Abstract
The hypothalamo–pituitary–adrenal (HPA) axis plays a major role in the regulation of responses to stress. Human stress-related disorders such as chronic fatigue syndrome (CFS), fibromyalgia syndrome (FMS), chronic pelvic pain and post-traumatic stress disorder are characterized by alterations in HPA axis activity. However, the role of the HPA axis alterations in these stress-related disorders is not clear. Most studies have shown that the HPA axis is underactive in the stress-related disorders, but contradictory results have also been reported, which may be due to the patients selected for the study, the methods used for the investigation of the HPA axis, the stage of the syndrome when the tests have been done and the interpretation of the results. There is no structural abnormality in the endocrine organs which comprise the HPA axis, thus it seems that hypocortisolemia found in the patients with stress-related disorder is functional. It may be also an adaptive response of the body to chronic stress. In this review, tests used in the assessment of HPA axis function and the HPA axis alterations found in CFS and FMS are discussed in detail.
Introduction
The hypothalamo–pituitary–adrenal (HPA) axis plays a major role in the regulation of responses to stress. Circadian inputs, sleep, food intake and physiological stresses including hypoglycaemia, infection and hypotension are involved in the regulation of the HPA axis. Stimulation by these factors of the HPA axis involves CRH and arginine vasopressin (AVP) secretion from the hypothalamus and together they stimulate the anterior pituitary gland to secrete ACTH. ACTH is responsible for the secretion of cortisol from the adrenal glands (Melmed and Kleinberg Citation2003). Many immune signals such as IL-1, IL-6 and TNF-α may also stimulate the HPA axis. Cortisol, the end product of the glucocorticoid pathway, has negative feedback effects on CRH and ACTH secretion and on cytokine secretion (Sapolsky et al. Citation1987; Naitoh et al. Citation1988).
There may be alterations in HPA axis activity in stress-related disorders such as chronic fatigue syndrome (CFS) and fibromyalgia syndrome (FMS) (Naitoh et al. Citation1988; Griep et al. Citation1993; Heim et al. Citation2000; Cleare Citation2003). The extent to which these alterations in HPA axis activity are responsible for the development of stress-related disorders has not been clarified. The major point of controversy in this field is whether changes in the HPA axis are causal or consequential. In this review, tests for the assessment of HPA axis function and the alterations in HPA axis activity found in CFS and FMS are discussed. Heterogeneity of the tests and their different interpretations may be responsible for the variable results described in the literature. For this reason, investigation of the HPA axis is particularly discussed in detail.
The tests used for the investigation of hypothalamo–pituitary–adrenal axis insufficiency
The first step in the investigation of HPA axis insufficiency is to measure the basal levels of hormones including ACTH, cortisol and DHEA/DHEAS. Since the current data are not sufficient, the importance of DHEA/DHEAS in the diagnosis of adrenal failure is not well known and its measurement is not routine. On the other hand, except in the severe cases of adrenal insufficiency, not uncommonly basal ACTH or cortisol blood levels overlap values in healthy subjects and patients with mild or subclinical HPA axis insufficiency (Melmed and Kleinberg Citation2003; Stewart Citation2003). For this reason, measurement of basal ACTH and cortisol levels are only rarely useful to make a differential diagnosis between the normal HPA axis and mild HPA axis insufficiency. An alternative test to basal blood cortisol assay is measurement of the urinary free cortisol (UFC) or saliva cortisol level (Evans and Hunder Citation2000; Cleare et al. Citation2001a; Gaab et al. Citation2002). Measurement of UFC is not without problems. Free cortisol in the circulation constitutes only 2–3% of the total circulating cortisol. So measurement of UFC level may not reflect the cortisol status accurately. Collection of urine may not be complete and urine creatinine level should be also measured to correct for this. On the other hand, commercially available kits in the market for the measurement of UFC level may overestimate UFC in the urine (Murphy Citation2002). Although salivary cortisol is a promising screening test especially in the outpatient setting, further large studies of its diagnostic accuracy are needed (Putignano et al. Citation2001; Morris et al. Citation2006). Consequently, dynamic tests are frequently used to investigate the HPA axis.
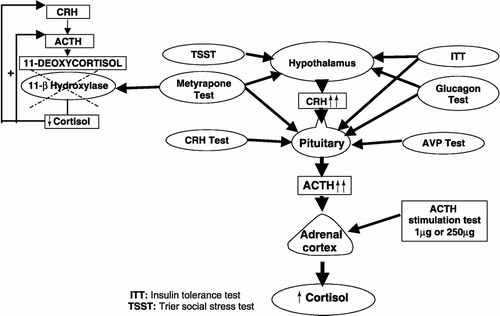
The most widely used tests to investigate the HPA axis, particulary in patients with CFS and FMS, are the insulin tolerance test (ITT), the short (standard or rapid) ACTH (250 μg) stimulation test, the low-dose ACTH (1 μg) stimulation test, the CRH stimulation test, the metyrapone test, glucagon test and AVP stimulation test. The sites of action of these tests are shown in and interpretation and limitations of the tests are shown in .
Table I. Summary of the stimulation tests used in the investigation of HPA axis underactivity.
Insulin tolerance test
The ITT has been accepted as the gold standard for the investigation of HPA axis insufficiency. ITT may cause serious complications and is contraindicated in the elderly, in patients with cerebrovascular disease and epilepsy or coronary artery disease. Moreover, it is time-consuming and close medical supervision is also required during the test. Hypoglycaemia (plasma glucose less than 2.2 nmol/l) is a strong stimulus for the secretion of ACTH and thereby of cortisol from the adrenal gland. Sometimes hypoglycaemia may be difficult to achieve during ITT. In patients with insulin resistance, such as in obesity and acromegaly, repeated and larger doses of insulin may be required. A peak cortisol value after ITT greater than 500 or 550 nmol/l is commonly used as an adequate response. In principle, measurement of the ACTH response instead of cortisol would be more physiological, but there are only few data regarding ACTH levels after ITT. In a recent study, peak ACTH levels after ITT were evaluated in 125 patients at risk for secondary adrenal insufficieny. It was indicated that there is much variability in the values and suggested that the peak ACTH concentration is a poor parameter for the evaluation of the HPA axis (Borm et al. Citation2003). Therefore, interpretation of HPA axis underactivation according to the ACTH response after ITT is not reliable and may result in misdiagnosis in secondary adrenal insufficiency.
