Abstract
Male rats show a differential adrenocortical response to stress before and after pubertal development, such that prepubertal animals have a more prolonged stress-induced corticosterone response compared to adults. Whether pubertal maturation affects other adrenocortical responses to stress is currently unknown. To address this question, we assessed stress-induced progesterone secretion in both intact and gonadectomized prepubertal (28 days of age) and adult (77 days of age) male rats either before or after exposure to a 30 min session of restraint stress. We found that prepubertal males show a greater and more prolonged stress-induced progesterone response compared to adults. We also found a similar effect in castrated prepubertal and adult males, indicating the differential stress-induced progesterone response is not gonadal in origin. We also examined progesterone receptor (PR) levels by immunohistochemistry in the paraventricular nucleus (PVN) of the hypothalamus, a key regulatory nucleus of the hypothalamic–pituitary–adrenal (HPA) axis, and found lower PR protein expression in the PVN of prepubertal compared to adult males. These data indicate that in addition to corticosterone, stress-induced adrenocortical progesterone levels are differentially affected by pubertal maturation. Furthermore, these data raise the possibility of different progesterone sensitivity of the PVN before and after puberty. The significance of this differential response is presently unknown. However, given the pleiotropic effects of progesterone on male physiology and behaviour, it is likely that the disparate post-stress exposure to progesterone affects the prepubertal and adult male differently.
Introduction
Prepubertal and adult male rats show a differential adrenocortical response to stress, such that prepubertal animals have a more prolonged stress-induced corticosterone response compared to adults. Specifically, following intermittent foot shock (Goldman et al. Citation1973), ether vapour (Vazquez and Akil Citation1993) or restraint (Romeo et al. Citation2004a), corticosterone levels of prepubertal males take at least 45–60 min longer to return to baseline compared to adults. However, it is currently unknown whether prepubertal and adult males demonstrate other differential adrenocortical responses to stress.
Progesterone is a steroid synthesized and released from both the gonad and adrenal cortex (Kalra and Kalra Citation1977). A number of studies have shown that, in addition to corticosterone, stress leads to increases in progesterone secretion in adult male rats as well (Persengiev et al. Citation1991; Andersen et al. Citation2004, Citation2005). Interestingly, castrated adult males continue to exhibit stress-induced progesterone secretion, indicating a role for the adrenal gland in the progesterone stress response (Schaeffer and Aron Citation1987). In females, stress-induced increases in progesterone secretion have also been observed (Boehm et al. Citation1982; Budec et al. Citation2002). Recently, we have shown that prepubertal and adult ovariectomized female rats show differential stress-induced progesterone secretion (Romeo et al. Citation2004b), indicating differential adrenocortical secretion of progesterone in pre-pubertal and adult females. Together, these data point to the possibility of a differential adrenocortical stress-induced progesterone response in prepubertal and adult males.
To characterize possible differences in stress-induced progesterone secretion in pre-pubertal and adult males, we assessed progesterone secretion either before or after a 30 min session of restraint stress. In a follow up experiment, we assessed stress-induced progesterone secretion in castrated prepubertal and adult males. Finally, we examined progesterone receptor (PR)-immunoreactivity in the paraventricular nucleus (PVN) of the hypothalamus, a key regulatory nucleus of the hypothalamic–pituitary–adrenal (HPA) axis (Herman et al. Citation2003), in gonadally intact, unstressed prepubertal and adult males. We show here that prepubertal males show a greater and more prolonged stress-induced progesterone response, but fewer PR positive cells in the PVN under basal conditions, compared to adults. These data indicate that pubertal development modulates both stress-induced progesterone secretion and possibly genomic-mediated progesterone signalling in the PVN.
