., Baier, H., & Gahtan, E. (2012). A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Frontiers in Behavioral Neuroscience, 6, 68. doi:10.3389/fnbeh.2012.00068
- Grossman, L., Utterback, E., Stewart, A., Gaikwad, S., Chung, K.M., Suciu, C., … Kalueff, A.V. (2010). Characterization of behavioral and endocrine effects of LSD on zebrafish. Behavioural Brain Research, 214, 277–284. doi:10.1016/j.bbr.2010.05.039
- Grzelak, A.K., Davis, D.J., Caraker, S.M., Crim, M.J., Spitsbergen, J.M., & Wiedmeyer, C.E. (2017). Stress leukogram induced by acute and chronic stress in zebrafish (Danio rerio). Comparative Medicine, 67, 263–269.
- Hammond, G.L. (2016). Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. Journal of Endocrinology, 230, R13–R25. doi:10.1530/JOE-16-0070
- Hartig, E.I., Zhu, S., King, B.L., & Coffman, J.A. (2016). Cortisol-treated zebrafish embryos develop into pro-inflammatory adults with aberrant immune gene regulation. Biology Open, 5, 1134–1141. doi:10.1242/bio.020065
- Healy, E., Jordan, S.N.A., Budd, P.S., Suffolk, R., Rees, J.L., & Jackson, I.J. (2001). Functional variation of MC1R alleles from red-haired individuals. Human Molecular Genetics, 10, 2397–2402. doi:10.1093/hmg/10.21.2397
- Hinsch, K., & Zupanc, G.K.H. (2007). Generation and long-term persistence of new neurons in the adult zebrafish brain: A quantitative analysis. Neuroscience, 146, 679–696. doi:10.1016/j.neuroscience.2007.01.071
- Hodes, G.E., Kana, V., Menard, C., Merad, M., & Russo, S.J. (2015). Neuroimmune mechanisms of depression. Nature Neuroscience, 18, 1386–1393. doi:10.1038/nn.4113
- Hollenberg, S.M., & Evans, R.M. (1988). Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell, 55, 899–906. doi:10.1016/0092-8674(88)90145-6
- Holsboer, F., & Ising, M. (2008). Central CRH system in depression and anxiety – Evidence from clinical studies with CRH1 receptor antagonists. European Journal of Pharmacology, 583, 350–357. doi:10.1016/j.ejphar.2007.12.032
- Hu, M.C., Chiang, E.F., Tong, S.K., Lai, W., Hsu, N.C., Wang, L.C., & Chung, B.C. (2001). Regulation of steroidogenesis in transgenic mice and zebrafish. Molecular and Cellular Endocrinology, 171, 9–14. doi:10.1016/S0303-7207(00)00385-3
- Huang, V., Butler, A.A., & Lubin, F.D. (2019). Telencephalon transcriptome analysis of chronically stressed adult zebrafish. Scientific Reports, 9, 1379. doi:10.1038/s41598-018-37761-7
- Huising, M.O., & Flik, G. (2005). The remarkable conservation of corticotropin-releasing hormone (CRH)-binding protein in the honeybee (Apis mellifera) dates the CRH system to a common ancestor of insects and vertebrates. Endocrinology, 146, 2165–2170. doi:10.1210/en.2004-1514
- Huising, M.O., Kruiswijk, C.P., van Schijndel, J.E., Savelkoul, H.F.J., Flik, G., & Verburg-van Kemenade, B.M.L. (2005). Multiple and highly divergent IL-11 genes in teleost fish. Immunogenetics, 57, 432–443. doi:10.1007/s00251-005-0012-2
- Huising, M.O., Metz, J.R., van Schooten, C., Taverne-Thiele, A.J., Hermsen, T., Verburg-van Kemenade, B.M., & Flik, G. (2004). Structural characterisation of a cyprinid (Cyprinus carpio L.) CRH, CRH-BP and CRH-R1, and the role of these proteins in the acute stress response. Journal of Molecular Endocrinology, 32, 627–648. doi:10.1677/jme.0.0320627
- Huising, M.O., van der Aa, L.M., Metz, J.R., de Fátima Mazon, A., Verburg-van Kemenade, B.M.L., & Flik, G. (2007). Corticotropin-releasing factor (CRF) and CRF-binding protein expression in and release from the head kidney of common carp: Evolutionary conservation of the adrenal CRF system. Journal of Endocrinology, 193, 349–357. doi:10.1677/JOE-07-0070
- Idalencio, R., Kalichak, F., Rosa, J.G.S., Oliveira, T.A.D., Koakoski, G., Gusso, D., … Barcellos, L.J.G. (2015). Waterborne risperidone decreases stress response in zebrafish. PLoS One, 10, e0140800. doi:10.1371/journal.pone.0140800
- Irons, T.D., MacPhail, R.C., Hunter, D.L., & Padilla, S. (2010). Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicology and Teratology, 32, 84–90. doi:10.1016/j.ntt.2009.04.066
- Ito, K., Barnes, P.J., & Adcock, I.M. (2000). Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Molecular and Cellular Biology, 20, 6891–6903. doi:10.1128/MCB.20.18.6891-6903.2000
