Abstract
Stress initiates a series of neuronal responses that prepare an organism to adapt to new environmental challenges. However, chronic stress may lead to maladaptive responses that can result in psychiatric syndromes such as anxiety and depressive disorders. Corticotropin-releasing factor (CRF) has been identified as a key neuropeptide responsible for initiating many of the endocrine, autonomic and behavioral responses to stress. The amygdala expresses high concentrations of CRF receptors and is itself a major extrahypothalamic source of CRF containing neurons. Within the amygdala, the basolateral nucleus (BLA) has an important role in regulating anxiety and affective responses. During periods of stress, CRF is released into the amygdala and local CRF receptor activation has been postulated as a substrate for stress-induced alterations in affective behavior. Previous studies have suggested that synaptic plasticity in the BLA contributes to mechanisms underlying long-term changes in the regulation of affective behaviors. Several studies have shown that acute glutamate receptor-mediated activation, by either GABA-mediated disinhibition or CRF-mediated excitation, induces long-term synaptic plasticity and increases the excitability of BLA neurons. This review summarizes some of the data supporting the hypotheses that stress induced plasticity within the amygdala may be a critical step in the pathophysiology of the development of chronic anxiety states. It is further proposed that such a change in the limbic neural circuitry is involved in the transition from normal vigilance responses to pathological anxiety, leading to syndromes such as panic and post-traumatic stress disorders.
Stress initiates a cascade of hormonal and behavioral responses that enable an organism to adapt to new environmental pressures. However, chronic stress may lead to pathological states, which can result in several psychiatric syndromes such as anxiety and depressive disorders. Corticotropin-releasing factor (CRF), a 41 amino acid neuropeptide, has been identified as a key neuropeptide responsible for initiating many of the endocrine, autonomic and behavioral responses to a variety of stressors. In addition to its effects on regulating adrenocorticotropin (ACTH) release (Vale et al. Citation1981; Rivier and Plotsky Citation1986), CRF is a putative neurotransmitter that is implicated in the stress/anxiety responses [see (Dunn and Berridge Citation1990)]. CRF injections into the cerebral ventricles induce anxiety-like responses in several animal tests of anxiety, such as the conflict test (Britton et al. Citation1986), social interaction test (Dunn and File Citation1987), acoustic startle (Swerdlow et al. Citation1986) and elevated plus maze (File et al. Citation1988). Transgenic mice that overproduce CRF shows increased anxiety-like behaviors that are reversed by central administration of CRF antagonists (Stenzel-Poore et al. Citation1994). Currently, there are at least two different CRF receptors identified (the CRF1 and the CRF 2 receptors). The anxiogenic behaviors produced by CRF in rats may be due to stimulation of the CRF1 receptor. Recent evidence suggests that dysfunction of systems regulating CRF function in the CNS contributes to the etiology of several psychiatric disorders such as anxiety, panic disorder, posttraumatic stress disorder (PTSD) and depression (Arborelius et al. Citation1999; Boyer Citation2000).
Neural substrates of anxiety
The neural pathways involved in generating and regulating anxiety-like responses are still poorly understood. A number of CNS structures have been implicated. A large number of studies suggest a primary role for the amygdala in anxiety responses (Hilton Citation1963; Kaada Citation1972; Davis Citation1992; Kapp et al. Citation1992; LeDoux Citation1992; Sanders and Shekhar Citation1995a,Citationb; Adolphs et al. Citation2002; Davis and Myers Citation2002). In addition, the septo-hippocampal system (Gray Citation1996), and the brain stem centers of the global monoamine projections such as the locus coeruleus (LC) and the raphé nuclei appear to have important regulatory roles in these responses (Price and Amaral Citation1987; Charney et al. Citation1992; Price et al. Citation1995; Aston-Jones et al. Citation1996; Valentino et al. Citation2001; Baxter and Murray Citation2002; Linthorst et al. Citation2002; Lowry Citation2002; Keck et al. Citation2005). Thus, a network of CNS sites extending from the neocortex (particularly frontal and temporal regions) down to the brainstem monoamine pathways appear to form an intricately connected neural axis that may regulate anxiety and panic responses (Shekhar et al. Citation2002). A regulatory dysfunction at one or more of the key sites in this network could lead to an abnormal pattern of affective responses and the development of recurrent emotional disorders.
Role of the amygdala in anxiety states
The amygdala is particularly thought to be critical for providing affective salience to sensory information (Adolphs Citation1999). Recent fMRI studies report that increased amygdala activation is associated with several anxiety and mood states (Adolphs et al. Citation2001; Baxter and Murray Citation2002; Anand and Shekhar Citation2003). The activity of the amygdala is regulated by a balance between glutamate induced excitation and GABA-mediated inhibition (Rainnie et al. Citation1991a,Citationb), and this is particularly important for the regulation of anxiety responses (Sajdyk and Shekhar Citation1997a,Citationb). Experimental evidence suggests that the amygdala may be a critical site for the anti-stress effects of the benzodiazepine (BZs) group of drugs (Petersen et al. Citation1985; Sanders and Shekhar Citation1995a,Citationb). The role of the specific nuclei of the amygdala in generating negative affective responses has been further characterized by studying the development of conditioned fear responses (Davis Citation1992; LeDoux Citation1992). Within the amygdala, the basolateral (BLA) and the central (CE) nuclei have long been known to regulate affective responses (Hilton Citation1963; Kaada Citation1972; Davis Citation1992; Kapp et al. Citation1992; LeDoux Citation1992; Sanders and Shekhar Citation1995a,Citationb; Adolphs et al. Citation2002; Davis and Myers Citation2002). The CE appears to be the primary efferent site of the amygdala in eliciting conditioned fear responses (LeDoux et al. Citation1988; Kapp et al. Citation1992). Disrupting the function of the BLA inhibits both the acquisition of conditioned fear/negative affective responses as well as retrieval of information necessary for the expression of emotion (Campeau and Davis Citation1995). Hence, it has been proposed that the BLA serves as a major integrator and relay center for the sensory and memory information necessary for anxiety responses (Campeau and Davis Citation1995). While there are many excellent reviews of the function of the different regions of the amygdala in conditioned fear (Davis et al. Citation1997a,Citationb; LeDoux Citation2003), reward salience (Holland and Gallagher Citation2004), drug abuse (Koob Citation1999; See et al. Citation2003) and other behavioral disturbances (Stevens Citation1999; Post Citation2002), the present review will specifically focus on the BLA and its role in anxiety-like behaviors.