Materials and methods
Animals and housing
For all experiments, male Sprague Dawley rats were obtained from Charles River (Kingston, NY). Animals were housed 2–3 per cage (same age cage mates) in clear polycarbonate cages with wood chip bedding and maintained on a 12 h light–dark schedule (lights on at 0900 h EST). All animals had ad libitum access to food and water and the vivarium was maintained at 21 ± 2°C. All procedures were carried out in accordance with the guidelines established by the NIH Guide for the Care and Use of Laboratory Animals and the Animal Experimentation Guidelines from the Committee on Animal Research of The Rockefeller University.
Experimental design
Three experiments were conducted. Experiments 1 and 2 assessed stress-induced progesterone secretion in gonadally intact prepubertal and adult males and castrated prepubertal and adult males, respectively. Experiment 3 was a separate, but related experiment, in which PR-immunoreactive (ir) cell density was examined in the PVN of unstressed, gonadally intact prepubertal and adult males. In experiment 1, prepubertal (28 days of age) and adult (77 days of age) males were weighed and rapidly decapitated by a guillotine either before (basal) or after 30 min session of restraint stress. Three time points after a 30 min restraint stress session were examined: immediately after termination of the stressor (i.e. time 0) or 30 or 120 min after the stress session (n = 5–6/age and time point). These time points were chosen based on a previous study that showed stress-induced corticosterone levels were different in prepubertal and adult animals at the 30 min post-stress time point and returned to baseline at 120 min following termination of the stressor (Romeo et al. Citation2004a). The rats were restrained in wire mesh restrainers, which were closed and secured at both ends with binder clips. Immediately after termination of the stressor, the animals were returned to their home cages. At the appropriate time points, trunk blood samples were collected in BD Vacutainer K3 EDTA-coated test tubes and spun down in a refrigerated centrifuge. All animals were killed between 1300 and 1700 h. Plasma was removed and stored at − 20°C until the radioimmunoassay for progesterone was performed (see below). Adrenal glands were also collected, cleaned of fat and weighed.
In experiment 2, prepubertal (21 days of age) and adult (70 days of age) males were castrated under sodium pentobarbital anaesthesia (50 mg/kg, ip) and 1 week after castration (i.e. at 28 or 77 days of age), were rapidly decapitated by a guillotine at the appropriate time point and blood samples and adrenal glands were collected and treated in the same manner as described in experiment 1 (n = 4–5/age and time point).
In experiment 3, prepubertal (28 days of age) and adult (77 days of age) males (n = 4) were perfused after an overdose of sodium pentobarbital (100 mg/kg) with 50 ml of 0.9% heparinized saline followed by 50 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were then post-fixed for 4 h in 4% paraformaldehyde with 0.125% glutaraldehyde and then stored in 30% sucrose at 4°C. Coronal brain sections were made on a cryostat (30 μm) and stored at − 20°C in cryoprotectant until processed for immunohistochemistry (see below).
Radioimmunoassay
Plasma progesterone concentrations were measured separately in experiments 1 and 2. Within each experiment, samples were assayed in duplicate in a single assay using the Coat-a-Count Progesterone Kit (Diagnostic Products, Los Angeles, CA). For experiment 1, the lower limit of detectability (LLD) of the assay was 0.093 ng/ml and the intra-assay coefficient of variation (CV) was 6.6%, while LLD and CV for experiment 2 was 0.059 ng/ml and 3.0%, respectively. The inter-assay CV was 11.8%.