- James, S.L., Abate, D., Abate, K.H., Abay, S.M., Abbafati, C., Abbasi, N., … Murray, C.J.L. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet, 392, 1789–1858. doi:10.1016/S0140-6736(18)32279-7
- Jesuthasan, S. (2012). Fear, anxiety, and control in the zebrafish. Developmental Neurobiology, 72, 395–403. doi:10.1002/dneu.20873
- Jesuthasan, S.J., & Mathuru, A.S. (2008). The alarm response in zebrafish: Innate fear in a vertebrate genetic model. Journal of Neurogenetics, 22, 211–228. doi:10.1080/01677060802298475
- Jimenez-Gonzalez, A., Garcia-Concejo, A., Lopez-Benito, S., Gonzalez-Nunez, V., Arevalo, J.C., & Rodriguez, R.E. (2016). Role of morphine, miR-212/132 and mu opioid receptor in the regulation of Bdnf in zebrafish embryos. Biochimica et Biophysica Acta (BBA) - General Subjects, 1860, 1308–1316. doi:10.1016/j.bbagen.2016.03.001
- Johansson, L., Guo, X., Waern, M., Östling, S., Gustafson, D., Bengtsson, C., & Skoog, I. (2010). Midlife psychological stress and risk of dementia: A 35-year longitudinal population study. Brain, 133, 2217–2224. doi:10.1093/brain/awq116
- Joëls, M., & Baram, T.Z. (2009). The neuro-symphony of stress. Nature Reviews Neuroscience, 10, 459–466. doi:10.1038/nrn2632
- Kafkafi, N., Agassi, J., Chesler, E.J., Crabbe, J.C., Crusio, W.E., Eilam, D., … Benjamini, Y. (2018). Reproducibility and replicability of rodent phenotyping in preclinical studies. Neuroscience & Biobehavioral Reviews, 87, 218–232. doi:10.1016/j.neubiorev.2018.01.003
- Kalueff, A.V., Echevarria, D.J., Homechaudhuri, S., Stewart, A.M., Collier, A.D., Kaluyeva, A.A., … Song, C. (2016). Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquatic Toxicology, 170, 297–309. doi:10.1016/j.aquatox.2015.08.007
- Kalueff, A.V., Echevarria, D.J., & Stewart, A.M. (2014). Gaining translational momentum: More zebrafish models for neuroscience research. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 55, 1–6. doi:10.1016/j.pnpbp.2014.01.022
- Kalueff, A.V., Gebhardt, M., Stewart, A.M., Cachat, J.M., Brimmer, M., Chawla, J.S., … Schneider; Zebrafish Neuroscience Research Consortium. (2013). Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish, 10, 70–86. doi:10.1089/zeb.2012.0861
- Kalueff, A.V., Stewart, A.M., & Gerlai, R. (2014). Zebrafish as an emerging model for studying complex brain disorders. Trends in Pharmacological Sciences, 35, 63–75. doi:10.1016/j.tips.2013.12.002
- Karlén, J., Frostell, A., Theodorsson, E., Faresjö, T., & Ludvigsson, J. (2013). Maternal influence on child HPA axis: A prospective study of cortisol levels in hair. Pediatrics, 132, E1333–E1340. doi:10.1542/peds.2013-1178
- Keck, M.E., & Holsboer, F. (2001). Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides, 22, 835–844. doi:10.1016/S0196-9781(01)00398-9
- Kenney, J.W., Scott, I.C., Josselyn, S.A., & Frankland, P.W. (2017). Contextual fear conditioning in zebrafish. Learning & Memory, 24, 516–523. doi:10.1101/lm.045690.117
- Khor, B.S., Jamil, M.F., Adenan, M.I., & Shu-Chien, A.C. (2011). Mitragynine attenuates withdrawal syndrome in morphine-withdrawn zebrafish. PLoS One, 6, e28340. doi:10.1371/journal.pone.0028340
- Kirsten, K., Pompermaier, A., Koakoski, G., Mendonça-Soares, S., da Costa, R.A., Maffi, V.C., Kreutz, L.C., & Barcellos, L.J.G. (2020). Acute and chronic stress differently alter the expression of cytokine and neuronal markers genes in zebrafish brain. Stress, 12, 1–6. doi:10.1080/10253890.2020.1724947
- Kishimoto, T., Radulovic, J., Radulovic, M., Lin, C.R., Schrick, C., Hooshmand, F., … Spiess, J. (2000). Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nature Genetics, 24, 415–419. doi:10.1038/74271
- Kleinhappel, T.K., Pike, T.W., & Burman, O.H.P. (2019). Stress-induced changes in group behaviour. Scientific Reports, 9, 17200. doi:10.1038/s41598-019-53661-w
- Kokel, D., Bryan, J., Laggner, C., White, R., Cheung, C.Y.J., Mateus, R., … Peterson, R.T. (2010). Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nature Chemical Biology, 6, 231–237. doi:10.1038/nchembio.307
