Abstract
A single 24 h period of maternal deprivation (MD) in rats has been shown to induce, in adulthood, a number of abnormalities in brain and behaviour that also occur in patients with schizophrenia. However, the short-term behavioural effects of MD have not been studied in detail. Since patients with schizophrenia are characterized by a retardation of normal development, we aimed in the present study to investigate the development of control rats and rats that were exposed to MD on postnatal day 9. Compared to control animals, MD rats showed (1) a reduction in body weight, (2) an increased in reversal latency in negative geotaxis, (3) a delayed eye opening, (4) a delayed emergence of walking and rearing; and (5) a delayed emergence of the behavioural response to amphetamine (amph). On the other hand, MD and control rats responded similarly to the non-competitive NMDA antagonist MK801. These data clearly show that early MD delays development, especially of the dopaminergic system and confirm our hypothesis that MD may represent an interesting animal model for the neurodevelopmental hypothesis of schizophrenia.
Introduction
The neurodevelopmental hypothesis of schizophrenia states that this disease results from a disturbance in the normal development of the central nervous system, resulting from a complex gene—environment interaction (Weinberger Citation1987; Pilowsky et al. Citation1993). Although there is a large body of evidence to support the neurodevelopmental hypothesis of schizophrenia (Ellenbroek and Cools Citation1998), probably the most convincing data come from large scale prospective epidemiological studies, such as the two birth-cohort studies performed in the UK in 1946 the National Survey of Health and Development (NSHD) and 1958 National Child Development Study (NCDS). The NSHD was a study of all children born in the UK during the week 3–9 March 1946 and the NCDS comprised about 98% of all the children born in the same week in 1958. These children were followed up throughout life and data have clearly shown that the children that developed schizophrenia were delayed in many developmental milestones, including sitting, standing, walking alone and talking (Jones et al. Citation1994; Jones Citation1997). Since then, these developmental disturbances, especially in early motor behaviour have been replicated in several independent studies, including a large birth cohort study in Finland (Isohanni et al. Citation2000) and New Zealand (Cannon et al. Citation2002). In the latter study, the authors did not find a similar retardation in children that later developed anxiety/depression or bipolar disorder, which is in good agreement with studies by Walker et al. (Citation1994). It thus seems that retardation in development and early motor behaviour is one of the first symptoms of schizophrenia, occurring long before the onset of psychotic symptoms.
The neurodevelopmental hypothesis also led to a plethora of new models to investigate aspects of schizophrenia in animals. Until the middle of the 1980s virtually all animal models were based on the acute or (sub)chronic administration of psychotomimetic drugs such as amphetamine (amph) or phencyclidine (Ellenbroek et al. Citation2000; Ellenbroek and Cools Citation2002a). However, in more recent years, numerous animal models have focussed on the long-term consequences of early lesions or other environmental manipulations. The most prominent of these are early postnatal lesions of the ventral hippocampus (Lipska et al. Citation1993), the amygdalae (Daenen et al. Citation2003) or the prefrontal cortex (Schwabe et al. Citation2004). In addition the long-term changes after environmental challenges such as prenatal stress (Koenig et al. Citation2005), perinatal hypoxia (Boksa Citation2004b) or early postnatal maternal deprivation (MD) (Ellenbroek and Riva Citation2003) have been studied in relation to schizophrenia. Although, all these models show a number of similarities with schizophrenia, most studies have focussed on the long-term (post-pubertal) consequences of such early manipulations.
Several years ago we started to investigate the long-term consequences of a single 24 h period of MD. Since it was already well established that MD led to an enhanced sensitivity of the hypothalamus–pituitary–adrenal axis to stressors (Levine et al. Citation1991) as well as an increased behavioural response to amph (Zimmerberg and Shartrand Citation1992), i.e. features that also occur in patients with schizophrenia (Lieberman et al. Citation1987; Lammers et al. Citation1995), we set out to investigate whether MD also led to other abnormalities observed in schizophrenia. Indeed, we found that maternally deprived rats showed, in adulthood, disturbances in prepulse inhibition (Ellenbroek et al. Citation1998), latent inhibition (Ellenbroek and Cools Citation1995), auditory sensory gating and startle habituation (Ellenbroek et al. Citation2004), all abnormalities also observed in patients with schizophrenia (Adler et al. Citation1982; Braff et al. Citation1978, Citation1992). In addition to this, other similarities (behavioural, pharmacological and neurochemical, see Discussion) between MD and schizophrenia were also observed, as was recently reviewed (Ellenbroek and Riva Citation2003). Moreover, there is epidemiological evidence that early stressors, including maternal separation (Mednick Citation1970) and early parental loss (Agid et al. Citation1999) increase the risk for schizophrenia. All these data together have led us to propose that MD might be an interesting animal model to study specific aspects of schizophrenia. Using different strains of rats, we could show that these long-term consequences were due to a gene—environment interaction, as F344 and Lewis rats responded differently to an identical MD (Ellenbroek and Cools Citation2000). However, so far we focussed predominantly on the long-term consequences, and have only shown that the deficits in prepulse inhibition and in BDNF were not detectable before puberty at postnatal day 35 (Ellenbroek et al. Citation1998) or 21 (Roceri et al. Citation2002), respectively.
Given the similarities between the effects of MD and schizophrenia, we aimed to test the hypothesis that early MD would retard normal (motor) development. To that extent behaviour and aspects of general development were assessed in young maternally deprived and control pups. In addition, the sensitivity of these pups to the dopamine releasing drug amph and the NMDA receptor antagonist MK801 (both of which stimulate motor activity) was also investigated.
