Abstract
In a previous study, we examined hair cortisol concentrations (HCCs) in children when first entering elementary school (at 4 years). In this follow-up study, we examined their HCC when they entered third grade (at 6 years), where the more playful first grades proceed into a more formal learning setting. Participants were 30 6-year-old children (14 boys). Hair samples (≥5 cm) were collected 2 months after the summer holidays. Hair analysis was conducted using two 2-cm long segments, reflecting the first 2 months of school attendance in grade 3 (the scalp-near segment), and 2 months prior to the start in grade 3. Between these two sections, we left a gap of 1 cm to avoid overlap of periods (due to differences in hair growth rate). Children showed a significant increase in cortisol levels when they entered third grade. This increase was not associated with social fearfulness or academic achievement, but did show significant associations with inhibitory control: children with less inhibitory control had higher cortisol levels after entering third grade, and larger increases in cortisol than children with higher scores on inhibitory control. This suggests that the ability to inhibit or control impulsive responsivity is important for children’s stress regulation when making the transition to a more formal school environment.
Introduction
In a previous study, we showed that starting elementary school is accompanied by increased stress hormone levels in 4-year-old children, by analyzing their hair cortisol concentrations (HCCs; Groeneveld et al., Citation2013). Cortisol is a well-known stress hormone which in humans is the final product of activation of the hypothalamic-pituitary-adrenal axis. In the past, cortisol levels have mainly been determined in urine, blood or saliva. To assess cortisol levels over a prolonged period of time, repeated sampling is needed at different daily time points over several days. Currently, cortisol can also be determined in human scalp hair to determine long-term cortisol levels (Bates, Salsberry, & Ford, Citation2017; Russell, Koren, Rieder, & Van Uum, Citation2012; Stalder & Kirschbaum, Citation2012). Because hair grows at an average of 1 cm/month, assessment of hair cortisol can reflect changes over time. In our previous study, we showed that HCCs were higher after school entry than before, especially for fearful children. This finding supported our hypothesis that a rise in HCC can be specifically linked to a stress-related transition: the first entry into elementary school. In this follow-up study, we examined the same children’s HCC when they entered third grade at 6 years of age, where the more playful first grades proceed into a more formal learning setting.
Although researchers have used HCC widely to approximate stress levels in adults (Sauve, Koren, Walsh, Tokmakejian, & Van Uum, Citation2007; Stalder et al., Citation2017; Stalder & Kirschbaum, Citation2012; Staufenbiel, Penninx, Spijker, Elzinga, & Van Rossum, Citation2013), this measure is less often used in early childhood (Bates et al., Citation2017; Golub, Kuitunen-Paul, & Panaseth, Citation2019; Gray et al., Citation2018). The studies that measured HCC in children especially focused on chronic stress, not on stress during transitions (Yamada et al., Citation2007). Because HCC allows a retrospective assessment of cortisol exposure, this method seems very valuable when studying the effects of major transitions in life. Previous studies, using saliva measures, showed that it can be expected that the transition from grades is stressful (Bruce, Poggi Davis, & Gunnar, Citation2002). We expect that especially the transition from the more playful first grades into a phase with more formal requirements can be stressful for young children. In the third grade children have to focus on reading, writing, and math skills, working at their own desk, and experience ratings on tests (Smeets, Citation2014; Smeets & Resing, Citation2013). The main research question of this study is: Do cortisol levels increase when children make this transition to formal learning in grade 3? These increased stress levels might have a negative effect on the development of children. Although stress responses are necessary for survival, chronic exposure to stress can change from adaptive to maladaptive (De Kloet, Oitzl, & Joëls, Citation1999; Segerstrom & Miller, Citation2004). Previous studies have shown that higher stress levels in children are related to behavior changes, anxiety, sleep problems, illness, and cognitive functioning (Bäumler et al., Citation2014; Forbes et al., Citation2005; Golub et al., Citation2019; Kenny, Gallagher, Alvarez-Salvat, & Silsby, Citation2002; Lipton, Becker, & Kothare, Citation2008; Turner Cobb & Steptoe, Citation1998; Windle & Windle, Citation1996).
It can be expected that the transition to formal learning is more stressful for a subgroup of children, for example, children with a difficult temperament. In our previous study, we showed that especially children scoring high on social fearfulness showed an increase of cortisol levels when entering elementary school (Groeneveld et al., Citation2013), probably due to the new situation, with unfamiliar children and teachers. This temperamental characteristic has more often been linked to individual differences in children’s stress reactivity in young children in childcare (Talge, Donzella, & Gunnar, Citation2008; Vermeer & Groeneveld, Citation2017; Watamura, Donzella, Alwin, & Gunnar, Citation2003), although other studies did not find any associations between a fearful temperament and cortisol reactivity in childcare (Ahnert, Gunnar, Lamb, & Barthel, Citation2004; Groeneveld, Vermeer, Van IJzendoorn, & Linting, Citation2012; Watamura, Sebanc, & Gunnar, Citation2002) or during the start of a new school year (Davis, Donzella, Krueger, & Gunnar, Citation2001).