While the role of the amygdala in anxiety-like behaviors is generally supported, there are significant aversive behaviors that could be independent of the amygdala. For example, adaptation of hypothalamic–pituitary–adrenal responses to chronic restraint stress appears to be independent of the amygdala (Carter et al. Citation2004). Lesions of the amygdala are also not uniformly effective in disrupting all types of anxiety or emotional behaviors (Treit et al. Citation1998; Amaral Citation2002; Anderson and Phelps Citation2002; Blundell et al. Citation2003). Thus, this review will primarily focus on the specific anxiety-like behaviors such as non-cued, exploratory and conditioned anxiety models that have a strong implication of amygdala involvement.
CRF function in the amygdala and its role in emotional salience to stress cues
The amygdala is also a major extra-hypothalamic source of CRF-containing neurons and has high expression levels for the two cognate CRF receptors (Reul and Holsboer Citation2002). During periods of stress, CRF is released into the amygdala (Koob and Heinrichs Citation1999) and local CRF receptor activation has been postulated as a substrate for stress-induced alterations in affective behavior (Dunn and Berridge Citation1990; Lee et al. Citation1994, Citation1996; Lee and Davis Citation1997; Sajdyk et al. Citation1999; Sajdyk and Gehlert Citation2000). Our previous studies have suggested that the BLA is a critical site of synaptic plasticity that contributes to changes in affective behavior, and we have shown that acute glutamate receptor mediated activation, by either GABA disinhibition or CRF excitation, induces long-term synaptic plasticity and increases the excitability of BLA neurons (Sanders et al. Citation1995; Sajdyk et al. Citation1999; Sajdyk and Gehlert Citation2000; Shekhar et al. Citation2003).
Not all the studies implicating CRF in the amygdala in stress and anxiety are pharmacological or genetic manipulation studies. The increases in anxiety-like responses following restraint stress appear to be mediated by CRF receptor activation in the amygdala (Gehlert et al. Citation2005). Restraint or drug withdrawal stress releases CRF in the amygdala (Merlo Pich et al. Citation1995), as does neonatal stress (Cratty et al. Citation1995) and CRF mRNA is increased in the amygdala following stress (Herringa et al. Citation2004). Chronic, repeated stress results in specific synaptic plasticity within the amygdala (Vyas et al. Citation2002). These data suggest that a stress-induced increase of CRF release in the amygdala may contribute to altered emotional states often associated with chronic stress.
The basolateral complex, comprising the BLA and the lateral nucleus, receives highly processed multimodal information from several cortical regions including the visual, auditory, somatosensory and olfactory cortex. The basolateral complex is believed to process these inputs and allocate emotional salience to the sensory inputs (LeDoux; McDonald Citation1998; Pitkanen et al. Citation2000). The process of allocating emotional salience to sensory stimuli within the BLA is thought to play an integral role in emotional learning, and consequently any disruption of this nucleus disrupts the memory enhancing effects of emotionally salient cues (Ferry et al. Citation1999; Roozendaal et al. Citation2001; McGaugh and Roozendaal Citation2002; Goosens and Maren Citation2003; LaLumiere et al. Citation2003; Roesler et al. Citation2003). Furthermore, accumulating evidence suggests that CRF release within the amygdala and subsequent activation of its cognate receptors (especially in the BLA) may be critical for stress or negative cue-induced behavioral responses (Heilig et al. Citation1994; Lee et al. Citation1994; Gray and Bingaman Citation1996; Sajdyk et al. Citation1999; Roozendaal et al. Citation2002; Shekhar et al. Citation2003). Therefore, activation of CRF receptors in the BLA, of which the CRF1 receptor seems to be the primary subtype (Rainnie et al. Citation1992; Sanchez et al. Citation1999; Van Pett et al. Citation2000), appears critical for attributing emotional salience to a variety of cues eliciting “anxiety” or negative emotions (Sajdyk et al. Citation1999; Roozendaal et al. Citation2002; Rainnie et al. Citation2004).
The exact source of CRF input to the BLA is still somewhat unclear. There are CRF positive cell bodies in the Ce and the bed nucleus of the stria terminalis (BNST), both of which project to the BLA and other autonomic regulatory areas (See ; (Swanson et al. Citation1983; Van Bockstaele et al. Citation1999; Van Pett et al. Citation2000). Thus, it is likely that the bulk of the CRF input to the BLA comes from within the extended amygdala. There are sparse CRF cells in the cortex and dentate regions (Palkovits et al. Citation1983), which could also contribute CRF input to the BLA.
Figure 1 A schematic representation of the sites of action of the CRF system within the amygdala and within an extended “emotional” network that may be involved in normal stress responses and may be critical in the development of chronic stress induced anxiety states. Many other regions modulated by the basolateral amygdala such as the nucleus accumbens and orbitofrontal cortex are not shown for the sake of simplicity and the limited focus on regions critical for anxiety-like behaviors. The central nucleus and the bed nucleus of the stria terminalis are presented as a single group since they have similar afferents from the BLA and efferent projections out of the extended amygdala. Projection neurons are presented as circles within the different brain regions. Abbreviations: BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CE, central nucleus of the amygdala; CRF, corticotrophin releasing factor; CRF1 and 2, CRF receptor types1 and 2; GABA, gamma aminobutyric acid; Glu, glutamate; 5-HT, serotonin; mPFC, medial prefrontal cortex; NE, norepinephrine.
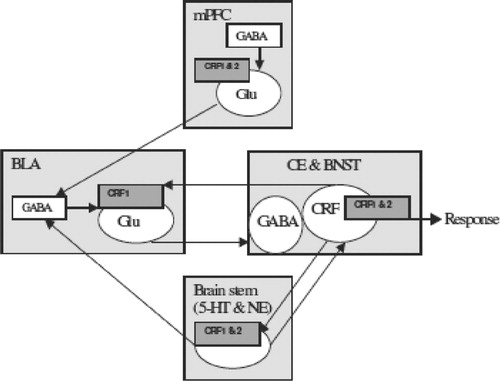
Once sensory stimuli have acquired emotional salience, via neural mechanisms involving the BLA, this information is then relayed to efferent target sites within the amygdala (see ), specifically the Ce and BNST (McDonald Citation1998; Pitkanen et al. Citation2000) for some types of anxiety behaviors, and elsewhere for other functions, such as nucleus accumbens for positive reward cues (Louilot and Besson Citation2000; Floresco et al. Citation2001), hippocampus for memory consolidation (Akirav and Richter-Levin Citation2002; McGaugh et al. Citation2002; Roozendaal et al. Citation2002, Citation2003) and frontal cortex for stimulus coding (Schoenbaum et al. Citation1998; Schoenbaum Citation2003). These other functions of the BLA are outside the focus of the current review and information can be obtained from several excellent reviews (Baxter and Murray Citation2002; McGaugh Citation2002; Holland and Gallagher Citation2004).