- Kokel, D., & Peterson, R.T. (2008). Chemobehavioural phenomics and behaviour-based psychiatric drug discovery in the zebrafish. Briefings in Functional Genomics and Proteomics, 7, 483–490. doi:10.1093/bfgp/eln040
- Koob, G.F. (2009). Brain stress systems in the amygdala and addiction. Brain Research, 1293, 61–75. doi:10.1016/j.brainres.2009.03.038
- Kopp, C., Vogel, E., Rettori, M.C., Delagrange, P., & Misslin, R. (1999). The effects of melatonin on the behavioural disturbances induced by chronic mild stress in C3H/He mice. Behavioural Pharmacology, 10, 73–83. doi:10.1097/00008877-199902000-00007
- Krug, R.G., Lee, H.B., El Khoury, L.Y., Sigafoos, A.N., Petersen, M.O., & Clark, K.J. (2018). The endocannabinoid gene faah2a modulates stress-associated behavior in zebrafish. PLoS One, 13, e0190897. doi:10.1371/journal.pone.0190897
- Kumari, Y., Choo, B.K.M., Shaikh, M.F., & Othman, I. (2019). Melatonin receptor agonist Piper betle L. ameliorates dexamethasone-induced early life stress in adult zebrafish. Experimental and Therapeutic Medicine, 18, 1407–1416. doi:10.3892/etm.2019.7685
- Kysil, E.V., Meshalkina, D.A., Frick, E.E., Echevarria, D.J., Rosemberg, D.B., Maximino, C., … Kalueff, A.V. (2017). Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish, 14, 197–208. doi:10.1089/zeb.2016.1415
- Kyzar, E.J., Collins, C., Gaikwad, S., Green, J., Roth, A., Monnig, L., … Kalueff, A.V. (2012). Effects of hallucinogenic agents mescaline and phencyclidine on zebrafish behavior and physiology. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 37, 194–202. doi:10.1016/j.pnpbp.2012.01.003
- Lakstygal, A.M., de Abreu, M.S., & Kalueff, A.V. (2018). Zebrafish models of epigenetic regulation of CNS functions. Brain Research Bulletin, 142, 344–351. doi:10.1016/j.brainresbull.2018.08.022
- Larson, E.T., O’Malley, D.M., & Melloni, R.H., Jr. (2006). Aggression and vasotocin are associated with dominant–subordinate relationships in zebrafish. Behavioural Brain Research, 167, 94–102. doi:10.1016/j.bbr.2005.08.020
- Law, W.Y., Chen, W.H., Song, Y.L., Dufour, S., & Chang, C.F. (2001). Differential in vitro suppressive effects of steroids on leukocyte phagocytosis in two teleosts, tilapia and common carp. General and Comparative Endocrinology, 121, 163–172. doi:10.1006/gcen.2000.7593
- Lederis, K., Fryer, J.N., Okawara, Y., Schönrock, C., & Richter, D. (1994). Corticotropin-releasing factors acting on the fish pituitary: Experimental and molecular analysis. In N. M. Sherwood, C. L. Hew, A. P. Farrell, & D. J. Randall (Eds.), Fish physiology (Vol. 13, pp. 67–100). San Diego, CA: Academic Press.
- Lee, S.P., Sung, I.-K., Kim, J.H., Lee, S.-Y., Park, H.S., & Shim, C.S. (2015). The effect of emotional stress and depression on the prevalence of digestive diseases. Journal of Neurogastroenterology and Motility, 21, 273–282. doi:10.5056/jnm14116
- Liu, Y.-W., Liu, W.-H., Wu, C.-C., Juan, Y.-C., Wu, Y.-C., Tsai, H.-P., … Tsai, Y.-C. (2016). Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Research, 1631, 1–12. doi:10.1016/j.brainres.2015.11.018
- Logan, D.W., Bryson-Richardson, R.J., Pagán, K.E., Taylor, M.S., Currie, P.D., & Jackson, I.J. (2003). The structure and evolution of the melanocortin and MCH receptors in fish and mammals. Genomics, 81, 184–191. doi:10.1016/S0888-7543(02)00037-X
- London, S., & Volkoff, H. (2019). Effects of fasting on the central expression of appetite-regulating and reproductive hormones in wild-type and Casper zebrafish (Danio rerio). General and Comparative Endocrinology, 282, 113207. doi:10.1016/j.ygcen.2019.06.011
- Luca, R.M., & Gerlai, R. (2012). Animated bird silhouette above the tank: Acute alcohol diminishes fear responses in zebrafish. Behavioural Brain Research, 229, 194–201. doi:10.1016/j.bbr.2012.01.021
- Lupien, S.J., Juster, R.-P., Raymond, C., & Marin, M.-F. (2018). The effects of chronic stress on the human brain: From neurotoxicity, to vulnerability, to opportunity. Frontiers in Neuroendocrinology, 49, 91–105. doi:10.1016/j.yfrne.2018.02.001