In addition to temperament, academic skills can also be an important factor in explaining individual differences in cortisol levels in a school setting. It could be argued that especially low achievers show an increase in cortisol levels when they enter third grade. In a study conducted with adolescents, it was shown that those students who were at the bottom of the scholastic hierarchy in the classroom, defined as students with less academic success and more troublesome behaviors, had higher cortisol levels (West, Sweeting, Young, & Kelly, Citation2010) compared to their peers.
Finally, behavioral inhibition, i.e. the ability to regulate and control behavioral impulses, might be related to higher cortisol levels after the transition to third grade at age six. This executive cognitive ability is important since other executive functions appear to depend on it: working memory, self-regulation of affect, motivation, and arousal, internalization of speech, and reconstitution (Barkley, Citation1997). Individual differences in children’s inhibitory control have been found to be related to internalizing and externalizing behavior of children (Eisenberg et al., Citation2001) and teacher-child conflict (Berry, Citation2012). Moreover, it has been shown that inhibitory control is related to (salivary) cortisol responses in preschoolers (Blair, Granger, & Razza, Citation2005). Overactivity of the stress systems may impact the development of prefrontal regulatory systems, and as a consequence increases the risk for both attention- and emotion-regulatory problems (for a review, see Loman & Gunnar, Citation2010). With the transition to formal learning, it seems important to be able to inhibit behavioral responses that enable children to direct their attention and behavior. Thus, higher levels of inhibitory control would be expected to be associated with lower cortisol levels when making the transition to formal learning.
To summarize, in this study, we measure HCC alterations in young children before and after a potential stressful transition, that is, their entry into formal learning at school. We hypothesize that children’s HCCs are higher after entry in third grade than before entry. Furthermore, we study whether alterations in HCC are moderated by temperament (social fearfulness), academic skills, and executive functioning (inhibitory control).
Method
Subjects
This study is a follow-up of the (Groeneveld et al., Citation2013) study that started in 2012. A total of 284 families from Leiden (the Netherlands) were invited by postal mail to participate. Recruitment material was in Dutch. Registration for the study was closed after agreement from 50 families. In 2012, eight children were excluded for analyses, (n = 4 hair was too short and n = 4 extremely high cortisol values with > 3 SD above the mean). The remaining 42 families were again invited to participate in the study of 2014. An additional 9 families declined participation (n = 3 moved/not able to contact, n = 3 did not want to cut hair, n = 3 were too busy). After the home visits, another three children were excluded (n = 2 hair too short, n = 1 extremely high cortisol values with > 3 SD above the mean). This resulted in a final sample of 30 children (45% boys). There were no significant differences between the families who participated in the follow-up study and those who declined (e.g. maternal or paternal education or age, child fearfulness, time spent in childcare, or children’s HCC). In the follow-up study, children’s age ranged from 6.5 to 6.8 years during the home visit (M = 6.6, SD = 0.08). Parents’ educational level was coded as the number of years of education after age 6. Mean educational level across mothers and fathers was 14.90 years (SD = 1.90). Twenty four children had two Dutch parents with the Dutch nationality, six children had one Dutch parent, and one parent with a different nationality (Egyptian, Afghan, Hungarian, Chilean, German, and Japanese).
Home visit
All children were visited at home, in a 2-week period, approximately 2 months after they started in grade 3. Prior to the home-visit, parents received a questionnaire, with questions about the background of the parents and the children and the temperament of the children. During the home visit children’s hair was collected. In addition, children administered tasks to measure their academic skills and a computerized inhibition control task. All procedures were carried out with adequate understanding and written consent of the parents. Ethical approval for this study was provided by the Research Ethics Committee of the Institute of Education and Child Studies of Groeneveld et al., Citation2013.
Temperament
The Child Behavior Questionnaire (CBQ) short form was used to measure the child’s temperament (Rothbart, Ahadi, & Hershey, Citation1994; Rothbart, Ahadi, Hershey, & Fisher, Citation2001). Parents were asked to fill out the CBQ based on their child’s behavior during the last 6 months. The questionnaire was sent to them a few weeks before the home visit. The CBQ is a 7-point rating scale for the assessment of different aspects of temperament in children aged 3–7 years. For this study, we used the subscale fearfulness (12 items, Cronbach’s alpha = .86). An example item is “How often was your child afraid of the dark?” answers can range from “1 never” to “7 always”. Higher scores on the fear scale represent more fearfulness in children. In 2012, parents were also asked to fill out the CBQ about their child when they were 4 years of age. Scores of the two questionnaires were significantly correlated, r = .71, p < .01.