Several lines of converging evidence support the role of the extended amygdala target areas in the expression of some forms of aversive emotional responses, particularly anxiety-like behaviors (LeDoux et al. Citation1988; LeDoux Citation1992; Campeau and Davis Citation1995; Davis Citation1998; Hall et al. Citation2001; Royer and Pare Citation2002; Kalin et al. Citation2004). Significantly, recent evidence suggests that CRF receptor activation within the BNST and Ce also plays a role in modulating negative affect (Davis et al. Citation1997; Merali et al. Citation1998; Richter-Levin and Akirav Citation2000; Yilmazer-Hanke et al. Citation2002; Bale and Vale Citation2004; Campbell et al. Citation2004).
Internal circuitry of BLA
Based on a number of anatomical similarities, the BLA has been proposed to closely resemble the adjacent neocortex (McDonald and Pearson Citation1989; McDonald Citation1992). Hence, the BLA consists of two major classes of neurons, the large pyramidal (projection) cells and the smaller non-pyramidal cells (McDonald Citation1982, Citation1992a,Citationb). The non-pyramidal cells of the BLA are similar to their cortical counterparts and are immuno-positive for GABA, acetylcholine and a variety of peptides such as somatostatin (SOM), neuropeptide-Y (NPY), vasoactive intestinal peptide (VIP) and cholecystokinin (CCK) (McDonald and Pearson Citation1989; Roberts Citation1992). It appears that in the BLA, similar to the cortex, the non-pyramidal cells impinge on the pyramidal cells and modulate the activity of these projection neurons (McDonald and Mascagni Citation2001a,Citationb,Citationc). In contrast, projection neurons of the BLA are excitatory in nature and utilize the excitatory amino acid (EAA) glutamate as their primary neurotransmitter (McDonald Citation1996).
The BLA receives substantial extrinsic EAA inputs from cortical and subcortical structures, as well as intrinsic EAA inputs from the lateral nucleus of the amygdala (LA) (Price and Amaral Citation1987; McDonald Citation1996). Not surprisingly, therefore, both pyramidal neurons and non-pyramidal neurons of the BLA express the N-methyl-d-aspartate (NMDA) and the non-NMDA subtypes of EAA receptors (McDonald Citation1994). Moreover, stimulation of either extrinsic or intrinsic afferent pathways elicits a fast-excitatory postsynaptic potential (f-EPSP) i.e. mediated by non-NMDA receptor activation and a slow-EPSP that is mediated by NMDA receptor activation in pyramidal neurons of the BLA (Rainnie et al. Citation1991b). The BLA is also rich in GABAA and GABAB receptors. Similar to the glutamate mediated EPSPs, stimulation of the afferent pathways to the BLA also elicit GABAA receptor-mediated fast inhibitory postsynaptic potentials (f-IPSPs) and GABAB receptor-mediated slow inhibitory postsynaptic potentials (s-IPSPs) in the BLA projection neurons (Rainnie et al. Citation1991a). Thus, a coordinated balance of glutamate-mediated excitation and GABA-mediated inhibition of the BLA projection neurons appear to be critical for regulation of behavioral responses. Disruption of this balance appears to result.
Significantly, electrophysiological studies have demonstrated that the firing activity of the pyramidal neurons is tonically regulated by inhibitory GABAergic interneurons of the BLA (Gean and Chang Citation1991; Rainnie et al. Citation1991a,Citationb; Smith and Dudek Citation1996; Mahanty and Sah Citation1998; Rainnie Citation1999). These interneurons constitute only 15% of the total neuronal population in the BLA (McDonald Citation1982, Citation1992a,Citationb) and yet a network of at least four distinct subclasses of interneurons exist, based on their expression of calcium binding protein and neuropeptide content (McDonald and Betette Citation2001; McDonald Citation2001a,Citationb,Citationc, Citation2002; Mascagni and McDonald Citation2003; Muller et al. Citation2003). In addition to their unique combination of protein/neuropeptide content, these interneurons also appear to be functionally distinct. Hence, each class of interneuron can be further differentiated by its efferent connectivity (McDonald and Betette Citation2001; McDonald and Mascagni Citation2002; McDonald et al. Citation2002; Muller et al. Citation2003a,Citationb), and by its neurotransmitter receptor profile (Morales and Bloom Citation1997; McDonald and Mascagni Citation2001a,Citationb,Citationc; Blurton-Jones and Tuszynski Citation2002).
This complex arrangement of inhibitory neurons may be key to understanding the function of the BLA in emotional regulation. Deficits in this inhibitory modulation may be linked to the etiology and maintenance of several pathophysiological conditions (Rainnie et al. Citation1991a,Citationb; Sanders and Shekhar Citation1991; Rainnie et al. Citation1992a,Citationb; Davis et al. Citation1994; Pitkanen et al. Citation1997; Benes and Berretta Citation2001). In particular, blocking the GABAergic inhibition in the BLA with bicuculline methiodide (BMI) results in anxiety-like responses (Sanders and Shekhar Citation1991; Sajdyk and Shekhar Citation1997a,Citationb). Moreover, repeated disruption of the inhibition of the BLA leads to the development of long-term synaptic facilitation and a behavioral state of chronic anxiety and susceptibility to panic-like reactions (Sanders et al. Citation1995; Sajdyk and Shekhar Citation2000). Furthermore, increased stimulation of the BLA by repeatedly injecting the CRF receptor agonist urocortin I (UCN I) leads to a persistent state of anxiety- or panic-like symptoms in the rat (Sajdyk et al. Citation1999; Shekhar et al. Citation2003a,Citationb).
Although, the work cited above demonstrates that an obvious link exists between the steady state activity of the BLA circuitry and expression of anxiety, little is known of the mechanism by which the inhibitory tone is altered in the BLA during pathological anxiety. For example, we recently demonstrated that UCN I-induced stimulation of the BLA results in persistent expression of anxiety-like behaviors that is associated with a reduction in the steady state inhibition of BLA pyramidal cells by GABAergic interneurons, and that this effect was associated with a reduction in GABAA receptor-mediated IPSPs in projection neurons (Rainnie et al. Citation2004). However, the mechanism responsible for this reduction in inhibition has not yet been determined. Further characterizing the pattern of activation of specific neuronal populations within the amygdala following treatments that lead to anxiety-like and depression-like behaviors in the rat would be the next critical step in elucidating the role of the amygdala in these behaviors.