Material and methods
Rats and housing
Male and female (nulliparous) Wistar rats were obtained from the Central Animal Laboratory of the Radboud University of Nijmegen. The rats were housed in pairs of one male and one female in a standard Macrolon® cage (26 × 42 × 15 cm) in a temperature controlled room (22 ± 2°C) on a standard 12 h light/dark (light on at 07.00 a.m.) cycle. Food and water were available ad libitum. Two weeks later the males were removed and the females were checked twice daily (08.00 and 17.00 h) for delivery. The day of delivery was designated postnatal day 0. Within 24 h after birth, the litters were culled to eight animals (six males and two females) and animals were weighed. MD took place at postnatal day 9. The mothers were removed from the cage around 10.00 h and placed in a single cage, housed in the same room as the pups. The pups were weighed and left in the home-cage at room temperature. In control (Ctrl) litters, mothers were removed at postnatal day 9, pups were weighed and mothers returned immediately (within 3 min). At postnatal 10 (24 h later) the deprived pups were weighed again and the mothers placed back. In the control litter the mothers were again removed, pups weighed and mothers placed back. On different days after the MD several experiments were performed as described below. Since experiments in the past have shown that prior manipulations of young rats can influence their speed of development (Degen et al. Citation2005), each rat was used for only one behavioural paradigm. All experiments were performed in accordance with (inter)national laws and institutional guidelines.
Negative geotaxis
Rats were weighed and placed in the centre of a 25° inclined wooden platform (27 × 23 cm), head facing downward. The platform was covered with fine sandpaper. Reversal time was defined as the time the animal needed to complete a 135° turn of the spine from the original position, thus head facing upwards. For statistical comparison, reversal time was analysed with a two-way analysis of variance (ANOVA) with factors early manipulation (MD vs. Ctrl) and age.
Eye opening
Eyes of both males and females were examined on postnatal days 9–11, 13, 15–18. Any degree of eyelid separation was scored as a positive eye opening. For statistical comparison, the percentages of MD and Ctrl animals with eyes opened were compared with a χ2 test over all days and for each day separately.
Open field behaviour
Open field behaviour was measured in a black arena of 50 × 50 cm with black walls of 60 cm height. Rats from postnatal days 12, 14, 16, 18 and 21 were injected with either saline, amph (1 mg/kg) or MK-801 (0.1 mg/kg, both from Sigma/RBI, Zwijdrecht, the Netherlands) subcutaneously in the neck 5 min prior to being placed in the arena and behaviour was recorded for 1 h and videos were analysed by an observer blind to the treatment using a well defined ethogram (). Since the same control group was used for both the amph and the MK-801 treated rats, we first analysed the duration of the different behaviours for only the saline treated animals with a two-way ANOVA (with factors early manipulation and age) to investigate the effects of MD. In this respect, it is important to note that all the experiments were done in the same time period and rats for the saline, amph and MK-801 treatments were obtained from the same litters (ensuring that animals within each treatment group all came from different litters). After this analysis, the effects of amph and MK801 were separately analysed using a three way ANOVA with factors early manipulation (MD vs. Ctrl), drug treatment (saline vs. amph, or saline vs. MK801) and age. Where appropriate, a subsequent two-way and/or one-way ANOVA was performed to identify the source of the significance (especially focussing on the possible differential response of MD and Ctrl rats to amph or MK-801).
Results
Body weight
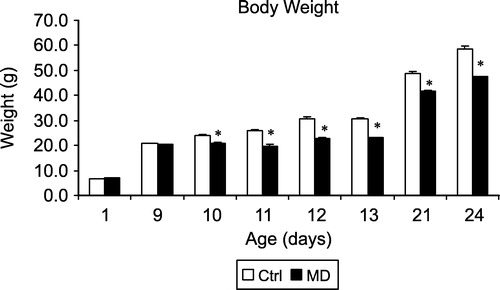
Body weights of all the rats from postnatal day 1–24 are shown in . The data also include rats that were not used for the present experiments (especially at postnatal days 1, 9, 10 and 24). A two-way ANOVA showed a significant effect of early treatment (F(1,1366) = 264.9, p < 0.001) and of days (F(7,1366) = 2166, p < 0.001) as well as a significant interaction between early treatment and days (F(7,1366) = 264.9, p < 0.001). Post hoc t-tests and inspection of showed that MD and Ctrl rats had similar body weights on postnatal days 1 and 9, but were significantly reduced in weight at all subsequent time points. The data, therefore, indicate that MD led to a significant reduction in body weight from pnd10 until the end of the measurement at pnd24.
Figure 1 Body weight of MD and Ctrl rats from postnatal one to postnatal day 24. The data are a summary of a large series of animals. The total number are: pnd1: Ctrl N = 159, MD N = 151; pnd9: Ctrl N = 196, MD N = 183; pnd10: Ctrl N = 188, MD N = 166; pnd11: Ctrl N = 16, MD N = 16; pnd12: Ctrl N = 16, MD N = 16; pnd13: Ctrl N = 16, MD N = 16; pnd9: Ctrl N = 175, MD N = 155; pnd24: Ctrl N = 16, MD N = 13. Values are mean ± SEM. * represents a significant difference between MD and Ctrl groups.
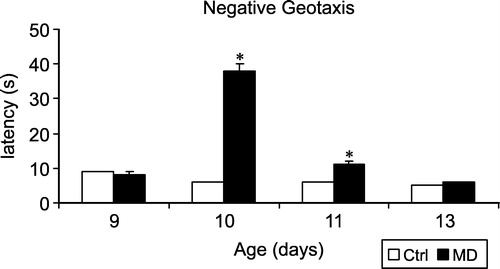
Negative geotaxis
Reversal time in the negative geotaxis test is represented in . The two-way ANOVA showed a significant effect of early treatment (F(1,505) = 110.3, p < 0.001) and of days (F(3,505) = 78.0, p < 0.001), as well as a significant interaction between early treatment and days (F(3,505) = 94.7, p < 0.001). Post-hoc t-test showed that MD and Ctrl rats did not differ on postnatal day 9, but MD rats had significantly longer latencies on postnatal day 10 and 11. On postnatal day 13, MD and Ctrl rats were not significantly different anymore. In other words, MD led to an increase in the negative geotaxis latency on postnatal days 10 and 11, but not 13.