Academic skills
The Rapid Automatized Naming (RAN; CB&WL [Continu Benoemen en Woorden Lezen]; Van den Bos & Lutje Spelberg, Citation2007) task was used to measure children’s academic skills. The CB task consists of four components: colors, letters, numbers, and objects. For each component, children were asked to name 50 symbols or objects as rapid as they can, from paper forms. The time was recorded per component using a stopwatch. A mean score of the time of these four components represents children’s academic skills. Higher scores represent lower academic skills, since the children took longer to finish the naming tasks. The RAN is a reliable instrument for children aged 5–16 years, judged by the COTAN (Dutch Commission for Test Materials of the Dutch Institute of Psychologists that test the quality of psychodiagnostic instruments).
Inhibitory control
A computerized Go/NoGo task was administered during the home visit to measure inhibitory control on a laptop. In this task, children had to catch all the mice that appeared on the screen (Go-stimuli) by pressing a red button. When a cat appeared, children had to inhibit their reaction, and should not press the button (NoGo-stimuli). The task consisted of a practice session, in which five mice and five cats were presented (in alternating order), and a test session, in which 30 mice and 10 cats were displayed in random order. Only during the practice session, the child received feedback. Commission errors (responses to NoGo-stimuli) were used as a measure for a lack of inhibitory control (Groot, De Sonneville, Stins, & Boomsma, Citation2004). To generate a measure for inhibitory control the sum score of the commission errors was subtracted from the total number of NoGo-stimuli (10 – number of commission errors), with higher scores representing more inhibitory control.
Scalp hair collection
Hair was collected during home visits, which were planned approximately 2 months after the children had started school. Visits were conducted by the first author or trained (under)graduate students. Hair collection was performed as described previously (Manenschijn, Koper, Lamberts, & van Rossum, Citation2011). A sample containing around 300 hairs was cut from the posterior vertex as close to the scalp as possible. The sample was taped to a piece of paper, the scalp end marked, and stored in an envelope at room temperature until analysis.
Prior to the home-visit, parents received a questionnaire, with questions about age, educational level, and birth country of the parents, and the following information concerning the participating child: after-school care, hair color, hair washing frequency, use (and type) of corticosteroids during last 6 months, other medication use, and chronic diseases.
Hair processing and analysis
Hair analysis was conducted using two 2-cm-long segments, reflecting the first 2 months of school attendance in grade 3 (the scalp-near segment), and 2 months prior to the start in grade 3. Between these two sections, we left a gap of 1 cm to avoid overlap of periods (due to differences in hair growth rate). As described previously (Manenschijn et al., Citation2011), we weighed and cut the hair segments into small pieces in glass vials using surgical scissors. Cortisol was extracted by 16 h incubation in one mL methanol at 52 °C while gently shaking. Subsequently, the methanol was transferred to disposable glass vials, and evaporated under a constant stream of nitrogen. The samples were then dissolved in 250 μL phosphate-buffered saline, pH 8.0, and vortexed for one min. Prior to analysis, samples were vortexed again for 30 s. Cortisol concentrations were measured using a commercially available salivary cortisol ELISA kit (DRG Instruments GmbH, Marburg, Germany) according to the manufacturer’s directions. We applied a correction factor to take background signal into account. As stated by the manufacturer, antibody cross-reactivity is as follows: corticosterone 29%, cortisone 3%, 11-deoxycortisol <1%, 17-OHP <0.5%, prednisone <0.1%. Intra-assay variation was 2.6% and the inter-assay variation was 6.7%, as stated by the manufacturer.
Data analysis
Because the distributions of the cortisol measurements were positively skewed, log10 transformations were used for analysis. After log10 transformation, HCC did not deviate from normality. To test whether HCC differed before and after school entry, we conducted a repeated measure MANCOVA with time as a within-subject variable. To analyze associations with temperament, academic skills, and inhibitory control, multivariate regression analyses were conducted, using interaction terms.
Results
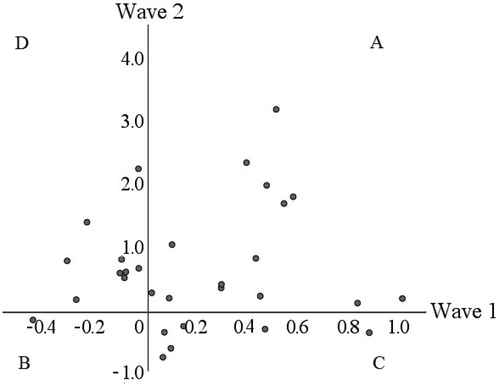
Descriptive statistics of HCC during wave 1 (transition to grade 1) and wave 2 (transition to grade 3) are shown in . HCC before and after entering a new grade showed stability across time (wave 1 r = .80, p < .001; wave 2 r = .52, p = .004). In addition, HCC prior to entering third grade (wave 2, pre HCC) were related to HCC in wave 1 (pre r = .36, p = .05; post r = .37, p = .04), while HCC after entering third grade (wave 2, post HCC) were not related to HCC in wave 1. HCCs in wave 2 were not significantly related to any of the child characteristics (gender, hair color, hair washing frequency, use of corticosteroids, other medication, hours in group care), or parent characteristics (educational levels fathers and mothers).