- López-Patiño, M.A., Gesto, M., Conde-Sieira, M., Soengas, J.L., & Míguez, J.M. (2014). Stress inhibition of melatonin synthesis in the pineal organ of rainbow trout (Oncorhynchus mykiss) is mediated by cortisol. The Journal of Experimental Biology, 217, 1407. doi:10.1242/jeb.087916
- Malkoski, S.P., & Dorin, R.I. (1999). Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Molecular Endocrinology, 13, 1629–1644. doi:10.1210/mend.13.10.0351
- Manuel, R., Gorissen, M., Zethof, J., Ebbesson, L.O., van de Vis, H., Flik, G., & van den Bos, R. (2014). Unpredictable chronic stress decreases inhibitory avoidance learning in Tuebingen long-fin zebrafish: Stronger effects in the resting phase than in the active phase. Journal of Experimental Biology, 217, 3919–3928. doi:10.1242/jeb.109736
- Marcon, M., Herrmann, A.P., Mocelin, R., Rambo, C.L., Koakoski, G., Abreu, M.S., … Piato, A.L. (2016). Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology, 233, 3815–3824. doi:10.1007/s00213-016-4408-5
- Marcon, M., Mocelin, R., Benvenutti, R., Costa, T., Herrmann, A.P., de Oliveira, D.L., … Piato, A. (2018). Environmental enrichment modulates the response to chronic stress in zebrafish. The Journal of Experimental Biology, 221, jeb176735. doi:10.1242/jeb.176735
- Marsh, D.J., Hollopeter, G., Huszar, D., Laufer, R., Yagaloff, K.A., Fisher, S.L., … Palmiter, R.D. (1999). Response of melanocortin-4 receptor-deficient mice to anorectic and orexigenic peptides. Nature Genetics, 21, 119–122. doi:10.1038/5070
- Marsland, A.L., Walsh, C., Lockwood, K., & John-Henderson, N.A. (2017). The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. doi:10.1016/j.bbi.2017.01.011
- Martin, C.R., Osadchiy, V., Kalani, A., & Mayer, E.A. (2018). The brain-gut-microbiome axis. Cellular and Molecular Gastroenterology and Hepatology, 6, 133–148. doi:10.1016/j.jcmgh.2018.04.003
- Maximino, C., de Brito, T.M., da Silva Batista, A.W., Herculano, A.M., Morato, S., & Gouveia, A. (2010). Measuring anxiety in zebrafish: A critical review. Behavioural Brain Research, 214, 157–171. doi:10.1016/j.bbr.2010.05.031
- Maximino, C., Lima, M.G., Costa, C.C., Guedes, I.M.L., & Herculano, A.M. (2014). Fluoxetine and WAY 100,635 dissociate increases in scototaxis and analgesia induced by conspecific alarm substance in zebrafish (Danio rerio Hamilton 1822). Pharmacology Biochemistry and Behavior, 124, 425–433. doi:10.1016/j.pbb.2014.07.003
- Maximino, C., Marques de Brito, T., Dias, C.A.G.D.M., Gouveia, A., Jr., & Morato, S. (2010). Scototaxis as anxiety-like behavior in fish. Nature Protocols, 5, 209–216. doi:10.1038/nprot.2009.225
- Mazeaud, M.M., Mazeaud, F., & Donaldson, E.M. (1977). Primary and secondary effects of stress in fish: Some new data with a general review. Transactions of the American Fisheries Society, 106, 201–212. doi:10.1577/1548-8659(1977)106<201:PASEOS>2.0.CO;2
- McEwen, B.S. (2017). Neurobiological and systemic effects of chronic stress. Chronic Stress, 1, 1–17. doi:10.1177/2470547017692328
- McEwen, B.S., & Stellar, E. (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. doi:10.1001/archinte.1993.00410180039004
- McGonigle, P. (2014). Animal models of CNS disorders. Biochemical Pharmacology, 87, 140–149. doi:10.1016/j.bcp.2013.06.016
- McHenry, M.J., Feitl, K.E., Strother, J.A., & Van Trump, W.J. (2009). Larval zebrafish rapidly sense the water flow of a predator’s strike. Biology Letters, 5, 477–479. doi:10.1098/rsbl.2009.0048
- McMahon, M., Gerich, J., & Rizza, R. (1988). Effects of glucocorticoids on carbohydrate metabolism. Diabetes/Metabolism Reviews, 4, 17–30. doi:10.1002/dmr.5610040105
- McQuade, R., & Young, A.H. (2000). Future therapeutic targets in mood disorders: The glucocorticoid receptor. British Journal of Psychiatry, 177, 390–395. doi:10.1192/bjp.177.5.390
- Metz, J.R., Peters, J.J., & Flik, G. (2006). Molecular biology and physiology of the melanocortin system in fish: A review. General and Comparative Endocrinology, 148, 150–162. doi:10.1016/j.ygcen.2006.03.001
- Mineur, Y.S., Belzung, C., & Crusio, W.E. (2006). Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behavioural Brain Research, 175, 43–50. doi:10.1016/j.bbr.2006.07.029