ACTH stimulation test
The ACTH stimulation test has been commonly used for a long time to investigate the HPA axis. It has been proposed as an alternative test to the ITT. Adrenal failure may be primary or secondary. In primary adrenal failure, adrenocortical tissue is not able to synthesize and secrete an adequate amount of cortisol. Because of loss of the negative feedback effect of cortisol on the hypothalamus and pituitary, ACTH is maximally increased in the circulation and exogenous ACTH does not further stimulate the adrenocortical tissue. Long-term secondary adrenal insufficiency due to decreased CRH or ACTH secretion results in adrenal atrophy and in reduced ACTH receptor expression in the adrenal gland, since ACTH up-regulates its own receptor (Lebrethon et al. Citation1994). The ACTH stimulation test is not able to differentiate primary from secondary adrenal failure. But it is very sensitive in the diagnosis of HPA insufficiency. Although there is no agreement regarding the peak cortisol level after the short ACTH test at which it would be possible to differentiate normal and subnormal cortisol responses, a peak cortisol response of 550 nmol/l at any time during the test is generally accepted as a normal response (Borst et al. Citation1982; Stewart et al. Citation1988; Kelestimur et al. Citation1995; Clark et al. Citation1998).
An ACTH dose of 1 μg has been suggested to be the lowest dose that can cause a maximal cortisol response and it may be more useful than the 250 μg ACTH test in the assessment of HPA axis insufficiency, particularly in secondary adrenal failure and in detecting subtle HPA axis abnormalities (Clayton Citation1996; Rasmuson et al. Citation1996; Hudson and Cleare Citation1999; Dickstein Citation2003). Although there is not a certain cut-off limit for clinical use, a cortisol response higher than 500 nmol/l provides almost 100% sensitivity and a specifity of 80–100% (Shankar et al. Citation1997). We have recently reported that the 1 μg ACTH test is more sensitive than the 250 μg ACTH test or the ITT in the investigation of the HPA axis to demonstrate subnormal adrenocortical function (Kelestimur et al. Citation1994; Gulmez et al. Citation1996; Dokmetas et al. Citation2000; Kirnap et al. Citation2001). The dose of ACTH used in the short ACTH (250 μg) test is at least 1000 times higher than needed for maximal adrenal stimulation and this dose provides a very high blood ACTH concentration which may induce false positive cortisol responses and may result in underdiagnosis of HPA axis insufficiency (Landon et al. Citation1967; Dokmetas et al. Citation2000). In contrast, a maximal adrenal response can be obtained with much smaller doses of ACTH and it was reported that this may reveal more subtle disturbances in the HPA axis (Rasmuson et al. Citation1996). In secondary adrenal failure due to a pituitary or a hypothalamic disorder, the 1 μg ACTH test has been suggested to be more sensitive than the 250 μg ACTH test in the assessment of HPA axis underactivation and the low-dose test has been found to be more strongly correlated with results from the ITT than is the standard dose ACTH test (Tordjman et al. Citation1995; Dokmetas et al. Citation2000).
Metyrapone test
Another test used in the assessment of HPA axis underactivation is the metyrapone test. Metyrapone inhibits the 11β-hydroxylase enzyme, which converts 11-deoxycortisol to cortisol in the adrenal gland and consequently metyrapone results in increased ACTH secretion from the pituitary. In healthy subjects, adequate adrenal function is confirmed by an 11-deoxycortisol value greater than 200 nmol/l regardless of the cortisol level after metyrapone administration (Orth and Kovacs Citation1998). The fall in cortisol level after metyrapone stimulates ACTH release from the pituitary, which stimulates steroid precursors proximal to the blocked enzyme and results in increased 11-deoxycortisol level. In secondary adrenal failure, 11-deoxycortisol will not be increased because of insufficient ACTH. Adrenal failure may be diagnosed if the 11-deoxycortisol response is less than 200 nmol/l and simultaneously measured serum cortisol is low. Although the metyrapone test is really useful and has been used more commonly in the past, the limited availability of metyrapone itself and the 11-deoxycortisol assay prevent the common use of the test. Also, the data regarding 11-deoxycortisol levels after metyrapone administration in healthy subjects are not sufficient. It has been reported that an overnight single-dose administration of metyrapone (30 mg/kg) is able to stimulate ACTH release similar to the ITT and the metyrapone test identifies more patients with possible ACTH deficiency than the ITT (Staub et al. 1979; Courtney et al. Citation2000). Fiad et al. (Citation1994) reported that the metyrapone test gave similar information to the ITT about the HPA axis in patients with secondary adrenal failure due to pituitary adenoma, post-glucocorticoid treatment, idiopathic hypopituitarism, Sheehan's syndrome and pituitary apoplexia. The mechanisms by which metyrapone stimulates CRH and/or ACTH secretion is remarkably different than the effects of the ITT and the other tests. Metyrapone acts by inhibiting the negative feedback effect of cortisol and allows the hypothalamus and pituitary to release more CRH and ACTH. In contrast, the ITT stimulates the hypothalamus or pituitary by hypoglycaemia which is a strong stimulus for HPA axis activation. It has been speculated that the negative feedback stimulus may be weaker than the insulin-induced hypoglycaemia, thus some patients may respond normally to the stronger stimulus of hypoglycemia but fail to respond to the weaker metyrapone-induced stimulus (Courtney et al. Citation2000).
CRH stimulation test
The CRH stimulation test has also been used in the assessment of HPA axis underactivation. It may be useful in the differential diagnosis of secondary adrenal failure, particularly to differentiate hypothalamic from pituitary disease (Arlt and Allolio Citation2003). CRH is expensive and not commonly available. On the other hand, CRH-stimulated ACTH and/or cortisol levels in normal individuals and in patients with adrenal failure are still not well standardized (Grinspoon and Biller Citation1994). The type of CRH, which may be human or ovine, test protocols, differences in sampling and particularly the lack of generally accepted cut-off levels make the CRH test less reliable.