Table 1. Descriptive statistics of HCC in wave 1 (transition grade 1) and wave 2 (transition grade 3).
Change in HCC
A repeated measures MANCOVA on children’s HCC yielded a significant main effect of time (Pillais, F[1, 29] = 5.22, p = .03, η2partial = .15). Children’s HCC were significantly lower during the holiday (M = 12.61 SD = 10.11) than after the start in third grade (M = 16.54, SD = 12.78). The Ratio of Change (RC) ((post HCC – pre HCC)/pre HCC) was not correlated between the two waves (r = .11, p = 57), which means that an increase in HCC when entering first grade was not associated with an increase in HCC when entering third grade. Nevertheless, almost half of the children (46.7%) showed an increase in HCC after entering both first and third grade ().
Child characteristics
The correlations between HCC in wave 2 and social fearfulness, academic achievement, and inhibitory control are shown in . There were no significant correlations between these child characteristics and pre HCC, post HCC, or change in HCC. In addition, we found no interaction effect for social fearfulness (B = –0.17, SEB = 0.19, β = –0.15, p = .37, R2 = .32) or academic skills (B = –0.01, SEB = 0.01, β = –0.25, p = .14, R2 = .34) when predicting post HCC.
Table 2. Descriptive statistics of child characteristics and correlations with hair cortisol concentrations wave 2.
For inhibitory control, significant main effects and an interaction effect were present. In it is shown that pre HCC and inhibitory control predicted post HCC (R2 = 0.36, p < .01). The interaction between pre HCC and inhibitory control significantly improved the model (ΔR2 = .14, p < .05). For graphic concerns, we made a median split in inhibitory control (Median = 8). In , it is shown that children scoring low on inhibitory control show a large increase in their HCC after entering grade 3 (Pillais, F[1, 14] = 14.70, p < .01, η2partial = .51), while HCC from children scoring high on inhibitory control do not change (Pillais, F[1, 14] = 0.02, p = .89, η2partial < .01).
Figure 2. HCC levels of children scoring high or low on inhibitory control. HCC: hair cortisol concentrations.
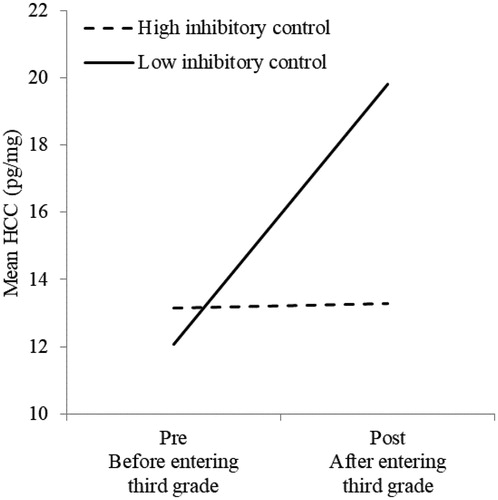
Table 3. Inhibitory control predicting post HCC.
Discussion
In this study, we showed that on average HCCs increase when children enter third grade at 6 years of age. This increase was not associated with social fearfulness or academic achievement, but we did find associations with inhibitory control: children with less inhibitory control had higher cortisol levels after entering third grade, and larger increases in cortisol, than children with higher scores on inhibitory control.
As expected, children show an increase in HCC when they entered grade 3, where the more playful first grades proceed into a more formal learning setting. This corresponds to our earlier finding of HCC increases after entering grade one (Groeneveld et al., Citation2013). HCCs in this follow-up study were lower in terms of absolute values (pre and post) than in the previous study. Because we measured differences between the manufacturer’s standards (optimized for measuring cortisol in saliva) and our own standard curve (prepared in PBS, like the HCC samples), we computed a correction factor and corrected the absorbance values of the samples based on the standards from the kit. This is something we have decided to apply with increased knowledge over time and could probably be one of the reasons for the difference with the earlier study.