Eye opening
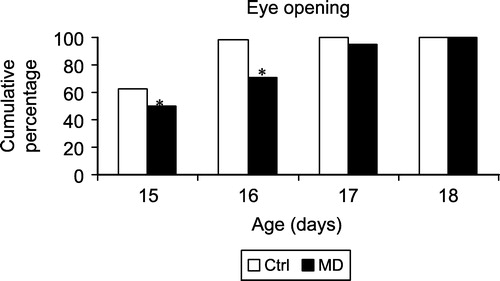
On postnatal days 9–13 none of the Ctrl and MD rats had opened their eyes (data not shown). The cumulative percentage of animals that opened their eyes between postnatal day 15 and 18 is shown in . It is clear that Ctrl rats were somewhat faster in opening their eyes than rats that were exposed to MD. A χ2 analysis confirmed that there was a significantly different distribution between the MD and the Ctrl rats (χ2 = 17.1, p < 0.001). When analysed per day, a significant difference was observed between MD and Ctrl rats on postnatal day 15 (χ2 = 6.8, p < 0.01) and 16 (χ2 = 17.7, p < 0.001), with a tendency to be different on postnatal day 17 (χ2 = 3.1, p = 0.08). Thus, MD led to a delay in eye opening.
Open field behaviour—effects of MD
Before presenting the effects of amph and MK801, the effects of MD per se will first be described ( and ). Since several of the behaviours listed in occurred only rarely, we will limit our discussion to walking, sitting, rearing (wall) and grooming. When the results of only the saline-treated rats were investigated with a two-way ANOVA (with factors early manipulation and age), there was a significant effect of early manipulation for rearing (F(1,49) = 7.3; p < 0.01) and grooming (F(1,49) = 6.1; p < 0.02). In addition, there was a significant effect of age for walking (F(4,49) = 6.9; p < 0.001), rearing (F(4,49) = 4.4; p < 0.004) and grooming (F(4,49) = 5.4; p < 0.001). Finally, there was a significant interaction between early manipulation and age with respect to grooming (F(4,49) = 3.2; p < 0.02). In order to identify the source of the statistical significance for early manipulation, the data were separately analysed per day. The one-way ANOVA revealed that MD rats showed significantly less rearing on postnatal days 12, 14 and 18, whereas grooming was significantly reduced in the MD rats on postnatal days 12 and 16.
Figure 4 The effects of amphetamine (amph) on the duration of various components of motor behaviour in the open field of MD and Ctrl rats. Each group consisted of 5–7 animals and each day new animals were used. Values are mean ± SEM. * represents a significant difference between MD and Ctrl groups. ‡ represents a significant difference between amphetamine and saline treatment.
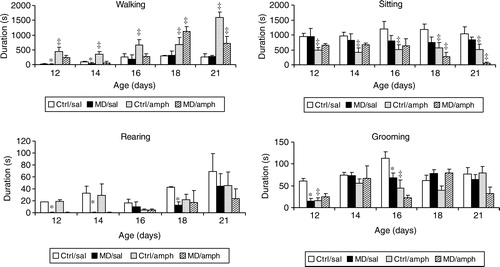
Figure 5 The effects of MK801 (MK) on the duration of various components of motor behaviour in the open field of MD and Ctrl rats. Each group consisted of 5–7 animals and each day new animals were used. Values are mean ± SEM. * represents a significant difference between MD and Ctrl groups. ‡ represents a significant difference between MK801 and saline treatment.
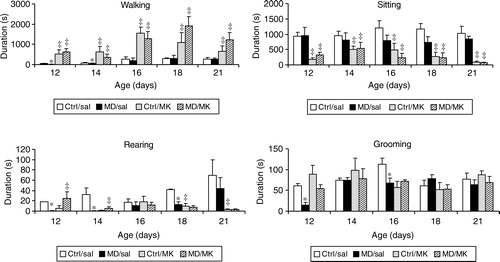
Open field behaviour—effects of amphetamine
For walking (, upper left corner), the three-way ANOVA showed a significant effect of early manipulation (F(1,100) = 7.1, p < 0.01), of drug treatment (F(1,100) = 65.7, p < 0.001) and of age (F(4,100) = 17.6, p < 0.001). In addition a significant two-way interaction was observed for early manipulation—drug treatment (F(1,100) = 4.9, p < 0.05), for early manipulation—age (F(4,100) = 4.1, p < 0.005) and for drug treatment—age (F(4,100) = 6.3, p < 0.001), as well as a significant three-way interaction (F(4,100) = 3.7, p < 0.01).
Given the early manipulation—drug treatment interaction, we investigated the effects of amph in the Ctrl and the MD rats separately. In the Ctrl animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,52) = 39.9, p < 0.001) and of age (F(4,52) = 8.7, p < 0.001) as well as a significant interaction (F(4,52) = 5.1, p < 0.002). When analysed separately for each age, we found that amph significantly increased the duration of walking on all postnatal days. In the MD animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,48) = 29.4, p < 0.001) and of age (F(4,48) = 19.3, p < 0.001) as well as a significant interaction (F(4,48) = 6.7, p < 0.001). Separated for age, a one-way ANOVA showed that amph only significantly increased the duration of walking on postnatal days 18 and 21.
For sitting (, upper right corner), the three-way ANOVA showed a tendency for early manipulation (F(1,100) = 2.7, p = 0.09), a significant effect of drug treatment (F(1,100) = 65.7, p < 0.001) but no significant effect of age (F(4,100) < 1.0). In addition a significant two-way interaction was observed for early manipulation—drug treatment (F(1,100) = 4.8, p < 0.05), but not for early manipulation—age (F(4,100) = 1.1, p>0.3) nor for drug treatment—age (F(4,100) < 1.0). The three-way interaction was also not significant (F(4,100) = 1.2, p>0.3).