Trier social stress test
The Trier social stress test (TSST, ) is a method which induces acute psychosocial stress in human volunteers. The test is composed of a 5-min public speech followed by 10 min of mental arithmetic performed before an evaluating panel of two people and leads to increase in cortisol level, heart rate and blood pressure in healthy participants (Kirschbaum et al. Citation1993). Although it is not commonly used by many centers, it seems to be a physiological and promising test. But more studies are needed to establish the value of the test in the assessment of abnormal HPA axis function.
Arginine vasopressin
Despite limited experience, administration of AVP may be useful in the clinical investigation of HPA axis function. Although a weaker secretagogue than CRH, it acts at the anterior pituitary to induce the release of ACTH (Salata et al. Citation1988). Compared with CRH, relatively few studies have examined its role in HPA axis dysfunction and probably AVP is a less potent stimulus than CRH. AVP challenge was used in a study including patients with CFS, and a decreased ACTH response has been interpreted as an indirect marker of low ambient CRH secretion (Altemus et al. Citation2001). When AVP is secreted in the presence of CRH, a synergism between the two peptides leads to greater ACTH release than either one alone can achieve. The AVP infusion test is performed as intravenous administration of AVP (1 mIU/kg) for 60 min (Michelson et al. Citation1994).
Glucagon stimulation test
The glucagon stimulation test (GST) is also used to assess the HPA axis. The mechanism of glucagon-induced cortisol release is not well known. Glucagon stimulates corticotroph and somatotroph cells to secrete ACTH and growth hormone after intramuscular (i.m.) or subcutaneous (s.c.), but not intravenous (i.v.) administration (Ghigo et al. Citation1994; Arvat et al. Citation1999). Cortisol secretion after glucagon administration is probably ACTH-dependent and ACTH secretion itself may be due to glucagon-induced catecholamine secretion (Goodwin et al. Citation1976). The ITT or standard ACTH stimulation tests are shorter and more practical to perform when compared to the i.m. glucagon stimulation (IMGST); glucagon may be used either subcutaneously or intramuscularly for the assessment of HPA function. In a study by Leong et al. (Citation2001), an audit of 500 consecutive GSTs performed in patients with hypothalamic–pituitary disease was reported and they suggested that the medical supervision required was minimal, side effects encountered during the test were mild and GST is a simple and safe method in the assesment of GH and ACTH secretion and it is a suitable alternative when the ITT is contra-indicated (Leong et al. Citation2001).
Overview
None of the tests above mentioned is powerful enough to diagnose HPA axis underactivity correctly in all the patients and particularly to locate the level of the defect underlying the disease. The pathophysiology of HPA axis underactivation may be due to an anatomical defect such as damage to the hypothalamus, pituitary surgery, post-partum pituitary necrosis, radiotherapy on the hypothalamo–pituitary region, adrenal gland destruction due to tuberculosis or autoimmune adrenalitis, or it may be functional in which there is no anatomical defect anywhere in the HPA axis such as in CFS and FMS. Whether there are some patients who have a normal cortisol response to one test but a subnormal cortisol response to another test or vice versa is not established. We can speculate that there may be some patients characterized by a normal cortisol response to ITT but a subnormal cortisol response to metyrapone. In other words, the patient may have a normal cortisol response to insulin-induced hypoglycaemia but a subnormal cortisol response to the lack of negative feedback effect of cortisol on the hypothalamus and/or pituitary due to administration of metyrapone. We do not know to what extent it is logical to use the same tests to assess the HPA axis in different pathophysiological conditions. Obviously, we need new methods to assess HPA axis underactivation due to different causes including stress-related disorders which are characterized by “functional” but not structural changes.
Stress-related disorders
Stress-related disorders include CFS and FMS and share several symptoms such as pain, fatigue and disturbed sleep (Griep et al. Citation1998; Heim et al. Citation2000; Cleare Citation2004). An organic abnormality should be ruled out before a diagnosis of one of these disorders is given. The clinical picture of these disorders resemble each other, and one of the most important common features is alterations in the HPA axis. Hypocortisolemia is a common endocrine abnormality, at least in a subgroup of patients with stress-related disorders. The possible underlying mechanisms of hypocortisolism can be summarized as follows: (1) decreased release or biosynthesis of the respective hormone at different levels of the HPA axis (CRH/AVP from the hypothalamus, ACTH from the pituitary, cortisol from the adrenal glands); (2) hypersecretion of one of the hormones with a subsequent down-regulation of the relevant target receptors; (3) increased sensitivity to the negative feedback action of glucocorticoids; (4) decreased availability of free cortisol; and (5) decreased effects of cortisol on the target tissues suggesting a relative cortisol resistance (Heim et al. Citation2000; Raison and Miller Citation2003). It is important to mention that different stress-related disorders and even patient subgroups within a certain disorder are characterized by different hypocortisolim patterns (Heim et al. Citation2000).
Whether HPA axis alterations, particularly hypocortisolemia, play a role in the pathophysiology of stress-related disorders or they are a final result of these disorders is still not clarified. More than 10 years ago, Hellhammer and Wade proposed that hypocortisolemia or HPA axis underactivation might develop after prolonged periods of stress together with a hyperactivity of the HPA axis and excessive glucocorticoid release (Hellhammer and Wade Citation1993). HPA axis underactivation is also associated with an increased sensititivity of the anterior pituitary to the negative feedback effect of circulating glucocorticoids and this abnormality was suggested as a contributing factor to the development of hypocortisolemia (Houshyar et al. Citation2001). Fries et al. suggested that chronic stress will eventually result in a hypoactive HPA axis and that hypocortisolemia is a protective response dampening chronic HPA axis activity (Fries et al. Citation2005). But there are no sufficient prospective data in humans in which the HPA axis has been investigated in healthy subjects who had normal HPA axis initially and developed HPA axis underactivation after having been subjected to chronic stress. Recently in two prospective studies, including patients after EBV infection and after surgery that are at high risk of developing CFS, it has been reported that development of fatigue six months after the event was not associated with any HPA axis changes (Candy et al. Citation2003; Rubin et al. Citation2005). In contrast to these findings, in another prospective study including healthy subjects Gaab et al. (Citation2006) reported that long-lasting stress could lead to dissociation of the cognitive appraisal of acute stress from the cortisol response, which was reduced, perhaps as a result of HPA adaptation (Gaab et al. Citation2006). Therefore, to understand whether HPA axis dysregulation is causal or consequential in stress-related disorders further prospective studies are warranted. In other words, whether it is the patients who initially have normal HPA axis activity, or the patients who have HPA axis underactivity that develop stress-related disorder is not yet clear. In this review, HPA axis alterations in stress-related disorders including CFS and FMS are addressed.