- Mocelin, R., Herrmann, A.P., Marcon, M., Rambo, C.L., Rohden, A., Bevilaqua, F., … Piato, A.L. (2015). N-acetylcysteine prevents stress-induced anxiety behavior in zebrafish. Pharmacology Biochemistry and Behavior, 139, 121–126. doi:10.1016/j.pbb.2015.08.006
- Mommsen, T.P., Vijayan, M.M., & Moon, T.W. (1999). Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Reviews in Fish Biology and Fisheries, 9, 211–268. doi:10.1023/A:1008924418720
- Morash, M.G., MacDonald, A.B., Croll, R.P., & Anini, Y. (2009). Molecular cloning, ontogeny and tissue distribution of zebrafish (Danio rerio) prohormone convertases: pcsk1 and pcsk2. General and Comparative Endocrinology, 162, 179–187. doi:10.1016/j.ygcen.2009.03.013
- Mueller, T. (2012). What is the thalamus in zebrafish? Frontiers in Neuroscience, 6, 64–64. doi:10.3389/fnins.2012.00064
- Müller, M.B., & Wurst, W. (2004). Getting closer to affective disorders: The role of CRH receptor systems. Trends in Molecular Medicine, 10, 409–415. doi:10.1016/j.molmed.2004.06.007
- Nesan, D., & Vijayan, M.M. (2016). Maternal cortisol mediates hypothalamus-pituitary-interrenal axis development in zebrafish. Scientific Reports, 6, 22582. doi:10.1038/srep22582
- Nestler, E.J., & Hyman, S.E. (2010). Animal models of neuropsychiatric disorders. Nature Neuroscience, 13, 1161–1169. doi:10.1038/nn.2647
- Nguyen, M., Stewart, A.M., & Kalueff, A.V. (2014). Aquatic blues: Modeling depression and antidepressant action in zebrafish. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 55, 26–39. doi:10.1016/j.pnpbp.2014.03.003
- Nirwane, A., Sridhar, V., & Majumdar, A. (2016). Neurobehavioural changes and brain oxidative stress induced by acute exposure to GSM900 mobile phone radiations in zebrafish (Danio rerio). Toxicological Research, 32, 123–132. doi:10.5487/TR.2016.32.2.123
- Norton, W., & Bally-Cuif, L. (2010). Adult zebrafish as a model organism for behavioural genetics. BMC Neuroscience, 11, 90. doi:10.1186/1471-2202-11-90
- Oberlander, T.F., Weinberg, J., Papsdorf, M., Grunau, R., Misri, S., & Devlin, A.M. (2008). Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics, 3, 97–106. doi:10.4161/epi.3.2.6034
- Ogwang, S.P. (2008). Inspection of a novel object by wild and laboratory Zebrafish (Danio rerio H.) in the presence and absence of alarm substance (Master’s thesis). Department of Biology, Fisheries Biology and Management, University of Bergen, Bergen.
- Oliveira, T.A., Koakoski, G., Kreutz, L.C., Ferreira, D., Rosa, J.G.S.D., de Abreu, M.S., … Barcellos, L.J.G. (2013). Alcohol impairs predation risk response and communication in zebrafish. PLoS One, 8, e75780. doi:10.1371/journal.pone.0075780
- Pagniello, K.B., Bols, N.C., & Lee, L.E. (2002). Effect of corticosteroids on viability and proliferation of the rainbow trout monocyte/macrophage cell line, RTS11. Fish & Shellfish Immunology, 13, 199–214. doi:10.1006/fsim.2001.0395
- Palić, D., Andreasen, C.B., Ostojić, J., Tell, R.M., & Roth, J.A. (2007). Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. Journal of Immunological Methods, 319, 87–97. doi:10.1016/j.jim.2006.11.003
- Panula, P., Chen, Y.-C., Priyadarshini, M., Kudo, H., Semenova, S., Sundvik, M., & Sallinen, V. (2010). The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiology of Disease, 40, 46–57. doi:10.1016/j.nbd.2010.05.010
- Pavlidis, M., Theodoridi, A., & Tsalafouta, A. (2015). Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 60, 121–131. doi:10.1016/j.pnpbp.2015.02.014
- Piato, Â.L., Capiotti, K.M., Tamborski, A.R., Oses, J.P., Barcellos, L.J.G., Bogo, M.R., … Bonan, C.D. (2011). Unpredictable chronic stress model in zebrafish (Danio rerio): Behavioral and physiological responses. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35, 561–567. doi:10.1016/j.pnpbp.2010.12.018
- Piato, A.L., Rosemberg, D.B., Capiotti, K.M., Siebel, A.M., Herrmann, A.P., Ghisleni, G., … Bonan, C.D. (2011). Acute restraint stress in zebrafish: Behavioral parameters and purinergic signaling. Neurochemical Research, 36, 1876–1886. doi:10.1007/s11064-011-0509-z
- Pickering, A.D. (1998). Stress responses of farmed fish. In K. D. Black & A. D. Pickering (Eds.), Biology of farmed fish (pp. 222–255). Sheffield: Sheffield Academic Press.