In view of the significant early manipulation—drug treatment interaction, we investigated the effects of amph in the Ctrl and the MD rats separately. In the Ctrl animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,52) = 19.6, p < 0.001) but not of age (F(4,52) < 1.0) nor a significant interaction (F(4,52) < 1.0). Separated for age, a one-way ANOVA showed that amph significantly reduced the duration of sitting at all postnatal days in Ctrl rats. In the MD animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,48) = 18.0, p < 0.001) but not of age (F(4,48) = 1.4, p>0.2) nor a significant interaction (F(4,48) = 1.1, p>0.3). Separated for age, a one-way ANOVA showed that amph significantly reduced the duration of sitting on postnatal days 18 and 21 in MD rats.
For rearing, the three-way ANOVA showed a significant effect of early manipulation (F(1,100) = 11.5, p < 0.001), and of age (F(4,100) = 5.9, p < 0.001) but not of drug treatment (F(4,100) = 2.2, p>0.1). There was no significant two-way interaction observed for early manipulation—drug treatment, of early manipulation—age (F(4,100) < 1.0), nor of drug treatment—age (all F(4,100) < 1.0). The three-way interaction was also not significant (F(4,100) < 1.0). Since there were no significant drug effects, nor interactions, we did not analyse the effects of amph in the Ctrl and MD rats separately.
For grooming, the three-way ANOVA showed no significant effect of early manipulation (F(1,100) = 2.5, p = 0.12), a significant effect of drug treatment (F(1,100) = 13.3, p < 0.001) and a significant effect of age (F(4,100) = 5.1, p < 0.001). In addition, no significant two-way interaction was observed for early manipulation—drug treatment (F(1,100) = 1.6, p>0.2). The early manipulation—age interaction was almost significant (F(4,100) = 2.1, p = 0.08) but the drug treatment—age was not (F(4,100) = 1.3, p>0.2). The three-way interaction was also not significant (F(4,100) = 1.2, p>0.3).
Given the significant drug effect, we investigated the effects of amph in the Ctrl and the MD rats separately. In the Ctrl animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,52) = 13.5, p < 0.001) and of age (F(4,52) = 3.7, p < 0.01). The interaction approached significance (F(4,52) = 2.3, p = 0.08). Separated for age, a one-way ANOVA showed that amph significantly reduced the duration of rearing at postnatal days 12 and 16. In the MD animals, a two-way ANOVA showed no significant effect of drug treatment (F(1,48) = 2.6, p>0.1) but a significant effect of age (F(4,48) = 5.1, p < 0.005). The interaction was not significant (F(4,48) = 1.31, p>0.28).
In summary, amph increased walking in Ctrl rats on all postnatal days, whereas in MD rats this drug only increased walking on postnatal days 18 and 21. This was accompanied by a similar reduction in sitting behaviour. Amph did not increase rearing or affect grooming in either Ctrl or MD rats.
Open field behaviour—effects of MK801
The effects of saline and MK801 (0.1 mg/kg s.c.) on the behaviour in the open field test are shown in . As with amph only the duration of walking, sitting, rearing and grooming will be discussed.
For walking, the three-way ANOVA showed no significant effect of early manipulation (F(1,101) < 1.0), but a significant effect of drug treatment (F(1,101) = 55.9, p < 0.001) and of age (F(4,101) = 5.6, p < 0.001). None of the two or three way interactions were significant.
Given the significant effect of drug treatment, we also investigated the effects of MK801 in the Ctrl and the MD rats separately. In the Ctrl animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,51) = 21.6, p < 0.001) and a trend towards a significant effect of age (F(4,51) = 2.3, p = 0.07) but no significant interaction (F(4,51) = 1.1, p>0.35). Separated for age, a one-way ANOVA showed that MK801 significantly increased the duration of walking on all postnatal days. In the MD animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,50) = 34.8, p < 0.001) and of days (F(4,50) = 4.5, p < 0.005) but no significant interaction (F(4,50) = 2.0, p>0.1). Separated for age, a one-way ANOVA showed that MK801 significantly increased the duration of walking on all postnatal days.
For sitting, the three-way ANOVA showed no significant effect of early manipulation (F(1,101) < 1.0), but a significant effect of drug treatment (F(1,101) = 76.3, p < 0.001) and of age (F(4,101) = 2.7, p < 0.05). None of the two or three way interactions were significant.
Given the significant drug effect, we also investigated the effects of MK801 in the Ctrl and the MD rats separately. In the Ctrl animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,51) = 60.0, p < 0.001) but no significant effect of age (F(4,51) = 1.3, p>0.27) and no significant interaction (F(4,51) < 1.0). Separated for age, a one-way ANOVA showed that MK801 significantly reduced the duration of sitting on all postnatal days in Ctrl animals. In the MD animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,50) = 25.6, p < 0.001) but not of age (F(4,50) = 1.4, p>0.25) and no significant interaction (F(4,50) < 1.0). Separated for age, a one-way ANOVA showed that MK801 significantly decreased the duration of sitting on postnatal days 12, 18 and 21, with a strong tendency (p < 0.07) at postnatal day 14.
For rearing, the three-way ANOVA showed a significant effect of early manipulation (F(1,101) = 4.7, p < 0.05), of drug treatment (F(1,101) = 12.4, p < 0.001) and of age (F(4,101) = 2.7, p < 0.05). In addition, there was a significant early manipulation—drug treatment interaction (F(1,101) = 8.1, p < 0.005) and a significant drug treatment—age interaction (F(4,101) = 5.5, p < 0.001). The early manipulation—age interaction (F(4,101) < 1.0) as well as the three-way interaction (F(1,101) < 1.0) was not significant.