Chronic fatigue syndrome
CFS is a debilitating disease is characterized by severe and persistent fatigue of at least six month's duration, cognitive and psychological problems, sleep disturbances, post-exertion malaise, joint pain, muscle pain, adenopathy and sore throat which are not atributable to known clinical conditions (Fukuda et al. Citation1994). The etiology of CFS is still unknown and heterogenous. Since most of the symptoms and findings seen in CFS are common in adrenocortical insufficiency and steroid withdrawal syndrome, hypothetically HPA axis underactivation may be responsible for the clinical picture of CFS. Actually, CFS is associated with some abnormalities in the HPA axis which have been suggested as an underlying pathophysiological mechanism. The studies in which the HPA axis in CFS has been investigated are shown in .
Table II. Dynamic studies on the HPA axis in patients with CFS.
In a study by Ottenweller et al. (Citation2001), hormonal responses including ACTH secretion with exercise were investigated in 20 patients with CFS and 15 controls. Baseline ACTH and cortisol levels were similar between the patients and sedentary controls. The plasma ACTH response to exercise was lower in CFS patients than in the controls. ACTH level normalized the day after exercise. Thus, exercise might uncover subtle endocrine abnormalities in patients with CFS. Since the abnormal hormonal changes were not persistent, it seems that HPA axis changes are the results rather than causes of CFS. But the cortisol response to exercise was not assessed in that study (Ottenweller et al. Citation2001). The ACTH response to CRH has been reported as blunted in CFS. Demitrack et al. (Citation1991) assessed the HPA axis in detail in CFS patients and reported a decreased drive at the level of hypothalamic CRH which resulted in a mild hypocortisolemia (Demitrack et al. Citation1991).
Cleare et al. (Citation2004) assessed both basal and CRH stimulated DHEA and DHEAS levels before and after low dose oral hydrocortisone treatment (5 or 10 mg) for one month in 16 patients with CFS without depression in a double-blind crossover study (Cleare et al. Citation2004). Basal levels of DHEA, but not DHEAS, were higher in the patient group than in the controls. Levels of both DHEA and DHEAS were lower in the patients after hydrocortisone treatment. DHEA levels correlated with the degree of self reported disability. Basal cortisol levels were found as higher in the patients. Hydrocortisone therapy resulted in an increased DHEA response to CRH, which was most marked in those who were responsive to the therapy.
In another study by Cleare et al. (Citation2001) HPA axis dysfunction in CFS and the effects of low dose hydrocortisone therapy were investigated in 37 medication-free patients with CFS but no comorbid psychiatric disorders and in 28 healthy controls. They measured ACTH and cortisol responses to human CRH, the insulin stress test and d-fenfluramine. They found that ACTH responses to pituitary and hypothalamic challenges were intact in CFS and suggested that hypocortisolism found in CFS might be secondary to reduced adrenal gland output. Low-dose hydrocortisone therapy was associated with reversal of the HPA axis dysfunction in the minority of patients with CFS in whom treatment was beneficial (Cleare et al. Citation2001b).
In a randomized, double-blind, placebo-controlled study in patients with CFS, low dose hydrocortisone treatment (16 mg/m2 of body surface area per day, 20–30 mg every morning at about 8 am and 5 mg every day at 2 pm, for 12 weeks) was associated with improvement in symptoms of CFS. The patients who received hydrocortisone had a greater improvement in mean wellness score, but not in other self-rating scales. Although the dose administered was low, hydrocortisone treatment resulted in adrenal supression (McKenzie et al. Citation1998). We have recently investigated the HPA axis with the low-dose (1 μg) ACTH test and metyrapone test in 20 patients with CFS and 15 healthy subjects. We found that peak cortisol responses to the 1 μg ACTH test were significantly lower in patients with CFS than in healthy subjects. Approximately 40% of the patients with CFS had a lower peak cortisol response to the 1 μg ACTH test than the lowest peak cortisol response measured in healthy subjects. After metyrapone administration, 11-deoxycortisol levels in patients with CFS were significantly lower than the values in healthy subjects. Both tests suggest that the HPA axis is certainly underactivated in CFS. On the other hand, we measured adrenal size including width of the adrenal body and limbs by computed tomography (CT) scanning both in patients and controls. We found that adrenal gland size was similar between the patients and controls (Izgi et al. Citation2005). In contrast, Scott et al. (Citation1999a,Citationb) found 50% reduction in the size of the adrenal glands of CFS patients indicating significant adrenal atrophy.
Gaab et al. (Citation2004) assessed the relationship between patient characteristics and HPA axis functioning using a neuroendocrine challenge test, ITT. ACTH, salivary free cortisol and total plasma cortisol levels after ITT were measured and correlations of patient characteristics with integrated responses were calculated for all endocrine parameters. They found that the patients with CFS had a significantly reduced area under the ACTH response curve (AUC) in the ITT. Plasma cortisol or salivary cortisol responses to ITT were similar between the patients and controls. On the other hand, integrated responses of ACTH after ITT were strongly associated with the duration of CFS. There was a significant negative association between the integrated ACTH response and severity of fatigue symptoms. Significant negative correlations were also found between the integrated ACTH response and scores on the Hamilton scale of depression and anxiety (Gaab et al. Citation2004). AVP secreted from the hypothalamus is able to stimulate ACTH secretion from the anterior pituitary and it acts synergistically with CRH, which is physiologically the most important factor in the synthesis and secretion of ACTH at the pituitary level. Patients with CFS have a reduced ACTH response to AVP infusion and a more rapid cortisol response to the infusion. These results were interpreted as evidence suggesting that CFS patients had a reduced hypothalamic CRH secretion and for this reason impaired hypothalamic CRH secretion may account for HPA axis underactivation of CFS. Moreover, primary adrenocortical insufficiency may be ruled out by this finding as a cause of endocrine abnormalities seen in CFS (Altemus et al. Citation2001). In a recent study, the cortisol response to low-dose (1.25 μg) and high-dose (225 μg) ACTH was assessed in patients with CFS and healthy controls. No significant difference for salivary and plasma cortisol after either low-dose and high-dose ACTH stimulation was detected between the groups (Gaab et al. Citation2003). It has been demonstrated that 1 μg ACTH significantly elevates DHEA levels, with no difference in output between CFS and healthy subjects. The DHEA/cortisol ratio decreased in response to 1 μg ACTH stimulation in healthy subjects but not in the CFS patients. This study suggests that CFS may be characterized by an inappropriate response to stress (Scott et al. Citation2000). UFC and cortisone levels were measured from 0600 to 2100 h in CFS patients to investigate the diurnal rhythm. Urinary cortisol and cortisone levels were found to be reduced throughout the day. UFC and cortisone concentrations showed a significant normal diurnal rhythm which was not different from that of controls (Jerjes et al. Citation2006). Despite contradictory results, most of the studies show that CFS is characterized by hypocortisolemia. But the current data are not sufficient enough to explain the exact role of hypocortisolemia. Whether it plays a role in the development of CFS, or occurs during the natural history of the syndrome or it is the final result of the syndrome is not clarified yet. It seems that HPA dysfunction in CFS is not the main abnormality but once established it may affect the nature of the syndrome (Cleare Citation2004).