Progesterone receptor immunohistochemistry and microscopy analysis
Free-floating sections were washed five times for 10 min in 0.1 M PB and incubated for 10 min in 0.05% H2O2 in 0.1 M phosphate buffered saline (PBS). Sections were then washed three times for 10 min in 0.1 M PB with 0.1% Triton X-100 (PBT), blocked in 5% bovine serum albumin (BSA) in PBT for 1 h, and incubated in rabbit anti-PR (1:2500; DakoCytomation, Glostrup, Denmark) in 5% BSA in PBT for 48 h at 4°C. This antibody recognizes both the A and B forms of the PR (Traish and Wotiz Citation1990), and previous studies in rats have indicated that preadsorption of this PR antibody abolishes all immunoreactivity (Wagner et al. Citation1998). Sections were then washed five times for 10 min in PBT and incubated in biotinylated goat anti-rabbit IgG (1:200; Vector, Burlingame, CA) in PBT for 1 h at room temperature (RT). Sections were then washed three times for 10 min in PBT and incubated in avidin-biotin horseradish peroxidase complex (1:200; Vectastain ABC Kit, Vector) in PBT for 1 h at RT. After three 10 min washes in PBS, horseradish peroxidase was visualized with 0.02% 3,3′diaminobenzidine (DAB) in a 3 M sodium acetate buffer containing 2.5% nickel sulfate and 0.05% H2O2. Sections were then washed five times for 10 min in PBS, mounted onto Fisher Brand Plus slides (Fisher Scientific, Pittsburgh, PA), dried, dehydrated in increasing concentrations of alcohol (70, 95 and 100%), cleared in xylenes, and coverslipped with DPX Mountant (Sigma, St Louis, MO). Processing tissue in the absence of the primary antibody resulted in no detectable immunostaining by the secondary antibody.
The areal density (cells per unit area) of PR-ir cell profiles was quantified in the PVN. Brain sections were inspected under light microscopy using a Nikon Labophot microscope. Bilateral counts from two sections separated by 90 μm, and anatomically matched across animals, were used for analysis. Brain sections corresponding only to plates 25 and 26 in The Rat Brain in Stereotaxic Atlas (Paxinos and Watson Citation1986) were used to ensure similar anatomical levels of the PVN were analyzed among animals. The PVN was centered in the field of view at 4 × and the magnification was increased to 20 × . PR-ir cells that fell within a superimposed ocular grid (40,000 μm2) were counted. The ocular grid covered both the magnocellular and parvocellular regions of the PVN, and hence, the areal densities presented represent both values for the combined magnocellular and parvocellular subdivisions of the PVN.
Statistical analysis
Plasma progesterone concentrations in experiments 1 and 2 were analyzed by a two-way analysis of variance (ANOVA; age × time point). Significant interactions were analyzed with Tukey's HSD tests. Adrenal gland data were expressed as percent body weight. For analysis, these non-parametric data were first arcsine transformed and then analyzed using two-way ANOVAs. PR-ir cell counts in experiment 3 were analyzed by a t-test. Differences were considered significant when P < 0.05. All data are reported as means ± SEM.
Results
Experiment 1
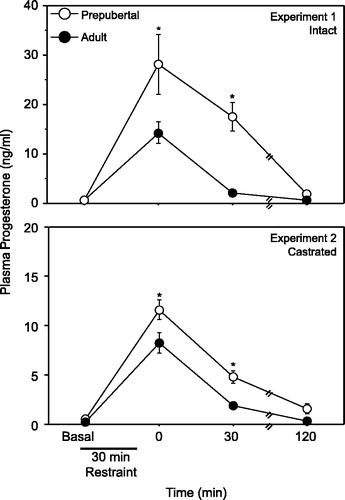
Statistical analyses revealed a significant interaction of age and time point on progesterone secretion (F(3, 37) = 4.403, P < 0.05). Specifically, although both prepubertal and adult males exhibited significantly higher concentrations of progesterone 0 and 30 min after termination of the stressor, prepubertal males demonstrated significantly higher stress-induced progesterone secretion compared to adults at these two time points (, upper panel). Values in both prepubertal and adult males returned to baseline 120 min following the termination of the restraint stress session.
Figure 1 Mean ( ± SEM) plasma progesterone in gonadally intact (upper panel) and castrated (lower panel) prepubertal (28 days old) and adult (77 days old) males either before or after a 30 min restraint stress. Asterisks indicate a significant difference between prepubertal and adult males (P < 0.05).
Interestingly, when normalized to body weights, there was a significant main effect of age on adrenal weight such that prepubertal males had significantly heavier paired adrenal weights compared to adults (F(1,37) = 164.620, P < 0.05; ). There were no significant effects of stress or interaction between age and stress on adrenal weight.