- Pippal, J.B., Cheung, C.M.I., Yao, Y.-Z., Brennan, F.E., & Fuller, P.J. (2011). Characterization of the zebrafish (Danio rerio) mineralocorticoid receptor. Molecular and Cellular Endocrinology, 332, 58–66. doi:10.1016/j.mce.2010.09.014
- Radahmadi, M., Alaei, H., Sharifi, M.R., & Hosseini, N. (2015). Effects of different timing of stress on corticosterone, BDNF and memory in male rats. Physiology & Behavior, 139, 459–467. doi:10.1016/j.physbeh.2014.12.004
- Rambo, C.L., Mocelin, R., Marcon, M., Villanova, D., Koakoski, G., de Abreu, M.S., … Bonan, C.D. (2017). Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiology & Behavior, 171, 50–54. doi:10.1016/j.physbeh.2016.12.032
- Ramsay, J.M., Feist, G.W., Varga, Z.M., Westerfield, M., Kent, M.L., & Schreck, C.B. (2009). Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture, 297, 157–162. doi:10.1016/j.aquaculture.2009.08.035
- Reichmann, F., & Holzer, P. (2016). Neuropeptide Y: A stressful review. Neuropeptides, 55, 99–109. doi:10.1016/j.npep.2015.09.008
- Resnick, S.G., Bond, G.R., & Mueser, K.T. (2003). Trauma and posttraumatic stress disorder in people with schizophrenia. Journal of Abnormal Psychology, 112, 415–423. doi:10.1037/0021-843X.112.3.415
- Richardson, J., Lundegaard, P.R., Reynolds, N.L., Dorin, J.R., Porteous, D.J., Jackson, I.J., & Patton, E.E. (2008). mc1r pathway regulation of zebrafish melanosome. Zebrafish, 5, 289–295. doi:10.1089/zeb.2008.0541
- Rieger, S., Wang, F., & Sagasti, A. (2011). Time-lapse imaging of neural development: Zebrafish lead the way into the fourth dimension. Genesis, 49, 534–545. doi:10.1002/dvg.20729
- Rihel, J., & Schier, A.F. (2012). Behavioral screening for neuroactive drugs in zebrafish. Developmental Neurobiology, 72, 373–385. doi:10.1002/dneu.20910
- Roebuck, M.M., Jones, C.T., Robinson, J.S., Mitchell, M.D., & Thorburn, G.D. (1984). ACTH control of steroid secretion from adrenal cells of the developing rhesus monkey (Macaca mulatta). Acta Endocrinologica, 105, 545–551. doi:10.1530/acta.0.1050545
- Ruddick, J.P., Evans, A.K., Nutt, D.J., Lightman, S.L., Rook, G.A.W., & Lowry, C.A. (2006). Tryptophan metabolism in the central nervous system: Medical implications. Expert Reviews in Molecular Medicine, 8, 1–27. doi:10.1017/S1462399406000068
- Ruuskanen, J.O., Peitsaro, N., Kaslin, J.V.M., Panula, P., & Scheinin, M. (2005). Expression and function of α2-adrenoceptors in zebrafish: Drug effects, mRNA and receptor distributions. Journal of Neurochemistry, 94, 1559–1569. doi:10.1111/j.1471-4159.2005.03305.x
- Sallin, P., & Jaźwińska, A. (2016). Acute stress is detrimental to heart regeneration in zebrafish. Open Biology, 6, 160012. doi:10.1098/rsob.160012
- Sarkar, D.K., Kuhn, P., Marano, J., Chen, C., & Boyadjieva, N. (2007). Alcohol exposure during the developmental period induces β-endorphin neuronal death and causes alteration in the opioid control of stress axis function. Endocrinology, 148, 2828–2834. doi:10.1210/en.2006-1606
- Saverino, C., & Gerlai, R. (2008). The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behavioural Brain Research, 191, 77–87. doi:10.1016/j.bbr.2008.03.013
- Schaaf, M.J.M., Champagne, D., van Laanen, I.H.C., van Wijk, D.C.W.A., Meijer, A.H., Meijer, O.C., … Richardson, M.K. (2008). Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish. Endocrinology, 149, 1591–1599. doi:10.1210/en.2007-1364
- Schaaf, M.J.M., Chatzopoulou, A., & Spaink, H.P. (2009). The zebrafish as a model system for glucocorticoid receptor research. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 153, 75–82. doi:10.1016/j.cbpa.2008.12.014
- Scheinman, R.I., Gualberto, A., Jewell, C.M., Cidlowski, J.A., & Baldwin, A.S. (1995). Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Molecular and Cellular Biology, 15, 943–953. doi:10.1128/MCB.15.2.943
- Schioth, H.B., Chhajlani, V., Muceniece, R., Klusa, V., & Wikberg, J.E. (1996). Major pharmacological distinction of the ACTH receptor from other melanocortin receptors. Life Sciences, 59, 797–801. doi:10.1016/0024-3205(96)00370-0