Because of the contraversial results of the studies regarding HPA axis dysfunction in CFS, it is difficult and too early to recommend routine use of corticosteroids. There have been three randomized placebo-controlled trials using low dose hydrocortisone treatment (McKenzie et al. Citation1998; Cleare et al. Citation1999) and combination therapy with low dose hydrocortisone and fludrocortisone (Blockmans et al. Citation2003) in CFS. These researches also have concluded that steroids are not yet the treatment of choice in CFS.
Fibromyalgia syndrome (FMS)
FMS is a nonarticular rheumatological syndrome characterized by chronic widespread musculoskeletal aches, pain and stiffness accompanied by fatigue, anxiety, headache and poor sleep (Wolfe et al. Citation1990). The pathophysiology of FMS is not well understood but it has been suggested that neuroendocrine abnormalities may play a role in its development. The HPA axis has been investigated in detail by various investigators. In one of these studies, ACTH and epinephrine responses to hypoglycaemia were assessed by using a graded hypoglycaemic–hyperinsulinemic clamp technique and significant (30%) decreases in ACTH and epinephrine responses were found in premenopausal women with FMS (Adler et al. Citation1999). A delayed ACTH response was also reported after IL-6 administration (Torpy et al. Citation2000). Exercise was used as a stimulator in the assessment of the HPA axis and the HPA axis response was found to be normal or reduced in FMS (van Denderen et al. Citation1992; Gursel et al. Citation2001). We have investigated the HPA axis with the ITT, the standard dose (250 μg) ACTH test and low dose (1 μg) ACTH test in 16 patients with FMS and 16 healthy subjects. Peak cortisol responses to the low dose ACTH test, standard dose ACTH test and ITT were lower in patients with FMS than in the control group. Six (37.5%) of the 16 patients with FMS had peak cortisol responses to the low dose ACTH test lower than the lowest peak cortisol response of 555 nmol/l obtained in controls after the low dose test, or lower than 550 nmol/l which is widely accepted as a normal cortisol response to the standard dose ACTH test or ITT. We suggested that the HPA axis was underactivated in FMS and some patients with FMS may have subnormal adrenocortical function. This study showed that the low dose test is more sensitive than standard dose ACTH test or ITT in patients with FMS in detecting subtle adrenocortical insufficiency, which may progress to frank adrenocortical insufficiency unless glucocorticoid replacement is given. But there are not enough data regarding the effectiveness of glucocorticoid treatment in patients with FMS characterized by subnormal HPA axis activity (Kirnap et al. Citation2001). That study clearly shows that pharmacological tests may underestimate the subtle HPA axis underactivation and more physiological tests should be performed. We have recently investigated the HPA axis in 22 patients with FMS and in 15 age-, sex- and body mass index-matched controls with the 1 μg ACTH stimulation test and the metyrapone test. Peak cortisol level was significantly lower in the patients with FMS than the peak cortisol level in the control subjects. About 10 (45%) of the patients had peak cortisol responses to the 1 μg ACTH test lower than the lowest peak cortisol detected in healthy controls. After the metyrapone test, the 11-deoxycortisol level in FMS patients was significantly lower than in healthy controls. About 95% of the patients with FMS had a lower 11-deoxycortisol level after metyrapone than the lowest 11-deoxycortisol level after metyrapone detected in healthy controls (Calis et al. Citation2004).
The measurement of adrenal size by CT in the disorders affecting the HPA axis may be useful in the differential diagnosis of hypocortisolemia. Adrenal gland size is reduced in patients with secondary adrenal failure due to long-term ACTH or CRH deficiency. The adrenal gland is enlarged in patients with an overactivated HPA axis due to active infection when compared to healthy subjects and adrenal enlargement is reduced after appropriate therapy (Kelestimur et al. Citation1994; Gulmez et al. Citation1996; Yildiz et al. Citation2005). We have found that adrenal size in patients with FMS was similar to that found in healthy subjects (Calis et al. Citation2004). The finding of normal adrenal size suggests that there is no severe CRH-ACTH deficiency to cause adrenal shrinkage detectable by adrenal CT scan. Cortisol level may be related to the presence of depression. In fact, the patients with FMS who have high Beck depression inventory (BDI) scores had significantly lower cortisol levels than controls and low cortisol levels have been suggested as a biological factor that contributes to depressive symptoms in FMS (Gür et al. Citation2004). The HPA axis has been investigated by basal cortisol measurement only and no dynamic test was done in that study. As has been mentioned above, differing results for HPA axis activity were reported in patients with FMS. But most of the studies have shown reduced activity of the HPA axis ().
Table III. Dynamic studies on the HPA axis in patients with FMS.
Similar to the information available for CFS, current data are not sufficient enough to recommend corticosteroid replacement as a general therapy in FMS. Geenen et al. Citation2002 mentioned that hormonal supplementation is an option in patients with clinically overt hormone deficiency. However, further placebo-controlled studies are warranted.