Long-term synaptic plasticity in the BLA
The basal activity of the projection neurons in the BLA is maintained by a balance between the excitatory (both non-NMDA and NMDA-mediated) input, and local inhibitory input from GABAergic non-pyramidal cells (GABAA and GABAB receptor-mediated inhibition). Upon stimulation of the afferent inputs to the BLA, NMDA receptor-mediated excitation of BLA projection neurons temporally overlaps with the GABAA receptor-mediated inhibition and, as a consequence, is generally unable to drive the cell towards NMDA receptor-mediated stimulation (Rainnie et al. Citation1991a,Citationb). This GABAA receptor-mediated inhibition of NMDA receptor activation is a key step in limiting the NMDA receptor-mediated calcium flux into the amygdala projection neurons and thus preventing the initiation of plasticity within these cells. However, If for any reason this inhibitory GABAergic tone onto BLA projection neurons is reduced, this could unmask the NMDA receptor-mediated excitation, leading not only to greater activation of the cell but also to long-term changes in synaptic plasticity that are dependent on NMDA receptor activation (Rainnie et al. Citation1991b).
The BLA exhibits a high degree of synaptic plasticity such as long-term potentiation (LTP), which is believed to be a cellular substrate for synaptic changes that may occur during learning, and which results from a cascade of events that are initially triggered by EAA receptor activation (Collingridge et al. Citation1983; Brown et al. Citation1988; Collingridge and Bliss Citation1995). Although, most studies of LTP have been conducted in the hippocampus (Brown et al. Citation1988), the amygdala, and in particular the BLA and LA, (Maren Citation1999) are also known to exhibit LTP, both in vitro (Chapman et al. Citation1990; Chapman and Bellavance Citation1992), and in vivo (Clugnet and LeDoux Citation1990; McKernan and Shinnick-Gallagher Citation1997). These forms of long-term synaptic changes are thought to be critical in learning and memory and are primarily brought about by an EAA receptor-mediated cascade of events (Collingridge et al. Citation1983; Brown et al. Citation1988; Collingridge and Bliss Citation1995).
There is much work being done to understand the intra-cellular mechanisms involved in the development of synaptic plasticity in the amygdala. Most studies support the role of the NMDA receptors in the initiation of the synaptic plasticity (Miserendino et al. Citation1990; Gewirtz and Davis Citation1997; Rogan et al. Citation1997; Adamec Citation1998; Maren Citation1999; Rainnie et al. Citation2004) although exceptions have been noted (Chapman and Bellavance Citation1992). There have been many intracellular second messenger cascades implicated further in the development of plasticity and LTP within the amygdala including activation of several types of kinases, including mitogen-activated protein (MAP) kinase (Huang et al. Citation2000; Schafe et al. Citation2000), calcium calmodulin kinases (Wei et al. Citation2002; Rainnie et al. Citation2004), protein kinase A (Huang and Kandel Citation1998; Huang et al. Citation2000), and extracellular signal-regulated kinase (Schafe et al. Citation2000). These kinases affect other signaling molecules such as cAMP response element-binding protein (CREB) and growth factors (Lamprecht et al. Citation1997; Hall et al. Citation2001; Josselyn et al. Citation2001; Rattiner et al. Citation2005), which eventually induce specific genomic changes (Lin et al. Citation2003a,Citationb). Such molecular mechanisms of amygdala synaptic plasticity are reviewed elegantly elsewhere (Blitzer et al. Citation2005; Levenson and Sweatt Citation2005; Xia and Storm Citation2005).
Proposed intrinsic BLA circuit regulating anxiety responses
Based on the neuroanatomical and functional organization of the BLA outlined above, a simple BLA circuit can be proposed that is capable of integrating incoming sensory information and assigning the appropriate salience and vigilance to that sensory information, as shown in .
Figure 2 A schematic drawing showing a highly simplified circuit within the BLA regulating emotional responses. The BLA is represented as a series of inhibitory cells ( − ) arranged around the projection neurons to integrate two sets of inputs: a stream of processed and direct sensory information (+; directly excitatory on the projection neurons) and the cortical input about predicted ability to cope with the given sensory inputs (inhibitory to the projection neurons; probably via the local inhibitory inputs). During chronic stress, in vulnerable individuals, the balance of these two inputs onto the projection neuron is disrupted, resulting in pathological anxiety and mood responses. Ascending serotonin and norepinephrine pathways provide modulatory tone that regulates this balance of stimulus and coping inputs, thus capable of up- or down-regulating the individuals ability to adapt to stress. The disruption of these modulatory mechanisms in extreme stress or in vulnerable populations, e.g. such as those with genetic polymorphisms in the monoamine modulatory systems, may also result in pathological stress reactivity. While this is clearly an oversimplification, similar principles can be applied to model the BLA function with multiple feedback loops containing many neurotransmitters and neuropeptides. Abbreviations: 5-HT, serotonin; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CB, calbindin; CCK, cholecystokinin; CE, central nucleus of the amygdala; Cg, cingulate cortex; CR, calretinin; DRN, dorsal raphe nucleus; GABA, gamma-aminobutyric acid; HC, hippocampus; LA, lateral amygdala; LC, locus coeruleus; NE, norepinephrine; PFC, prefrontalcortex; PRh, perirhinal cortex; PV, parvalbumin; SOM, somatostatin; VIP, vasoactive intestinal peptide.