- Schmidt, M.V., Scharf, S.H., Sterlemann, V., Ganea, K., Liebl, C., Holsboer, F., & Müller, M.B. (2010). High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology, 35, 635–643. doi:10.1016/j.psyneuen.2009.10.002
- Schmidt, R., Strähle, U., & Scholpp, S. (2013). Neurogenesis in zebrafish – From embryo to adult. Neural Development, 8, 3. doi:10.1186/1749-8104-8-3
- Seth, A., Stemple, D.L., & Barroso, I. (2013). The emerging use of zebrafish to model metabolic disease. Disease Models & Mechanisms, 6, 1080–1088. doi:10.1242/dmm.011346
- Shams, S., Chatterjee, D., & Gerlai, R. (2015). Chronic social isolation affects thigmotaxis and whole-brain serotonin levels in adult zebrafish. Behavioural Brain Research, 292, 283–287. doi:10.1016/j.bbr.2015.05.061
- Shams, S., Seguin, D., Facciol, A., Chatterjee, D., & Gerlai, R. (2017). Effect of social isolation on anxiety-related behaviors, cortisol, and monoamines in adult zebrafish. Behavioral Neuroscience, 131, 492–504. doi:10.1037/bne0000220
- Song, C., Liu, B.-P., Zhang, Y.-P., Peng, Z., Wang, J., Collier, A.D., … Kalueff, A.V. (2018). Modeling consequences of prolonged strong unpredictable stress in zebrafish: Complex effects on behavior and physiology. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 81, 384–394. doi:10.1016/j.pnpbp.2017.08.021
- Speedie, N., & Gerlai, R. (2008). Alarm substance induced behavioral responses in zebrafish (Danio rerio). Behavioural Brain Research, 188, 168–177. doi:10.1016/j.bbr.2007.10.031
- Spencer, K.A. (2017). Developmental stress and social phenotypes: Integrating neuroendocrine, behavioural and evolutionary perspectives. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160242. doi:10.1098/rstb.2016.0242
- Stankiewicz, A.M., Swiergiel, A.H., & Lisowski, P. (2013). Epigenetics of stress adaptations in the brain. Brain Research Bulletin, 98, 76–92. doi:10.1016/j.brainresbull.2013.07.003
- Steenbergen, P.J., Richardson, M.K., & Champagne, D.L. (2011). The use of the zebrafish model in stress research. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35, 1432–1451. doi:10.1016/j.pnpbp.2010.10.010
- Stewart, A., Gaikwad, S., Kyzar, E., Green, J., Roth, A., & Kalueff, A.V. (2012). Modeling anxiety using adult zebrafish: A conceptual review. Neuropharmacology, 62, 135–143. doi:10.1016/j.neuropharm.2011.07.037
- Stewart, A.M., Braubach, O., Spitsbergen, J., Gerlai, R., & Kalueff, A.V. (2014). Zebrafish models for translational neuroscience research: From tank to bedside. Trends in Neurosciences, 37, 264–278. doi:10.1016/j.tins.2014.02.011
- Stewart, A.M., Gaikwad, S., Kyzar, E., & Kalueff, A.V. (2012). Understanding spatio-temporal strategies of adult zebrafish exploration in the open field test. Brain Research, 1451, 44–52. doi:10.1016/j.brainres.2012.02.064
- Stewart, A.M., Gerlai, R., & Kalueff, A.V. (2015). Developing higher-throughput zebrafish screens for in-vivo CNS drug discovery. Frontiers in Behavioral Neuroscience, 9, 14. doi:10.3389/fnbeh.2015.00014
- Stewart, A.M., Yang, E., Nguyen, M., & Kalueff, A.V. (2014). Developing zebrafish models relevant to PTSD and other trauma- and stressor-related disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 55, 67–79. doi:10.1016/j.pnpbp.2014.08.003
- Stocco, D.M., & Clark, B.J. (1996). Regulation of the acute production of steroids in steroidogenic cells. Endocrine Reviews, 17, 221–244. doi:10.1210/edrv-17-3-221
- Stolte, E.H., Nabuurs, S.B., Bury, N.R., Sturm, A., Flik, G., Savelkoul, H.F., & Lidy Verburg-van Kemenade, B.M. (2008). Stress and innate immunity in carp: Corticosteroid receptors and pro-inflammatory cytokines. Molecular Immunology, 46, 70–79. doi:10.1016/j.molimm.2008.07.022
- Sumpter, J.P. (1997). The endocrinology of stress. Fish Stress and Health in Aquaculture, 819, 95–118.
- Sumpter, J.P., Pottinger, T.G., Rand-Weaver, M., & Campbell, P.M. (1994). The wide-ranging effects of stress on fish. In K. G. Davey, R. E. Peter, & S. S. Tobe (Eds.), Perspectives in endocrinology (pp. 535–538). Ottawa: National Research Council of Canada.