Conclusions
Although most of the studies have shown that the HPA axis is underactive, contradictory results have been found in different studies regarding the HPA axis in patients with CFS and FMS. These contradictory results may be due to the patients selected for the study, the methods used for the investigation of the HPA axis, the stage of the syndrome when the tests have been done and the interpretation of the results. There is no structural abnormality in the endocrine organs that comprise the HPA axis; therefore, hypocortisolemia found in the patients with stress-related disorder reflects dysfunction. Pharmacological tests used in the assessment of the HPA axis may be misleading and more physiological tests should be chosen. Despite contradictory results, most of the studies show that stress-related disorders are characterized by HPA dysfunction and hypocortisolemia is the most common neuroendocrine abnormality. But the current data are insufficient to explain the pathophysiology of HPA dysfunction, the location of the defect and the exact role of the hypocortisolemia. Whether this plays a role in the development of the stress-related disorders, or occurs during the natural history of the disorders, or is the final result of the disorder is not yet clarified. The mechanisms leading to hypocortisolemia may be also an adaptive response of the body to chronic stress. Further studies are required to understand the underlying defect in HPA axis alterations seen in stress-related disorders.
References
- Adler GK, Kinsley BT, Hurwitz S, Mossey CJ, Goldenberg DL. Reduced hypothalamic–pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med 1999; 106(5)534–543
- Altemus M, Dale JK, Michelson D, Demitrack MA, Gold PW, Straus SE. Abnormalities in response to vasopressin infusion in chronic fatigue syndrome. Psychoneuroendocrinology 2001; 26(2)175–188
- Arlt W, Allolio B. Adrenal insufficiency. Lancet 2003; 361(9372)1881–1893
- Arvat E, Maccagno B, Di Vito L, Gottero C, Talliano M, Ghigo E. ACTH-releasing effect of glucagon in humans. Interaction with hCRH, AVP and hexarelin. J Endocrinol Invest 1999; 22((10 Suppl) 82)24
- Blockmans D, Persoons P, Van Houdenhove B, Lejeune M, Bobbaers H. Combination therapy with hydrocortisone and fludrocortisone does not improve symptoms in chronic fatigue syndrome: A randomized, placebo-controlled, double-blind, crossover study. Am J Med 2003; 114(9)736–741
- Borm K, Slawik M, Seiler L, Flohr F, Petrick M, Honegger J, Reincke M. Is the plasma ACTH concentration a reliable parameter in the insulin tolerance test?. Eur J Endocrinol 2003; 149(6)535–541
- Borst GC, Michenfelder HJ, O'Brian JT. Discordant cortisol response to exogenous ACTH and insulin-induced hypoglycemia in patients with pituitary disease. N Engl J Med 1982; 306(24)1462–1464
- Calis M, Gokce C, Ates F, Ulker S, Izgi HB, Demir H, Kirnap M, Sofuoglu S, Durak AC, Tutus A, Kelestimur F. Investigation of the hypothalamo–pituitary–adrenal axis (HPA) by 1 microg ACTH test and metyrapone test in patients with primary fibromyalgia syndrome. J Endocrinol Invest 2004; 27(1)42–46
- Candy B, Chalder T, Cleare AJ, Peakman A, Skowera A, Wessely S, Weinman J, Zuckerman M, Hotopf M. Predictors of fatigue following the onset of infectious mononucleosis. Psychol Med 2003; 33(5)847–855
- Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short synacthen test: Implications for the investigation of hypothalamic–pituitary disorders. Clin Endocrinol (Oxf) 1998; 49(3)287–292
- Clayton RN. Short synacthen test versus insulin stress test for assessment of the hypothalamo [correction of hypothalmo]–pituitary–adrenal axis: Controversy revisited. Clin Endocrinol (Oxf) 1996; 44: 147–149
- Cleare AJ. The neuroendocrinology of chronic fatigue syndrome. Endocr Rev 2003; 24(2)236–252
- Cleare AJ. The HPA axis and the genesis of chronic fatigue syndrome. Trends Endocrinol Metab 2004; 15(5)55–59
- Cleare AJ, Heap E, Malhi GS, Wessely S, O'Keane V, Miell J. Low-dose hydrocortisone in chronic fatigue syndrome: A randomised crossover trial. Lancet 1999; 353(9151)455–458
- Cleare AJ, Blair D, Chambers S, Wessely S. Urinary free cortisol in chronic fatigue syndrome. Am J Psychiatry 2001a; 158(4)641–643
- Cleare AJ, Miell J, Heap E, Sookdeo S, Young L, Malhi GS, O'Keane V. Hypothalamo–pituitary–adrenal axis dysfunction in chronic fatigue syndrome, and the effects of low-dose hydrocortisone therapy. J Clin Endocrinol Metab 2001b; 86(8)3545–3554
- Cleare AJ, O'Keane V, Miell JP. Levels of DHEA and DHEAS and responses to CRH stimulation and hydrocortisone treatment in chronic fatigue syndrome. Psychoneuroendocrinology 2004; 29: 724–732
- Courtney CH, McAllister AS, McCance DR, Hadden DR, Leslie H, Sheridan B, Atkinson AB. The insulin hypoglycaemia and overnight metyrapone tests in the assessment of the hypothalamic–pituitary–adrenal axis following pituitary surgery. Clin Endocrinol (Oxf) 2000; 53: 309–312
- Demitrack MA, Dale JK, Straus SE, Laue L, Listwak SJ, Kruesi MJ, Chrousos GP, Gold PW. Evidence for impaired activation of the hypothalamic–pituitary–adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab 1991; 73(6)1224–1234
- Dickstein G. The assessment of the hypothalamo–pituitary–adrenal axis in pituitary disease: Are there short cuts?. J Endocrinol Invest 2003; 26(7 Suppl)25–30
- Dokmetas HS, Colak R, Kelestimur F, Selcuklu A, Unluhizarci K, Bayram F. A comparison between the 1-microg adrenocorticotropin (ACTH) test, the short ACTH (250 microg) test, and the insulin tolerance test in the assessment of hypothalamo–pituitary–adrenal axis immediately after pituitary surgery. J Clin Endocrinol Metab 2000; 85(10)3713–3719