When studying child characteristics, we focused on inhibitory control, temperament, and academic skills. First, we found that children scoring low on inhibitory control showed an increase in their HCC after entering grade 3, while HCCs from children scoring high on inhibitory control do not change. This implies that especially children who find it difficult to inhibit reactions experience the transition to formal learning as more stressful. This seems logical because the dynamics change from the more playful first years, to the more scholastic environment. For example, in third grade the time children spend playing inside and outside decreases considerably, and at the same time they are requested to sit still at their desk, and focus on fine motoric skills, learning how to write, read, and do math (Smeets, Citation2014; Smeets & Resing, Citation2013). On the other hand, it might also be possible that cortisol levels influenced the inhibitory control levels of the children. It has been shown that overactivity of the stress systems may impact the development of prefrontal regulatory systems, and as a consequence increase the risk for emotion-regulatory problems (for a review, see Loman & Gunnar, Citation2010), but these studies are primarily based on animal-models (Brake, Zhang, Diorio, Meaney, & Gratton, Citation2004), or on children with severe early life stress (Nemeroff, Citation2004), so the relevance for humans is not clear yet. Previous studies with humans, focusing on the effect of cortisol levels on inhibition showed a positive association: higher cortisol levels increase inhibitory control (Shields, Bonner, & Moons, Citation2015). But these studies mainly focus on short term increases in (salivary) cortisol. A meta-analysis has shown that cortisol improves inhibition from 15 min to 135 min post-administration, but cortisol begins to impair inhibition after 136 min (Shields et al., Citation2015). This could imply that the increases in cortisol levels with the transfer to third grade have resulted in impaired inhibition. A pretest measure of inhibition scores before entry in third grade should be conducted to test causality.
Secondly, we tested the moderation effect of social fearfulness, because this temperamental subscale was a significant moderator in our previous study (Groeneveld et al., Citation2013). High fearful children showed the highest rise in HCC after school entry in grade 1. In this study, it seemed conceivable that the same pattern would be present when entering grade 3, with new unfamiliar teachers, and more focus on formal learning. This hypothesis was however not confirmed. The increase in cortisol levels of fearful children was comparable to the increases of HCC of their less fearful peers. It is possible that, although children entered a new classroom with new peers and teachers, the presence of familiar peers and the familiar school environment and routine is a buffer for these children.
Third, no association was found between cortisol levels and academic skills, although some studies did find this association for older children (West et al., Citation2010). Stress may interfere with the academic performance by diverting attention from cognitive tasks to worry and feelings of being overwhelmed (Matheny, Aycock, & McCarthy, Citation1993). It might be possible that children were not yet aware of (and thus stressed by) their lower academic skills in the first weeks after they entered grade 3. This effect might be present a few months later when children have experienced more academic tests. In addition, parental educational levels were quite high. A larger, more diverse sample including families from lower SES is needed to further explore these associations.
To conclude, in this study, we showed that cortisol levels increase when children make the transition to a school phase with formal learning. It is important to study this transition, since higher levels of stress in the school and classroom are related to more mental health problems, adjustment problems in school, and lower academic achievement (Kaplan, Liu, & Kaplan, Citation2005; Kenny et al., Citation2002, Windle & Windle, Citation1996). It might be helpful for children to learn strategies to cope with the transition, or even to cope with stress. Previous studies have shown a positive effect of interventions to decrease stress in children (Bothe, Grignon, & Olness, Citation2014; Haraldsson et al., Citation2008). Bothe et al., (Citation2014) showed that a 10-min daily stress management technique was helpful for reducing anxiety scores and improving the ability to relax in school-age children. These daily sessions included deep breathing, movement, and guided imagery and were provided by the teacher, in the classroom for 4 months. In addition, there are several other resources that seem to help students to cope with stress, such as improved social skills, problem-solving orientation, and social support. An important source of support in the educational setting is the teacher. In childcare studies, it has been shown that higher caregiver sensitivity and quality of care are associated with lower diurnal cortisol production in preschoolers (Vermeer & Groeneveld, Citation2017). In addition, lowering teachers’ occupational stress may be effective, since this occupational stress has been linked with children’s physiological stress regulation as well (Oberle & Schonert-Reichl, Citation2016).
We showed that especially children scoring low on inhibitory control showed these increased cortisol levels. This means that this group of children deserves extra attention in schools: how can we support these children to regulate their inhibitory control (for specific tasks) and how can we adapt the school environment to support these children? Several interventions use self-regulation techniques such as relaxation, yoga, and imagery to effectively improve well-being, health-related issues, and cognitive or emotional control in children (Bell & Deater-Deckard, Citation2007; Bothe et al., Citation2014; Ehud, An, & Avshalom, Citation2010; Goldbeck & Schmid, Citation2003; Lee & Olness, Citation1996). The Bothe et al., (Citation2014) intervention with the 10-min daily stress management technique also included these self-regulation techniques. It would be interesting to test whether this intervention might positively affect children’s inhibitory control as well. In addition, the school environment might also be adapted, for example by giving children more room in the third grade to move around. It has been shown that that physical activity can positively affect important brain areas, which might lead to increases in working memory, planning skills, and cognitive control in the short and long term (Davis et al., Citation2011; Hillman et al., Citation2009; Mullender-Wijnsma et al., Citation2015). A recent intervention in third-grade children, including physically active academic lessons, showed an increase in mathematics and reading scores of children who participated in the intervention compared to children in a control group who received regular lessons (Mullender-Wijnsma et al., Citation2015). These physically active academic lessons might be especially effective for children scoring low on inhibitory control. More research is however needed for this group of children.