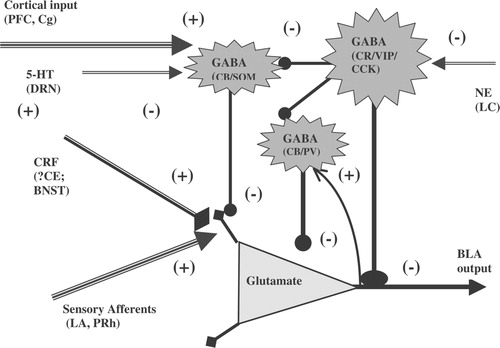
Under basal conditions, glutamatergic projection neurons of the BLA are tonically inhibited by local GABAergic interneurons and in a majority of BLA projection neurons baseline recordings exhibit rhythmic spontaneous inhibitory post-synaptic potentials (IPSPs) (Rainnie et al. Citation1991a). The occurrence of rhythmic spontaneous IPSPs in BLA projection neurons is thought to facilitate the synchronized output of ensembles of these neurons in response to sensory input (Rainnie Citation1999). Hence, highly processed sensory information is relayed to BLA projection neurons via excitatory afferent pathways from the lateral amygdala (LA), perirhinal cortex (PRh) and the hippocampal formation (Price and Amaral Citation1987; Amaral et al. Citation1992; McDonald Citation1998). These excitatory inputs activate both the glutamatergic projection neurons and also the local GABAergic interneurons, most likely via a collateral excitatory pathway (see ). Thus, stimulation of primary sensory afferents to the BLA results in a direct glutamate-mediated excitation and a secondary indirect GABA-mediated inhibition of projection neurons, such that there is a rapid activation followed by inhibition of the projection neurons (Rainnie et al. Citation1991a,Citationb). At the same time, the BLA receives concurrent information about the organism's ability to “cope” with the sensory stimuli from the executive centers of the prefrontal circuit. The input from the prefrontal cortex is thought to gate information flow within the BLA in such a way as to facilitate attentional bias to salient sensory input (Rosenkranz and Grace Citation2001, Citation2002) and initiate the activation of downstream structures involved in maintaining vigilance or “anxiety” states, or conversely inhibit this process when the input is not salient. At this stage, stress-induced activation of CRF in the amygdala could shift the network towards greater excitability and make it more resistant to prefrontal inhibition, thus reducing the ability to cope with the stimulus (Rogan et al. Citation1997; Maroun and Richter-Levin Citation2003). Such a mechanism is supported by the fact that studies involving stimulating the mPFC and recording from either interneurons or projection neurons of the amygdala found increased EPSPs in the interneurons and increased IPSPs in the projection neurons following mPFC stimulation. Furthermore, the IPSPs in the projection neurons were blocked by pretreatment with a GABAA receptor antagonist while the temporal relation of the stimulation to the increase in the occurrence of IPSPs was consistent with activation of an interneuron (Rosenkranz and Grace Citation2002). Some of the downstream structures that could be activated by salient sensory stimuli include the BNST for arousal/anxiety, the CE for fear and autonomic responses, the hippocampus for “contextual” memory, the medial nucleus of the amygdala (ME) for reproductive and endocrine responses, and nucleus accumbens (NA) for reward. Thus, the differential effects of the BLA on these different output pathways are likely to influence the physiological and behavioral responses to specific salient sensory stimuli, and modulate the animal's behavior.
The neural network of the BLA is extremely plastic with the potential for LTP following repeated exposure to excitatory stimuli. Thus, under chronic stress, when aversive stimuli repeatedly activate afferent pathways to the BLA, long-term plastic changes could occur within the local BLA circuitry such that the tonic inhibition is reduced. Then, seemingly non-salient stimuli could activate downstream structures and elicit anxiety, fear, or neuroendocrine responses, i.e. result in pathological anxiety responses. Such a mechanism is plausible and has been supported by several of our previous studies (Sanders and Shekhar Citation1991; Rainnie et al. Citation1992a, Citationb; Sanders and Shekhar Citation1995a, Citationb; Sajdyk et al. Citation1999; Sajdyk and Shekhar Citation2000) as well as by others (Clugnet and LeDoux Citation1990; McKernan and Shinnick-Gallagher Citation1997; Mahanty and Sah Citation1998; Maren Citation1999; Huang et al. Citation2000; Goosens and Maren Citation2002; Lin et al. Citation2003a; Citationb). In addition, this balance of excitatory and inhibitory tone in the BLA may be modulated by tonic serotonergic (5-HT) input (Cheng et al. Citation1998; Rainnie Citation1999; Chen et al. Citation2003) from the dorsal raphe nucleus (Abrams et al. Citation2005), and norepinephrine input (Ellis and Kesner Citation1983; Koob Citation1999; McGaugh Citation2002; Charney Citation2003; LaLumiere et al. Citation2003; Morilak et al. Citation2003) from the locus coeruleus (Fallon et al. Citation1978).
Plasticity in the BLA and its role in other behaviors
While the above discussion has focused on the role of plasticity within the amygdala, and the resulting enhancement of glutamate-mediated excitation and reduction of GABA-mediated inhibition, on the development of anxiety responses, similar mechanisms have been implicated in other models such as kindling and epilepsy (Post Citation2002; Wig et al. Citation2002) or memory consolidation. This is in fact supported by existing evidence, and the amygdala plasticity that results in enhanced anxiety-like behaviors, when pushed to extremes could easily result in seizures and provide models of epilepsy (Sanders et al. Citation1995; Adamec Citation2001). However, epileptogenesis generally results in spontaneous firing of the neurons, reduced number of GABAergic neurons (Callahan et al. Citation1991) and other structural abnormalities (Asprodini et al. Citation1992) not usually seen with synaptic plasticity that results in anxiety-like behaviors. Thus, while plasticity is a broad neural mechanism that underlies acquisition of a variety of new responses within the CNS, there are emerging differences in the cellular substrates of plasticity leading to the different types of behaviors.
Conclusion
Based on the above data, it is evident that the BLA has an important role in regulating anxiety and affective responses. Existing data summarized in this review support the hypotheses that stress induced plasticity within the amygdala may be a critical step in the pathophysiology of the development of chronic anxiety states, and this change in the limbic neural circuitry is involved in the transition from normal vigilance responses to pathological anxiety, which then often leads to syndromes such as panic and post-traumatic stress disorders.
Acknowledgements
The authors are grateful to the technical assistance of Ms Stephanie Fitz. This work was supported by grants RO1s MH065702 and MH52619.