- Evans JM, Hunder GG. Polymyalgia rheumatica and giant cell arteritis. Rheum Dis Clin North Am 2000; 26(3)493–515
- Fiad TM, Kirby JM, Cunningham SK, McKenna TJ. The overnight single-dose metyrapone test is a simple and reliable index of the hypothalamic–pituitary–adrenal axis. Clin Endocrinol (Oxf) 1994; 40(5)603–609
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology 2005; 30(10)1010–1016
- Fukuda K, Strauss SE, Hickie H. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann Intern Med 1994; 121(12)953–959
- Gaab J, Huster D, Peisen R, Engert V, Heitz V, Schad T, Schurmeyer TH, Ehlert U. Hypothalamic–pituitary–adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological, and pharmacological stimulation. Psychosom Med 2002; 64(6)951–962
- Gaab J, Huster D, Peisen R, Engert V, Heitz V, Schad T, Schurmeyer T, Ehlert U. Assessment of cortisol response with low-dose and high-dose ACTH in patients with chronic fatigue syndrome and healthy comparison subjects. Psychosomatics 2003; 44(2)113–119
- Gaab J, Engert V, Heitz V, Schad T, Schurmeyer TH, Ehlert U. Associations between neuroendocrine responses to the insulin tolerance test and patient characteristics in chronic fatigue syndrome. J Psychosom Res 2004; 56(4)419–424
- Gaab J, Sonderegger L, Scherrer S, Ehlert U. Psychoneuroendocrine effects of cognitive-behavioral stress management in a naturalistic setting—a randomized controlled trial. Psychoneuroendocrinology 2006; 31(4)428–438
- Geenen R, Jacobs JW, Bijlsma JW. Evaluation and management of endocrine dysfunction in fibromyalgia. Rheum Dis Clin North Am 2002; 28(2)389–404
- Ghigo E, Bartolotta E, Imperiale E, Bellone J, Cardinale G, Aimaretti G, Valetto MR, Cherubini V, Maccario M, Cocchi D. Glucagon stimulates GH secretion after intramuscular but not intravenous administration. Evidence against the assumption that glucagon per se has a GH-releasing activity. J Endocrinol Invest 1994; 17(11)849–854
- Goodwin PM, Capildeo R, Harrop JS, Marks V, Rose FC. The metabolic and hormonal response to glucagon. Part 1. Normal subjects. J Neurol Sci 1976; 27(3)373–380
- Griep EN, Boersma JW, de Kloet ER. Altered reactivity of the hypothalamic–pituitary–adrenal axis in the primary fibromyalgia syndrome. J Rheumatol 1993; 20: 469–474
- Griep EN, Boersma JW, Lentjes EG, Prins AP, van der Korst JK, de Kloet ER. Function of the hypothalamic–pituitary–adrenal axis in patients with fibromyalgia and low back pain. J Rheumatol 1998; 25(7)1374–1381
- Grinspoon SK, Biller BM. Clinical review 62: Laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab 1994; 79(4)923–931
- Gulmez I, Kelestimur F, Durak AC, Ozesmi M. Changes in the size of adrenal glands in acute pulmonary tuberculosis with therapy. Endocr J 1996; 43(5)573–576
- Gür A, Cevik R, Nas K, Coplan L, Sarac S. Cortisol and hypothalamic–pituitary–gonadal axis hormones in follicular-phase women with fibromyalgia and chronic fatigue syndrome and effect of depressive symptoms on these hormones. Arthritis Res Ther 2004; 6(3)232–238
- Gursel Y, Ergin S, Ulus Y, Erdogan MF, Yalcin P, Evcik D. Hormonal responses to exercise stress test in patients with fibromyalgia syndrome. Clin Rheumatol 2001; 20(6)401–405
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 2000; 25(1)1–35
- Hellhammer DH, Wade S. Endocrine correlates of stress vulnerability. Psychother Psychosom 1993; 60(1)8–17
- Houshyar H, Galigniana MD, Pratt WB, Woods JH. Differential responsivity of the hypothalamic–pituitary–adrenal axis to glucocorticoid negative-feedback and corticotropin releasing hormone in rats undergoing morphine withdrawal: Possible mechanisms involved in facilitated and attenuated stress responses. J Neuroendocrinol 2001; 13(10)875–886
- Hudson M, Cleare AJ. The 1 microg short synacthen test in chronic fatigue syndrome. Clin Endocrinol (Oxf) 1999; 51(5)625–630
- Izgi HB, Gökce C, Calis M, Turan T, Kirnap M, Sofuoglu S, Durak AC, Tutus A, Kelestimur F. Investigation of the hypothalamo–pituitary adrenal axis by low dose (1μg) adrenocorticotrophic hormone tests and metyrapone tests in patients with chronic fatigue syndrome. Endocrinologist 2005; 15: 89–92
- Jerjes WK, Peters TJ, Taylor NF, Wood PJ, Wessely S, Cleare AJ. Diurnal excretion of urinary cortisol, cortisone, and cortisol metabolites in chronic fatigue syndrome. J Psychosom Res 2006; 60(2)145–153
- Kavelaars A, Kuis W, Knook L, Sinnema G, Heijnen CJ. Disturbed neuroendocrine–immune interactions in chronic fatigue syndrome. J Clin Endocrinol Metab 2000; 85(2)692–696
- Kelestimur F, Unlu Y, Ozesmi M, Tolu I. A hormonal and radiological evaluation of adrenal gland in patients with acute or chronic pulmonary tuberculosis. Clin Endocrinol (Oxf) 1994; 41(1)53–56
- Kelestimur F, Akgun A, Gunay O. A comparison between short synacthen test and depot synacthen test in the evaluation of cortisol reserve of adrenal gland in normal subjects. J Endocrinol Invest 1995; 18(11)823–826
- Kirnap M, Colak R, Eser C, Ozsoy O, Tutus A, Kelestimur F. A comparison between low-dose (1 microg), standard-dose (250 microg) ACTH stimulation tests and insulin tolerance test in the evaluation of hypothalamo–pituitary–adrenal axis in primary fibromyalgia syndrome. Clin Endocrinol (Oxf) 2001; 55(4)455–459
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993; 28: 76–81
- Landon J, James VH, Wharton MJ, Friedman M. Threshold adrenocortical sensitivity in man and its possible application to corticotrophin bioassay. Lancet 1967; 2(7518)697–700