To summarize, this study showed that cortisol levels increase when children make the transition to formal learning, especially children scoring low on inhibitory control. It is important to study this transition to grade 3 and possible resources to regulate the stress responses of children, because this transition is the basis of the academic career of all children, and stress can have a negative effect on this career.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Marleen G. Groeneveld
Marleen Groeneveld is an assistant professor, working at the Societal Challenges Lab at Leiden University, the Netherlands. Her research projects focus on children’s stress and wellbeing, gender socialization, and the quality of care children receive in different contexts: in families, at childcare, and at school.
Mesut Savas
Mesut Savas is an Internal Medicine resident and PhD candidate at the Erasmus University Medical Center (Rotterdam, The Netherlands). His research is focused on endogenous and exogenous glucocorticoids in obesity and stress-related diseases.
Elisabeth F. C. van Rossum
Elisabeth van Rossum is internist-endocrinologist and professor in the field of obesity and biological stress research at the Erasmus University Medical Center, Rotterdam, the Netherlands. She is co-founder of the Obesity Center CGG (Centrum Gezond Gewicht) and member of the Young Academy of the Royal Dutch Academy of Sciences (KNAW).
Harriet J. Vermeer
Harriet Vermeer is an associate professor at the Institute of Education and Child Studies at Leiden University. Her research mainly focuses on nonmaternal care and children’s stress, wellbeing and development.
References
- Ahnert, L., Gunnar, M.R., Lamb, M.E., & Barthel, M. (2004). Transition to child care: Associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Development, 75, 639–650. doi:10.1111/j.1467-8624.2004.00698.x
- Barkley, R.A. (1997). Behavioral inhibition, sustained attention, and executive functions: Construction a unifying theory of ADHA. Psychological Bulletin, 121, 65–94. doi:10.1037/0033-2909.121.1.65
- Bates, R., Salsberry, P., & Ford, J. (2017). Measuring stress in young children using hair cortisol: The state of the science. Biological Research for Nursing, 19, 499–510. doi:10.1177/1099800417711583
- Bäumler, D., Voigt, B., Miller, R., Stalder, T., Kirschbaum, C., & Kliegel, M. (2014). The relation of the cortisol awakening response and prospective memory functioning in young children. Biological Psychology, 99, 41–46. doi:10.1016/j.biopsycho.2014.02.011
- Bell, M.A., & Deater-Deckard, K. (2007). Biological systems and the development of self-regulation: Integrating behavior, genetics, and psychophysiology. Journal of Developmental & Behavioral Pediatrics, 28, 409–420. doi:10.1097/DBP.0b013e3181131fc7
- Berry, D. (2012). Inhibitory control and teacher-child conflict: Reciproval associations across the elementary-school years. Journal of Applied Developmental Psychology, 33, 66–76. doi:10.1016/j.appdev.2011.10.002
- Blair, C., Granger, D., & Razza, R.P. (2005). Cortisol reactivity is positively related to executive function in preschool children attending Head Start. Child Development, 76, 554–567. doi:10.1111/j.1467-8624.2005.00863.x
- Bothe, D.A., Grignon, J.B., & Olness, K.N. (2014). The effects of a stress management intervention in elementary school children. Journal of Developmental and Behavioral Pediatrics : JDBP, 35, 62–67. doi:10.1097/DBP.0000000000000016
- Brake, W.G., Zhang, T.Y., Diorio, J., Meaney, M.J., & Gratton, A. (2004). Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. European Journal of Neuroscience, 19, 1863–1874. doi:10.1111/j.1460-9568.2004.03286.x
- Bruce, J., Poggi Davis, E., & Gunnar, M.R. (2002). Individual differences in children’s cortisol response to the beginning of a new school year. Psychoneuroendocrinology, 27, 635–650. doi:10.1016/S0306-4530(01)00031-2
- Davis, C.L., Tomporowski, P.D., McDowell, J.E., Austin, B.P., Miller, P.H., Yanasak, N.E., … Naglieri, J.A. (2011). Exercise improves executive function and achievement and alters brain activation in overweight children: A randomized, controlled trial. Health Psychology, 30, 91–98. doi:10.1037/a0021766
- Davis, E.P., Donzella, B., Krueger, W.K., & Gunnar, M.R., (2001). The start of a new school year: Individual differences in salivary cortisol response in relation to child temperament. Developmental Psychobiology, 35, 188–196. doi:10.1002/(SICI)1098-2302(199911)35:3 < 188::AID-DEV3 > 3.0.CO;2-K