Table I. Mean (±SEM) % adrenal/body weight of prepubertal (28 days old) and adult (77 days old) males in experiments 1 and 2.
Experiment 2
Similar to experiment 1, an ANOVA revealed a significant interaction of age and time point on progesterone secretion (F(3,26) = 4.527, P < 0.05). Specifically, although both castrated prepubertal and adult males exhibited significantly higher concentrations of circulating progesterone 0 and 30 min after termination of restraint, prepubertal males demonstrated significantly higher stress-induced progesterone secretion compared to adults at these time points (, lower panel). Values in both prepubertal and adult males both returned to baseline 120 min following the termination of the restraint stress session.
Again, similar to experiment 1, there was a significant main effect of age on adrenal weight–body weight ratio such that castrated prepubertal males had significantly heavier paired adrenal weights compared to castrated adults (F(1,26) = 73.820, P < 0.05; ). There were no significant effects of stress or interaction between age and stress on adrenal weight.
Experiment 3
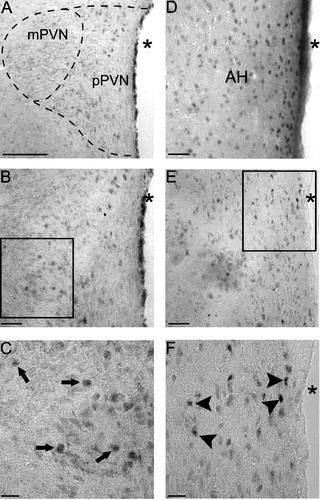
Analysis of the PR-ir cells per 40,000 μm2 revealed that adult males had a significantly higher number of PR-ir cells in the PVN compared to prepubertal males (t = − 2.729, P < 0.05, ). Qualitatively, PR-ir was observed in both the magnocellular and parvocellular regions of the PVN in both prepubertal and adult animals (). Furthermore, it appears qualitatively that immunostaining in the PVN was much less intense with many fewer immunopositive cells compared to that found in the anterior hypothalamus (AH) (), an area replete with PR-ir cells, suggesting relatively low levels of PR in the male PVN.
Figure 2 Mean ( ± SEM) PR-ir cell number (cells/40,000 μm2) in the PVN of gonadally intact, unstressed prepubertal and adult male rats. Asterisk indicates a significant difference between prepubertal and adult males (P < 0.05).
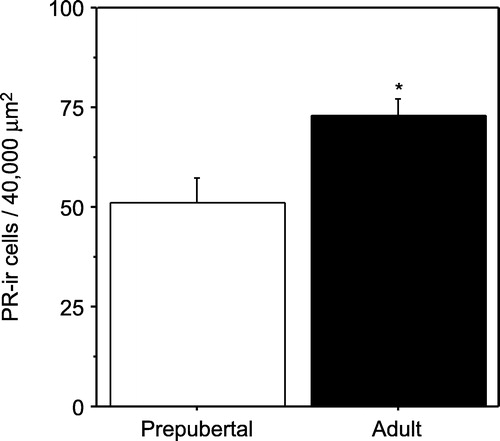
Figure 3 Photomicrographs of PR-ir cells in the PVN of prepubertal and adult males, and the AH (AH; panel D) of an adult male for comparison. (A) low power (10 × ) picture of the PVN and magnocellular and parvocellular subdivisions of the PVN in a prepubertal male. A higher power (20 × ) picture of the PVN in a prepubertal (B) or adult (E) male, while boxes are the areas of higher magnification (40 × ) in the prepubertal (C) and adult (F) male shown below. Arrows in panel C represent immunopositive cells in the prepubertal PVN, while arrowheads in panel F represent immunopositive cells in the adult PVN. (A) Bar = 150 μm, (B, D and E) Bar = 50 μm and (C and F) Bar = 25 μm. Abbreviations: AH, anterior hypothalamus; mPVN, magnocellular subdivision of the PVN; pPVN, parvocellular subdivision of the PVN; asterisks indicate the third ventricle.