Given the significant effect of drug treatment and the significant interaction between early manipulation and drug treatment, we also investigated the effects of MK801 in the Ctrl and the MD rats separately. In the Ctrl animals, a two-way ANOVA showed a significant effect of drug treatment (F(1,51) = 17.0, p < 0.001) but no significant effect of age (F(4,51) = 1.7, p>0.17). The interaction, on the other hand, was significant (F(4,51) = 2.7, p < 0.05). Separated for age, a one-way ANOVA showed that MK801 significantly reduced the duration of rearing on all postnatal days except postnatal day 16 in Ctrl animals. In the MD animals, a two-way ANOVA showed no significant effect of drug treatment (F(1,50) < 1.0) nor/ of age (F(4,50) = 1.5, p>0.2). However, the interaction was significant (F(4,50) = 3.4, p < 0.02). Separated for age, a one-way ANOVA showed that MK801 significantly increased the duration of rearing on postnatal days 12 and 14 in MD rats.
For grooming, the three-way ANOVA showed a significant effect of early manipulation (F(1,101) = 5.0, p < 0.05), but not of drug treatment (F(1,101) < 1.0). The effect of age was almost significant (F(4,101) = 2.47, p = 0.06). In addition, there was a significant drug treatment—day interaction (F(4,101) = 2.8, p < 0.05). The other two and three way interactions were not significant. Since there was no significant drug effect, we did not analyse the effects of MK801 for Ctrl and MD rats separately.
In summary MK801 increased walking and reduced sitting in both Ctrl and MD rats at all postnatal days tested. This drug also reduced rearing in both Ctrl and MD rats, but did not significantly affect grooming.
Discussion
Our main hypothesis was that early MD would retard normal (motor) development. The data obtained indeed seem to confirm this hypothesis. In short, we found that, compared to Ctrl rats, MD rats showed [1] a reduction in body weight from postnatal day 10 until at least day 24 (the last day of measurement); [2] a prolonged reversal time in the negative geotaxis on postnatal days 10 and 11; [3] a retardation of eye opening at postnatal days 15 and 16; [4] a reduction in walking on postnatal days 12–14, and rearing on postnatal days 12–18; and [5] a reduced response to amph on postnatal days 12–16.
The reduction in body weight after MD is in line with our own previous papers (Husum et al. Citation2002; Ellenbroek et al. Citation2004), as well as with those of others (Workel et al. Citation1997; Penke et al. Citation2001). In a previous paper, we pointed out that the relative reduction in body weight was larger at weaning (postnatal 21) than immediately after the deprivation on postnatal day 10 (Ellenbroek et al. Citation2004). In the present paper, we extended this by also looking at intermediate days. Interestingly, the largest relative decrease in body weight was seen on postnatal days 11 (24%) and 12 (26%), whereas it was only 14% at postnatal day 10. Moreover, MD rats at postnatal day 11 weighed less than those at postnatal day 10 (). This suggests that changes in maternal behaviour or milk production have occurred, which last beyond the 24 h period of deprivation. In agreement with this, we recently showed that the long-term consequences of MD are reduced when the maternally deprived rats are being fostered by non-deprived mothers (Ellenbroek and Cools Citation2002b).
Negative geotaxis is an early righting reflex that appears to be particularly sensitive to early manipulations. Thus prenatal treatment with drugs such as alcohol (Hannigan Citation1995), 2,4-dichlorophenoxyacetic acid (Bortolozzi et al. Citation1999) and 1,1,1-trichloroethane (Coleman et al. Citation1999) selectively retard the development of negative geotaxis. On the other hand, prenatal treatment with drugs like 5-bromo-2′-deoxyuridine (Kuwagata and Nagao Citation1998), phenytoin (McCartney et al. Citation1999) or maternal exposure to picrotoxin during early lactation (Baso et al. Citation2003) improves negative geotaxis. In addition, prenatal stress (Patin et al. Citation2004), neonatal hypoxia (Lubics et al. Citation2005) and neonatal dexamethasone (Gramsbergen and Mulder Citation1998; Ferguson et al. Citation2001) also retard the development of the negative geotaxis reflex. Prenatal stress (Koenig et al. Citation2005) neonatal hypoxia (Boksa Citation2004a,Citationc), and MD (Ellenbroek and Riva Citation2003) have all been proposed to model specific aspects of schizophrenia. In agreement with this, we found a significant increase in the reversal latency on postnatal days 10 and 11 in MD rats as well. However, from the data, it is not clear whether this increase really represents a delay in maturation. In fact, the rats at postnatal day 9 already had a fast geotaxis reflex. In this respect, it is important to realise that during the MD, the rats were kept at room temperature, implying that at the time of the geotaxis experiment on postnatal day 10, they were hypothermic, in relation to the non-deprived rats, which might explain the large increase. Although the MD rats also showed a significantly longer latency at postnatal day 11, this increase was much smaller than at postnatal day 10 (). The data on negative geotaxis, therefore, do not really allow a conclusion with respect to the retardation of development. Although differences in the protocol may be of importance too (Kreider and Blumberg Citation1999), a more important factor is presumably the time of measurement. As shown by others, negative geotaxis starts to occur on postnatal day 4 and is almost complete by postnatal day 9 (Patin et al. Citation2004), and may, therefore, not be an ideal parameter to assess after the MD (which starts at postnatal day 9).
The effects of MD on eye opening provide more convincing evidence for a delay in development. Thus, whereas virtually all control rats had opened their eyes on postnatal 16 (99%), 29% of all MD rats still had their eyes closed. Although, the delay may seem to be small, 24 h during this developmental period may be of crucial importance. Indeed most of the pre- and early postnatal manipulations that affect negative geotaxis do not delay eye opening (Gramsbergen and Mulder Citation1998; Baso et al. Citation2003), with the exception of neonatal hypoxia (Lubics et al. Citation2005) and prenatal stress (Patin et al. Citation2004), both of which also delay eye opening by about 24 h.