- De Kloet, E.R., Oitzl, M.S., & Joëls, M. (1999). Stress and cognition: Are corticosteroids good or bad guys? Trends in Neuroscience, 22, 422–426. doi:10.1016/s0166-2236(99)01438-1
- Ehud, M., An, B.D., & Avshalom, S. (2010). Here and now: Yoga in Israeli schools. International Journal of Yoga, 3, 42–47. doi:10.4103/0973-6131.72629
- Eisenberg, N., Cumberland, A., Spinrad, T.L., Fabes, R.A., Shepard, S.A., Reiser, M., Murphy, B.C., Losova, S.H., & Guthrie, I.K. (2001). The relations of regulation and emotionality to children's externalizing and internalizing problem behavior. Child Development, 72, 1112–1134. doi:10.1111/1467-8624.00337
- Forbes, E.E., Williamson, D.E., Ryan, N.D., Birmaher, B., Axelson, D.A., & Dahl, R.E., (2005). Peri-sleep-onset cortisol levels in children and adolescents with affective disorders. Biological Psychiatry, 59, 24–30. doi:10.1016/j.biopsych.2005.06.002
- Goldbeck, L., & Schmid, K. (2003). Effectiveness of autogenic relaxation training on children and adolescents with behavioral and emotional problems. Journal of the American Academy of Child & Adolescent Psychiatry, 42, 1046–1054. doi:10.1097/01.CHI.0000070244.24125.F
- Golub, Y., Kuitunen-Paul, S., & Panaseth, K. (2019). Salivary and hair cortisol as biomarkers of emotional and behavioral symptoms in 6-9 year old children. Physiology & Behavior, 209, 1–10. doi:10.1016/j.physbeh.2019.112584
- Gray, N.A., Dhana, A., Van Der Vyver, L., Van Wyk, J., Khumalo, N.P., & Stein, D.J. (2018). Determinants of hair cortisol concentration in children: A systematic review. Psychoneuroendocrinology, 87, 204–214. doi:10.1016/j.psyneuen.2017.10.022
- Groeneveld, M.G., Vermeer, H.J., Linting, M., Noppe, G., van Rossum, E.F.C., & van IJzendoorn, M.H. (2013). Children’s hair cortisol as a biomarker of stress at school entry. Stress, 16, 711–715. doi:10.3109/10253890.2013.817553
- Groeneveld, M.G., Vermeer, H.J., Van IJzendoorn, M.H., & Linting, M. (2012). Stress, cortisol and well-being of caregivers and children in home-based child care: A case for differential susceptibility. Child: Care, Health and Development, 38, 251–260. doi:10.1111/j.1365-2214.2010.01194.x
- Groot, A.S., De Sonneville, L.M.J., Stins, J.F., & Boomsma, D.I. (2004). Familial influences on sustained attention and inhibition in preschoolers. Journal of Child Psychology and Psychiatry, 45, 306–314. doi:10.1111/j.1469-7610.2004.00222.x
- Haraldsson, K.S., Lindgren, E.-C.M., Fridlund, B.G.A., Baigi, A.M.A.E., Lydell, M.C., & Marklund, B.R.G. (2008). Evaluation of a school-based health promotion programme for adolescents aged 12-15 years with focus on well-being related to stress. Public Health, 122, 25–33. doi:10.1016/j.puhe.2007.04.016
- Hillman, C.H., Pontifex, M.B., Raine, L.B., Castelli, D.M., Hall, E.E., & Kramer, A.F. (2009). The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience, 159, 1044–1054. doi:10.1016/j.neuroscience.2009.01.057
- Kaplan, D.S., Liu, R.X., & Kaplan, H.B. (2005). School related stress in early adolescence and academic performance three years later: The conditional influence of self expectations. Social Psychology of Education, 5, 3–17. doi:10.1007/s11218-004-3129-5
- Kenny, M.E., Gallagher, L.A., Alvarez-Salvat, R., & Silsby, J. (2002). Sources of support and psychological distress among academically successful inner-city youth. Adolescence, 37, 161–182.
- Lee, L.H., & Olness, K.N. (1996). Effects of self-induced mental imagery on autonomic reactivity in children. Journal of Developmental & Behavioral Pediatrics, 17, 323–327. doi:10.1097/00004703-199610000-00006
- Lipton, J., Becker, R.E., & Kothare, S.V. (2008). Insomnia of childhood. Current Opinion in Pediatrics, 20, 641–649. doi:10.1097/MOP.0b013e32831897cb
- Loman, M.M., & Gunnar, M.R. (2010). Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews, 34, 867–876. doi:10.1016/j.neubiorev.2009.05.007
- Manenschijn, L., Koper, J.W., Lamberts, S.W.J., & van Rossum, E.F.C. (2011). Evaluation of a method to measure long term cortisol levels. Steroids, 76, 1031–1036. doi:10.1016/j.steroids.2011.04.005
- Matheny, K.B., Aycock, D.W., & McCarthy, C.J. (1993). Stress in school-aged children and youth. Educational Psychology Review, 5, 109–134. doi:10.1007/BF01323156
- Mullender-Wijnsma, M.J., Hartman, E., de Greeff, J.W., Bosker, R.J., Doolaard, S., & Visscher, C. (2015). Improving academic performance of school-age children by physical activity in the classroom: 1-year program evaluation. Journal of School Health, 85, 365–371. doi:10.1111/josh.12259
- Nemeroff, C.B. (2004). Neurobiological consequences of childhood trauma. The Journal of Clinical Psychiatry, 65, 18–28.