- Lebrethon MC, Naville D, Begeot M, Saez JM. Regulation of corticotropin receptor number and messenger RNA in cultured human adrenocortical cells by corticotropin and angiotensin II. J Clin Invest 1994; 93(4)1828–1833
- Leong KS, Walker AB, Martin I, Wile D, Wilding J, MacFarlane IA. An audit of 500 subcutaneous glucagon stimulation tests to assess growth hormone and ACTH secretion in patients with hypothalamic–pituitary disease. Clin Endocrinol (Oxf) 2001; 54(4)463–468
- McKenzie R, O'Fallon A, Dale J, Demitrack M, Sharma G, Deloria M, Garcia-Borreguero D, Blackwelder W, Straus SE. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: A randomized controlled trial. JAMA 1998; 280(12)1061–1066
- Melmed S, Kleinberg D. Anterior pituitary. Williams textbook of endocrinology10th ed. Saunders, Philadephia 2003; 177–281
- Michelson D, Stone L, Galliven E, Magiakou MA, Chrousos GP, Sternberg EM, Gold PW. Multiple sclerosis is associated with alterations in hypothalamic–pituitary–adrenal axis function. J Clin Endocrinol Metab 1994; 79(3)848–853
- Morris DG, Grossman AB, Nieman LK. Cushing's syndrome. Endocrinology5th ed. Elsevier, Philadelphia 2006; 438
- Murphy BE. Urinary free cortisol determinations: What they measure. Endocrinologist 2002; 12: 143–150
- Naitoh Y, Fukata J, Tominaga T, Nakai Y, Tamai S, Mori K, Imura H. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun 1988; 155(3)1459–1463
- Orth DN, Kovacs WJ. Metyrapone test: The pituitary response to hypocortisolemia. Williams Textbook of Endocrinology, Saunders, Philadelphia 1998; 620–621
- Ottenweller JE, Sisto SA, McCarty RC, Natelson BH. Hormonal responses to exercise in chronic fatigue syndrome. Neuropsychobiology 2001; 43(1)34–41
- Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, Zappulli D, Cavagnini F. Salivary cortisol measurement in normal-weight, obese and anorexic women: Comparison with plasma cortisol. Eur J Endocrinol 2001; 145: 165–171
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003; 160: 1554–1565
- Rasmuson S, Olsson T, Hagg E. A low dose ACTH test to assess the function of the hypothalamic–pituitary–adrenal axis. Clin Endocrinol (Oxf) 1996; 44(2)151–156
- Riedel W, Layka H, Neeck G. Secretory pattern of GH, TSH, thyroid hormones, ACTH, cortisol, FSH, and LH in patients with fibromyalgia syndrome following systemic injection of the relevant hypothalamic-releasing hormones. Z Rheumatol 1998; 57(Suppl 2)81–87
- Riedel W, Schlapp U, Leck S, Netter P, Neeck G. Blunted ACTH and cortisol responses to systemic injection of corticotropin-releasing hormone (CRH) in fibromyalgia: Role of somatostatin and CRH-binding protein. Ann NY Acad Sci 2002; 966: 483–490
- Rubin GJ, Hotopf M, Papadopoulos A, Cleare A. Salivary cortisol as a predictor of postoperative fatigue. Psychosom Med 2005; 67(3)441–447
- Salata RA, Jarrett DB, Verbalis JG, Robinson AG. Vasopressin stimulation of adrenocorticotropin hormone (ACTH) in humans. In vivo bioassay of corticotropin-releasing factor (CRF) which provides evidence for CRF mediation of the diurnal rhythm of ACTH. J Clin Invest 1988; 81(3)766–774
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 1987; 238(4826)522–524
- Scott LV, Medbak S, Dinan TG. Blunted adrenocorticotropin and cortisol responses to corticotropin-releasing hormone stimulation in chronic fatigue syndrome. Acta Psychiatr Scand 1998a; 97(6)450–457
- Scott LV, Medbak S, Dinan TG. The low dose ACTH test in chronic fatigue syndrome and in health. Clin Endocrinol (Oxf) 1998b; 48(6)733–737
- Scott LV, Medbak S, Dinan TG. Desmopressin augments pituitary-adrenal responsivity to corticotropin-releasing hormone in subjects with chronic fatigue syndrome and in healthy volunteers. Biol Psychiatry 1999a; 45(11)1447–1454
- Scott LV, Teh J, Reznek R, Martin A, Sohaib A, Dinan TG. Small adrenal glands in chronic fatigue syndrome: A preliminary computer tomography study. Psychoneuroendocrinology 1999b; 24(7)759–768
- Scott LV, Svec F, Dinan T. A preliminary study of dehydroepiandrosterone response to low-dose ACTH in chronic fatigue syndrome and in healthy subjects. Psychiatry Res 2000; 97(1)21–28
- Shankar RR, Jakacki RI, Haider A, Lee MW, Pescovitz OH. Testing the hypothalamic–pituitary–adrenal axis in survivors of childhood brain and skull-based tumors. J Clin Endocrinol Metab 1997; 82(6)1995–1998
- Stewart PM. The adrenal cortex. Williams textbook of endocrinology10th ed. Saunders, Philadelphia 2003; 491–551
- Stewart PM, Corrie J, Seckl JR, Edwards CR, Padfield PL. A rational approach for assessing the hypothalamo–pituitary–adrenal axis. Lancet 1988; 1(8596)1208–1210
- Tordjman K, Jaffe A, Grazas N, Apter C, Stern N. The role of the low dose (1 microgram) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J Clin Endocrinol Metab 1995; 80(4)1301–1305
- Torpy DJ, Papanicolaou DA, Lotsikas AJ, Wilder RL, Chrousos GP, Pillemer SR. Responses of the sympathetic nervous system and the hypothalamic–pituitary–adrenal axis to interleukin-6: A pilot study in fibromyalgia. Arthritis Rheum 2000; 43(4)872–880
- van Denderen JC, Boersma JW, Zeinstra P, Hollander AP, van Neerbos BR. Physiological effects of exhaustive physical exercise in primary fibromyalgia syndrome (PFS): Is PFS a disorder of neuroendocrine reactivity?. Scand J Rheumatol 1992; 21(1)35–37
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum 1990; 33(2)160–172, Report of the Multicenter Criteria Committee
- Yildiz O, Gokce C, Alp E, Durak AC, Aygen B, Kelestimur F, Doganay M. Investigation of the hypothalamo–pituitary–adrenal axis and changes in the size of adrenal glands in acute brucellosis. Endocr J 2005; 52(2)183–188