References
- Abrams JK, Johnson PL, Hay-Schmidt A, Mikkelsen JD, Shekhar A, Lowry CA. Serotonergic systems associated with arousal and vigilance behaviors following administration of anxiogenic drugs. Neuroscience 2005; 133: 983–997
- Adamec RE. Evidence that NMDA-dependent limbic neural plasticity in the right hemisphere mediates pharmacological stressor (FG-7142)-induced lasting increases in anxiety-like behavior. Study 1—role of NMDA receptors in efferent transmission from the cat amygdala. J Psychopharmacol 1998; 12: 122–128
- Adamec RE. Partial kindling and behavioral pathologies. Int Rev Neurobiol 2001; 45: 409–434
- Adolphs R. The human amygdala and emotion. The Neuroscientist 1999; 5: 125–137
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci 2001; 13: 232–240
- Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci 2002; 14: 1264–1274
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. J Neurosci 2002; 22: 9912–9921
- Amaral DG. The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biol Psychiatry 2002; 51: 11–17
- Amaral DG PJ, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. Amygdala: Neurobiological aspects of emotion, memory and mental dysfunction, J Aggleton. Weily-Liss, New York 1992; 1–66
- Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: Special emphasis on the amygdala. Ann NY Acad Sci 2003; 985: 370–388
- Anderson AK, Phelps EA. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J Cogn Neurosci 2002; 14: 709–720
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropinreleasing factor in depression and anxiety disorders. J Endocrinol 1999; 160: 1–12
- Asprodini EK, Rainnie DG, Shinnick-Gallagher P. Epileptogenesis reduces the sensitivity of presynaptic gamma-aminobutyric acidB receptors on glutamatergic afferents in the amygdala. J Pharmacol Exp Ther 1992; 262: 1011–1021
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res 1996; 107: 379–402
- Bale TL, Vale WW. CRF and CRF receptors: Role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 2004; 44: 525–557
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci 2002; 3: 563–573
- Benes FM, Berretta S. GABAergic interneurons: Implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 2001; 25: 1–27
- Blitzer RD, Iyengar R, Landau EM. Postsynaptic signaling networks: Cellular cogwheels underlying long-term plasticity. Biol Psychiatry 2005; 57: 113–119
- Blundell P, Hall G, Killcross S. Preserved sensitivity to outcome value after lesions of the basolateral amygdala. The J Neurosci 2003; 23: 7702–7709
- Blurton-Jones M, Tuszynski MH. Estrogen receptor-beta colocalizes extensively with parvalbumin-labeled inhibitory neurons in the cortex, amygdala, basal forebrain, and hippocampal formation of intact and ovariectomized adult rats. J Comp Neurol 2002; 452: 276–287
- Boyer P. Do anxiety and depression have a common pathophysiological mechanism?. Acta Psychiatr Scand 2000; Supplementum: 24–29
- Britton KT, Lee G, Vale W, Rivier J, Koob GF. Corticotropin releasing factor (CRF) receptor antagonist blocks activating and ‘anxiogenic’ actions of CRF in the rat. Brain Res 1986; 369: 303–306
- Brown TH, Chapman PF, Kairiss EW, Keenan CL. Long-term synaptic potentiation. Science 1988; 242: 724–728
- Callahan PM, Paris JM, Cunningham KA, Shinnick-Gallagher P. Decrease of GABA-immunoreactive neurons in the amygdala after electrical kindling in the rat. Brain Res 1991; 555: 335–339
- Campbell BM, Morrison JL, Walker EL, Merchant KM. Differential regulation of behavioral, genomic, and neuroendocrine responses by CRF infusions in rats. Pharmacol Biochem Behav 2004; 77: 447–455
- Campeau S, Davis M. Involvement of the central nucleus and basolateral complex of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. J Neurosci 1995; 15: 2301–2311
- Carter RN, Pinnock SB, Herbert J. Does the amygdala modulate adaptation to repeated stress?. Neuroscience 2004; 126: 9–19
- Chapman PF, Bellavance LL. Induction of long-term potentiation in the basolateral amygdala does not depend on NMDA receptor activation. Synapse 1992; 11: 310–318
- Chapman PF, Kairiss EW, Keenan CL, Brown TH. Long-term synaptic potentiation in the amygdala. Synapse 1990; 6: 271–278
- Charney DS. Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand 2003; Suppl: 38–50
- Charney DS, Woods SW, Krystal JH, Nagy LM, Heninger GR. Noradrenergic neuronal dysregulation in panic disorder: The effects of intravenous yohimbine and clonidine in panic disorder patients. Acta Psychiatr Scand 1992; 86(273)282
- Chen A, Hough CJ, Li H. Serotonin type II receptor activation facilitates synaptic plasticity via N-methyl-d-aspartate-mediated mechanism in the rat basolateral amygdala. Neuroscience 2003; 119: 53–63
- Cheng LL, Wang SJ, Gean PW. Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci 1998; 10: 2163–2172
- Clugnet MC, LeDoux JE. Synaptic plasticity in fear conditioning circuits: Induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci 1990; 10: 2818–2824
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci 1995; 18: 54–56
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 1983; 334: 33–46
- Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res 1995; 675: 297–302
- Davis M. The role of the amygdala in fear-potentiated startle: Implications for animal models of anxiety. Trends Pharmacol Sci 1992; 13: 35–41
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety?. Biol Psychiatry 1998; 44: 1239–1247
- Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: Clinical implications for exposure therapy. Biol Psychiatry 2002; 52: 998–1007
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci 1994; 17: 208–214
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: Differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci 1997a; 352: 1675–1687
- Davis M, Walker DL, Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann NY Acad Sci 1997b; 821: 305–331
- Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotrophin releasing factor administration: Is CRF a mediator of anxiety or stress responses?. Brain Res Rev 1990; 15: 71–100
- Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Hormone Behav 1987; 21: 193–202
- Ellis ME, Kesner RP. The noradrenergic system of the amygdala and aversive information processing. Behav Neurosci 1983; 97: 399–415
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol 1978; 180: 509–532
- Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: A critical involvement of the amygdala. Biol Psychiatry 1999; 46: 1140–1152
- File SE, Johnston AL, Baldwin HA. Anxiolytic and anxiogenic drugs: Changes in behavioural and endocrine responses. Stress Med 1988; 4: 221–230
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci 2001; 21: 6370–6376
- Gean PW, Chang FC. Bursting discharges in disinhibited amygdala slices: The role of excitatory amino acid receptors. Neuropharmacology 1991; 30: 797–802
- Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. Stress and central urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol 2005; 509: 145–153
- Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature 1997; 388: 471–474
- Goosens KA, Maren S. Long-term potentiation as a substrate for memory: Evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus 2002; 12: 592–599
- Goosens KA, Maren S. Pretraining NMDA receptor blockade in the basolateral complex, but not the central nucleus, of the amygdala prevents savings of conditional fear. Behav Neurosci 2003; 117: 738–750