- Oberle, E., & Schonert-Reichl, K.A. (2016). Stress contagion in the classroom? The link between classroom teacher burnout and morning cortisol in elementary school students. Social Science & Medicine, 159, 30–37. doi:10.1016/j.socscimed.2016.04.031
- Rothbart, M.K., Ahadi, S.A., & Hershey, K.L. (1994). Temperament and social behavior in childhood. Merrill-Palmer Quarterly, 40, 21–39.
- Rothbart, M.K., Ahadi, S.A., Hershey, K.L., & Fisher, P.A. (2001). Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development, 72, 1394–1408. doi:10.1111/1467-8624.00355
- Russell, E., Koren, G., Rieder, M., & Van Uum, S. (2012). Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology, 37, 589–601. doi:10.1016/j.psyneuen.2011.09.009
- Sauve, B., Koren, G., Walsh, G., Tokmakejian, S., & Van Uum, S.H. (2007). Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical & Investigative Medicine, 30, 183–191. doi:10.25011/cim.v30i5.2894
- Segerstrom, S.C., & Miller, G.E. (2004). Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130, 1–27. doi:10.1037/0033-2909.130.4.601
- Shields, G.S., Bonner, J.C., & Moons, W.G. (2015). Does cortisol influence core executive functions? A meta-analysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology, 58, 91–103. doi:10.1016/j.psyneuen.2015.04.017
- Smeets J. (2014). Zo werkt het met jonge kinderen in groep 3! [This is how it works with young children in grade 3!] Retrieved from: http://docplayer.nl/368497-Zo-werkt-het-met-jonge-kinderen-in-groep-3-jose-smeets-ipabo-amsterdam
- Smeets, J., & Resing, W. (2013). Overgang van najaarsleerling naar groep 3 nader onderzocht. [Transition from fall students to grade 3, further investigated]. Tijdschrift Voor Orthopedagogiek, 52, 442–453.
- Stalder, T., & Kirschbaum, C. (2012). Analysis of cortisol in hair – State of the art and future directions. Brain, Behavior, and Immunity, 26, 1019–1029. doi:10.1016/j.bbi.2012.02.002
- Stalder, T., Steudte-Schmiedgen, S., Alexander, N., Klucken, T., Vater, A., Wichmann, S., … Miller, R. (2017). Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology, 77, 261–274. doi:10.1016/j.psyneuen.2016.12.017
- Staufenbiel, S.M., Penninx, B.W., Spijker, A.T., Elzinga, B.M., & Van Rossum, E.F. (2013). Hair cortisol, stress exposure, and mental health in humans: A systematic review. Psychoneuroendocrinology, 38, 1220–1235. doi:10.1016/j.psyneuen.2012.11.015
- Talge, N.M., Donzella, B., & Gunnar, M.R. (2008). Fearful temperament and stress reactivity among preschool-aged children. Infant and Child Development, 17, 427–445. doi:10.1002/icd.585
- Turner Cobb, J.M., & Steptoe, A. (1998). Psychosocial influences on upper respiratory infectious illness in children. Journal of Psychosomatic Research, 45, 319–330. doi:10.1016/S0022-3999(97)00311-5
- Vermeer, H.J., & Groeneveld, M.G. (2017). Children’s physiological responses to childcare. Current Opinion in Psychology, 15, 201–206. doi:10.1016/j.copsyc.2017.03.006
- Van den Bos, K., & Lutje Spelberg, H.C. (2007). Continu Benoemen en Woorden Lezen (CB&WL). Amsterdam: BoomTestuitgevers.
- Watamura, S.E., Donzella, B., Alwin, J., & Gunnar, M.R. (2003). Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: Age differences and behavioral correlates. Child Development, 74, 1006–1020. doi:10.1111/1467-8624.00583
- Watamura, S.E., Sebanc, A.M., & Gunnar, M.R. (2002). Rising cortisol at childcare: Relations with nap, rest, and temperament. Developmental Psychobiology, 40, 33–42. doi:10.1002/dev.10011
- West, P., Sweeting, H., Young, R., & Kelly, S. (2010). The relative importance of family socioeconomic status and school-based peer hierarchies for morning cortisol in youth: An exploratory study. Social Science & Medicine, 70, 1246–1253. doi:10.1016/j.socscimed.2009.12.006
- Windle, M., & Windle, R.C. (1996). Coping strategies, drinking motives, and stressful life events among middle adolescents: Associations with emotional and behavioral problems and with academic functioning. Journal of Abnormal Psychology, 105, 551–560. doi:10.1037//0021-843x.105.4.551
- Yamada, J., Stevens, B., de Silva, N., Gibbins, S., Beyene, J., Taddio, A., … Koren, G. (2007). Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology, 92, 42–49. doi:10.1159/000100085