As with eye opening, MD rats showed signs of a retardation of motor development in the open field test. Thus maternally deprived rats showed significantly less walking and rearing on postnatal days 12 and 14. Especially the differences in rearing (reduced in MD rats on postnatal day 12, 14 and 18) is interesting, in view of the data presented by Eilam and Golani (Citation1988), showing that normal development of motor behaviour in rats follows a series of very strict rules, starting with sideward (scanning) movements, followed by horizontal forward (walking) movements and the development is completed by vertical (rearing) movements (Eilam and Golani Citation1988). Given the fact that MD rats showed no rearing at all at postnatal days 12 and 14, and a reduction in postnatal days 16 and 18, this is a clear indication that MD retards normal (motor) development.
Finally, we found that both amph and MK801 increased locomotor activity, and decreased sitting in MD and Ctrl rats. However, whereas MK801 was equally effective in both groups of rats, there was a significant drug treatment—early manipulation interaction in the case of amph. Close inspection of showed that whereas amph increased the duration of walking (and concomitantly decreased the duration of sitting) at all days (from postnatal day 12 to 21) in ctrl rats, it was only effective on postnatal days 18 and 21 in MD rats.
Amph is known to increase extracellular dopamine concentrations in the brain, by blocking the dopamine transporter and by enhancing dopamine release through a process known as reverse transport (Sulzer et al. Citation2005). The increased locomotor activity seen after amph administration is generally considered to be due to an increase in extracellular dopamine, especially in the nucleus accumbens (Broekkamp et al. Citation1975; Kuczenski and Segal Citation1990). The differential response of amph in MD vs. Ctrl rats thus suggests a difference in the development of the mesolimbic dopaminergic system. The ontogeny of many aspects of the dopaminergic system in rats has been described, including the number of dopamine transporters (Coulter et al. Citation1996; Galineau et al. Citation2004), the dopamine D1 and D2 receptor densities (Jung and Bennett Citation1996) and the methamphetamine-induced dopamine release (Tsuchida et al. Citation1998). All these data clearly show that during postnatal 9 and 10 (when MD takes places) the dopaminergic system is still developing, with levels being much less than in adulthood. Given the fact that MD acutely leads to high levels of corticosterone (Suchecki et al. Citation1993), and that high levels of corticosterone are detrimental to cell development (Gould and Tanapat Citation1999), it is tempting to speculate that MD stops or retards the normal development of the dopaminergic system through its actions on the HPA axis. In agreement with this, MD has been shown to reduce neurogenesis (Park et al. Citation2002) and to increase cell death (Zhang et al. Citation2002). Although it has been shown that early environmental manipulations (such as repeated maternal separation) can reduce the number of dopamine transporters (Meaney et al. Citation2002), so far this has only been observed in adulthood. In fact it is unlikely that our MD procedure will also lead to a reduction in dopamine transporters in adulthood, as we did not find any differences in amph response in rats at postnatal day 18 and 21 (and 30, unpublished data), and it has been found that maternally deprived rats, in adulthood, are actually more sensitive to apomorphine (Ellenbroek and Cools Citation1995; Rots et al. Citation1996) and amph (Zimmerberg and Shartrand Citation1992).
Interestingly, MD rats did not differ from Ctrl rats in their behavioural response to MK801. This implies first of all that even at pnd12, MD rats can show walking and rearing, when stimulated with a drug, and secondly that the differential sensitivity of MD and Ctrl rats to drugs, depends on the type of pharmacological challenge. In contrast to amph, MK801 selectively targets the glutamatergic system, by non-competitively blocking the NMDA receptor. It has been shown that this receptor is already functionally active at a very young age. Indeed it has been shown that a single injection of MK801 at postnatal day 7 can lead to long lasting changes in neurochemistry and behaviour, such as a reduction in prepulse inhibition (Harris et al. Citation2003). In addition, even prenatal injections of MK801 induce long lasting changes (Ikonomidou et al. Citation1999). Thus, one might hypothesize that the development of the NMDA receptors is already in such an advanced state at postnatal day 9, when MD commences, that this stressor cannot affect its development. However, additional experiments are needed to test this hypothesis.
In summary, the present set of data show that a single 24 h period of MD on postnatal day 9 leads to a long lasting reduction in body weight, a delay in eye opening, in walking and in rearing and in a delayed behavioural response to amph. As discussed in the introduction, early MD leads to a large number of abnormalities in adulthood, that show similarity with known signs and symptoms of schizophrenia, including reductions in acoustic sensory gating, startle habituation, prepulse inhibition and an increased stress response (Ellenbroek and Riva Citation2003). In addition, both MD rats and patients with schizophrenia have reduced hippocampal levels of brain derived neurotrophic factor (Roceri et al. Citation2002; Weinberger Citation1999), neuropeptide Y (Frederiksen et al. Citation1991; Husum et al. Citation2002), polysialilated-neuronal cell adhesion molecule (Barbeau et al. Citation1995; Foley et al. Citation2000) and mRNA levels for the NR1 subunit of the NMDA receptor (Roceri et al. Citation2002; Harrison et al. Citation2003; Meador-Woodruff et al. Citation2005). Although none of the items, by themselves, are specific or pathognomic for schizophrenia, together they substantiate the claim that MD might represent an interesting animal model for several aspects of schizophrenia.
The present data, which show that MD also leads to a retardation of normal development, seem to further substantiate the claim that early MD might represent an interesting animal model for schizophrenia. Although rats show a different development after birth than humans, for example, with respect to eye opening, the retardation in walking and rearing in rats is reminiscent of the retarded development of motor behaviour in children that develop schizophrenia (Jones et al. Citation1994; Jones Citation1997).
Acknowledgements
We would like to thank L. Lubbers and J. Maier for their skilful help during the experiments. This work was in part financed by a joint grant from NWO-ZONMW/KOSEF (2005/00445/IB).