- Gray J. The neuropsychology of anxiety: An inquiry into the function of the septohippocampal system. Oxford University Press, Oxford 1982; 10: 155–168
- Gray TS, Bingaman EW. The amygdala: Corticotropin-releasing factor, steroids, and stress. Crit Rev Neurobiol 1996; 10: 155–168
- Hall J, Thomas KL, Everitt BJ. Fear memory retrieval induces CREB phosphorylation and Fos expression within the amygdala. Eur J Neurosci 2001; 13: 1453–1458
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: Role in emotional integration. Trends Neurosci 1994; 17: 80–85
- Herringa RJ, Nanda SA, Hsu DT, Roseboom PH, Kalin NH. The effects of acute stress on the regulation of central and basolateral amygdala CRF-binding protein gene expression. Brain Res Mol Brain Res 2004; 131: 17–25
- Hilton SMZA. Amygdaloid region for defence reactions and its efferent pathway to the brainstem. J Physiol 1963; 160–173
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol 2004; 14: 148–155
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron 1998; 21: 169–178
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesisdependent late phase of long-term potentiation. J Neurosci 2000; 20(6317)6325
- Josselyn SA, Shi C, Carlezon WA, Jr, Neve RL, Nestler EJ, Davis M. Long-term memory is facilitated by cAMP response element-binding protein overexpression in the amygdala. J Neurosci 2001; 21: 2404–2412
- Kaada B. Stimulation and regional ablation of the amygdaloid complex with reference to functional representation. The neurobiology of the amygdala, B Eleftheriou. Plenum Press, New York 1972; 205–281
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J Neurosci 2004; 24: 5506–5515
- Kapp BS WP, Supple WF, Pascoe JP. Amygdaloid contribution to conditioned arousal and sensory information processing. The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction, J Aggleton. Weily-Liss, New York 1992; 229–254
- Keck ME, Sartori SB, Welt T, Muller MB, Ohl F, Holsboer F, Landgraf R, Singewald N. Differences in serotonergic neurotransmission between rats displaying high or low anxiety/ depression-like behaviour: Effects of chronic paroxetine treatment. J Neurochem 2005; 92: 1170–1179
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry 1999; 46: 1167–1180
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res 1999; 848: 141–152
- LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci 2003; 23: 6754–6758
- Lamprecht R, Hazvi S, Dudai Y. cAMP response element-binding protein in the amygdala is required for long- but not short-term conditioned taste aversion memory. J Neurosci 1997; 17: 8443–8450
- LeDoux JE. Emotion and the amygdala. The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction, J Aggleton. Weily-Liss, New York 1992; 339–351
- LeDoux JE. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 2003; 23: 727–738
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988; 8: 2517–2529
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci 1997; 17: 6434–6446
- Lee Y, Schulkin J, Davis M. Effect of corticosterone on the enhancement of the acoustic startle reflex by corticotropin releasing factor (CRF). Brain Res 1994; 666: 93–98
- Lee Y, Lopez DE, Meloni EG, Davis M. A primary acoustic startle pathway: Obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci 1996; 16: 3775–3789
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci 2005; 6: 108–118
- Lin C-H, Yeh S-H, Lu H-Y, Gean P-W. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. The J Neurosci 2003a; 23: 8310–8317
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol 2003b; 63: 44–52
- Linthorst AC, Penalva RG, Flachskamm C, Holsboer F, Reul JM. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur J Neurosci 2002; 16: 2441–2452
- Louilot A, Besson C. Specificity of amygdalostriatal interactions in the involvement of mesencephalic dopaminergic neurons in affective perception. Neuroscience 2000; 96: 73–82
- Lowry CA. Functional subsets of serotonergic neurones: Implications for control of the hypothalamic–pituitary–adrenal axis. J Neuroendocrinol 2002; 14: 911–923
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 1998; 394: 683–687
- Maren S. Long-term potentiation in the amygdala: A mechanism for emotional learning and memory. Trends Neurosci 1999; 22: 561–567
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of longterm potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci 2003; 23: 4406–4409
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res 2003; 976: 171–184
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: A golgi study in the rat. J Comp Neurol 1982; 212: 293–312
- McDonald AJ. Cell types and intrinsic connections of the amygdala. Amygdala: Neurobiological aspects of emotion, memory and mental dysfunction, J Aggleton. Weily-Liss, New York 1992a; 67–96
- McDonald AJ. Projection neurons of the basolateral amygdala: A correlative golgi and retrograde tract tracing study. Brain Res Bull 1992b; 28: 179–185
- McDonald AJ. Neuronal localization of glutamate receptor subunits in the basolateral amygdala. NeuroReport 1994; 6: 13–16
- McDonald AJ. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: Colocalization of excitatory amino acids and projections to the limbic circuit. J Comp Neurol 1996; 365: 367–379
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol 1998; 55: 257–332
- McDonald AJ, Betette RL. Parvalbumin-containing neurons in the rat basolateral amygdala: Morphology and co-localization of Calbindin-D (28 k). Neuroscience 2001; 102: 413–425
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience 2001a; 105: 681–693
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: High concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience 2001b; 107: 641–652
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience 2001c; 105: 681–693
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res 2002; 943: 237–244
- McDonald AJ, Pearson JC. Coexistence of GABA and peptide immunoreactivity in non-pyramidal neurons of the basolateral amygdala. Neurosci Lett 1989; 100: 53–58
- McDonald AJ, Muller JF, Mascagni F. GABAergic innervation of alpha type II calcium/calmodulin-dependent protein kinase immunoreactive pyramidal neurons in the rat basolateral amygdala. J Comp Neurol 2002; 446: 199–218
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci 2002; 25: 456
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 2002; 12: 205–210
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: Interaction with other brain systems. Neurobiol Learn Mem 2002; 78: 539–552
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 1997; 390: 607–611
- Merali Z, McIntosch J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala. J Neurosci 1998; 18: 4758–4788
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci 1995; 15: 5439–5447
- Miserendino MJ, Sananes CB, Melia KR, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature 1990; 345: 716–718
- Morales M, Bloom FE. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J Neurosci 1997; 17: 3157–3167
- Morilak DA, Cecchi M, Khoshbouei H. Interactions of norepinephrine and galanin in the central amygdala and lateral bed nucleus of the stria terminalis modulate the behavioral response to acute stress. Life Sci 2003; 73: 715–726
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol 2003a; 456: 217–236
- Muller JF, Mascagni F, McDonald AJ. Synaptic output of somatostatin—immunoreactive interneurons in the basolateral amygdala. AbstractViewer/Itinerary Planner. ScholarOne, New Orleans, LA 2003b, Program No. 679.676.: 2003. Society for Neuroscience. Online.