Discussion
These data indicate that prepubertal male rats show a greater and more prolonged stress-induced progesterone secretory response following a 30 min session of restraint compared to adults. Furthermore, the data derived from the gonadectomized males suggest that the progesterone response is derived from the adrenal cortex, and is not gonadal in origin. Interestingly, plasma progesterone concentrations were generally lower in gonadectomized prepubertal and adult males (experiment 2), suggesting a contribution of the gonad to circulating plasma progesterone secretion in males (Kalra and Kalra Citation1977). However, gonadectomized prepubertal rats continued to show a greater stress-induced progesterone response compared to gonadectomized adults. The data indicate, therefore, that pubertal maturation significantly affects adrenocortical stress-induced progesterone secretion.
The results from the present experiments are in agreement with a recent report that ovariectomized prepubertal females show a greater and more prolonged progesterone response compared to ovariectomized adults (Romeo et al. Citation2004b). Thus, the findings in females (Romeo et al. Citation2004b) and the current data, indicate that differential stress-induced progesterone secretion in pre- and post-pubertal animals is independent of both the sex of the rat and the presence of the gonads.
Interestingly, once normalized to body weight, the adrenal glands of intact and castrated prepubertal males were heavier than in adult males. The data suggest that the relative production and storage of adrenal steroids, namely corticosterone (Romeo et al. Citation2004a) and progesterone (present study), may be greater in the prepubertal than in adult males. However, these adrenal wet weight data were obtained from entire adrenal glands, and thus, it is difficult to determine whether these differences are due to the adrenal cortex and/or medulla. Future experiments will need to determine steroidal content of the adrenal cortex and relative expression of steroid synthesizing enzymes, such as 3β-hydroxysteroid dehydrogenase and 11β-hydroxylase, at these two developmental stages.
In addition to the endocrine data, these experiments also show the presence of PR in the male PVN, albeit at lower levels than that observed in the AH. This is the first report to our knowledge demonstrating PR-ir in the male PVN. Interestingly, relatively low or no PR protein has been observed in the adult female PVN of many species (Blaustein et al. Citation1988; Numan et al. Citation1999; Francis et al. Citation2002; Caba et al. Citation2003). Thus, it is possible a sex difference exists in the level of PR expression in the PVN. Additional studies will be needed to explore this issue. The present data also indicate that PR-ir cell number in the PVN is higher in the adult compared to prepubertal males. Therefore, the data shows that pubertal maturation not only influences stress-induced progesterone secretion, but also raises the possibility that the PVN may be more progesterone responsive in the adult compared to the prepubertal male. Progesterone has been shown to reduce stress responsiveness in adult male rats (Patchev et al. Citation1996). However, it is presently unknown whether progesterone modulates HPA axis reactivity in the prepubertal animal. Thus, future studies will need to investigate the possible interaction of progesterone and pubertal development on HPA axis reactivity in males.
Progesterone is a potent modulator of neurobiological function (McEwen et al. Citation1991) and certain behaviours in males (Witt et al. Citation1994). Furthermore, progesterone is a precursor to many neuroactive steroids produced in the nervous system that also have a myriad of neurophysiological and behavioural effects (Oettel and Mukhopadhyay Citation2004), including modulation of the HPA axis (Patchev et al. Citation1994; Patchev and Almeida Citation1996; Patchev et al. Citation1996). The physiological and behavioural significance of a greater and more prolonged stress-induced progesterone response in prepubertal compared to adult males is presently unknown. However, it is likely that the differential exposure of the prepubertal and adult brain to progesterone, and its metabolites, following stressors are likely to modulate the physiology and behaviour of the organism differently before and after pubertal maturation.
Acknowledgements
This work was supported by grants MH065749 to R.D.R. and MH41256, MH58911 and NS07080 to B.S.M.