- Surjit, M., Ganti, K.P., Mukherji, A., Ye, T., Hua, G., Metzger, D., … Chambon, P. (2011). Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell, 145, 224–241. doi:10.1016/j.cell.2011.03.027
- Tea, J., Alderman, S.L., & Gilmour, K.M. (2019). Social stress increases plasma cortisol and reduces forebrain cell proliferation in subordinate male zebrafish (Danio rerio). The Journal of Experimental Biology, 222, jeb194894. doi:10.1242/jeb.194894
- Teles, M.C., Cardoso, S.D., & Oliveira, R.F. (2016). Social plasticity relies on different neuroplasticity mechanisms across the brain social decision-making network in zebrafish. Frontiers in Behavioral Neuroscience, 10, 16. doi:10.3389/fnbeh.2016.00016
- Tokarz, J., Norton, W., Möller, G., Hrabé de Angelis, M., & Adamski, J. (2013). Zebrafish 20β-hydroxysteroid dehydrogenase type 2 is important for glucocorticoid catabolism in stress response. PLoS One, 8, e54851. doi:10.1371/journal.pone.0054851
- Tran, S., Chatterjee, D., & Gerlai, R. (2014). Acute net stressor increases whole-body cortisol levels without altering whole-brain monoamines in zebrafish. Behavioral Neuroscience, 128, 621–624. doi:10.1037/bne0000005
- Tran, S., & Gerlai, R. (2015). Thirty-second net stressor task in adult zebrafish. Bio-Protocol, 5, e1413. doi:10.21769/BioProtoc.1413
- van den Bos, R., Mes, W., Galligani, P., Heil, A., Zethof, J., Flik, G., & Gorissen, M. (2017). Further characterisation of differences between TL and AB zebrafish (Danio rerio): Gene expression, physiology and behaviour at day 5 of the larval stage. PLoS One, 12, e0175420. doi:10.1371/journal.pone.0175420
- Van Der Boon, J., Van Den Thillart, G.E.E.J.M., & Addink, A.D.F. (1991). The effects of cortisol administration on intermediary metabolism in teleost fish. Comparative Biochemistry and Physiology Part A: Physiology, 100, 47–53. doi:10.1016/0300-9629(91)90182-C
- Vera-Chang, M.N., Moon, T.W., & Trudeau, V.L. (2019). Ancestral fluoxetine exposure sensitizes zebrafish to venlafaxine-induced reductions in cortisol and spawning. Endocrinology, 160, 2137–2142. doi:10.1210/en.2019-00281
- Vera-Chang, M.N., St-Jacques, A.D., Gagné, R., Martyniuk, C.J., Yauk, C.L., Moon, T.W., & Trudeau, V.L. (2018). Transgenerational hypocortisolism and behavioral disruption are induced by the antidepressant fluoxetine in male zebrafish Danio rerio. Proceedings of the National Academy of Sciences of the United States of America, 115, E12435–E12442. doi:10.1073/pnas.1811695115
- Vital, C., & Martins, E.P. (2011). Strain differences in zebrafish (Danio rerio) social roles and their impact on group task performance. Journal of Comparative Psychology, 125, 278–285. doi:10.1037/a0023906
- Wang, R., & Belosevic, M. (1995). The in vitro effects of estradiol and cortisol on the function of a long-term goldfish macrophage cell line. Developmental & Comparative Immunology, 19, 327–336. doi:10.1016/0145-305X(95)00018-O
- Wendelaar Bonga, S.E. (1997). The stress response in fish. Physiological Reviews, 77, 591–625. doi:10.1152/physrev.1997.77.3.591
- Wong, R.Y., Oxendine, S.E., & Godwin, J. (2013). Behavioral and neurogenomic transcriptome changes in wild-derived zebrafish with fluoxetine treatment. BMC Genomics., 14, 348. doi:10.1186/1471-2164-14-348
- Wood, S.K., Walker, H.E., Valentino, R.J., & Bhatnagar, S. (2010). Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: Role of corticotropin-releasing factor. Endocrinology, 151, 1795–1805. doi:10.1210/en.2009-1026
- Wright, D., Ward, A.J.W., Croft, D.P., & Krause, J. (2006). Social organization, grouping, and domestication in fish. Zebrafish, 3, 141–155. doi:10.1089/zeb.2006.3.141
- Yalcin, I., Belzung, C., & Surget, A. (2008). Mouse strain differences in the unpredictable chronic mild stress: A four-antidepressant survey. Behavioural Brain Research, 193, 140–143. doi:10.1016/j.bbr.2008.04.021
- Yang, L.-K., Zhang, Z.-R., Wen, H.-S., & Tao, Y.-X. (2019). Characterization of channel catfish (Ictalurus punctatus) melanocortin-3 receptor reveals a potential network in regulation of energy homeostasis. General and Comparative Endocrinology, 277, 90–103. doi:10.1016/j.ygcen.2019.03.011
- Yang-Yen, H.F., Chambard, J.C., Sun, Y.L., Smeal, T., Schmidt, T.J., Drouin, J., & Karin, M. (1990). Transcriptional interference between c-Jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein-protein interaction. Cell, 62, 1205–1215. doi:10.1016/0092-8674(90)90396-V
- Zaletel, I., Filipović, D., & Puškaš, N. (2016). Chronic stress, hippocampus and parvalbumin-positive interneurons: What do we know so far? Reviews in the Neurosciences, 27, 397–409. doi:10.1515/revneuro-2015-0042
- Zisapel, N. (2018). New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. British Journal of Pharmacology, 175, 3190–3199. doi:10.1111/bph.14116
- Ziv, L., Muto, A., Schoonheim, P.J., Meijsing, S.H., Strasser, D., Ingraham, H.A., … Baier, H. (2013). An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Molecular Psychiatry, 18, 681–691. doi:10.1038/mp.2012.64
- Zupanc, G.K.H., Hinsch, K., & Gage, F.H. (2005). Proliferation, migration, neuronal differentiation, and long‐term survival of new cells in the adult zebrafish brain. The Journal of Comparative Neurology, 488, 290–319. doi:10.1002/cne.20571