- Palkovits M, Brownstein MJ, Vale W. Corticotropin releasing factor (CRF) immunoreactivity in hypothalamic and extrahypothalamic nuclei of sheep brain. Neuroendocrinology 1983; 37: 302–305
- Petersen EN, Braestrup C, Scheel-Kruger J. Evidence that the anticonflict effect of midazolam in amygdala is mediated by the specific benzodiazepine receptors. Neurosci Lett 1985; 53: 285–288
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: An emerging framework for understanding functions of the amygdala. Trends Neurosci 1997; 20: 517–523
- Pitkanen A, Jolkkonen E, Kemppainen S. Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphol (Warsz) 2000; 59: 1–23
- Post RM. Do the epilepsies, pain syndromes, and affective disorders share common kindling-like mechanisms?. Epilepsy Res 2002; 50: 203–219
- Price JL, Amaral DG. The limbic region II: The amygdaloid complex. Handbook of chemical neruoanatomy, A Bjorklund, TH Hokfelt, LW Swanson. Elsevier, Amsterdam 1987; 279–388
- Price LH, Barr LC, Goodman WK. Pharmacological challenges in anxiety disorder. Psychopharmacology: The fourthe generation of progression, FE Bloom, DJ Kupfer. Raven Press, New York 1995; 1311–1323
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol 1999; 82: 69–85
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol 1991a; 66: 999–1009
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. J Neurophysiol 1991b; 66: 986–998
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Kindling-induced long-lasting changes in synaptic transmission in the basolateral amygdala. J Neurophysiol 1992a; 67: 443–454
- Rainnie DG, Fernhout BJ, Shinnick-Gallagher P. Differential actions of corticotropin releasing factor on basolateral and central amygdaloid neurones, in vitro. J Pharmacol Exp Therap 1992b; 263: 846–858
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci 2004; 24: 3471–3479
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdaladependent learning. Neuroscientist 2005; 11: 323–333
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2002; 2: 23–33
- Richter-Levin G, Akirav I. Amygdala-hippocampus dynamic interaction in relation to memory. Mol Neurobiol 2000; 22: 11–20
- Rivier CL, Plotsky PM. Mediation by corticotropin releasing factor (CRF) of adenohypophysial hormone secretion. Ann Rev Physiol 1986; 48: 475–494
- Roberts. Neuropeptides: Cellular morphology, major pathways, and functional considerations. The amygdala: Neurobiological aspects of emotion, memory and mental dysfunction, J Aggleton. Weily-Liss, New York 1992; 115–142
- Roesler R, Schroder N, Vianna MR, Quevedo J, Bromberg E, Kapczinski F, Ferreira MB. Differential involvement of hippocampal and amygdalar NMDA receptors in contextual and aversive aspects of inhibitory avoidance memory in rats. Brain Res 2003; 975: 207–213
- Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative longterm potentiation in the amygdala. Nature 1997; 390: 604–607
- Roozendaal B, de Quervain DJ, Ferry B, Setlow B, McGaugh JL. Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. J Neurosci 2001; 21: 2518–2525
- Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA 2002; 99: 13908–13913
- Roozendaal B, Griffith QK, Buranday J, De Quervain DJ, McGaugh JL. The hippocampus mediates glucocorticoid-induced impairment of spatial memory retrieval: Dependence on the basolateral amygdala. Proc Natl Acad Sci USA 2003; 100: 1328–1333
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 2001; 21: 4090–4103
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 2002; 22: 324–337
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 2002; 115: 455–462
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptors in the basolateral amygdala regulate anxiety responses in the social interaction test. Brain Res 1997a; 764: 262–264
- Sajdyk TJ, Shekhar A. Excitatory amino acid receptor antagonists block the cardiovascular and anxiety responses elicited by gamma-aminobutyric acidA receptor blockade in the basolateral amygdala of rats. J Pharmacol Exp Therap 1997b; 283: 969–977
- Sajdyk TJ, Gehlert DR. Astressin, a corticotropin releasing factor antagonist, reverses the anxiogenic effects of urocortin when administered into the basolateral amygdala. Brain Res 2000; 877: 226–234
- Sajdyk TJ, Shekhar A. Sodium lactate elicits anxiety in rats after repeated GABA receptor blockade in the basolateral amygdala. Eur J Pharmacol 2000; 394: 265–273
- Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res 1999; 100: 207–215
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol 1999; 408: 365–377
- Sanders SK, Shekhar A. Blockade of GABAA receptors in the region of the anterior basolateral amygdala of rats elicits increases in heart rate and blood pressure. Brain Res 1991; 567: 101–110
- Sanders SK, Shekhar A. GABAA receptors in the basolateral amygdala of rats regulate “anxiety”. Pharmacol Biochem Behav 1995a; 701–706
- Sanders SK, Shekhar A. Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry 1995b; 37: 473–476
- Sanders SK, Morzorati SL, Shekhar A. Priming of experimental anxiety by repeated subthreshold GABA blockade in the rat amygdala. Brain Res 1995; 699(250)259
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci 2000; 20: 8177–8187
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci 1998; 1: 155–159
- Schoenbaum GSB, Saddoris PM, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron 2003; 39: 855–867
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Ann NY Acad Sci 2003; 985: 294–307
- Shekhar A, Ball S, Sajdyk TJ, Goddard AW. Neurobiology of panic disorder. Trends Econ Neurobiol 2002; 36–41
- Shekhar A, Sajdyk TJ, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann NY Acad Sci 2003a; 985: 308–325
- Shekhar A, Sajdyk T, Gehlert DR, Rainnie DG. The amygdala, panic disorder, and cardiovascular responses. Ann NY Acad Sci 2003b; 985: 308–325
- Smith BN, Dudek FE. Amino acid-mediated regulation of spontaneous synaptic activity patterns in the rat basolateral amygdala. J Neurophysiol 1996; 76: 1958–1967
- Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: A genetic model of anxiogenic behavior. J Neurosci 1994; 2579–2584
- Stevens JR. Epilepsy, schizophrenia, and the extended amygdala. Ann NY Acad Sci 1999; 877: 548–561
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: An immunohistochemical study. Neuroendocrinology 1983; 36: 165–186
- Swerdlow NR, Geyer MA, Vale WW, Koob GF. Corticotropin-releasing factor potentiates acoustic startle in rats: Blockade by chlordiazepoxide. Psychopharmacology 1986; 88: 147–152
- Treit D, Aujla H, Menard J. Does the bed nucleus of the stria terminalis mediate fear behaviors?. Behav Neurosci 1998; 112: 379–386
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and betaendorphin. Science 1981; 213: 1394–1397
- Valentino RJ, Rudoy C, Saunders A, Liu XB, Van Bockstaele EJ. Corticotropinreleasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience 2001; 106: 375–384
- Van Bockstaele EJ, Peoples J, Valentino RJ. A.E. Bennett Research Award. Anatomic basis for differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol Psychiatry 1999; 46: 1352–1363
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 2000; 428(191)212
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818
- Wei F, Qiu CS, Liauw J, Robinson DA, Ho N, Chatila T, Zhuo M. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci 2002; 5: 573–579
- Wig GS, Barnes SJ, Pinel JP. Conditioning of a flavor aversion in rats by amygdala kindling. Behav Neurosci 2002; 116: 347–350
- Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci 2005; 6: 267–276
- Yilmazer-Hanke DM, Faber-Zuschratter H, Linke R, Schwegler H. Contribution of amygdala neurons containing peptides and calcium-binding proteins to fear-potentiated startle and exploration-related anxiety in inbred Roman high- and low-avoidance rats. Eur J Neurosci 2002; 15: 1206–1218