References
- Andersen ML, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res 2004; 37: 791–797
- Andersen ML, Martins PJ, D'Almedia V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res 2005; 14: 83–90
- Blaustein JD, King JC, Toft DO, Turcotte J. Immunocytochemical localization of estrogen-induced progestin receptors in guinea pig brain. Brain Res 1988; 474: 1–15
- Boehm N, Plas-Roser S, Roos M, Aron C. How different procedures of blood removal affect blood progesterone concentrations in the cyclic female rat. J Steroid Biochem 1982; 16: 339–342
- Budec M, Koko V, Milovanovic TLB-P, Petkovic A. Acute ethanol treatment increases level of progesterone in ovariectomized rats. Alcohol 2002; 26: 173–178
- Caba M, Rovirosa MJ, Beyer C, Gonzalez-Mariscal G. Immunocytochemical detection of progesterone receptor in the female rabbit forebrain: Distribution and regulation by oestradiol and progesterone. J Neuroendocrinol 2003; 15: 855–864
- Francis K, Meddle SL, Bishop VR, Russell JA. Progesterone receptor expression in the pregnant and parturient rat hypothalamus and brainstem. Brain Res 2002; 927: 18–26
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary–adrenal system of the rat. Neuroendocrinology 1973; 12: 199–211
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamic–pituitary–adrenocortical responsiveness. Front Neuroendocrinol 2003; 24: 151–180
- Kalra PS, Kalra SP. Circadian properties of serum androgens, progesterone, gonadotropins and luteinizing hormone-releasing hormone in male rats: The effects of hypothalamic deafferentation, castration, and adrenalectomy. Endocrinology 1977; 101: 1821–1827
- McEwen BS, Coirini H, Westlind-Danielsson A, Frankfurt M, Gould E, Schumacher M, Woolley CS. Steroid hormones as mediators of neural plasticity. J Steroid Biochem Mol Biol 1991; 39: 223–232
- Numan M, Roach JK, del Cerro MC, Guillamon A, Segovia S, Sheehan TP, Numan MJ. Expression of intracellular progesterone receptors in rat brain during different reproductive states, and involvement in maternal behavior. Brain Res 1999; 830: 358–371
- Oettel M, Mukhopadhyay AK. Progesterone: The forgotten hormone in men?. Aging Male 2004; 7: 236–257
- Patchev VK, Almeida OF. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci 1996; 16: 7077–7084
- Patchev VK, Shoaib M, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994; 62: 265–271
- Patchev VK, Hassan AHS, Holsboer F, Almeida OF. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 1996; 15: 533–540
- Paxinos G, Watson C. The rat brain in stereotaxic atlas2nd ed. Academic Press, London 1986
- Persengiev S, Kanchev L, Vezenkova G. Circadian patterns of melatonin, corticosterone, and progesterone in male rats subjected to chronic stress: Effect of constant illumination. J Pineal Res 1991; 11: 57–62
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology 2004a; 79: 125–132
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology 2004b; 80: 387–393
- Schaeffer C, Aron C. Stress-related effects on the secretion of progesterone by the adrenals in castrated male rats presented to a stimulus male. Involvement of oestrogen. Acta Endocrinol 1987; 114: 440–445
- Traish AM, Wotiz HH. Monoclonal and Polyclonal antibodies to human progesterone receptor peptide-(533–547) recognize a specific site in unactivated (8S) and activated (4S) progesterone receptor and distinguish between intact and proteolyzed receptors. Endocrinology 1990; 127: 1167–1175
- Vazquez DM, Akil H. Pituitary–adrenal response to ether vapor in the weanling animal: Characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res 1993; 34: 646–653
- Wagner CK, Nakayama AY, De Vries GJ. Potential role of maternal progesterone in the sexual differentiation of the brain. Endocrinology 1998; 139: 3658–3661
- Witt DM, Young LJ, Crews D. Progesterone and sexual behavior in males. Psychoneuroendocrinology 1994; 19: 553–562