References
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17649–17654
- Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, Troudart T, Bloch M, Heresco LU, Lerer B. Environment and vulnerability to major psychiatric illness: A case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry 1999; 4(2)163–172
- Barbeau D, Liang JJ, Robitalille Y, Quirion R, Srivastava LK. Decreased expression of the embryonic form of the neural cell adhesion molecule in schizophrenic brains. Proc Natl Acad Sci USA 1995; 92(7)2785–2789
- Baso ACZ, Goulart FC, Teodorov E, Felicio LF, Bernardi MM. Effects of maternal exposure to picrotoxin during lactation on physical and reflex development, square crossing and sexual behaviour of rat offspring. Pharmacol Biochem Behav 2003; 75(4)733–740
- Boksa P. Animal models of obstetric complications in relation to schizophrenia. Brain Res Rev 2004a; 45(1)1–17
- Boksa P. Animal models of obstetric complications in relation to schizophrenia. Brain Res Rev 2004b; 45(1)1–17
- Boksa P. Lasting effects of birth insult on dopamine function in animal models: Implications for schizophrenia and other disorders. Int J Neuropsychopharm 2004c; 7S76
- Bortolozzi AA, Duffard RO, de Duffard ME. Behavioral alterations induced in rats by a pre- and postnatal exposure to 2,4-dichlorophenoxyacetic acid. Neurotoxicol Teratol 1999; 21(4)451–465
- Braff D, Stone C, Callaway E, Geyer MA, Glick ID, Bali L. Prestimulus effects of human startle reflex in normals and schizophrenics. Psychophysiology 1978; 15339–15343
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 1992; 49(3)206–215
- Broekkamp CL, Pijnenburg AJ, Cools AR, van RJ. The effect of microinjections of amphetamine into the neostriatum and the nucleus accumbens on self-stimulation behaviour. Psychopharmacologia 1975; 42(2)179–183
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, Poulton R. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder—results from a longitudinal birth cohort. Arch Gen Psychiatry 2002; 59(5)449–456
- Coleman CN, Mason T, Hooker EP, Robinson SE. Developmental effects of intermittent prenatal exposure to 1,1,1-trichloroethane in the rat. Neurotoxicol Teratol 1999; 21(6)699–708
- Coulter CL, Happe HK, Murrin LC. Postnatal development of the dopamine transporter: A quantitative autoradiographic study. Dev Brain Res 1996; 92172–92181
- Daenen EWPM, Wolterink G, Van der Heyden JA, Kruse CG, Van Ree JM. Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur Neuropsychopharmacol 2003; 13(3)187–197
- Degen SB, Ellenbroek BA, Wiegant VM, Cools AR. The development of various somatic markers is retarded in an animal model for schizophrenia, namely apomorphine-susceptible rats. Behav Brain Res 2005; 157(2)369–377
- Eilam D, Golani I. The ontogeny of exploratory-behavior in the house rat (Rattus-Rattus)—The mobility gradient. Dev Psychobiol 1988; 21(7)679–710
- Ellenbroek BA, Cools AR. Maternal separation reduces latent inhibition in the conditioned taste aversion paradigm. Neurosci Res Comm 1995; 17(1)27–33
- Ellenbroek BA, Cools AR. The neurodevelopmental hypothesis of schizophrenia: Clinical evidence and animal models. Neurosci Res Comm 1998; 22(3)127–136
- Ellenbroek BA, Cools AR. The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology 2000; 23(1)99–106
- Ellenbroek BA, Cools AR. Animal models for schizophrenia. Textbook of biological psychiatry, H D'Haenen, JA den Boer, H Westenberg, P Willner. John Wiley & Sons, Chichester 2002a; 567–580
- Ellenbroek BA, Cools AR. Early maternal deprivation and prepulse inhibition—The role of the postdeprivation environment. Pharmacol Biochem Behav 2002b; 73(1)177–184
- Ellenbroek BA, de Bruin NMWJ, van Den Kroonenburg PT, van Luijtelaar ELJM, Cools AR. The effects of early maternal deprivation on auditory information processing in adult Wistar rats. Biol Psychiatry 2004; 55(7)701–707
- Ellenbroek BA, Riva MA. Early maternal deprivation as an animal model for schizophrenia. Clin Neurosci Res 2003; 3(4–5)297–302
- Ellenbroek BA. Simulation models for schizophrenia. Atypical antipsychotics, BA Ellenbroek, AR Cools. Birkhauser Verlag, Basel 2000; 121–142
- Ellenbroek BA, van den Kroonenberg PTJM, Cools AR. The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res 1998; 30(2)251–260
- Ferguson SA, Paule MG, Holson RR. Neonatal dexamethasone on day 7 in rats causes behavioral alterations reflective of hippocampal, but not cerebellar, deficits. Neurotoxicol Teratol 2001; 23(1)57–69
- Foley A, Ellenbroek B, Lubbers L, Cools A, Regan C. Role of NCAM polysialylation in the developmental emergence of learning mechanisms. Behav Pharmacol 2000; 11(3)337
- Frederiksen SO, Ekman R, Gottfries CG, Widerlov EJ, Jonsson S. Reduced concentrations of galanin, arginine vasopressin, neuropeptide Y and peptide YY in the temporal cortex but not in the hypothalamus of brains from schizophrenics. Acta Psychiatr Scand 1991; 83(4)273–277
- Galineau L, Kodas E, Guilloteau D, Vilar MP, Chalon S. Ontogeny of the dopamine and serotonin transporters in the rat brain: An autoradiographic study. Neurosci Lett 2004; 363(3)266–271
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry 1999; 461472–461479
- Gramsbergen A, Mulder EJH. The influence of betamethasone and dexamethasone on motor development in young rats. Pediatr Res 1998; 44(1)105–110
- Hannigan JH. Effects of prenatal exposure to alcohol plus caffeine in rats—pregnancy outcome and early offspring development. Alcoholism-Clinical and Experimental Research 1995; 19(1)238–246
- Harris Harrison PJ, Law AJ, Eastwood SL. Glutamate receptors and transporters in the hippocampus in schizophrenia. 2003
- Husum H, Termeer E, Mathe AA, Bolwig TG, Ellenbroek BA. Early maternal deprivation alters hippocampal levels of neuropeptide Y and calcitonin-gene related peptide in adult rats. Neuropharmacology 2002; 42(6)798–806
- Ikonomidou Isohanni M, Jones P, Kemppainen L, Croudace T, Isohanni I, Veijola J, Rasanen S, Wahlberg KE, Tienari P, Rantakallio P. Childhood and adolescent predictors of schizophrenia in the Northern Finland 1966 Birth Cohort—A descriptive life-span model. Eur Arch Psychiatry Clin Neurosci 2000; 250(6)311–319
- Jones P. The early origins of schizophrenia. Br Med Bull 1997; 53(1)135–155
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994; 344(8934)1398–1402
- Jung AB, Bennett JP. Development of striatal dopaminergic function. I. Pre- and postnatal development of mRNA and binding sites for striatal D1 (D1a) and D2 (D2a) receptors. Dev Brain Res 1996; 109–120
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: Potential relevance to schizophrenia. Behav Brain Res 2005; 156(2)251–261
- Kreider JC, Blumberg MS. Geotaxis in 2-week-old Norway rats (Rattus norvegicus): A reevaluation. Dev Psychobiol 1999; 35(1)35–42
- Kuczenski R, Segal DS. In vivo measures of monoamines during amphetamine-induced behaviors in rats. Prog Neuropsychopharmacol Biol Psychiatr 1990, 14 Suppl-50
- Kuwagata M, Nagao T. Behavior and reproductive function of rat male offspring treated prenatally with 5-bromo-2′-deoxyuridine. Reprod Toxicol 1998; 12(5)541–549
- Lammers C-H, Garcia-Borreguero D, Schmider J, Gotthardt U, Dettling M, Holsboer F, Heuser IJE. Combined dexamethasone/corticotropin-releasing hormone test in patients with schizophrenia and in normal controls II. Biol Psychiatr 1995; 38803–38807
- Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol 1991; 24(8)547–558
- Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology 1987; 91415–91533
- Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: A potential animal model of schizophrenia. Neuropsychopharmacology 1993; 967–975
- Lubics A, Reglodi D, Tamas A, Kiss P, Szalai M, Szalontay L, Lengvari I. Neurological reflexes and early motor behaviour in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res 2005; 157(1)157–165
- McCartney MA, Scinto PL, Wang SS, Altan S. Developmental effects of phenytoin may differ depending on sex of offspring. Neurotoxicol Teratol 1999; 21(2)119–128
- Meador-Woodruff J, Beneyto M, Kristiansen LV, McCullumsmith RE, Scarr E, Dean B. NMDA receptor subunit and associated PSD protein transcripts in hippocampus in schizophrenia and bipolar disorder. Schizophr Bull 2005; 31(2)291
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: A neurobiological mechanism for vulnerability to drug abuse?. Psychoneuroendocrinology 2002; 27(1–2)127–138
- Mednick SA. Breakdown in individuals at high risk for schizophrenia: Possible predispositional perinatal factors. Mental Hygiene 1970; 5450–5463
- Park HJ, Lim S, Lee HS, Lee HJ, Yoo YM, Lee HJ, Kim SA, Yin CS, Seo JC, Chung JH. Acupuncture enhances cell proliferation in dentate gyrus of maternally-separated rats. Neurosci Lett 2002; 319(3)153–156
- Patin V, Vincent A, Lordi B, Caston J. Does prenatal stress affect the motoric development of rat pups?. Brain Res Dev Brain Res 2004; 149(2)85–92
- Penke Z, Felszeghy K, Fernette B, Sage D, Nyakas C, Burlet A. Postnatal maternal deprivation produces long-lasting modifications of the stress response, feeding and stress-related behaviour in the rat. Eur J Neurosci 2001; 14747–14755
- Pilowsky LS, Kerwin RW, Murray RM. Schizophrenia: A neurodevelopmental perspective. Neuropsychopharmacology 1993; 9(1)83–91
- Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry 2002; 7(6)609–616
- Rots NY, de JJ, Workel JO, Levine S, Cools AR, de KE. Neonatal maternally deprived rats have as adults elevated basal pituitary-adrenal activity and enhanced susceptibility to apomorphine. J Neuroendocrinol 1996; 8(7)501–506
- Schwabe K, Enkel T, Klein S, Schutte M, Koch M. Effects of neonatal lesions of the medial prefrontal cortex on adult rat behaviour. Behav Brain Res 2004; 153(1)21–34
- Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology 1993; 57(2)204–212
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol 2005; 75406–75433
- Tsuchida K, Akiyama K, Sakai K, Ujike H, Li X, Kuroda S. Ontogeny of striatal dopamine relase in rats after acutre administration of methamphetamine. Pharmacol Biochem Behav 1998; 53(3)575–580
- Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20(3)441–451
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44(7)660–669
- Weinberger DR. Cell biology of the hippocampal formation in schizophrenia. Biol Psychiatry 1999; 45(4)395–402
- Workel JO, Oitzl MS, Ledeboer A, de KE. The Brown Norway rat displays enhanced stress-induced ACTH reactivity at day 18 after 24 h maternal deprivation at day 3. Brain Res Dev Brain Res 1997; 103(2)199–203
- Zhang LX, Levine S, Dent G, Zhan YT, Xing GQ, Okimoto D, Gordon MK, Post RM, Smith MA. Maternal deprivation increases cell death in the infant rat brain. Developmental Brain Research 2002; 133(1)1–11
- Zimmerberg B, Shartrand AM. Temperature-dependent effects of maternal separation on growth, activity, and amphetamine sensitivity in the rat. Dev Psychobiol 1992; 25(3)213–226